HLA-DRB1*14:54 Is Associated with Pulmonary Alveolar Proteinosis: A Retrospective Real-World Audit
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Diagnostic Criteria
2.3. DNA Extraction and PCR Amplification
2.4. DNA-Sequencing and HLA-DRB1 Genotyping
2.5. Statistics
3. Results
3.1. Study Populations
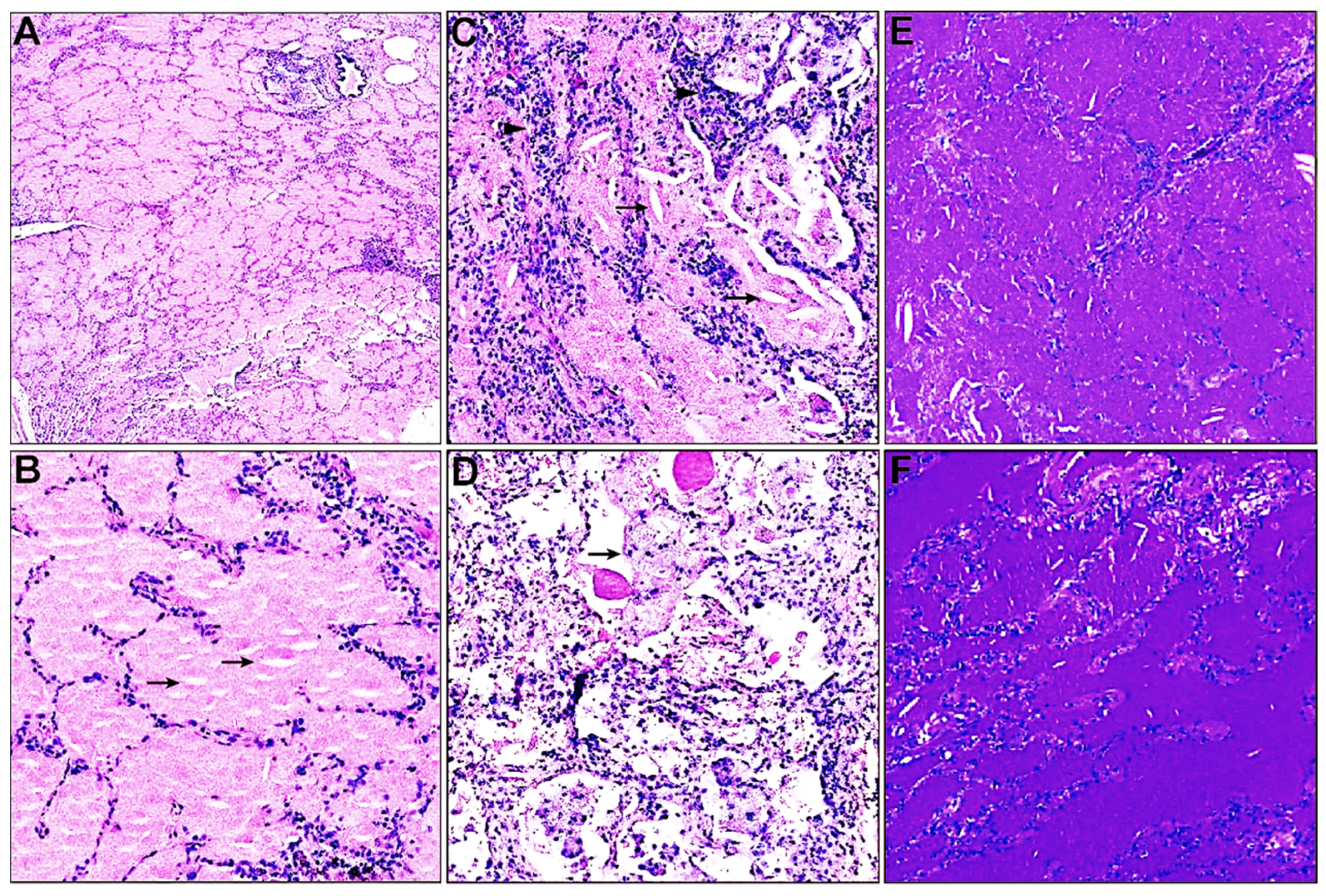
3.2. Histopathological Characteristics
3.3. HLA-DRB1 Allelic Polymorphism
3.4. Treatment Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
References
- Inoue, Y.; Trapnell, B.C.; Tazawa, R.; Arai, T.; Takada, T.; Hizawa, N.; Kasahara, Y.; Tatsumi, K.; Hojo, M.; Ichiwata, T.; et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am. J. Respir. Crit. Care Med. 2008, 177, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, B.C.; Nakata, K.; Bonella, F.; Campo, I.; Griese, M.; Hamilton, J.; Wang, T.; Morgan, C.; Cottin, V.; McCarthy, C. Pulmonary alveolar proteinosis. Nat. Rev. Dis. Primers 2019, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.; Carey, B.; Trapnell, B.C. Blood Testing for Differential Diagnosis of Pulmonary Alveolar Proteinosis Syndrome. Chest 2019, 155, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Takaki, M.; Tanaka, T.; Komohara, Y.; Tsuchihashi, Y.; Mori, D.; Hayashi, K.; Fukuoka, J.; Yamasaki, N.; Nagayasu, T.; Ariyoshi, K.; et al. Recurrence of pulmonary alveolar proteinosis after bilateral lung transplantation in a patient with a nonsense mutation in CSF2RB. Respir. Med. Case Rep. 2016, 19, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Nakagome, K.; Ohta, H.; Akasaka, K.; Uchida, Y.; Hashimoto, A.; Shiono, A.; Takada, T.; Nagata, M.; Tohyama, J.; et al. Elderly-onset hereditary pulmonary alveolar proteinosis and its cytokine profile. BMC Pulm Med. 2017, 17, 40. [Google Scholar] [CrossRef]
- Madden, K.; Chabot-Richards, D. HLA testing in the molecular diagnostic laboratory. Virchows Arch. 2019, 474, 139–147. [Google Scholar] [CrossRef]
- Anderson, K.; Carey, B.; Martin, A.; Roark, C.; Chalk, C.; Nowell-Bostic, M.; Freed, B.; Aubrey, M.; Trapnell, B.; Fontenot, A. Pulmonary alveolar proteinosis: An autoimmune disease lacking an HLA association. PLoS ONE 2019, 14, e0213179. [Google Scholar] [CrossRef]
- Sakaue, S.; Yamaguchi, E.; Inoue, Y.; Takahashi, M.; Hirata, J.; Suzuki, K.; Ito, S.; Arai, T.; Hirose, M.; Tanino, Y.; et al. Genetic determinants of risk in autoimmune pulmonary alveolar proteinosis. Nat. Commun. 2021, 12, 1032. [Google Scholar] [CrossRef]
- Lessard, C.J.; Sajuthi, S.; Zhao, J.; Kim, K.; Ice, J.A.; Li, H.; Ainsworth, H.; Rasmussen, A.; Kelly, J.A.; Marion, M.; et al. Identification of a Systemic Lupus Erythematosus Risk Locus Spanning ATG16L2, FCHSD2, and P2RY2 in Koreans. Arthritis Rheumatol. 2016, 68, 1197–1209. [Google Scholar] [CrossRef]
- Yasunami, M.; Nakamura, H.; Tokunaga, K.; Kawashima, M.; Nishida, N.; Hitomi, Y.; Nakamura, M. Principal contribution of HLA-DQ alleles, DQB1*06:04 and DQB1*03:01, to disease resistance against primary biliary cholangitis in a Japanese population. Sci. Rep. 2017, 7, 11093. [Google Scholar] [CrossRef]
- Chen, P.L.; Shih, S.R.; Wang, P.W.; Lin, Y.C.; Chu, C.C.; Lin, J.H.; Chen, S.C.; Chang, C.C.; Huang, T.S.; Tsai, K.S.; et al. Genetic determinants of antithyroid drug-induced agranulocytosis by human leukocyte antigen genotyping and genome-wide association study. Nat. Commun. 2015, 6, 7633. [Google Scholar] [CrossRef] [PubMed]
- Reveille, J.D. An update on the contribution of the MHC to AS susceptibility. Clin. Rheumatol. 2014, 33, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Holbert, J.M.; Costello, P.; Li, W.; Hoffman, R.M.; Rogers, R.M. CT features of pulmonary alveolar proteinosis. AJR Am. J. Roentgenol. 2001, 176, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Trapnell, B.C.; Tazawa, R.; Inoue, Y.; Akira, M.; Kogure, Y.; Tomii, K.; Takada, T.; Hojo, M.; Ichiwata, T.; et al. Comparative study of high-resolution CT findings between autoimmune and secondary pulmonary alveolar proteinosis. Chest 2009, 136, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Rivero, E.R.; Neves, A.C.; Silva-Valenzuela, M.G.; Sousa, S.O.; Nunes, F.D. Simple salting-out method for DNA extraction from formalin-fixed, paraffin-embedded tissues. Pathol. Res. Pract. 2006, 202, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Sayer, D.; Whidborne, R.; Brestovac, B.; Trimboli, F.; Witt, C.; Christiansen, F. HLA-DRB1 DNA sequencing based typing: An approach suitable for high throughput typing including unrelated bone marrow registry donors. Tissue Antigens 2001, 57, 46–54. [Google Scholar] [CrossRef]
- Jouneau, S.; Ménard, C.; Lederlin, M. Pulmonary alveolar proteinosis. Respirology 2020, 25, 816–826. [Google Scholar] [CrossRef]
- Salvator, H.; Tcherakian, C.; Maillard, N.; Milin, S.; Bergeron, A.; Bondeelle, L.; Meignin, V.; Nguyen, S.; Souchet, L.; Guenounou, S.; et al. Pulmonary Alveolar Proteinosis After Allogeneic Hematopoietic Stem-Cell Transplantation in Adults: A French Société Francophone de Greffe de Moelle et Thérapie Cellulaire Survey. Chest 2021, 160, 1783–1788. [Google Scholar] [CrossRef]
- Bertolizio, G.; Engelhardt, T.; Veyckemans, F. Congenital interstitial lung diseases: What the anesthesiologist needs to know. Paediatr. Anaesth. 2022, 32, 138–147. [Google Scholar] [CrossRef]
- Iftikhar, H.; Nair, G.B.; Kumar, A. Update on Diagnosis and Treatment of Adult Pulmonary Alveolar Proteinosis. Ther. Clin. Risk Manag. 2021, 17, 701–710. [Google Scholar] [CrossRef]
- Kumar, A.; Abdelmalak, B.; Inoue, Y.; Culver, D.A. Pulmonary alveolar proteinosis in adults: Pathophysiology and clinical approach. Lancet Respir. Med. 2018, 6, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.L.; Chen, M.J.; Lee, S.K.; Lin, C.C.; Tsai, M.J.; Chiu, H.M.; Jiang, S.; Chao, Y.C.; Chen, S.P.; Lin, S.; et al. New allele name of some HLA-DRB1*1401: HLA-DRB1*1454. Int. J. Immunogenet. 2009, 36, 119–120. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, J.; Mao, W.; Zhang, D.; Liu, M.; Shan, X.; Zhang, B.; Zhu, C.; Shen, J.; Deng, Z.; et al. HLA common and well-documented alleles in China. HLA 2018, 92, 199–205. [Google Scholar] [CrossRef]
- Luo, H.; Chen, M.; Yang, R.; Xu, P.C.; Zhao, M.H. The association of HLA-DRB1 alleles with antineutrophil cytoplasmic antibody-associated systemic vasculitis in Chinese patients. Hum. Immunol. 2011, 72, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Harman, K.; Mortimer, N.J.; Binda, V.; Black, M.M.; Kondeatis, E.; Vaughan, R.; Groves, R.W. Pemphigus vulgaris in White Europeans is linked with HLA Class II allele HLA DRB1*1454 but not DRB1*1401. J. Investig. Dermatol. 2010, 130, 311–314. [Google Scholar] [CrossRef]
- Khan, A.; Agarwal, R.; Aggarwal, A.N. Effectiveness of granulocyte-macrophage colony-stimulating factor therapy in autoimmune pulmonary alveolar proteinosis: A meta-analysis of observational studies. Chest 2012, 141, 1273–1283. [Google Scholar] [CrossRef]
- Tazawa, R.; Inoue, Y.; Arai, T.; Takada, T.; Kasahara, Y.; Hojo, M.; Ohkouchi, S.; Tsuchihashi, Y.; Yokoba, M.; Eda, R.; et al. Duration of benefit in patients with autoimmune pulmonary alveolar proteinosis after inhaled granulocyte-macrophage colony-stimulating factor therapy. Chest 2014, 145, 729–737. [Google Scholar] [CrossRef]
- Bonella, F.; Bauer, P.C.; Griese, M.; Ohshimo, S.; Guzman, J.; Costabel, U. Pulmonary alveolar proteinosis: New insights from a single-center cohort of 70 patients. Respir. Med. 2011, 105, 1908–1916. [Google Scholar] [CrossRef]
- Cummings, K.J.; Donat, W.E.; Ettensohn, D.B.; Roggli, V.L.; Ingram, P.; Kreiss, K. Pulmonary alveolar proteinosis in workers at an indium processing facility. Am. J. Respir. Crit. Care Med. 2010, 181, 458–464. [Google Scholar] [CrossRef]
- Xiao, Y.L.; Xu, K.F.; Li, Y.; Li, Y.; Li, H.; Shi, B.; Zhou, K.F.; Zhou, Z.Y.; Cai, H.R. Occupational inhalational exposure and serum GM-CSF autoantibody in pulmonary alveolar proteinosis. Occup. Environ. Med. 2015, 72, 504–512. [Google Scholar] [CrossRef]
- Ronsmans, S.; Nemery, B. The presence of autoimmune antibodies in pulmonary alveolar proteinosis does not necessarily imply idiopathic disease. Lancet Respir. Med. 2018, 6, e48. [Google Scholar] [CrossRef]
| Variable | aPAP (N = 46) | sPAP (N = 14) | p Value |
|---|---|---|---|
| Demographics | |||
| Age at diagnosis, yr a | 47.26 ± 10.910 | 47.14 ± 6.916 | 0.969 |
| Sex ratio | 1.7:1.0 | 2.5:1.0 | |
| male, no. (%) | 29 (63.0) | 10 (71.4) | 0.7980 |
| female, no. (%) | 17 (37.0) | 4 (25.6) | |
| Clinical symptoms, no. (%) | |||
| Cough and/or production of white frothy sputum | 29 (63.0) | 8 (57.1) | 0.690 |
| Dyspnea | 21 (45.7) | 10 (71.4) | 0.091 |
| Hypoxemia | 11 (23.9) | 7 (50.0) | 0.062 |
| Chest tightness or chest pain | 2 (4.3) | 6 (42.9) | 0.001 |
| Respiratory failure | 1 (2.2) | 2 (14.3) | 0.133 |
| FEV1/FVC b, no. (%) | |||
| ≥70% | 30 (65.2) | 6 (42.9) | >0.99 |
| <70% | 0 (0.0) | 0 (0.0) | |
| NA | 16 (34.8) | 8 (57.1) | / |
| PaO2 b, no. (%) | |||
| ≥70 mmHg | 15 (32.6) | 10 (71.4) | 0.1578 |
| <70 mmHg | 11 (23.9) | 2 (14.3) | |
| NA | 20 (43.5) | 2 (14.3) | / |
| SpO2 b, no. (%) | |||
| ≥94% | 17 (37.0) | 12 (85.7) | 0.502 |
| <94% | 2 (4.3) | 0 (0.0) | |
| NA | 27 (58.7) | 2 (14.3) | / |
| Disease severity score, no. (%) | |||
| 1 | 4 (8.7) | 0 (0.0) | 0.393 |
| 2 | 11 (23.9) | 10 (71.4) | |
| 3 | 9 (19.6) | 2 (14.3) | |
| 4 | 2 (4.3) | 0 (0.0) | |
| NA | 20 (43.5) | 2 (14.3) | / |
| Critical or serious illness during hospitalization c, no. (%) | |||
| Yes | 3 (6.5) | 6 (42.9) | 0.007 |
| No | 36 (78.2) | 8 (57.1) | |
| NA | 7 (15.2) | 0 (0.0) | / |
| HLA-DRB1 Alleles | Total (n = 56), No. (%) | aPAP (n = 36), No. (%) | sPAP (n = 20), No. (%) | p Value |
|---|---|---|---|---|
| 08:03 | 23 (41.1) | 9 (25.0) | 3 (15.0) | 0.593 |
| 14:54 | 7 (12.5) | 7 (19.4) | 0 (0.0) | 0.091 |
| 15:01 | 7 (12.5) | 2 (5.5) | 5 (25.0) | 0.091 |
| 16:02 | 5 (8.9) | 3 (8.3) | 2 (10.0) | 0.780 |
| 09:01 | 5 (8.9) | 2 (5.5) | 3 (15.0) | 0.485 |
| 13:12 | 3 (5.3) | 3 (8.3) | 0 (0.0) | 0.479 |
| 10:01 | 2 (3.6) | 1 (2.7) | 1 (5.0) | >0.999 |
| 04:05 | 2 (3.6) | 1 (2.7) | 1 (5.0) | >0.999 |
| 03:01 | 2 (3.6) | 1 (2.7) | 1 (5.0) | >0.999 |
| 11:01 | 2 (3.6) | 1 (2.7) | 1 (5.0) | >0.999 |
| 12:02 | 2 (3.6) | 1 (2.7) | 1 (5.0) | >0.999 |
| 01:01 | 1 (1.8) | 1 (2.7) | 0 (0.0) | >0.999 |
| 04:03 | 1 (1.8) | 1 (2.7) | 0 (0.0) | >0.999 |
| 07:01 | 1 (1.8) | 1 (2.7) | 0 (0.0) | >0.999 |
| 11:06 | 1 (1.8) | 1 (2.7) | 0 (0.0) | >0.999 |
| 12:01 | 1 (1.8) | 0 | 1 (5.0) | 0.3571 |
| 13:01 | 1 (1.8) | 0 | 1 (5.0) | 0.3571 |
| 14:05 | 1 (1.8) | 1 (2.7) | 0 (0.0) | >0.999 |
| HLA-DRB1 Alleles | Total (N = 28), No. (%) | aPAP (N = 18), No. (%) | sPAP (N = 10), No. (%) | p Value |
|---|---|---|---|---|
| 08:03 | 12 (42.9) | 9 (50.0) | 3 (30.0) | 0.434 |
| 14:54 | 7 (25.0) | 7 (38.9) | 0 (0.0) | 0.030 |
| 15:01 | 7 (25.0) | 2 (11.1) | 5 (50.0) | 0.067 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Liu, Q.; Wang, W.; Jiang, L. HLA-DRB1*14:54 Is Associated with Pulmonary Alveolar Proteinosis: A Retrospective Real-World Audit. Biomedicines 2023, 11, 2909. https://doi.org/10.3390/biomedicines11112909
Li M, Liu Q, Wang W, Jiang L. HLA-DRB1*14:54 Is Associated with Pulmonary Alveolar Proteinosis: A Retrospective Real-World Audit. Biomedicines. 2023; 11(11):2909. https://doi.org/10.3390/biomedicines11112909
Chicago/Turabian StyleLi, Mengqian, Qinglin Liu, Weiwen Wang, and Lili Jiang. 2023. "HLA-DRB1*14:54 Is Associated with Pulmonary Alveolar Proteinosis: A Retrospective Real-World Audit" Biomedicines 11, no. 11: 2909. https://doi.org/10.3390/biomedicines11112909
APA StyleLi, M., Liu, Q., Wang, W., & Jiang, L. (2023). HLA-DRB1*14:54 Is Associated with Pulmonary Alveolar Proteinosis: A Retrospective Real-World Audit. Biomedicines, 11(11), 2909. https://doi.org/10.3390/biomedicines11112909







