Changes in Whole Blood Polyamine Levels and Their Background in Age-Related Diseases and Healthy Longevity
Abstract
:1. Introduction
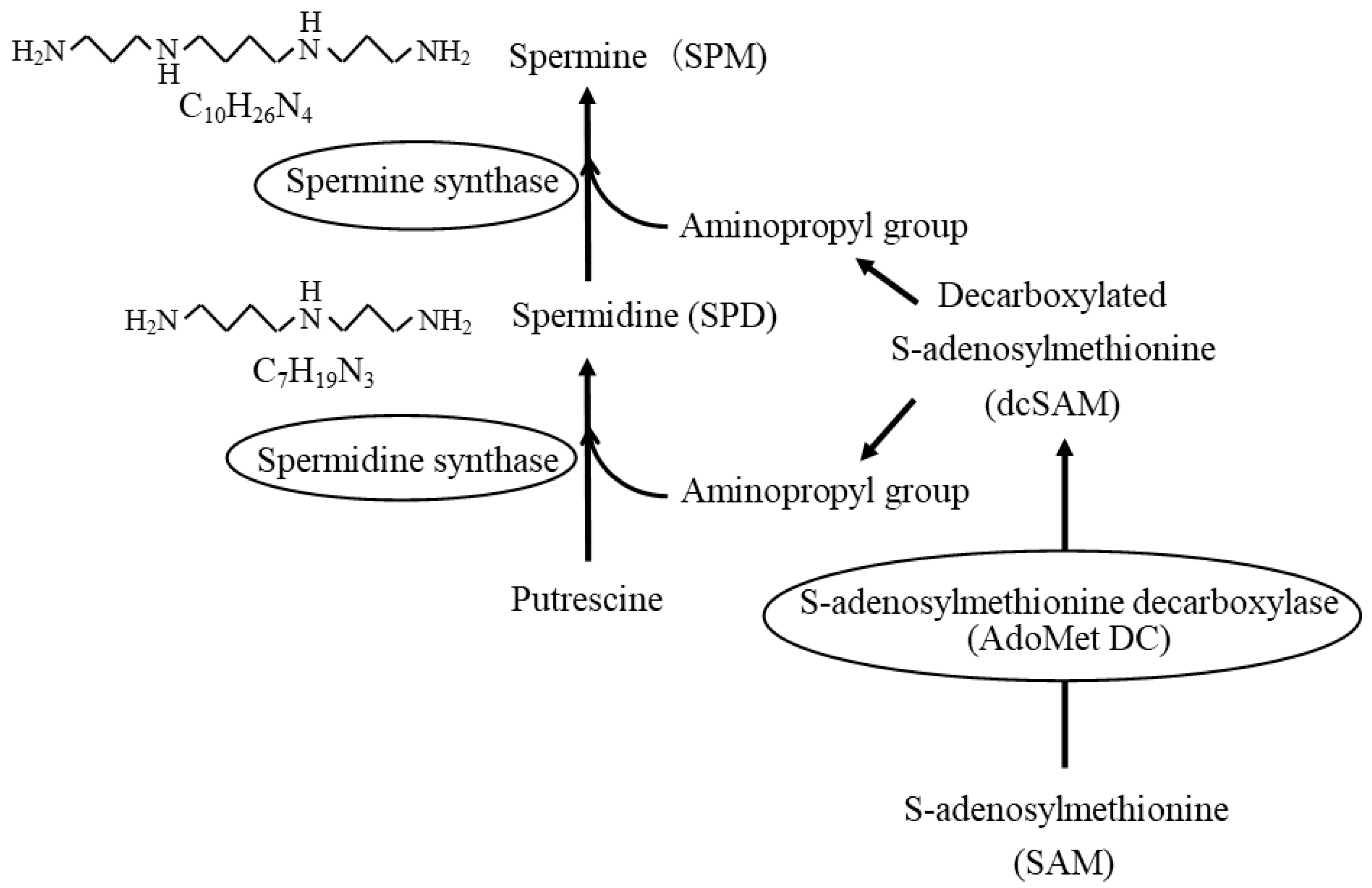
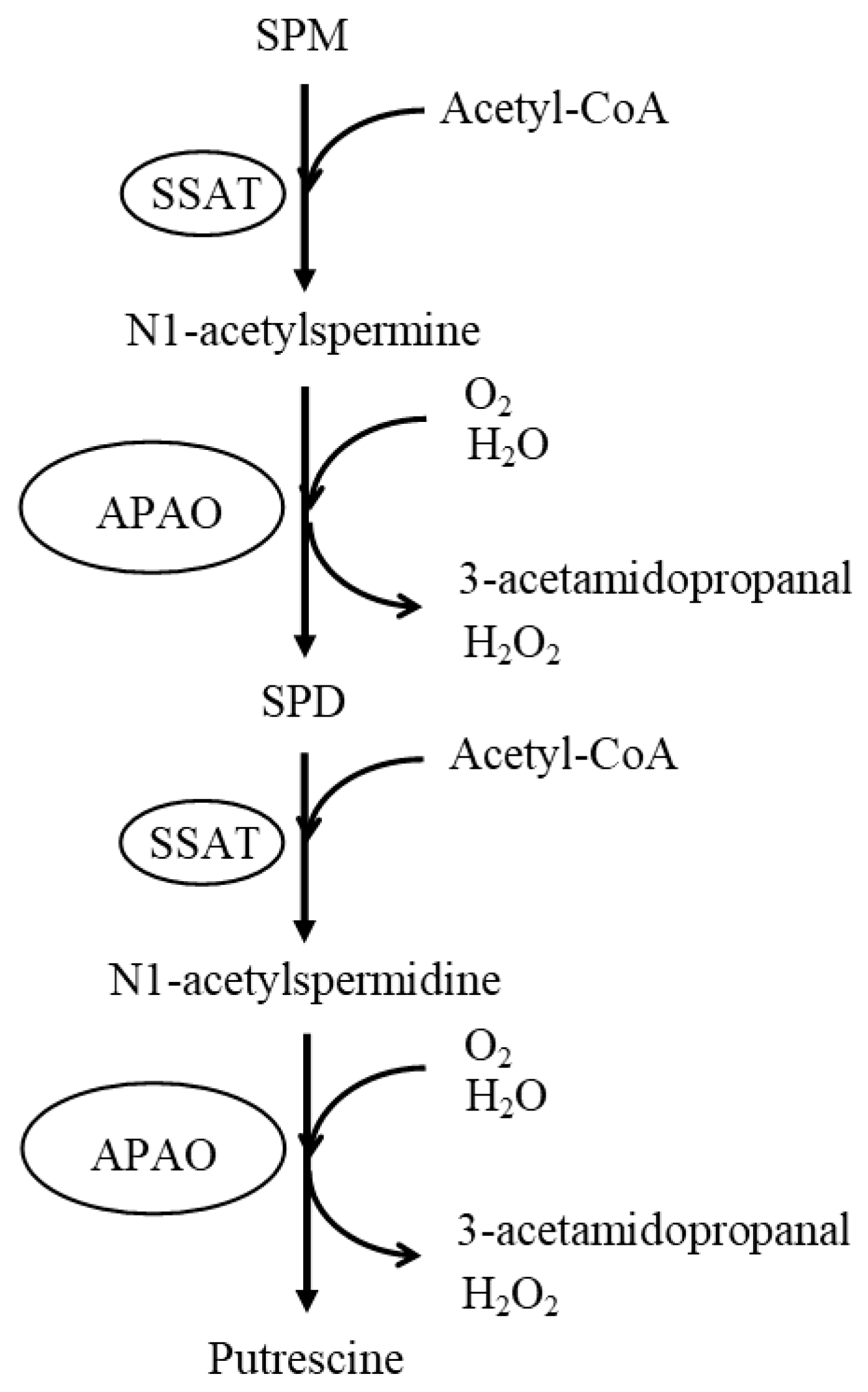
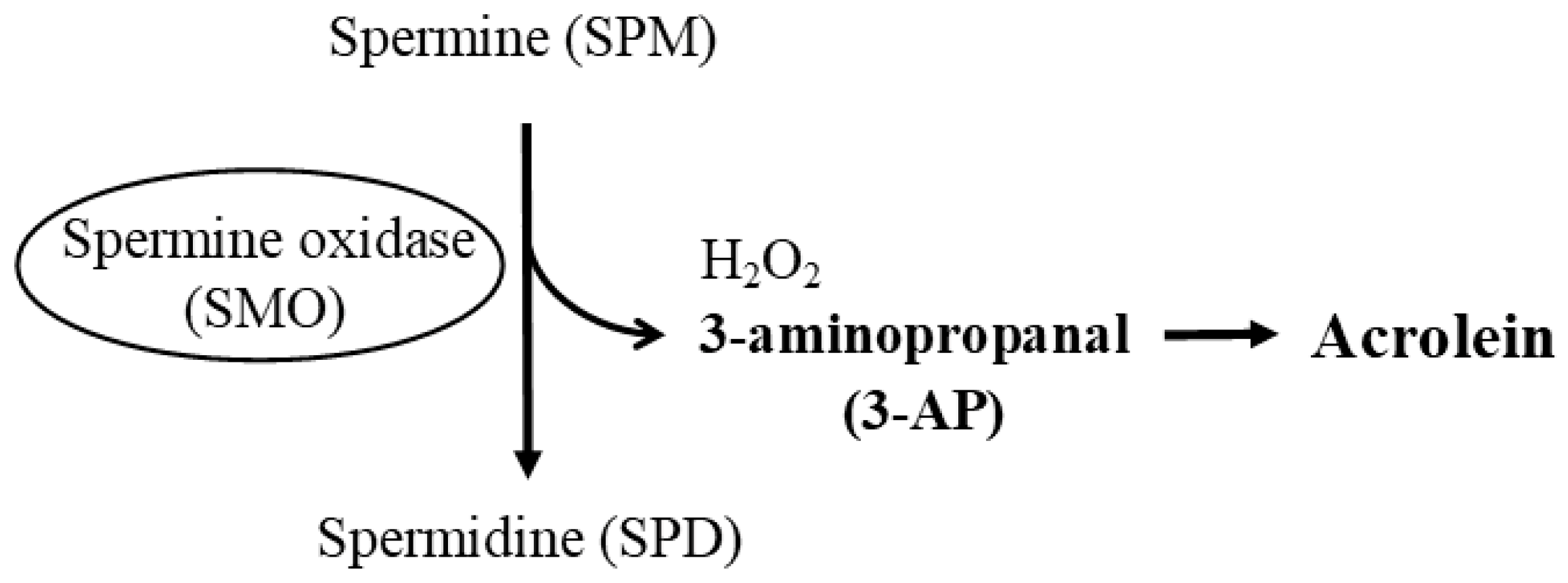
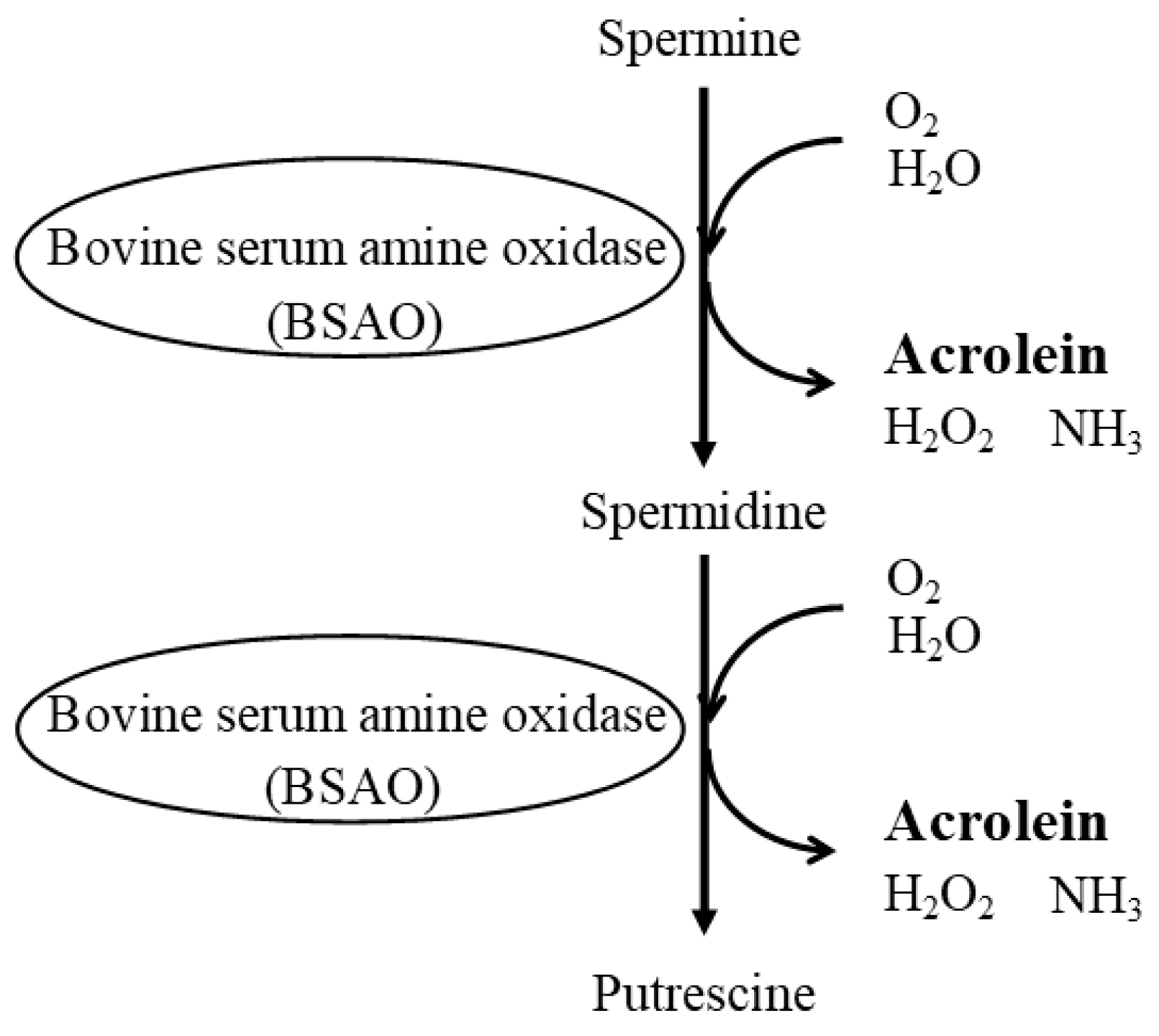
2. Polyamines
3. The Most Basic and Important Aspects of Conducting Polyamine Research
4. Age-Related Changes in Polyamine Concentrations
5. Age- and Disease-Related Changes in the Ratio of Spermine to Spermidine
6. Polyamines as Nutritional Contributors to the Prevention of Age- and Lifestyle-Related Disease Development
7. Biological Activity of Polyamines in Human Health and Disease
8. Conclusions of the Review and Issues to Be Addressed
Funding
Acknowledgments
Conflicts of Interest
References
- Soda, K.; Dobashi, Y.; Kano, Y.; Tsujinaka, S.; Konishi, F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp. Gerontol. 2009, 44, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Chae, Y.B.; Kim, M.M. Activation of p53 by spermine mediates induction of autophagy in HT1080 cells. Int. J. Biol. Macromol. 2014, 63, 56–63. [Google Scholar] [CrossRef]
- Zhang, H.; Alsaleh, G.; Feltham, J.; Sun, Y.; Napolitano, G.; Riffelmacher, T.; Charles, P.; Frau, L.; Hublitz, P.; Yu, Z.; et al. Polyamines Control eIF5A Hypusination, TFEB Translation, and Autophagy to Reverse B Cell Senescence. Mol. Cell 2019, 76, 110–125.e9. [Google Scholar] [CrossRef]
- Xu, T.T.; Li, H.; Dai, Z.; Lau, G.K.; Li, B.Y.; Zhu, W.L.; Liu, X.Q.; Liu, H.F.; Cai, W.W.; Huang, S.Q.; et al. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging 2020, 12, 6401–6414. [Google Scholar] [CrossRef]
- Soda, K. Overview of Polyamines as Nutrients for Human Healthy Long Life and Effect of Increased Polyamine Intake on DNA Methylation. Cells 2022, 11, 164. [Google Scholar] [CrossRef]
- Starks, R.; Kirby, P.; Ciliberto, M.; Hefti, M. Snyder-Robinson syndrome. Autops. Case Rep. 2018, 8, e2018031. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Khan, M.A.; Moskal, J.R. The concept of “aldehyde load” in neurodegenerative mechanisms: Cytotoxicity of the polyamine degradation products hydrogen peroxide, acrolein, 3-aminopropanal, 3-acetamidopropanal and 4-aminobutanal in a retinal ganglion cell line. Brain Res. 2007, 1145, 150–156. [Google Scholar] [CrossRef]
- Pegg, A.E. Toxicity of polyamines and their metabolic products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef]
- Goodwin, A.C.; Destefano Shields, C.E.; Wu, S.; Huso, D.L.; Wu, X.; Murray-Stewart, T.R.; Hacker-Prietz, A.; Rabizadeh, S.; Woster, P.M.; Sears, C.L.; et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15354–15359. [Google Scholar] [CrossRef]
- Xu, H.; Chaturvedi, R.; Cheng, Y.; Bussiere, F.I.; Asim, M.; Yao, M.D.; Potosky, D.; Meltzer, S.J.; Rhee, J.G.; Kim, S.S.; et al. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: Implications for gastric carcinogenesis. Cancer Res. 2004, 64, 8521–8525. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.C.; Piazuelo, M.B.; Luis, P.B.; Barry, D.P.; Allaman, M.M.; Asim, M.; Sebrell, T.A.; Finley, J.L.; Rose, K.L.; Hill, S.; et al. Spermine oxidase mediates Helicobacter pylori-induced gastric inflammation, DNA damage, and carcinogenic signaling. Oncogene 2020, 39, 4465–4474. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Sun, D.; Zhang, J.; Xue, R.; Janssen, H.L.A.; Tang, W.; Dong, L. Spermine oxidase is upregulated and promotes tumor growth in hepatocellular carcinoma. Hepatol. Res. 2018, 48, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Tepper, A.; Chu, G.; Klaren, V.N.A.; Kalin, J.H.; Molina-Ortiz, P.; Impagliazzo, A. Development and characterization of rabbit monoclonal antibodies that recognize human spermine oxidase and application to immunohistochemistry of human cancer tissues. PLoS ONE 2022, 17, e0267046. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.P.; Al-Greene, N.T.; Singh, K.; Coburn, L.A.; Sierra, J.C.; Verriere, T.G.; Luis, P.B.; Schneider, C.; Asim, M.; Allaman, M.M.; et al. Distinct Immunomodulatory Effects of Spermine Oxidase in Colitis Induced by Epithelial Injury or Infection. Front. Immunol. 2018, 9, 1242. [Google Scholar] [CrossRef]
- Igarashi, K.; Uemura, T.; Kashiwagi, K. Assessing acrolein for determination of the severity of brain stroke, dementia, renal failure, and Sjogren’s syndrome. Amino Acids 2020, 52, 119–127. [Google Scholar] [CrossRef]
- Alfarhan, M.; Liu, F.; Shan, S.; Pichavaram, P.; Somanath, P.R.; Narayanan, S.P. Pharmacological Inhibition of Spermine Oxidase Suppresses Excitotoxicity Induced Neuroinflammation in Mouse Retina. Int. J. Mol. Sci. 2022, 23, 2133. [Google Scholar] [CrossRef]
- Tabor, C.W.; Tabor, H.; Rosenthal, S.M. Purification of amine oxidase from beef plasma. J. Biol. Chem. 1954, 208, 645–661. [Google Scholar] [CrossRef]
- Holbert, C.E.; Dunworth, M.; Foley, J.R.; Dunston, T.T.; Stewart, T.M.; Casero, R.A., Jr. Autophagy induction by exogenous polyamines is an artifact of bovine serum amine oxidase activity in culture serum. J. Biol. Chem. 2020, 295, 9061–9068. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takabatake, Y.; Kimura, T.; Takahashi, A.; Namba, T.; Matsuda, J.; Minami, S.; Kaimori, J.Y.; Matsui, I.; Kitamura, H.; et al. Time-dependent dysregulation of autophagy: Implications in aging and mitochondrial homeostasis in the kidney proximal tubule. Autophagy 2016, 12, 801–813. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kitada, Y.; Naito, Y. Endothelial Function is improved by Inducing Microbial Polyamine Production in the Gut: A Randomized Placebo-Controlled Trial. Nutrients 2019, 11, 1118. [Google Scholar] [CrossRef]
- Pekar, T.; Wendzel, A.; Flak, W.; Kremer, A.; Pauschenwein-Frantsich, S.; Gschaider, A.; Wantke, F.; Jarisch, R. Spermidine in dementia: Relation to age and memory performance. Wien. Klin. Wochenschr. 2020, 132, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Sanayama, H.; Ito, K.; Ookawara, S.; Uemura, T.; Sakiyama, Y.; Sugawara, H.; Tabei, K.; Igarashi, K.; Soda, K. Whole Blood Spermine/Spermidine Ratio as a New Indicator of Sarcopenia Status in Older Adults. Biomedicines 2023, 11, 1403. [Google Scholar] [CrossRef]
- Soda, K.; Uemura, T.; Sanayama, H.; Igarashi, K.; Fukui, T. Polyamine-Rich Diet Elevates Blood Spermine Levels and Inhibits Pro-Inflammatory Status: An Interventional Study. Med. Sci. 2021, 9, 22. [Google Scholar] [CrossRef]
- Sternberg, Z.; Podolsky, R.; Nir, A.; Yu, J.; Nir, R.; Halvorsen, S.W.; Quinn, J.F.; Kaye, J.; Kolb, C. The Utility of Spermidine Serum Levels as a Biomarker of Alzheimer’s Disease a Pilot Study. Alzheimers Dis. Dement. 2021, 5, 119–125. [Google Scholar]
- Saiki, S.; Sasazawa, Y.; Fujimaki, M.; Kamagata, K.; Kaga, N.; Taka, H.; Li, Y.; Souma, S.; Hatano, T.; Imamichi, Y.; et al. A metabolic profile of polyamines in parkinson disease: A promising biomarker. Ann. Neurol. 2019, 86, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Akasaka, Y.; Ikegaya, H. Correlation of polyamines, acrolein-conjugated lysine and polyamine metabolic enzyme levels with age in human liver. Heliyon 2020, 6, e05031. [Google Scholar] [CrossRef]
- Sanayama, H.; Ito, K.; Ookawara, S.; Uemura, T.; Imai, S.; Kiryu, S.; Iguchi, M.; Sakiyama, Y.; Sugawara, H.; Morishita, Y.; et al. Positive Correlation between Relative Concentration of Spermine to Spermidine in Whole Blood and Skeletal Muscle Mass Index: A Possible Indicator of Sarcopenia and Prognosis of Hemodialysis Patients. Biomedicines 2023, 11, 746. [Google Scholar] [CrossRef]
- Gomes-Trolin, C.; Nygren, I.; Aquilonius, S.M.; Askmark, H. Increased red blood cell polyamines in ALS and Parkinson’s disease. Exp. Neurol. 2002, 177, 515–520. [Google Scholar] [CrossRef]
- Els, T.; Bruckmann, J.; Rohn, G.; Daffertshofer, M.; Monting, J.S.; Ernestus, R.I.; Hennerici, M. Spermidine: A predictor for neurological outcome and infarct size in focal cerebral ischemia? Stroke 2001, 32, 43–46. [Google Scholar] [CrossRef]
- Inoue, K.; Tsutsui, H.; Akatsu, H.; Hashizume, Y.; Matsukawa, N.; Yamamoto, T.; Toyo’oka, T. Metabolic profiling of Alzheimer’s disease brains. Sci. Rep. 2013, 3, 2364. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Igarashi, K. Polyamines and their metabolites as diagnostic markers of human diseases. Biomol. Ther. 2013, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.; Sousa-Victor, P. Regulation of inflammation as an anti-aging intervention. FEBS J. 2020, 287, 43–52. [Google Scholar] [CrossRef]
- Daimon, M.; Sugiyama, K.; Kameda, W.; Saitoh, T.; Oizumi, T.; Hirata, A.; Yamaguchi, H.; Ohnuma, H.; Igarashi, M.; Kato, T. Increased urinary levels of pentosidine, pyrraline and acrolein adduct in type 2 diabetes. Endocr. J. 2003, 50, 61–67. [Google Scholar] [CrossRef]
- Sakata, K.; Kashiwagi, K.; Sharmin, S.; Ueda, S.; Igarashi, K. Acrolein produced from polyamines as one of the uraemic toxins. Biochem. Soc. Trans. 2003, 31, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Tomitori, H.; Usui, T.; Saeki, N.; Ueda, S.; Kase, H.; Nishimura, K.; Kashiwagi, K.; Igarashi, K. Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke 2005, 36, 2609–2613. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Kanzaki, T.; Mizoi, M.; Nakamura, M.; Uemura, T.; Mimori, S.; Uju, Y.; Sekine, K.; Ishii, Y.; Yoshimi, T.; et al. Correlation between brain damage, associated biomarkers, and medication in psychiatric inpatients: A cross-sectional study. Clin. Chim. Acta 2017, 464, 50–56. [Google Scholar] [CrossRef]
- Kawabata, C.; Nagasawa, T.; Ono, M.; Tarumoto, N.; Katoh, N.; Hotta, Y.; Kawano, H.; Igarashi, K.; Shiokawa, K.; Nishimura, K. Plasma acrolein level in rheumatoid arthritis increases independently of the disease characteristics. Mod. Rheumatol. 2021, 31, 357–364. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; Ebner, N.; Dos Santos, M.R.; Ishida, J.; Hasenfuss, G.; von Haehling, S. Cachexia, muscle wasting, and frailty in cardiovascular disease. Eur. J. Heart Fail. 2020, 22, 2314–2326. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Chhetri, J.K.; de Souto Barreto, P.; Fougere, B.; Rolland, Y.; Vellas, B.; Cesari, M. Chronic inflammation and sarcopenia: A regenerative cell therapy perspective. Exp. Gerontol. 2018, 103, 115–123. [Google Scholar] [CrossRef]
- Sharif, S.; Van der Graaf, Y.; Cramer, M.J.; Kapelle, L.J.; de Borst, G.J.; Visseren, F.L.J.; Westerink, J.; SMART Study Group. Low-grade inflammation as a risk factor for cardiovascular events and all-cause mortality in patients with type 2 diabetes. Cardiovasc. Diabetol. 2021, 20, 220. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, K.; Igarashi, K. Molecular Characteristics of Toxicity of Acrolein Produced from Spermine. Biomolecules 2023, 13, 298. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. Functional roles of polyamines and their metabolite acrolein in eukaryotic cells. Amino Acids 2021, 53, 1473–1492. [Google Scholar] [CrossRef]
- Cipolla, B.; Guilli, F.; Moulinoux, J.P. Polyamine-reduced diet in metastatic hormone-refractory prostate cancer (HRPC) patients. Biochem. Soc. Trans. 2003, 31, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Araki, N.; Ohnishi, Y.; Kozaki, S. Effects of dietary polyamine deficiency on Trypanosoma gambiense infection in rats. Exp. Parasitol. 2001, 97, 95–101. [Google Scholar] [CrossRef]
- Soda, K. Anti-aging by polyamine. Food Style 21 2006, 10, 43–54. (In Japanese) [Google Scholar]
- Soda, K.; Kano, Y.; Sakuragi, M.; Takao, K.; Lefor, A.; Konishi, F. Long-term oral polyamine intake increases blood polyamine concentrations. J. Nutr. Sci. Vitaminol. 2009, 55, 361–366. [Google Scholar] [CrossRef]
- Schwarz, C.; Stekovic, S.; Wirth, M.; Benson, G.; Royer, P.; Sigrist, S.J.; Pieber, T.; Dammbrueck, C.; Magnes, C.; Eisenberg, T.; et al. Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging 2018, 10, 19–33. [Google Scholar] [CrossRef]
- Wirth, M.; Benson, G.; Schwarz, C.; Kobe, T.; Grittner, U.; Schmitz, D.; Sigrist, S.J.; Bohlken, J.; Stekovic, S.; Madeo, F.; et al. The effect of spermidine on memory performance in older adults at risk for dementia: A randomized controlled trial. Cortex 2018, 109, 181–188. [Google Scholar] [CrossRef]
- Senekowitsch, S.; Wietkamp, E.; Grimm, M.; Schmelter, F.; Schick, P.; Kordowski, A.; Sina, C.; Otzen, H.; Weitschies, W.; Smollich, M. High-dose spermidine supplementation does not increase spermidine levels in blood plasma and saliva of healthy adults: A randomized placebo-controlled pharmacokinetic and metabolomic study. Nutrients 2023, 15, 1852. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Nakamura, T.; Kasono, K.; Kawakami, M.; Konishi, F. Spermine, a natural polyamine, suppresses LFA-1 expression on human lymphocyte. J. Immunol. 2005, 175, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Caragine, T.; Wang, H.; Cohen, P.S.; Botchkina, G.; Soda, K.; Bianchi, M.; Ulrich, P.; Cerami, A.; Sherry, B.; et al. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: A counterregulatory mechanism that restrains the immune response. J. Exp. Med. 1997, 185, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Buttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Deng, C.; Lu, Q.; Richardson, B. Age-dependent DNA methylation changes in the ITGAL (CD11a) promoter. Mech. Ageing Dev. 2002, 123, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Kaplan, M.; Ray, D.; Ray, D.; Zacharek, S.; Gutsch, D.; Richardson, B. Demethylation of ITGAL (CD11a) regulatory sequences in systemic lupus erythematosus. Arthritis Rheum. 2002, 46, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Soda, K.; Konishi, F. Suppression of LFA-1 expression by spermine is associated with enhanced methylation of ITGAL, the LFA-1 promoter area. PLoS ONE 2013, 8, e56056. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Usui, S.; Aida, T.; Tachikawa, T.; Hu, G.F.; Sasaki, A.; Matsumura, T.; Todd, R.; Wong, D.T. Induction of epithelial differentiation and DNA demethylation in hamster malignant oral keratinocyte by ornithine decarboxylase antizyme. Oncogene 2001, 20, 24–33. [Google Scholar] [CrossRef]
- Yamamoto, D.; Shima, K.; Matsuo, K.; Nishioka, T.; Chen, C.Y.; Hu, G.F.; Sasaki, A.; Tsuji, T. Ornithine decarboxylase antizyme induces hypomethylation of genome DNA and histone H3 lysine 9 dimethylation (H3K9me2) in human oral cancer cell line. PLoS ONE 2010, 5, e12554. [Google Scholar] [CrossRef]
- Soda, K. Polyamine Metabolism and Gene Methylation in Conjunction with One-Carbon Metabolism. Int. J. Mol. Sci. 2018, 19, 3106. [Google Scholar] [CrossRef]
- Perez, R.F.; Tejedor, J.R.; Bayon, G.F.; Fernandez, A.F.; Fraga, M.F. Distinct chromatin signatures of DNA hypomethylation in aging and cancer. Aging Cell 2018, 17, e12744. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Chen, X.; Ning, C.; Zhu, Q.; Yao, Y.; Zhao, Y.; Luan, F. Methylation of the genes ROD1, NLRC5, and HKR1 is associated with aging in Hainan centenarians. BMC Med. Genom. 2018, 11, 7. [Google Scholar] [CrossRef] [PubMed]

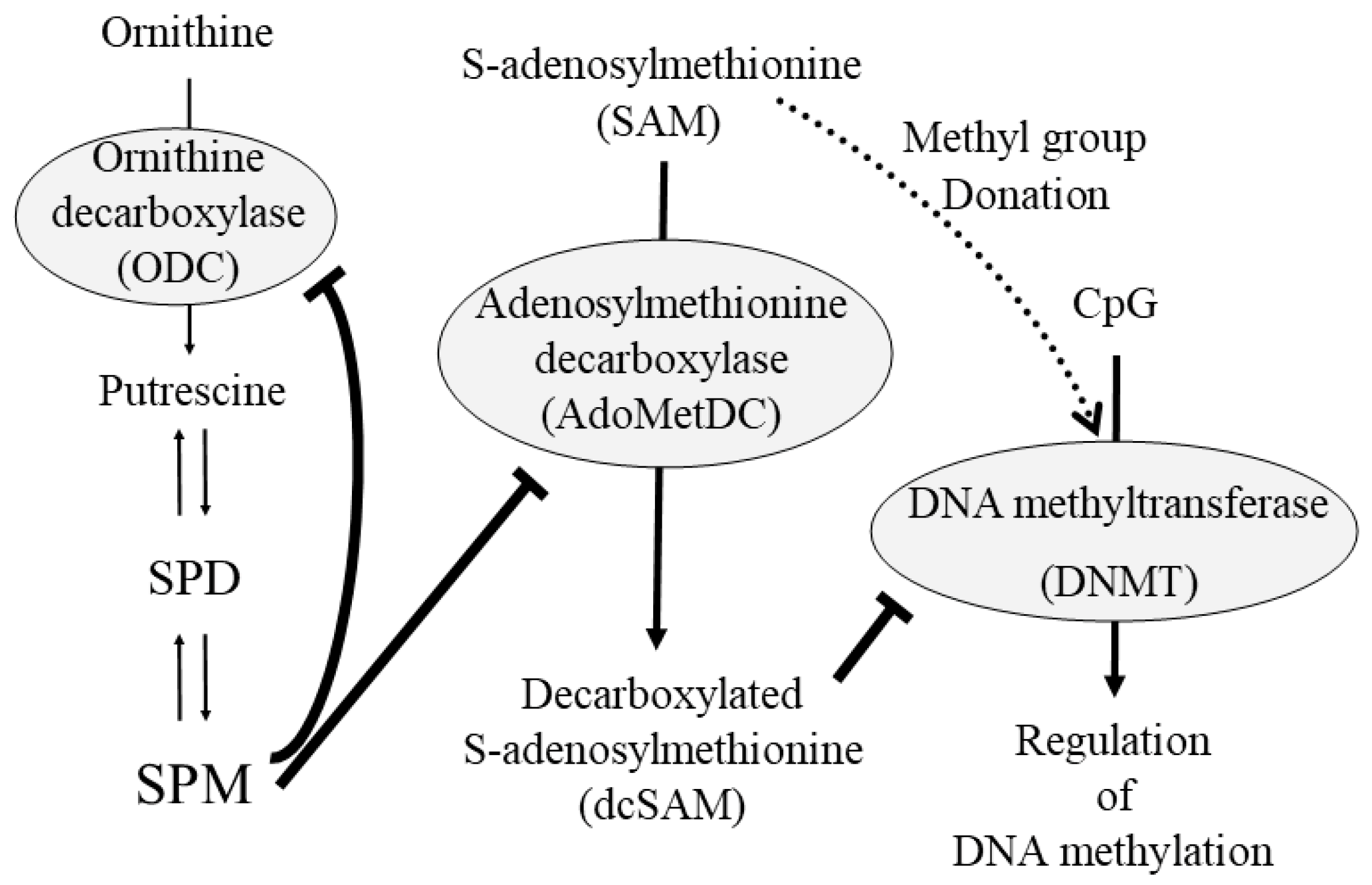
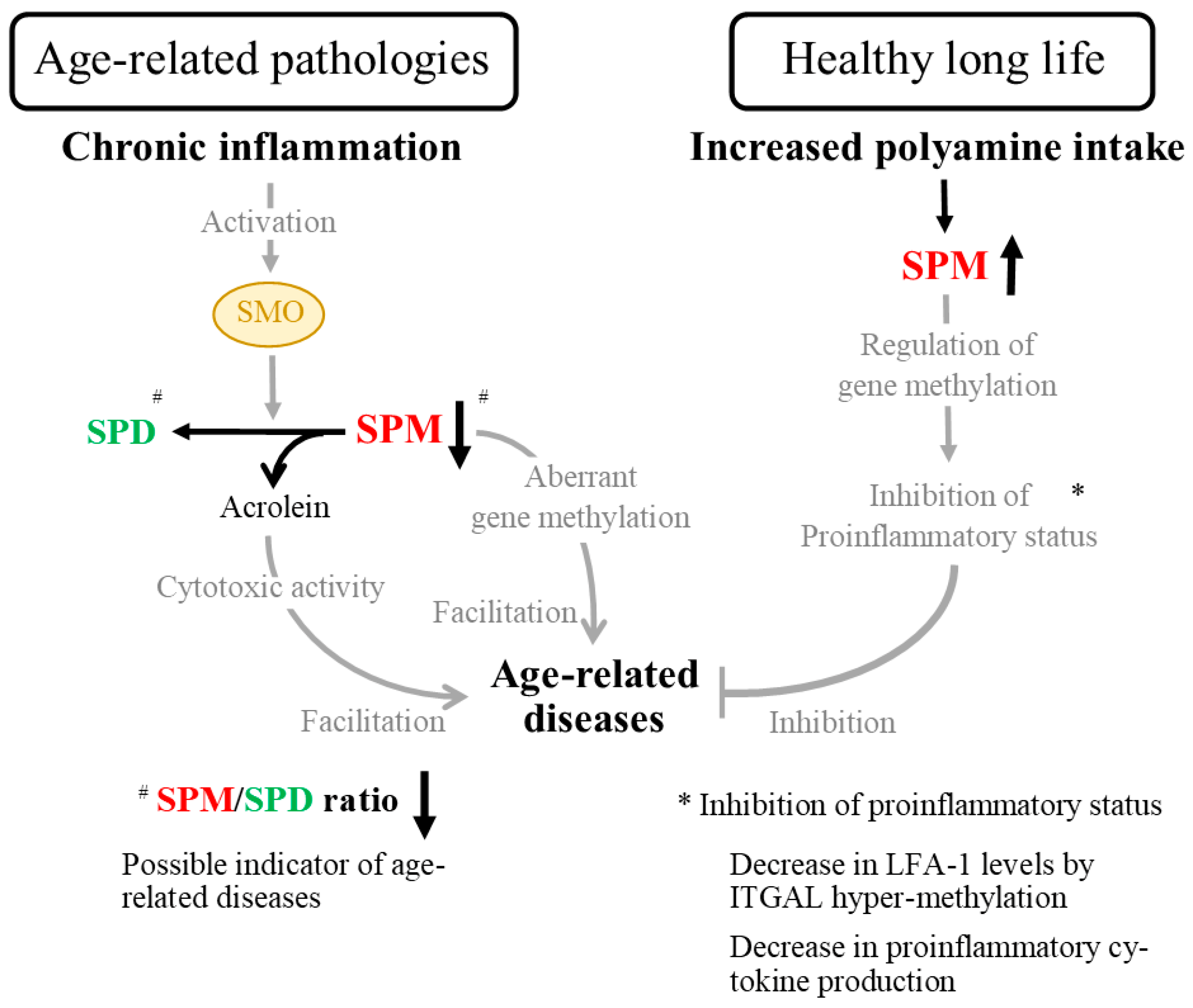
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soda, K. Changes in Whole Blood Polyamine Levels and Their Background in Age-Related Diseases and Healthy Longevity. Biomedicines 2023, 11, 2827. https://doi.org/10.3390/biomedicines11102827
Soda K. Changes in Whole Blood Polyamine Levels and Their Background in Age-Related Diseases and Healthy Longevity. Biomedicines. 2023; 11(10):2827. https://doi.org/10.3390/biomedicines11102827
Chicago/Turabian StyleSoda, Kuniyasu. 2023. "Changes in Whole Blood Polyamine Levels and Their Background in Age-Related Diseases and Healthy Longevity" Biomedicines 11, no. 10: 2827. https://doi.org/10.3390/biomedicines11102827
APA StyleSoda, K. (2023). Changes in Whole Blood Polyamine Levels and Their Background in Age-Related Diseases and Healthy Longevity. Biomedicines, 11(10), 2827. https://doi.org/10.3390/biomedicines11102827






