Influence of Different Types of β-Blockers on Mortality in Patients on Hemodialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data and Study Participants
2.2. Variables
2.3. Statistical Analyses
3. Results
3.1. Clinical Characteristics
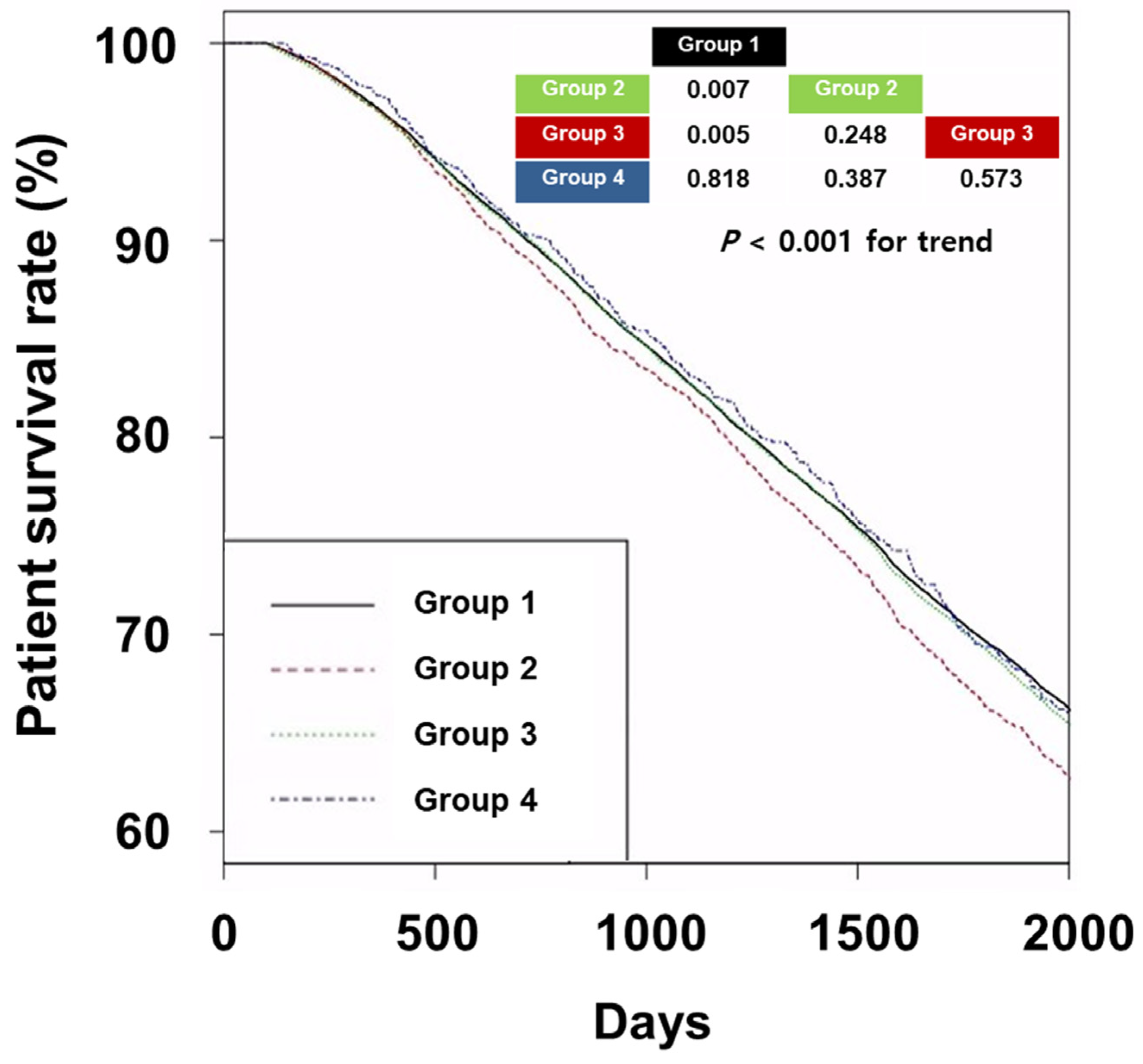
3.2. Survival Analyses
3.3. CVE Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ESRD Registry Committee: Korean Society of Nephrology. Current Renal Replacement Therapy in Korea. 2022. Available online: https://ksn.or.kr/bbs/index.php?code=report (accessed on 30 August 2023).
- US Renal Data System. USRDS 2020 Annual Data Report: Atlas of Chronic Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA. 2020. Available online: https://adr.usrds.org/2020 (accessed on 30 August 2023).
- Choi, H.; Kim, M.; Kim, H.; Pyo Lee, J.; Lee, J.; Tak Park, J.; Hoon Kim, K.; Sik Ahn, H.; Jae Hann, H.; Ryu, D.R. Excess mortality among patients on dialysis: Comparison with the general population in Korea. Kidney Res. Clin. Pract. 2014, 33, 89–94. [Google Scholar] [CrossRef] [PubMed]
- K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am. J. Kidney Dis. 2005, 45, S1–S153. [Google Scholar]
- Cheung, A.K.; Chang, T.I.; Cushman, W.C.; Furth, S.L.; Hou, F.F.; Ix, J.H.; Knoll, G.A.; Muntner, P.; Pecoits-Filho, R.; Sarnak, M.J.; et al. Executive summary of the KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021, 99, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Aoun, M.; Tabbah, R. Beta-blockers use from the general to the hemodialysis population. Nephrol. Ther. 2019, 15, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Lin, Y.T.; Liu, J.S.; Tsai, Y.C.; Kuo, M.C.; Chiu, Y.W.; Hwang, S.J.; Carrero, J.J. Comparative effectiveness of bisoprolol and carvedilol among patients receiving maintenance hemodialysis. Clin. Kidney J. 2021, 14, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Assimon, M.M.; Brookhart, M.A.; Fine, J.P.; Heiss, G.; Layton, J.B.; Flythe, J.E. A Comparative Study of Carvedilol Versus Metoprolol Initiation and 1-Year Mortality Among Individuals Receiving Maintenance Hemodialysis. Am. J. Kidney Dis. 2018, 72, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Shireman, T.I.; Mahnken, J.D.; Phadnis, M.A.; Ellerbeck, E.F. Effectiveness comparison of cardio-selective to non-selective β-blockers and their association with mortality and morbidity in end-stage renal disease: A retrospective cohort study. BMC Cardiovasc. Disord. 2016, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Wang, C.C.; Chen, T.H.; Hong, C.Y.; Sue, Y.M. Prognostic Benefits of Carvedilol, Bisoprolol, and Metoprolol Controlled Release/Extended Release in Hemodialysis Patients with Heart Failure: A 10-Year Cohort. J. Am. Heart Assoc. 2016, 5, e002584. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Lin, Y.T.; Kuo, M.C.; Liu, J.S.; Tsai, Y.C.; Chiu, Y.W.; Carrero, J.J. β-blocker dialyzability and the risk of mortality and cardiovascular events in patients undergoing hemodialysis. Nephrol. Dial. Transplant. 2020, 35, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.A.; Dixon, S.N.; Fleet, J.L.; Roberts, M.A.; Hackam, D.G.; Oliver, M.J.; Suri, R.S.; Quinn, R.R.; Ozair, S.; Beyea, M.M.; et al. β-Blocker dialyzability and mortality in older patients receiving hemodialysis. J. Am. Soc. Nephrol. 2015, 26, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.H.; Tu, K.C.; Hung, K.C.; Chuang, M.H.; Chen, J.Y. Impact of type of dialyzable beta-blockers on subsequent risk of mortality in patients receiving dialysis: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0279680. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Huang, J.; Xiao, J.; Ke, G.; Fu, P. Cardio-selective versus non-selective β-blockers for cardiovascular events and mortality in long-term dialysis patients: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0279171. [Google Scholar] [CrossRef] [PubMed]
- Tella, A.; Vang, W.; Ikeri, E.; Taylor, O.; Zhang, A.; Mazanec, M.; Raju, S.; Ishani, A. β-Blocker Use and Cardiovascular Outcomes in Hemodialysis: A Systematic Review. Kidney Med. 2022, 4, 100460. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Wright, H.M. Nebivolol: A highly selective beta1-adrenergic receptor blocker that causes vasodilation by increasing nitric oxide. Cardiovasc. Ther. 2008, 26, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.V.; Lanchote, V.L.; Moysés Neto, M.; da Costa, J.A.C.; Vieira, C.P.; Coelho, E.B. Influence of chronic kidney disease and haemodialysis treatment on pharmacokinetics of nebivolol enantiomers. Br. J. Clin. Pharmacol. 2016, 82, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, J.; Shepherd, G.; Hoffman, R.S.; Gosselin, S.; Roberts, D.M.; Li, Y.; Nolin, T.D.; Lavergne, V.; Ghannoum, M.; EXTRIP Workgroup. Extracorporeal treatment for poisoning to beta-adrenergic antagonists: Systematic review and recommendations from the EXTRIP workgroup. Crit. Care 2021, 25, 201. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Jhee, J.H.; Joo, Y.S.; Yang, K.H.; Jung, J.J.; Shin, J.H.; Han, S.H.; Yoo, T.H.; Kang, S.W.; Park, J.T. Clinical significance of hemodialysis quality of care indicators in very elderly patients with end stage kidney disease. J. Nephrol. 2022, 35, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Health Insurance Review & Assessment Service. 6th Hemodialysis Quality Assessment Program. Available online: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020002000100&brdScnBltNo=4&brdBltNo=6619#none (accessed on 30 August 2023).
- Daugirdas, J.T. Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J. Am. Soc. Nephrol. 1993, 4, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Lavie, C.J.; Fares, H.; Menezes, A.R.; O’Keefe, J.H. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol). Am. J. Cardiol. 2013, 111, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, M.; Miszczak-Kuban, J. Nonselective Beta-adrenergic blockade augments fasting hyperkalemia in hemodialysis patients. Nephron 2002, 91, 222–227. [Google Scholar] [CrossRef] [PubMed]

| Group 1 (n = 34,514) | Group 2 (n = 2789) | Group 3 (n = 15,808) | Group 4 (n = 1021) | p | |
|---|---|---|---|---|---|
| Age (years) | 60.5 ± 13.3 | 60.5 ± 12.5 | 59.7 ± 12.4 *# | 60.3 ± 12.3 | <0.001 |
| Sex (male, %) | 19,753 (57.2%) | 1737 (62.3%) | 10,157 (64.3%) | 647 (63.4%) | <0.001 |
| Hemodialysis vintage (months) | 53 ± 58 | 51 ± 56 | 50 ± 51 * | 44 ± 49 *#+ | <0.001 |
| Underlying causes of ESRD | <0.001 | ||||
| Diabetes mellitus | 13,942 (40.4%) | 1365 (48.9%) | 7957 (50.3%) | 504 (49.4%) | |
| Hypertension | 9095 (26.4%) | 681 (24.4%) | 4152 (26.3%) | 277 (27.1%) | |
| Glomerulonephritis | 3925 (11.4%) | 266 (9.5%) | 1438 (9.1%) | 96 (9.4%) | |
| Others | 3319 (9.6%) | 199 (7.1%) | 958 (6.1%) | 67 (6.6%) | |
| Unknown | 4233 (12.3%) | 278 (10.0%) | 1303 (8.2%) | 77 (7.5%) | |
| CCI score | 7.3 ± 2.9 | 7.9 ± 2.9* | 7.8 ± 2.8 * | 7.9 ± 2.6 * | <0.001 |
| Follow-up duration (months) | 62 ± 29 | 59 ± 28* | 60 ± 28 * | 60 ± 27 | <0.001 |
| Type of vascular access | <0.001 | ||||
| Arteriovenous fistula | 29,249 (84.7%) | 2366 (84.8%) | 13,658 (86.4%) | 866 (84.8%) | |
| Arteriovenous graft | 5265 (15.3%) | 423 (15.2%) | 2150 (13.6%) | 155 (15.2%) | |
| Kt/Vurea | 1.54 ± 0.27 | 1.51 ± 0.27* | 1.51 ± 0.27 * | 1.52 ± 0.30 | <0.001 |
| Ultrafiltration volume (L/session) | 2.22 ± 0.97 | 2.28 ± 0.94 * | 2.38 ± 0.92 *# | 2.33 ± 0.92 * | <0.001 |
| Hemoglobin (g/dL) | 10.7 ± 0.8 | 10.7 ± 0.7 | 10.6 ± 0.7 *# | 10.6 ± 0.8 * | <0.001 |
| Serum albumin (g/dL) | 3.99 ± 0.34 | 3.98 ± 0.34 | 3.99 ± 0.34 # | 3.98 ± 0.33 | 0.027 |
| Serum phosphorus (mg/dL) | 4.94 ± 1.37 | 5.05 ± 1.43 * | 5.01 ± 1.35 * | 4.93 ± 1.36 | <0.001 |
| Serum calcium (mg/dL) | 8.90 ± 0.84 | 8.89 ± 0.81 | 8.90 ± 0.80 | 8.77 ± 0.75 *#+ | <0.001 |
| Systolic blood pressure (mmHg) | 140 ± 16 | 143 ± 15 * | 144 ± 14 *# | 142 ± 14 *+ | <0.001 |
| Diastolic blood pressure (mmHg) | 78 ± 9 | 77 ± 10 * | 79 ± 9 *# | 77 ± 10 *+ | <0.001 |
| Serum creatinine (mg/dL) | 9.4 ± 2.8 | 9.5 ± 2.7 * | 9.7 ± 2.6 *# | 9.5 ± 2.8 | <0.001 |
| Use of RASB | 7932 (23.0%) | 1022 (36.6%) | 7016 (44.4%) | 443 (43.4%) | <0.001 |
| Use of statin | 8976 (26.0%) | 1103 (39.5%) | 5487 (34.7%) | 415 (40.6%) | <0.001 |
| Use of aspirin | 13246 (38.4%) | 1543 (55.3%) | 7893 (49.9%) | 535 (52.4%) | <0.001 |
| Use of clopidogrel | 4729 (13.7%) | 680 (24.4%) | 3314 (21.0%) | 216 (21.2%) | <0.001 |
| Use of ticlopidine | 560 (1.6%) | 64 (2.3%) | 336 (2.1%) | 22 (2.2%) | <0.001 |
| MI or CHF | 14021 (40.6%) | 1626 (58.3%) | 8366 (52.9%) | 501 (49.1%) | <0.001 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Group | ||||
| Ref: Group 1 | ||||
| Group 2 | 1.10 (1.04–1.17) | 0.002 | 1.07 (0.99–1.15) | 0.108 |
| Group 3 | 1.05 (1.02–1.09) | <0.001 | 1.01 (0.97–1.05) | 0.753 |
| Group 4 | 1.01 (0.92–1.12) | 0.795 | 0.98 (0.87–1.11) | 0.785 |
| Ref: Group 2 | ||||
| Group 3 | 0.96 (0.90–1.02) | 0.171 | 0.94 (0.87–1.02) | 0.158 |
| Group 4 | 0.92 (0.82–1.03) | 0.162 | 0.92 (0.80–1.06) | 0.262 |
| Ref: Group 3 | ||||
| Group 4 | 0.96 (0.87–1.07) | 0.467 | 0.98 (0.86–1.10) | 0.714 |
| Age (increase per 1 year) | 1.06 (1.06–1.06) | <0.001 | 1.06 (1.06–1.06) | <0.001 |
| Sex (ref: male) | 0.86 (0.84–0.89) | <0.001 | 0.74 (0.72–0.77) | <0.001 |
| Underlying cause of ESRD (ref: DM) | 0.81 (0.80–0.82) | <0.001 | 0.90 (0.88–0.91) | <0.001 |
| Vascular access (ref: AVF) | 1.51 (1.46–1.56) | <0.001 | 1.18 (1.13–1.23) | <0.001 |
| HD vintage (increase per 1 month) | 0.99 (0.99–1.01) | 0.097 | 1.00 (1.00–1.01) | <0.001 |
| CCI score (increase per 1 score) | 1.14 (1.13–1.14) | <0.001 | 1.06 (1.06–1.07) | <0.001 |
| UFV (increase per 1 kg/session) | 0.91 (0.90–0.93) | <0.001 | 1.07 (1.05–1.09) | <0.001 |
| Kt/Vurea (increase per 1 unit) | 0.91 (0.86–0.96) | <0.001 | 0.81 (0.76–0.88) | <0.001 |
| Hb (increase per 1 g/dL) | 0.86 (0.85–0.88) | <0.001 | 0.90 (0.88–0.92) | <0.001 |
| Salb (increase per 1 g/dL) | 0.37 (0.35–0.38) | <0.001 | 0.62 (0.59–0.65) | <0.001 |
| SCr (increase per 1 mg/dL) | 0.87 (0.86–0.87) | <0.001 | 0.93 (0.93–0.94) | <0.001 |
| Sph (increase per 1 mg/dL) | 0.85 (0.84–0.86) | <0.001 | 1.04 (1.03–1.06) | <0.001 |
| SCa (increase per 1 mg/dL) | 0.94 (0.92–0.95) | <0.001 | 1.06 (1.04–1.09) | <0.001 |
| SBP (increase per 1 mmHg) | 1.01 (1.01–1.01) | <0.001 | 1.01 (1.00–1.01) | <0.001 |
| DBP (increase per 1 mmHg) | 0.98 (0.98–0.99) | <0.001 | 1.00 (1.00–1.01) | 0.010 |
| Use of RASBs | 1.15 (1.12–1.18) | <0.001 | 1.01 (0.97–1.04) | 0.676 |
| Use of statin | 1.10 (1.07–1.13) | <0.001 | 0.93 (0.90–0.97) | <0.001 |
| Use of ticlopidine | 1.01 (0.91–1.11) | 0.892 | 1.06 (0.95–1.18) | 0.286 |
| Use of clopidogrel | 1.54 (1.49–1.59) | <0.001 | 1.15 (1.11–1.20) | <0.001 |
| Use of aspirin | 1.17 (1.14–1.20) | <0.001 | 0.96 (0.93–0.99) | 0.011 |
| MI or CHF | 1.50 (1.46–1.54) | <0.001 | 1.05 (1.02–1.09) | 0.005 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Cardioselectivity | ||||
| Ref: Group 1 | ||||
| Group 3 | 1.05 (1.02–1.09) | <0.001 | 1.01 (0.97–1.05) | 0.748 |
| Group 2 or 4 | 1.08 (1.02–1.14) | <0.001 | 1.04 (0.97–1.11) | 0.235 |
| Ref: Group 3 | ||||
| Group 2 or 4 | 1.02 (0.97–1.08) | 0.432 | 1.04 (0.97–1.11) | 0.332 |
| Dialyzability | ||||
| Ref: Group 1 | ||||
| Group 2 | 1.10 (1.04–1.17) | 0.002 | 1.07 (0.99–1.15) | 0.108 |
| Group 3 or 4 | 1.05 (1.02–1.08) | 0.001 | 1.01 (0.97–1.04) | 0.802 |
| Ref: Groups 2 | ||||
| Group 3 or 4 | 0.95 (0.90–1.02) | 0.149 | 0.94 (0.87–1.02) | 0.147 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-H.; Kim, B.-Y.; Son, E.-J.; Kim, G.-O.; Do, J.-Y. Influence of Different Types of β-Blockers on Mortality in Patients on Hemodialysis. Biomedicines 2023, 11, 2838. https://doi.org/10.3390/biomedicines11102838
Kang S-H, Kim B-Y, Son E-J, Kim G-O, Do J-Y. Influence of Different Types of β-Blockers on Mortality in Patients on Hemodialysis. Biomedicines. 2023; 11(10):2838. https://doi.org/10.3390/biomedicines11102838
Chicago/Turabian StyleKang, Seok-Hui, Bo-Yeon Kim, Eun-Jung Son, Gui-Ok Kim, and Jun-Young Do. 2023. "Influence of Different Types of β-Blockers on Mortality in Patients on Hemodialysis" Biomedicines 11, no. 10: 2838. https://doi.org/10.3390/biomedicines11102838
APA StyleKang, S.-H., Kim, B.-Y., Son, E.-J., Kim, G.-O., & Do, J.-Y. (2023). Influence of Different Types of β-Blockers on Mortality in Patients on Hemodialysis. Biomedicines, 11(10), 2838. https://doi.org/10.3390/biomedicines11102838






