Cerebrospinal Fluid and Serum Biomarker Insights in Aneurysmal Subarachnoid Haemorrhage: Navigating the Brain–Heart Interrelationship for Improved Patient Outcomes
Abstract
:1. Introduction
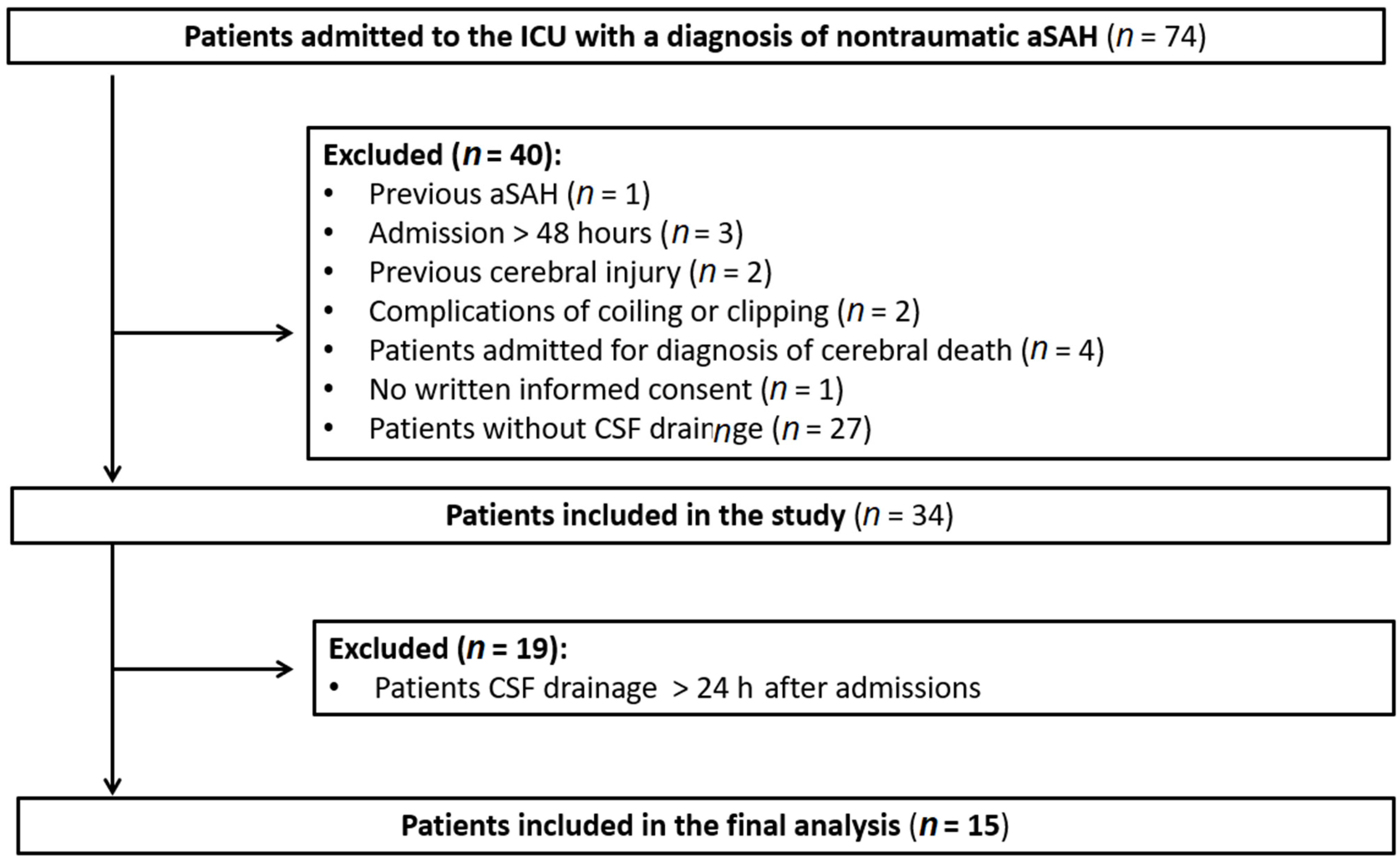
2. Materials and Methods
2.1. Patients
2.2. Clinical Evaluation
2.3. Blood and CSF Biomarkers
2.4. Treatment and Signal Monitoring
2.5. Endpoints and Definitions
3. Results
3.1. Patients Characteristics
3.2. Relationship between Cardiac Complications and DCI
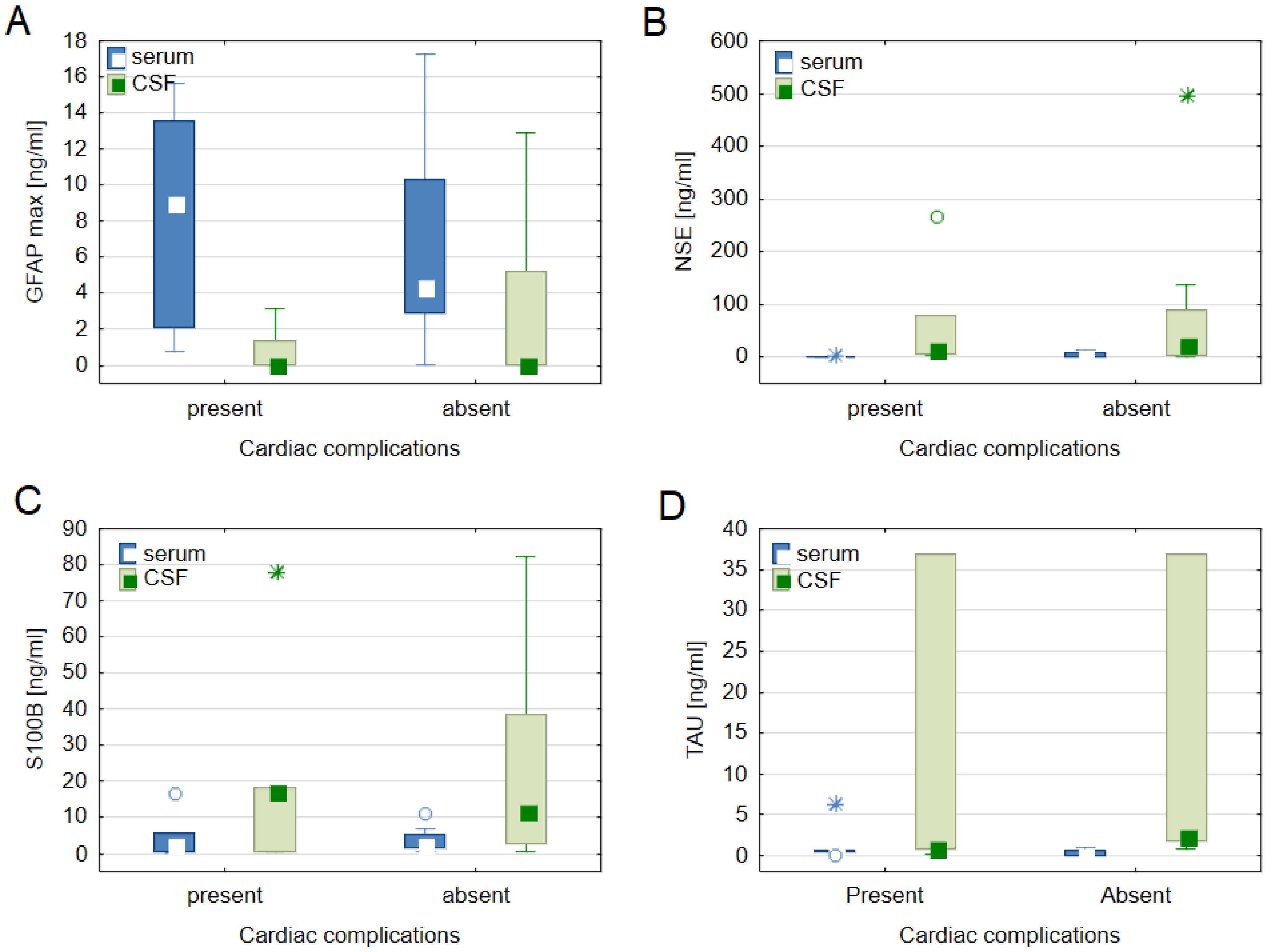
3.3. Biomarkers vs. Cardiac Complications
3.4. Cardiac Disease Biomarkers and Brain Damage Biomarkers
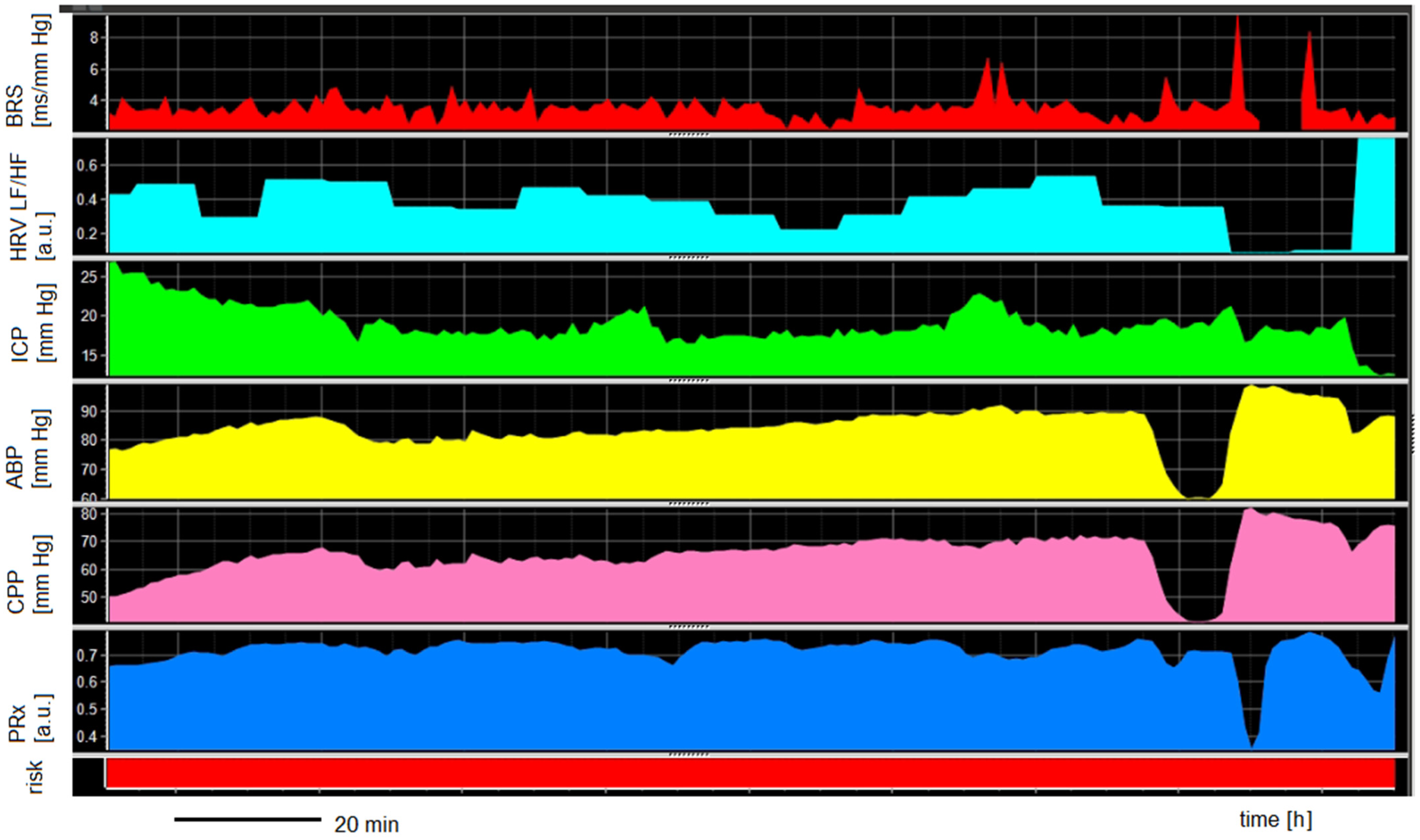
3.5. ANS and Cerebral Autoregulation vs. Severe Cardiac Complications
3.6. ANS and Cerebral Autoregulation vs. Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huhtakangas, J.; Lehto, H.; Seppä, K.; Kivisaari, R.; Niemelä, M.; Hernesniemi, J.; Lehecka, M. Long-Term Excess Mortality after Aneurysmal Subarachnoid Hemorrhage: Patients with Multiple Aneurysms at Risk. Stroke 2015, 46, 1813–1818. [Google Scholar] [CrossRef]
- Rinkel, G.J.E.; Algra, A. Long-Term Outcomes of Patients with Aneurysmal Subarachnoid Haemorrhage. Lancet Neurol. 2011, 10, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Greebe, P.; Rinkel, G.J.E.; Hop, J.W.; Visser-Meily, J.M.A.; Algra, A. Functional Outcome and Quality of Life 5 and 12.5 Years after Aneurysmal Subarachnoid Haemorrhage. J. Neurol. 2010, 257, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Kumar, J.K.; Shukla, D.; Bhat, D. Neurological, Neuropsychological, and Functional Outcome after Good Grade Aneurysmal Subarachnoid Hemorrhage. Neurol. India 2018, 66, 1713–1717. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Yan, J.; Rolland, W.B.; Soejima, Y.; Caner, B.; Zhang, J.H. Early Brain Injury, an Evolving Frontier in Subarachnoid Hemorrhage Research. Transl. Stroke Res. 2013, 4, 432–446. [Google Scholar] [CrossRef]
- Hravnak, M.; Frangiskakis, J.M.; Crago, E.A.; Chang, Y.; Tanabe, M.; John, G.I.; Horowitz, M.B. Elevated Cardiac Troponin I and Relationship to Persistence of Electrocardiographic and Echocardiographic Abnormalities After Aneurysmal Subarachnoid Hemorrhage. Stroke 2009, 40, 3478–3484. [Google Scholar] [CrossRef] [PubMed]
- Van Der Bilt, I.A.C.; Hasan, D.; Vandertop, W.P.; Wilde, A.A.M.; Algra, A.; Visser, F.C.; Rinkel, G.J.E. Impact of Cardiac Complications on Outcome after Aneurysmal Subarachnoid Hemorrhage: A Meta-Analysis. Neurology 2009, 72, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Crago, E.A.; Kerr, M.E.; Kong, Y.; Baldisseri, M.; Horowitz, M.; Yonas, H.; Kassam, A. The Impact of Cardiac Complications on Outcome in the SAH Population. Acta Neurol. Scand. 2004, 110, 248–253. [Google Scholar] [CrossRef]
- Elsharkawy, H.; Abd-Elsayed, A.; El-Hadi, S.; Provencio, J.; Tetzlaff, J. Fluctuating Electrocardiographic Changes Predict Poor Outcomes After Acute Subarachnoid Hemorrhage. Ochsner J. 2016, 16, 225. [Google Scholar]
- Burch, G.E.; Meyers, R.; Abildskov, J.A. A New Electrocardiographic Pattern Observed in Cerebrovascular Accidents. Circulation 1954, 9, 719–723. [Google Scholar] [CrossRef]
- Naidech, A.M.; Kreiter, K.T.; Janjua, N.; Ostapkovich, N.D.; Parra, A.; Commichau, C.; Fitzsimmons, B.F.M.; Connolly, E.S.; Mayer, S.A. Cardiac Troponin Elevation, Cardiovascular Morbidity, and Outcome after Subarachnoid Hemorrhage. Circulation 2005, 112, 2851–2856. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.-C.; Chang, C.-H.; Choi, B.-Y.; Kim, M.-S.; Kim, S.-W.; Kim, S.-H. Cardiac Troponin I Elevation in Patients with Aneurysmal Subarachnoid Hemorrhage. J. Korean Neurosurg. Soc. 2009, 46, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Talahma, M.; Alkhachroum, A.M.; Alyahya, M.; Manjila, S.; Xiong, W. Takotsubo Cardiomyopathy in Aneurysmal Subarachnoid Hemorrhage: Institutional Experience and Literature Review. Clin. Neurol. Neurosurg. 2016, 141, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.N.; Gross, B.A.; Rosalind Lai, P.M.; Moses, Z.B.; Du, R. Neurogenic Stress Cardiomyopathy After Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2015, 83, 880–885. [Google Scholar] [CrossRef]
- Abd, T.T.; Hayek, S.; Cheng, J.W.; Samuels, O.B.; Wittstein, I.S.; Lerakis, S. Incidence and Clinical Characteristics of Takotsubo Cardiomyopathy Post-Aneurysmal Subarachnoid Hemorrhage. Int. J. Cardiol. 2014, 176, 1362–1364. [Google Scholar] [CrossRef]
- Claassen, J.A.H.R.; Thijssen, D.H.J.; Panerai, R.B.; Faraci, F.M. Regulation of Cerebral Blood Flow in Humans: Physiology and Clinical Implications of Autoregulation. Physiol. Rev. 2021, 101, 1487–1559. [Google Scholar] [CrossRef]
- Havakuk, O.; King, K.S.; Grazette, L.; Yoon, A.J.; Fong, M.; Bregman, N.; Elkayam, U.; Kloner, R.A. Heart Failure-Induced Brain Injury. J. Am. Coll. Cardiol. 2017, 69, 1609–1616. [Google Scholar] [CrossRef]
- Gopinath, R.; Ayya, S.S. Neurogenic Stress Cardiomyopathy: What Do We Need to Know. Ann. Card. Anaesth. 2018, 21, 228. [Google Scholar] [CrossRef]
- Ziaka, M.; Exadaktylos, A. The Heart Is at Risk: Understanding Stroke-Heart-Brain Interactions with Focus on Neurogenic Stress Cardiomyopathy-A Review. J. Stroke 2023, 25, 39–54. [Google Scholar] [CrossRef]
- Gyldenholm, T.; Hvas, C.L.; Hvas, A.M.; Hviid, C.V.B. Serum Glial Fibrillary Acidic Protein (GFAP) Predicts Outcome after Intracerebral and Subarachnoid Hemorrhage. Neurol. Sci. 2022, 43, 6011–6019. [Google Scholar] [CrossRef]
- Helbok, R.; Schiefecker, A.; Delazer, M.; Beer, R.; Bodner, T.; Pfausler, B.; Benke, T.; Lackner, P.; Fischer, M.; Sohm, F.; et al. Cerebral Tau Is Elevated after Aneurysmal Subarachnoid Haemorrhage and Associated with Brain Metabolic Distress and Poor Functional and Cognitive Long-Term Outcome. J. Neurol. Neurosurg. Psychiatry 2015, 86, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; You, Y.; Ahn, H.J.; Park, J.S.; Jeong, W.; Min, J.H.; In, Y.N.; Yoo, I.; Cho, Y.; Ryu, S.; et al. Blood–Brain Barrier Disruption as a Cause of Various Serum Neuron-Specific Enolase Cut-off Values for Neurological Prognosis in Cardiac Arrest Patients. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Balança, B.; Ritzenthaler, T.; Gobert, F.; Richet, C.; Bodonian, C.; Carrillon, R.; Terrie, A.; Desmurs, L.; Perret-Liaudet, A.; Dailler, F. Significance and Diagnostic Accuracy of Early S100b Serum Concentration after Aneurysmal Subarachnoid Hemorrhage. J. Clin. Med. 2020, 9, 1746. [Google Scholar] [CrossRef]
- Jacob, R.; Khan, M. Cardiac Biomarkers: What Is and What Can Be. Indian. J. Cardiovasc. Dis. Women WINCARS 2018, 3, 240. [Google Scholar] [CrossRef]
- Budohoski, K.P.; Czosnyka, M.; Smielewski, P.; Varsos, G.V.; Kasprowicz, M.; Brady, K.M.; Pickard, J.D.; Kirkpatrick, P.J. Monitoring Cerebral Autoregulation after Subarachnoid Hemorrhage. Acta Neurochir. Suppl. 2016, 33, 449–456. [Google Scholar] [CrossRef]
- Sykora, M.; Diedler, J.; Rupp, A.; Turcani, P.; Rocco, A.; Steiner, T. Impaired Baroreflex Sensitivity Predicts Outcome of Acute Intracerebral Hemorrhage. Crit. Care Med. 2008, 36, 3074–3079. [Google Scholar] [CrossRef]
- Szabo, J.; Smielewski, P.; Czosnyka, M.; Jakubicek, S.; Krebs, S.; Siarnik, P.; Sykora, M. Heart Rate Variability Is Associated with Outcome in Spontaneous Intracerebral Hemorrhage. J. Crit. Care 2018, 48, 85–89. [Google Scholar] [CrossRef]
- Uryga, A.; Burzyńska, M.; Tabakow, P.; Kasprowicz, M.; Budohoski, K.P.; Kazimierska, A.; Smielewski, P.; Czosnyka, M.; Goździk, W. Baroreflex Sensitivity and Heart Rate Variability Are Predictors of Mortality in Patients with Aneurysmal Subarachnoid Haemorrhage. J. Neurol. Sci. 2018, 394, 112–119. [Google Scholar] [CrossRef]
- Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J. Neurosurg. 1988, 68, 985–986. [CrossRef]
- Fisher, C.M.; Kistler, J.P.; Davis, J.M. Relation of Cerebral Vasospasm to Subarachnoid Hemorrhage Visualized by Computerized Tomographic Scanning. Neurosurgery 1980, 6, 1–9. [Google Scholar] [CrossRef]
- Hijdra, A.; Brouwers, P.; Vermeulen, M.; Gijn, J. Van Grading the Amount of Blood on Computed Tomograms after Subarachnoid Hemorrhage. Stroke 1990, 21, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Savarraj, J.P.; Pervez, M.; Jones, W.; Park, J.; Jeon, S.B.; Kwon, S.U.; Chang, T.R.; Lee, K.; Kim, D.H.; et al. The Subarachnoid Hemorrhage Early Brain Edema Score Predicts Delayed Cerebral Ischemia and Clinical Outcomes. Neurosurgery 2018, 83, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.S.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef] [PubMed]
- Vergouwen, M.D.I.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.E.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.; et al. Definition of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage as an Outcome Event in Clinical Trials and Observational Studies: Proposal of a Multidisciplinary Research Group. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef]
- Davison, D.L.; Terek, M.; Chawla, L.S. Neurogenic Pulmonary Edema. Crit. Care 2012, 16, 212. [Google Scholar] [CrossRef]
- Czosnyka, M.; Smielewski, P.; Kirkpatrick, P.; Piechnik, S.; Laing, R.; Pickard, J.D. Continuous Monitoring of Cerebrovascular Pressure-Reactivity in Head Injury. Acta Neurochir. Suppl. 1998, 71, 74–77. [Google Scholar]
- Czosnyka, M.; Smielewski, P.; Kirkpatrick, P.; Menon, D.K.; Pickard, J.D. Monitoring of Cerebral Autoregulation in Head-Injured Patients. Stroke 1996, 27, 1829–1834. [Google Scholar] [CrossRef]
- Westerhof, B.E.; Gisolf, J.; Stok, W.J.; Wesseling, K.H.; Karemaker, J.M. Time-Domain Cross-Correlation Baroreflex Sensitivity: Performance on the EUROBAVAR Data Set. J. Hypertens. 2004, 22, 1–101. [Google Scholar] [CrossRef]
- Malik, M.; Bigger, J.; Camm, A.; Kleiger, R. Heart Rate Variability. Standards of Measurement, Physiological Interpretation, and Clinical Use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Quinn, T.J.; Dawson, J.; Walters, M.R.; Lees, K.R. Reliability of the Modified Rankin Scale: A Systematic Review. Stroke 2009, 40, 3393–3395. [Google Scholar] [CrossRef]
- McAteer, A.; Hravnak, M.; Chang, Y.; Crago, E.A.; Gallek, M.J.; Yousef, K.M. The Relationships Between BNP and Neurocardiac Injury Severity, Noninvasive Cardiac Output, and Outcomes After Aneurysmal Subarachnoid Hemorrhage. Biol. Res. Nurs. 2017, 19, 531. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, C.; Fei, J.; Luo, S.; You, C. The Elevation of Different Myocardial Biomarkers on Admission Is Associated with Disease Features and Different Outcomes in Aneurysmal Subarachnoid Hemorrhage. Sci. Rep. 2022, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zahid, T.; Eskander, N.; Emamy, M.; Ryad, R.; Jahan, N. Cardiac Troponin Elevation and Outcome in Subarachnoid Hemorrhage. Cureus 2020, 12, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Temes, R.E.; Tessitore, E.; Schmidt, J.M.; Naidech, A.M.; Fernandez, A.; Ostapkovich, N.D.; Frontera, J.A.; Wartenberg, K.E.; Di Tullio, M.R.; Badjatia, N.; et al. Left Ventricular Dysfunction and Cerebral Infarction from Vasospasm after Subarachnoid Hemorrhage. Neurocrit. Care 2010, 13, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Kaculini, C.; Sy, C.; Lacci, J.V.; Jafari, A.A.; Mirmoeeni, S.; Seifi, A. The Association of Takotsubo Cardiomyopathy and Aneurysmal Subarachnoid Hemorrhage: A U.S. Nationwide Analysis. Clin. Neurol. Neurosurg. 2022, 215, 107221. [Google Scholar] [CrossRef] [PubMed]
- Redfors, B.; Vedad, R.; Angerås, O.; Råmunddal, T.; Petursson, P.; Haraldsson, I.; Ali, A.; Dworeck, C.; Odenstedt, J.; Ioaness, D.; et al. Mortality in Takotsubo Syndrome Is Similar to Mortality in Myocardial Infarction—A Report from the SWEDEHEART Registry. Int. J. Cardiol. 2015, 185, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-C.; Gao, S.-J.; Zhuo, S.-L.; Weng, C.-L.; Feng, H.-W.; Lin, J.; Lin, X.-S.; Huang, L. Predictive Factors for Cerebrocardiac Syndrome in Patients with Severe Traumatic Brain Injury: A Retrospective Cohort Study. Front. Neurol. 2023, 14, 1192756. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Venkat, P.; Seyfried, D.; Chopp, M.; Yan, T.; Chen, J. Brain-Heart Interaction: Cardiac Complications After Stroke. Circ. Res. 2017, 121, 451–468. [Google Scholar] [CrossRef]
- Scheitz, J.F.; Sposato, L.A.; Schulz-Menger, J.; Nolte, C.H.; Backs, J.; Endres, M. Stroke-Heart Syndrome: Recent Advances and Challenges. J. Am. Heart Assoc. 2022, 11, e026528. [Google Scholar] [CrossRef]
- Sposato, L.A.; Hilz, M.J.; Aspberg, S.; Murthy, S.B.; Bahit, M.C.; Hsieh, C.Y.; Sheppard, M.N.; Scheitz, J.F. Post-Stroke Cardiovascular Complications and Neurogenic Cardiac Injury: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 2768–2785. [Google Scholar] [CrossRef]
- Marenholz, I.; Heizmann, C.W.; Fritz, G. S100 Proteins in Mouse and Man: From Evolution to Function and Pathology (Including an Update of the Nomenclature). Biochem. Biophys. Res. Commun. 2004, 322, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Vos, P.; Wunderlich, M.T.; De Bruijn, C.H.M.M.; Lamers, K.J.B. Release of Glial Tissue-Specific Proteins after Acute Stroke: A Comparative Analysis of Serum Concentrations of Protein S-100B and Glial Fibrillary Acidic Protein. Stroke 2000, 31, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Janigro, D.; Mondello, S.; Posti, J.P.; Unden, J. GFAP and S100B: What You Always Wanted to Know and Never Dared to Ask. Front. Neurol. 2022, 13, 835597. [Google Scholar] [CrossRef] [PubMed]
- Guzel, A.; Er, U.; Tatli, M.; Aluclu, U.; Ozkan, U.; Duzenli, Y.; Satici, O.; Guzel, E.; Kemaloglu, S.; Ceviz, A.; et al. Serum Neuron-Specific Enolase as a Predictor of Short-Term Outcome and Its Correlation with Glasgow Coma Scale in Traumatic Brain Injury. Neurosurg. Rev. 2008, 31, 439–445. [Google Scholar] [CrossRef]
- Zaheer, S.; Beg, M.; Rizvi, I.; Islam, N.; Ullah, E.; Akhtar, N. Correlation between Serum Neuron Specific Enolase and Functional Neurological Outcome in Patients of Acute Ischemic Stroke. Ann. Ind. Acad. Neurol. 2013, 16, 504. [Google Scholar] [CrossRef]
- Correia, M.; Silva, I.; Gabriel, D.; Simrén, J.; Carneiro, A.; Ribeiro, S.; Dória, H.M.; Varela, R.; Aires, A.; Minta, K.; et al. Early Plasma Biomarker Dynamic Profiles Are Associated with Acute Ischemic Stroke Outcomes. Eur. J. Neurol. 2022, 29, 1630–1642. [Google Scholar] [CrossRef]
- Dias, A.; Silva, L.; Moura, J.; Gabriel, D.; Maia, L.F. Fluid Biomarkers in Stroke: From Animal Models to Clinical Care. Acta Neurol. Scand. 2022, 146, 332–347. [Google Scholar] [CrossRef]
- Stammet, P. Blood Biomarkers of Hypoxic-Ischemic Brain Injury after Cardiac Arrest. Semin. Neurol. 2017, 37, 75–80. [Google Scholar] [CrossRef]
- Larsson, I.M.; Wallin, E.; Kristofferzon, M.L.; Niessner, M.; Zetterberg, H.; Rubertsson, S. Post-Cardiac Arrest Serum Levels of Glial Fibrillary Acidic Protein for Predicting Neurological Outcome. Resuscitation 2014, 85, 1654–1661. [Google Scholar] [CrossRef]
- Hanin, A.; Denis, J.A.; Frazzini, V.; Cousyn, L.; Imbert-Bismut, F.; Rucheton, B.; Bonnefont-Rousselot, D.; Marois, C.; Lambrecq, V.; Demeret, S.; et al. Neuron Specific Enolase, S100-Beta Protein and Progranulin as Diagnostic Biomarkers of Status Epilepticus. J. Neurol. 2022, 269, 3752–3760. [Google Scholar] [CrossRef]
- Meric, E.; Gunduz, A.; Turedi, S.; Cakir, E.; Yandi, M. The Prognostic Value of Neuron-Specific Enolase in Head Trauma Patients. J. Emerg. Med. 2010, 38, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Meynaar, I.A.; Oudemans-Van Straaten, H.M.; Wetering, J.; Verlooy, P.; Slaats, E.H.; Bosman, R.J.; Spoel, J.I.; Zandstra, D.F. Serum Neuron-Specific Enolase Predicts Outcome in Post-Anoxic Coma: A Prospective Cohort Study. Intensive Care Med. 2003, 29, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, M.T.; Lins, H.; Skalej, M.; Wallesch, C.W.; Goertler, M. Neuron-Specific Enolase and Tau Protein as Neurobiochemical Markers of Neuronal Damage Are Related to Early Clinical Course and Long-Term Outcome in Acute Ischemic Stroke. Clin. Neurol. Neurosurg. 2006, 108, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Abboud, T.; Mende, K.C.; Jung, R.; Czorlich, P.; Vettorazzi, E.; Priefler, M.; Kluge, S.; Westphal, M.; Regelsberger, J. Prognostic Value of Early S100 Calcium Binding Protein B and Neuron-Specific Enolase in Patients with Poor-Grade Aneurysmal Subarachnoid Hemorrhage: A Pilot Study. World Neurosurg. 2017, 108, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Moritz, S.; Warnat, J.; Bele, S.; Graf, B.M.; Woertgen, C. The Prognostic Value of NSE and S100B from Serum and Cerebrospinal Fluid in Patients with Spontaneous Subarachnoid Hemorrhage. J. Neurosurg. Anesthesiol. 2010, 22, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Tawk, R.G.; Grewal, S.S.; Heckman, M.G.; Rawal, B.; Miller, D.A.; Edmonston, D.; Ferguson, J.L.; Navarro, R.; Ng, L.; Brown, B.L.; et al. The Relationship Between Serum Neuron-Specific Enolase Levels and Severity of Bleeding and Functional Outcomes in Patients With Nontraumatic Subarachnoid Hemorrhage. Neurosurgery 2016, 78, 487–491. [Google Scholar] [CrossRef]
- Isgrò, M.A.; Bottoni, P.; Scatena, R. Neuron-Specific Enolase as a Biomarker: Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 125–143. [Google Scholar] [CrossRef]
- Kosik, K.S.; Finch, E.A. MAP2 and Tau Segregate into Dendritic and Axonal Domains after the Elaboration of Morphologically Distinct Neurites: An Immunocytochemical Study of Cultured Rat Cerebrum. J. Neurosci. 1987, 7, 3142–3153. [Google Scholar] [CrossRef]
- Schiefecker, A.J.; Dietmann, A.; Beer, R.; Pfausler, B.; Lackner, P.; Kofler, M.; Fischer, M.; Broessner, G.; Sohm, F.; Mulino, M.; et al. Neuroinflammation Is Associated with Brain Extracellular TAU-Protein Release After Spontaneous Subarachnoid Hemorrhage. Curr. Drug Targets 2017, 18, 1408–1416. [Google Scholar] [CrossRef]
- Heilig, M.; Rass, V.; Lindner, A.; Kofler, M.; Ianosi, B.A.; Gaasch, M.; Putnina, L.; Humpel, C.; Scherfler, C.; Zamarian, L.; et al. Brain Microdialysate Tau Dynamics Predict Functional and Neurocognitive Recovery after Poor-Grade Subarachnoid Haemorrhage. Brain Commun. 2023, 5, fcac342. [Google Scholar] [CrossRef]
- Zanier, E.R.; Longhi, L.; Fiorini, M.; Cracco, L.; Bersano, A.; Zoerle, T.; Branca, V.; Monaco, S.; Stocchetti, N. Increased Levels of CSF Heart-Type Fatty Acid-Binding Protein and Tau Protein after Aneurysmal Subarachnoid Hemorrhage. Acta Neurochir. Suppl. 2008, 102, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, G.S.; Schaf, D.V.; Oliveira, Á.R.; Gonçalves, C.A.; Belló-Klein, A.; Bordignon, S.; Bruch, R.S.; Campos, G.F.; Vassallo, D.V.; Souza, D.O.; et al. The Ischemic Rat Heart Releases S100B. Life Sci. 2005, 77, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Snyder-Ramos, S.A.; Gruhlke, T.; Bauer, H.; Bauer, M.; Luntz, A.P.; Motsch, J.; Martin, E.; Vahl, C.F.; Missler, U.; Wiesmann, M.; et al. Cerebral and Extracerebral Release of Protein S100B in Cardiac Surgical Patients. Anaesthesia 2004, 59, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, R.; Ferrari, M.; Börner, A.; Deufel, T.; Figulla, H.R. Serum Concentration of NSE and S-100b during LVAD in Non-Resuscitated Patients. Resuscitation 2008, 79, 46–53. [Google Scholar] [CrossRef]
- Truter, N.; Malan, L.; Essop, M.F. Glial Cell Activity in Cardiovascular Diseases and Risk of Acute Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H373–H390. [Google Scholar] [CrossRef] [PubMed]
- Saand, A.R.; Yu, F.; Chen, J.; Chou, S.H.Y. Systemic Inflammation in Hemorrhagic Strokes—A Novel Neurological Sign and Therapeutic Target? J. Cereb. Blood Flow. Metab. 2019, 39, 959–988. [Google Scholar] [CrossRef]
- Winek, K.; Soreq, H.; Meisel, A. Regulators of Cholinergic Signaling in Disorders of the Central Nervous System. J. Neurochem. 2021, 158, 1425–1438. [Google Scholar] [CrossRef]
- Bjerkne Wenneberg, S.; Löwhagen Hendén, P.M.; Oras, J.; Naredi, S.; Block, L.; Ljungqvist, J.; Odenstedt Hergès, H. Heart Rate Variability Monitoring for the Detection of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. Acta Anaesthesiol. Scand. 2020, 64, 945–952. [Google Scholar] [CrossRef]
- Su, I.C.; Li, C.H.; Wang, K.C.; Lai, D.M.; Huang, S.J.; Shieh, J.S.; Tu, Y.K. Prediction of Early Secondary Complications in Patients with Spontaneous Subarachnoid Hemorrhage Based on Accelerated Sympathovagal Ratios. Acta Neuroch. 2009, 151, 1631–1637. [Google Scholar] [CrossRef]
- Chiu, T.F.; Huang, C.C.; Chen, J.H.; Chen, W.L. Depressed Sympathovagal Balance Predicts Mortality in Patients with Subarachnoid Hemorrhage. Am. J. Emerg. Med. 2012, 30, 651–656. [Google Scholar] [CrossRef]
- Burzyńska, M.; Uryga, A.; Kasprowicz, M.; Czosnyka, M.; Goździk, W.; Robba, C. Cerebral Autoregulation, Cerebral Hemodynamics, and Injury Biomarkers, in Patients with COVID-19 Treated with Veno-Venous Extracorporeal Membrane Oxygenation. Neurocrit. Care 2023, 1, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.K.; Jaggi, A.S. Unraveling the Role of Adenosine in Remote Ischemic Preconditioning-Induced Cardioprotection. Life Sci. 2016, 155, 140–146. [Google Scholar] [CrossRef] [PubMed]
- English, S.W.; Fergusson, D.; Chassé, M.; Turgeon, A.F.; Lauzier, F.; Griesdale, D.; Algird, A.; Kramer, A.; Tinmouth, A.; Lum, C.; et al. Aneurysmal SubArachnoid Hemorrhage—Red Blood Cell Transfusion And Outcome (SAHaRA): A Pilot Randomised Controlled Trial Protocol. BMJ Open 2016, 6, PMC5168610. [Google Scholar] [CrossRef] [PubMed]
- Al-Khindi, T.; MacDonald, R.L.; Schweizer, T.A. Cognitive and Functional Outcome after Aneurysmal Subarachnoid Hemorrhage. Stroke 2010, 41, e519–e536. [Google Scholar] [CrossRef]
- Passier, P.E.C.A.; Visser-Meily, J.M.A.; Van Zandvoort, M.J.E.; Post, M.W.M.; Rinkel, G.J.E.; Van Heugten, C. Prevalence and Determinants of Cognitive Complaints after Aneurysmal Subarachnoid Hemorrhage. Cerebrovasc. Dis. 2010, 29, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Nobels-Janssen, E.; Abma, I.L.; Verhagen, W.I.M.; Bartels, R.H.M.A.; van der Wees, P.J.; Boogaarts, J.D. Development of a Patient-Reported Outcome Measure for Patients Who Have Recovered from a Subarachnoid Hemorrhage: The “Questionnaire for the Screening of Symptoms in Aneurysmal Subarachnoid Hemorrhage” (SOS-SAH). BMC Neurol. 2021, 21, 162. [Google Scholar] [CrossRef]
- Winek, K.; Lobentanzer, S.; Nadorp, B.; Dubnov, S.; Dames, C.; Jagdmann, S.; Moshitzky, G.; Hotter, B.; Meisel, C.; Greenberg, D.S.; et al. Transfer RNA Fragments Replace MicroRNA Regulators of the Cholinergic Poststroke Immune Blockade. Proc. Natl. Acad. Sci. USA 2020, 117, 32606–32616. [Google Scholar] [CrossRef]
| Characteristic | Total N = 15 | mRS 0–2 n = 6 | mRS 3–6 n = 9 | p-Value |
|---|---|---|---|---|
| Age | 67 ± 28 | 60 ± 18 | 74 ± 22 | 0.617 |
| Female | 8 (53%) | 4 (67%) | 3 (33%) | 0.230 |
| BMI | 27.8 ± 9.3 | 30.2 ±12.6 | 27.8 ± 3.0 | 0.643 |
| GCS | 12 ± 10 | 14 ± 1 | 6 ± 8 | 0.071 |
| MAP [mm Hg] | 65 ± 33 | 58 ± 32 | 66 ± 23 | 0.982 |
| HR [bpm] | 80 ± 11 | 58 ± 32 | 65 ± 23 | 0.857 |
| Temp [°C] | 37.0 ± 1.1 | 37.0 ± 0.4 | 37.6 ± 0.9 | 0.642 |
| Reactive pupils | 15 (100%) | 6 (100%) | 9 (100%) | ----- |
| Comorbidities | ||||
| Hypertension | 8 (53%) | 3 (50%) | 5 (55%) | 0.751 |
| Hyperlipidaemia | 7 (47%) | 2 (33%) | 5 (55%) | 0.377 |
| Current smoker | 10 (67%) | 4 (67%) | 6 (67%) | 0.713 |
| Alcoholism | 1 (7%) | 0 | 1 (11%) | 0.600 |
| Diabetes mellitus | 0 | 0 | 0 | ----- |
| Coronary heart disease | 3 (20%) | 0 | 3 (33%) | 0.184 |
| Aneurysm | ||||
| ICA | 3 (20%) | 2 (33%) | 1 (11%) | 0.565 |
| MCA | 2 (13%) | 1 (17%) | 1 (11%) | |
| ACA | 1 (7%) | 0 | 1 (11%) | |
| ACoA | 6 (40%) | 3 (50%) | 3 (33%) | |
| Posterior | 3 (20%) | 0 | 3 (33%) | |
| Clinical assessment | ||||
| mFisher | 4 | 4 ± 2 | 4 | 0.867 |
| Apache II | 18 ± 14 | 12 ± 6 | 20 ± 6 | 0.049 |
| Hijdra | 27 ± 17 | 22 ± 16 | 27 ± 11 | 0.529 |
| SEBES | 2 ± 3 | 2 ± 2 | 1 ± 3 | 0.857 |
| SEBES: Grade 0–2 | 11 (73%) | 5 (83%) | 6 (67%) | 0.461 |
| SEBES: Grades 3–4 | 4 (27%) | 1 (17%) | 3 (33%) | |
| WFNS | 4 ± 3 | 2 ± 1 | 5 ± 1 | 0.143 |
| WFNS: Grade I-III | 7 (47%) | 5 (83%) | 2 (22%) | 0.035 |
| WFNS: Grade IV-V | 8 (53%) | 1 (17%) | 7 (78%) | |
| ICH | 5 (33%) | 1 (17%) | 4 (44%) | 0.293 |
| IVH | 15 (100%) | 6 (100%) | 9 (100%) | ----- |
| Aneurysm treatment | ||||
| Clipping | 9 (60%) | 6 (100%) | 3 (33%) | 0.016 |
| Coiling | 3 (20%) | 0 | 3 (33%) | 0.184 |
| Conservative treatment | 3 (20%) | 0 | 3 (33%) | 0.184 |
| Outcome | ||||
| ICU stay [days] | 17 ± 14 | 13 ± 11 | 18 ± 15 | 0.170 |
| Hospital stay [days] | 18 ± 15 | 19 ± 3 | 18 ± 49 | 0.133 |
| Dead | 6 (40%) | 0 | 6 (67%) | 0.016 |
| Neurologic complications | ||||
| Pathological changes in CT after surgery | 12 (80%) | 4 (67%) | 8 (89%) | 0.340 |
| CV | 10 (67%) | 5 (83%) | 5 (55%) | 0.293 |
| DCI | 4 (27%) | 0 | 4 (44%) | 0.092 |
| Cardiac complications | ||||
| non-ischemic increased troponin levels | 8 (53%) | 3 (50%) | 5 (56%) | 0.622 |
| post-stroke AMI | 0 | 0 | 0 | ------ |
| LV dysfunction, HF and post-stroke TTS | 7 (47%) | 3 (50%) | 4 (44%) | 0.622 |
| ECG changes and arrhythmias | 13 (87%) | 6 (100%) | 7 (78%) | 0.342 |
| Pulmonary complications | ||||
| Pulmonary oedema | 5 (33%) | 2 (33%) | 3 (33%) | 0.706 |
| Pulmonary congestion | 4 (27%) | 1 (17%) | 3 (33%) | 0.461 |
| Characteristic | Total N = 15 | Severe Cardiac Complications n = 7 | Without Severe Cardiac Complications n = 8 | p-Value |
|---|---|---|---|---|
| Brain biomarkers | ||||
| serum GFAP day 1 [ng/mL] | 4.38 ± 8.77 | 4.94 ± 9.54 | 2.83 ± 7.39 | 0.396 |
| serum GFAP max [ng/mL] | 4.50 ± 9.27 | 9.05 ± 11.47 | 4.41 ± 7.41 | 0.778 |
| CSF GFAP day 1 [ng/mL] | 0.03 ± 3.12 | 0.03 ± 3.12 | 0.07 ± 5.61 | 0.231 |
| CSF GFAP max [ng/mL] | 0.05 ± 3.12 | 0.05 ± 1.34 | 0.07 ± 5.16 | 0.612 |
| serum S100B day 1 [ng/mL] | 0.93 ± 3.21 | 0.93 ± 0.79 | 2.03 ± 3.49 | 0.535 |
| serum S100B max [ng/mL] | 2.30 ± 4.76 | 2.30 ± 5.00 | 2.31 ± 3.74 | 0.778 |
| CSF S100B day 1 [ng/mL] | 17.76 ± 23.77 | 17.76 ± 21.37 | 12.89 ± 35.58 | 0.612 |
| CSF S100B max [ng/mL] | 13.41 ± 24.91 | 17.76 ± 17.72 | 11.66 ± 36.16 | 0.778 |
| serum NSE day 1 [ng/mL] | 1.40 ± 5.00 | 1.40 ± 0.39 | 1.99 ± 7.78 | 0.280 |
| serum NSE max [ng/mL] | 1.81 ± 4.67 | 1.40 ± 0.67 | 3.67 ± 7.79 | 0.054 |
| CSF NSE day 1 [ng/mL] | 16.49 ± 68.70 | 7.60 ± 19.46 | 40.47 ± 100.51 | 0.396 |
| CSF NSE max [ng/mL] | 16.49 ± 76.57 | 16.49 ± 76.17 | 23.15 ± 85.63 | 0.866 |
| a serum TAU day 1 [ng/mL] | 0.07 ± 0.19 | 0.21 ± 0.23 | 0.03 ± 0.04 | 0.009 |
| a serum TAU day max [ng/mL] | 0.44 ± 0.60 | 0.45 ± 0.21 | 0.06 ± 0.60 | 0.177 |
| a CSF TAU day 1 [ng/mL] | 2.05 ± 36.05 | 1.00 ± 36.05 | 2.33 ± 35.07 | 0.662 |
| a CSF TAU max [ng/mL] | 2.05 ± 36.05 | 1.00 ± 36.05 | 2.33 ± 35.07 | 0.662 |
| Cardiac biomarkers | ||||
| b BNP [pg/mL] (0–100) | 155 ± 119 | 155 ± 111 | 138 ± 116 | 0.792 |
| cTnI day 1 [ng/mL] (0–0.028) | 0.03 ± 0.66 | 0.04 ± 0.03 | 0.03 ± 1.55 | 0.955 |
| cTnI max [ng/mL] | 0.06 ± 1.97 | 0.06 ± 1.98 | 0.34 ± 2.08 | 0.955 |
| CK-MB [U/L] (0–25) | 28 ± 22 | 27 ± 25 | 32 ± 16 | 0.396 |
| Non-specific biomarkers | ||||
| CRP [mg/L] (0–5) | 5.20 ± 3.99 | 5.20 ± 6.98 | 4.35 ± 3.69 | 0.694 |
| D-dimer [ug/mL] (0–0.5) | 1.72 ± 3.16 | 2.35 ± 2.60 | 1.20 ± 4.06 | 0.120 |
| WBC [G/L] (4–10 103/ul) | 14.83 ± 9.17 | 13.52 ± 12.67 | 15.01 ± 6.81 | 0.994 |
| PCT [ng/mL] (0–0.05) | 0.11 ± 0.28 | 0.11 ± 0.27 | 0.13 ± 0.28 | 0.778 |
| LAC [mmol/L] (0.5–1.6) | 1.75 ± 1.20 | 1.75 ± 0.70 | 1.58 ± 1.64 | 0.866 |
| K [mmol/L] (3.5–5.0) | 3.91 ± 0.70 | 4.10 ± 0.81 | 3.77 ± 0.42 | 0.463 |
| Na [mmol/L] (136–146) | 139 ± 3 | 139 ± 3 | 139 ± 3 | 0.694 |
| Ht [L] (40–54) | 43 ± 5 | 43 ± 5 | 43 ± 4 | 0.788 |
| Glucose [mg/dL] (74–106) | 186 ± 62 | 154 ± 50 | 202 ± 40 | 0.120 |
| Albumin [G/L] (35–52) | 30 ± 7 | 30 ± 12 | 30 ± 5 | 0.866 |
| Cardiac Biomarkers | ||||
|---|---|---|---|---|
| Brain-Specific Biomarkers | cTnI Day 1 | cTnI Max | BNP a | CK-MB |
| serum GFAP day 1 | −0.14 | −0.23 | 0.04 | −0.04 |
| serum GFAP max | −0.06 | −0.15 | −0.04 | 0.06 |
| CSF GFAP day 1 | 0.05 | 0.10 | 0.31 | 0.16 |
| CSF GFAP max | 0.15 | 0.26 | 0.48 | 0.21 |
| serum S100B day 1 | 0.01 | 0.02 | 0.22 | 0.04 |
| serum S100B max | −0.04 | 0.07 | 0.55 | −0.08 |
| CSF S100B day 1 | −0.21 | −0.06 | 0.62 * | −0.29 |
| CSF S100B max | −0.13 | −0.02 | 0.62 * | −0.22 |
| serum NSE day 1 | −0.43 | −0.38 | −0.02 | −0.26 |
| serum NSE max | −0.43 | −0.38 | −0.02 | −0.26 |
| CSF NSE day 1 | 0.13 | 0.24 | 0.63 * | 0.15 |
| CSF NSE max | 0.47 | 0.57 * | 0.66 * | 0.16 |
| b serum TAU day 1 | 0.25 | 0.18 | −0.11 | 0.04 |
| b serum TAU max | 0.33 | 0.29 | 0.53 | −0.11 |
| b CSF TAU day 1 | 0.12 | 0.20 | 0.78 * | −0.16 |
| b CSF TAU max | 0.12 | 0.20 | 0.78 * | −0.17 |
| Parameter | Total Group * (N = 13) | Cardiac Complications (n = 7) | Without Cardiac Complications (n = 8) | p-Value |
|---|---|---|---|---|
| ABP day 1 [mm Hg] | 83.1 ± 15.6 | 82.7 ± 15.59 | 90.4 ± 13.1 | 0.665 |
| ABP day 3 [mm Hg] | 87.9 ± 7.3 | 91.6 ± 13.8 | 86.3 ± 10.6 | 0.366 |
| BRS day 1 [ms/mm Hg] | 8.1 ± 6.0 | 6.2 ± 3.8 | 9.5 ± 4.5 | 0.248 |
| BRS day 3 [ms/mm Hg] | 8.6 ± 6.2 | 5.2 ± 9.4 | 9.1 ± 12.2 | 0.533 |
| HRV LF/HF day 1 [a.u.] | 0.9 ± 0.7 | 0.9 ± 0.3 | 0.9 ± 0.5 | 0.841 |
| HRV LF/HF day 3 [a.u.] | 1.0 ± 0.4 | 0.8 ± 0.4 | 1.1 ± 0.5 | 0.628 |
| a ICP day 1 [mm Hg] | 7.7 ± 8.0 | 5.6 ± 2.7 | 8.9± 5.5 | 0.114 |
| a ICP day 3 [mm Hg] | 11.2 ± 9.1 | 15.9 ± 10.3 | 7.8 ± 9.4 | 0.190 |
| a CPP day 1 [mm Hg] | 70.7 ± 8.3 | 70.2 ± 30.5 | 70.7 ± 32.3 | 0.995 |
| a CPP day 3 [mm Hg] | 78 ± 8.6 | 84.8 ± 18.5 | 77.6 ± 2.6 | 0.730 |
| a PRx day 1 [a.u.] | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.200 |
| a PRx day 3 [a.u.] | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.8 ± 0.1 | 0.730 |
| ANS and Cerebral Autoregulation Parameters | ||||||
|---|---|---|---|---|---|---|
| BRS Day 1 | HRV LF/HF Day 1 | PRx a Day 1 | BRS Day 3 | HRV LF/HF Day 3 | PRx a Day 3 | |
| Brain-specific biomarkers | ||||||
| serum GFAP day 1 | −0.18 | −0.83 ** | −0.60 | 0.16 | −0.49 | −0.56 |
| serum GFAP max | −0.02 | −0.57 | −0.50 | 0.41 | −0.34 | −0.63 |
| CSF GFAP day 1 | −0.01 | −0.59 | 0.35 | 0.04 | −0.02 | 0.49 |
| CSF GFAP max | −0.11 | −0.51 | −0.11 | −0.25 | −0.05 | 0.72 * |
| serum S100B day 1 | −0.03 | −0.78 ** | 0.01 | 0.43 | −0.38 | 0.54 |
| serum S100B max | −0.19 | −0.66 * | 0.10 | 0.25 | −0.33 | 0.65 |
| CSF S100B day 1 | 0.14 | −0.29 | 0.56 | 0.34 | −0.03 | 0.53 |
| CSF S100B max | 0.03 | −0.03 | −0.21 | 0.56 | 0.18 | 0.01 |
| serum NSE day 1 | 0.30 | 0.33 | 0.90 * | 0.59 * | 0.32 | 0.07 |
| serum NSE max | 0.38 | −0.15 | 0.90 * | 0.57 * | 0.05 | 0.42 |
| CSF NSE day 1 | 0.45 | −0.26 | 0.80 | 0.19 | 0.03 | 0.58 |
| CSF NSE max | 0.26 | −0.25 | 0.05 | 0.04 | 0.04 | 0.03 |
| b serum TAU day 1 | −0.47 | −0.28 | −0.56 | −0.30 | −0.27 | 0.03 |
| b serum TAU max | 0.07 | 0.05 | −0.60 | −0.01 | −0.05 | −0.10 |
| b CSF TAU day 1 | 0.09 | −0.13 | 0.50 | 0.44 | −0.29 | 0.65 |
| b CSF TAU max | 0.09 | −0.13 | 0.50 | 0.44 | −0.29 | 0.65 |
| Cardiac-specific biomarkers | ||||||
| cTnI day 1 | 0.28 | 0.01 | 0.15 | −0.15 | 0.25 | 0.11 |
| cTnI max | 0.21 | 0.08 | 0.10 | −0.31 | 0.13 | 0.08 |
| c BNP | 0.57 | −0.14 | 0.01 | 0.10 | −0.03 | 0.90 * |
| CK-MB | −0.07 | −0.29 | 0.30 | −0.23 | −0.35 | −0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burzyńska, M.; Uryga, A.; Załuski, R.; Goździk, A.; Adamik, B.; Robba, C.; Goździk, W. Cerebrospinal Fluid and Serum Biomarker Insights in Aneurysmal Subarachnoid Haemorrhage: Navigating the Brain–Heart Interrelationship for Improved Patient Outcomes. Biomedicines 2023, 11, 2835. https://doi.org/10.3390/biomedicines11102835
Burzyńska M, Uryga A, Załuski R, Goździk A, Adamik B, Robba C, Goździk W. Cerebrospinal Fluid and Serum Biomarker Insights in Aneurysmal Subarachnoid Haemorrhage: Navigating the Brain–Heart Interrelationship for Improved Patient Outcomes. Biomedicines. 2023; 11(10):2835. https://doi.org/10.3390/biomedicines11102835
Chicago/Turabian StyleBurzyńska, Małgorzata, Agnieszka Uryga, Rafał Załuski, Anna Goździk, Barbara Adamik, Chiara Robba, and Waldemar Goździk. 2023. "Cerebrospinal Fluid and Serum Biomarker Insights in Aneurysmal Subarachnoid Haemorrhage: Navigating the Brain–Heart Interrelationship for Improved Patient Outcomes" Biomedicines 11, no. 10: 2835. https://doi.org/10.3390/biomedicines11102835
APA StyleBurzyńska, M., Uryga, A., Załuski, R., Goździk, A., Adamik, B., Robba, C., & Goździk, W. (2023). Cerebrospinal Fluid and Serum Biomarker Insights in Aneurysmal Subarachnoid Haemorrhage: Navigating the Brain–Heart Interrelationship for Improved Patient Outcomes. Biomedicines, 11(10), 2835. https://doi.org/10.3390/biomedicines11102835






