Pathomorphological Features of the Novel Coronavirus Disease in Patients with Systemic Amyloidosis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
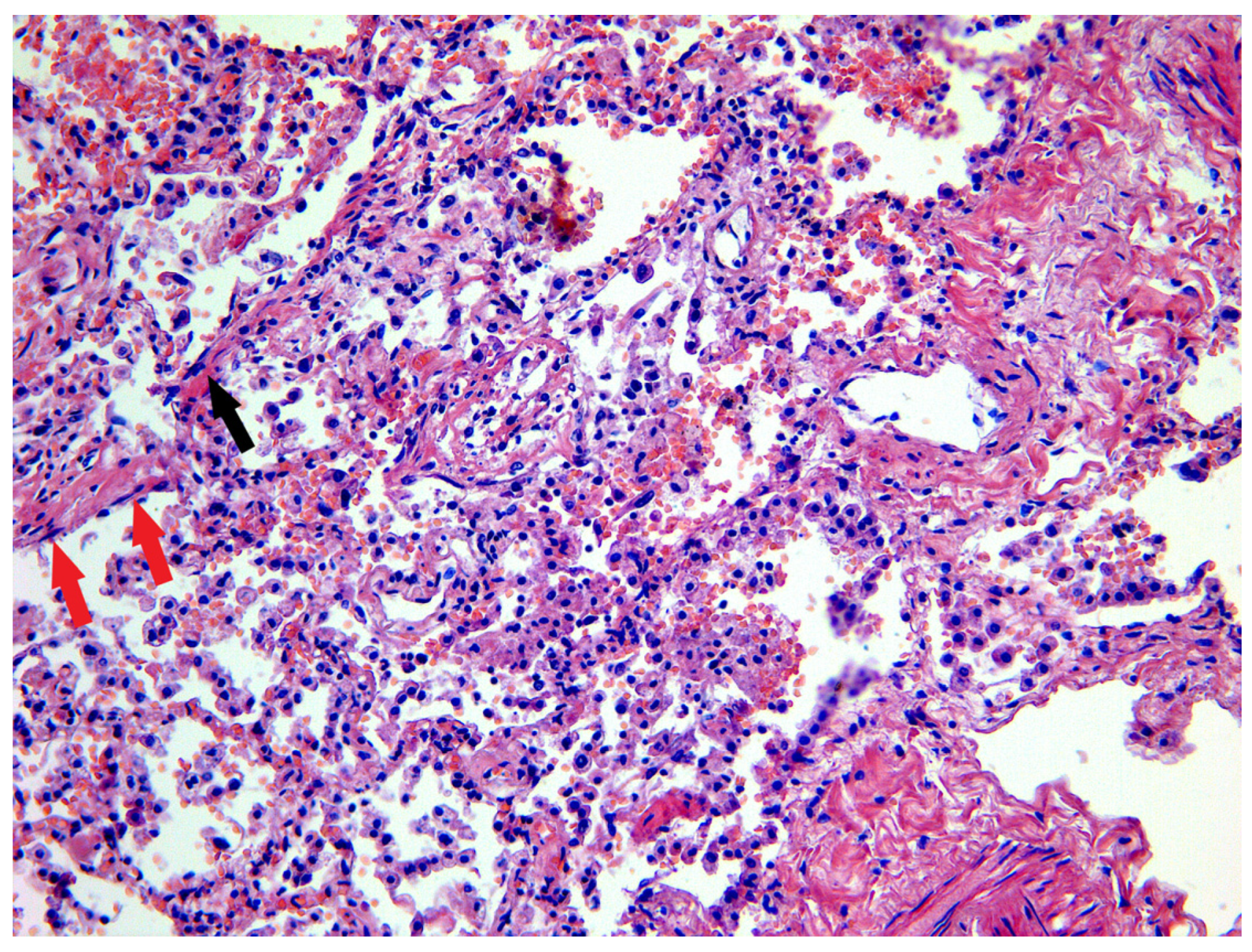
3.1. Lung and Upper Airway Findings
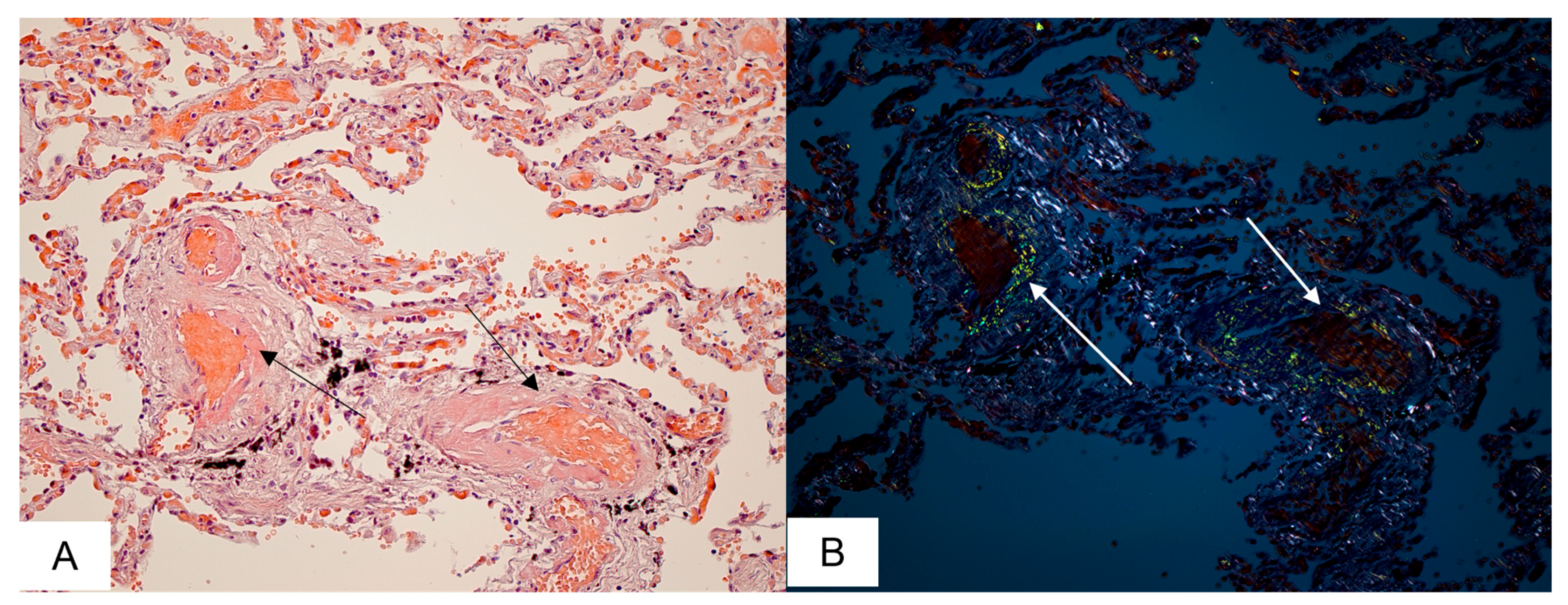
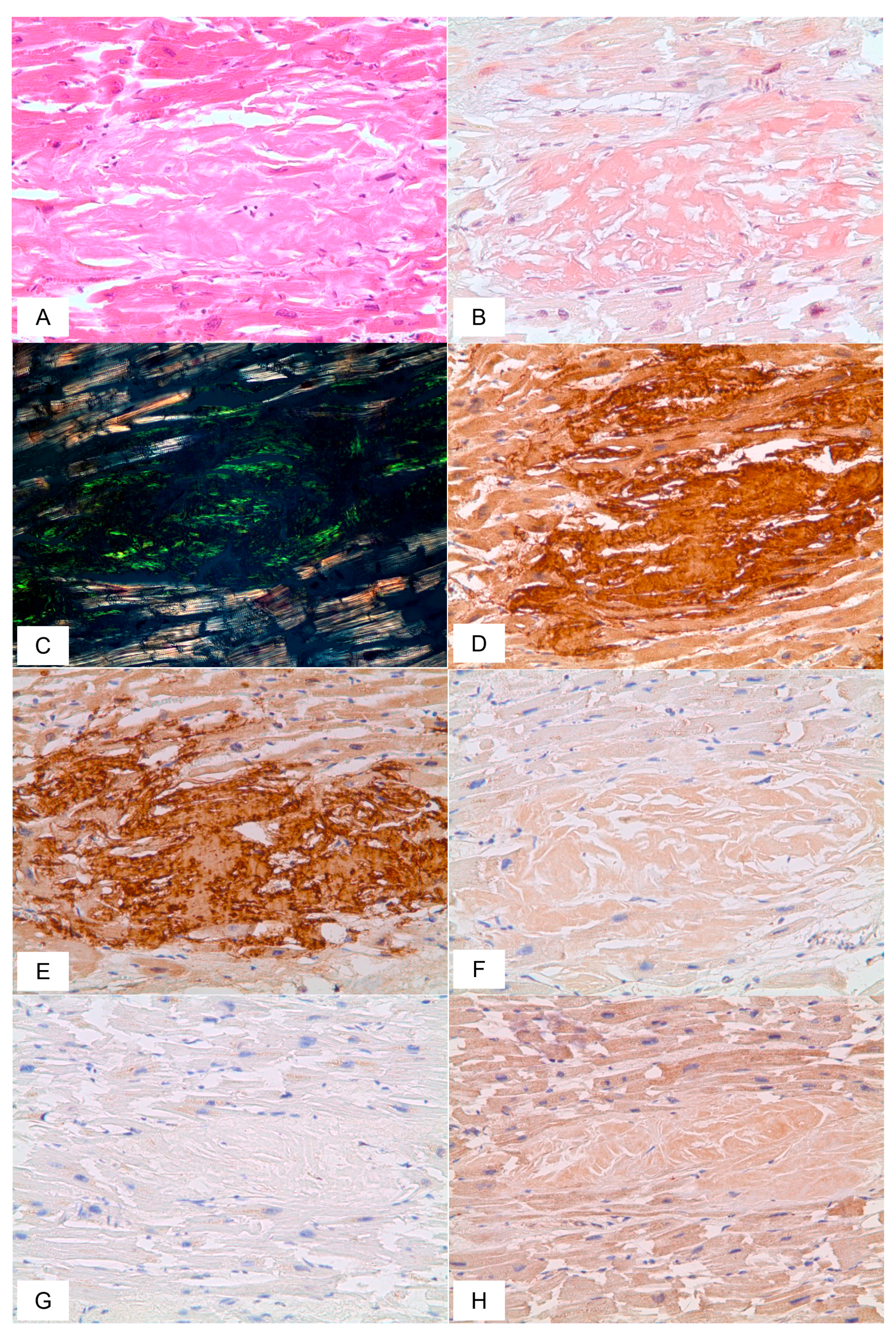
3.2. Cardiovascular System Findings
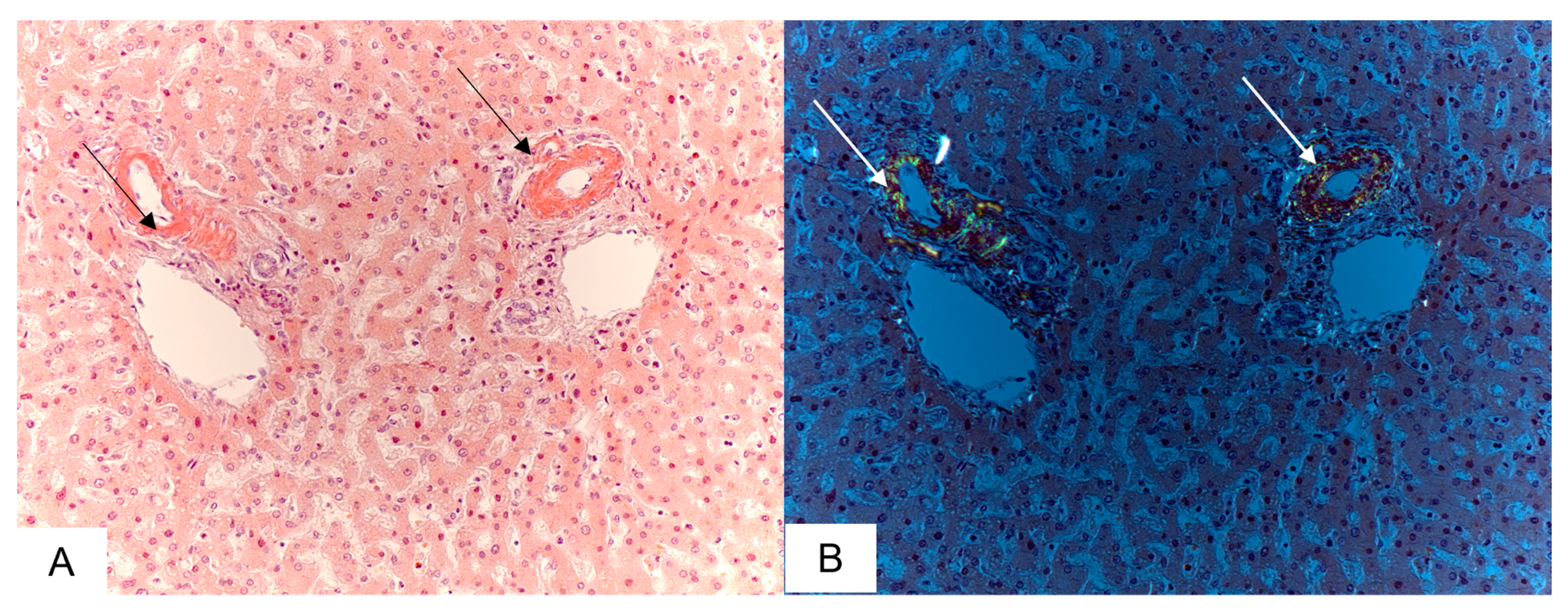
3.3. Liver Findings
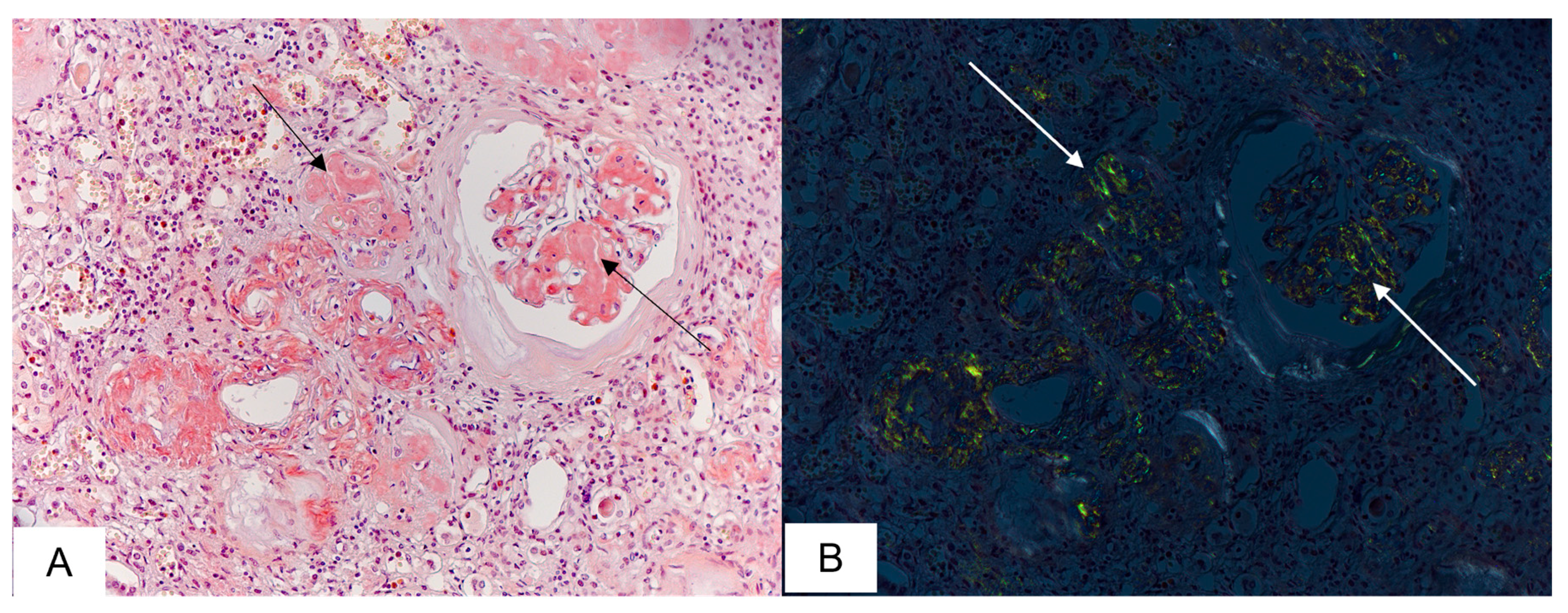
3.4. Kidney Findings
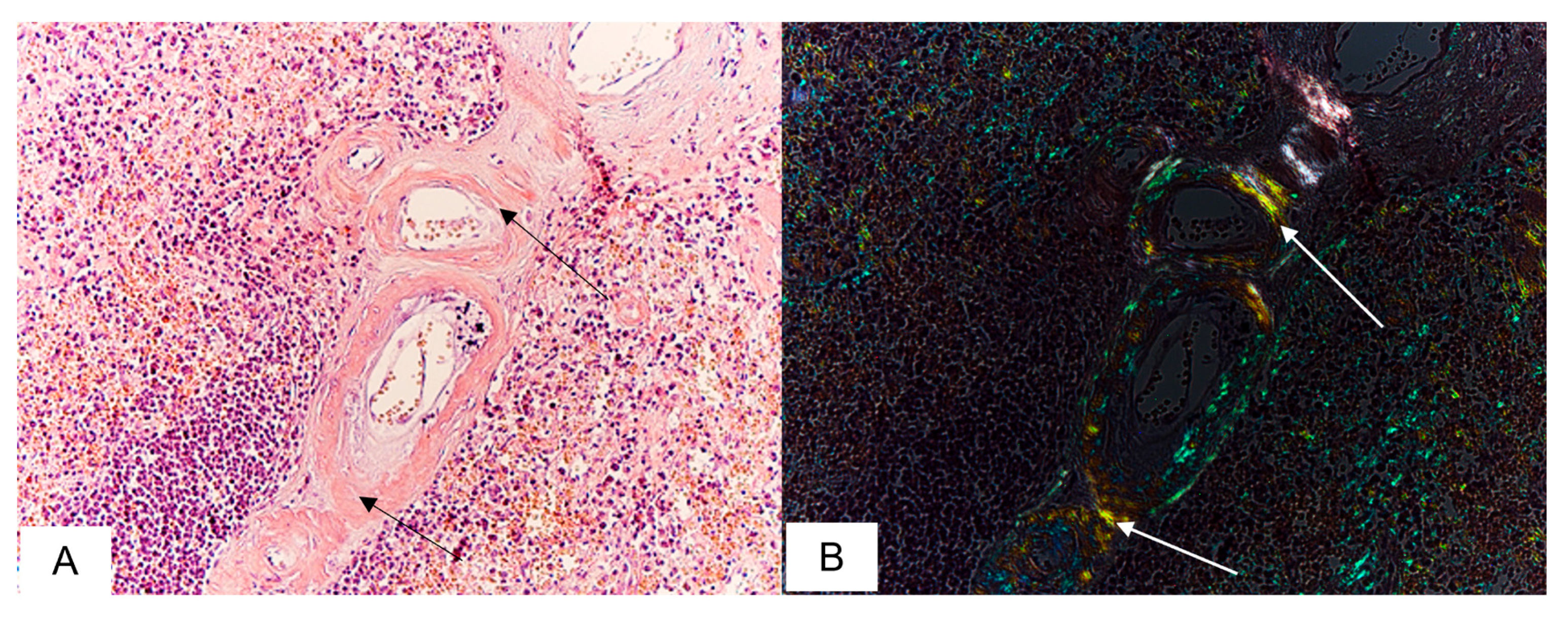
3.5. Spleen Findings
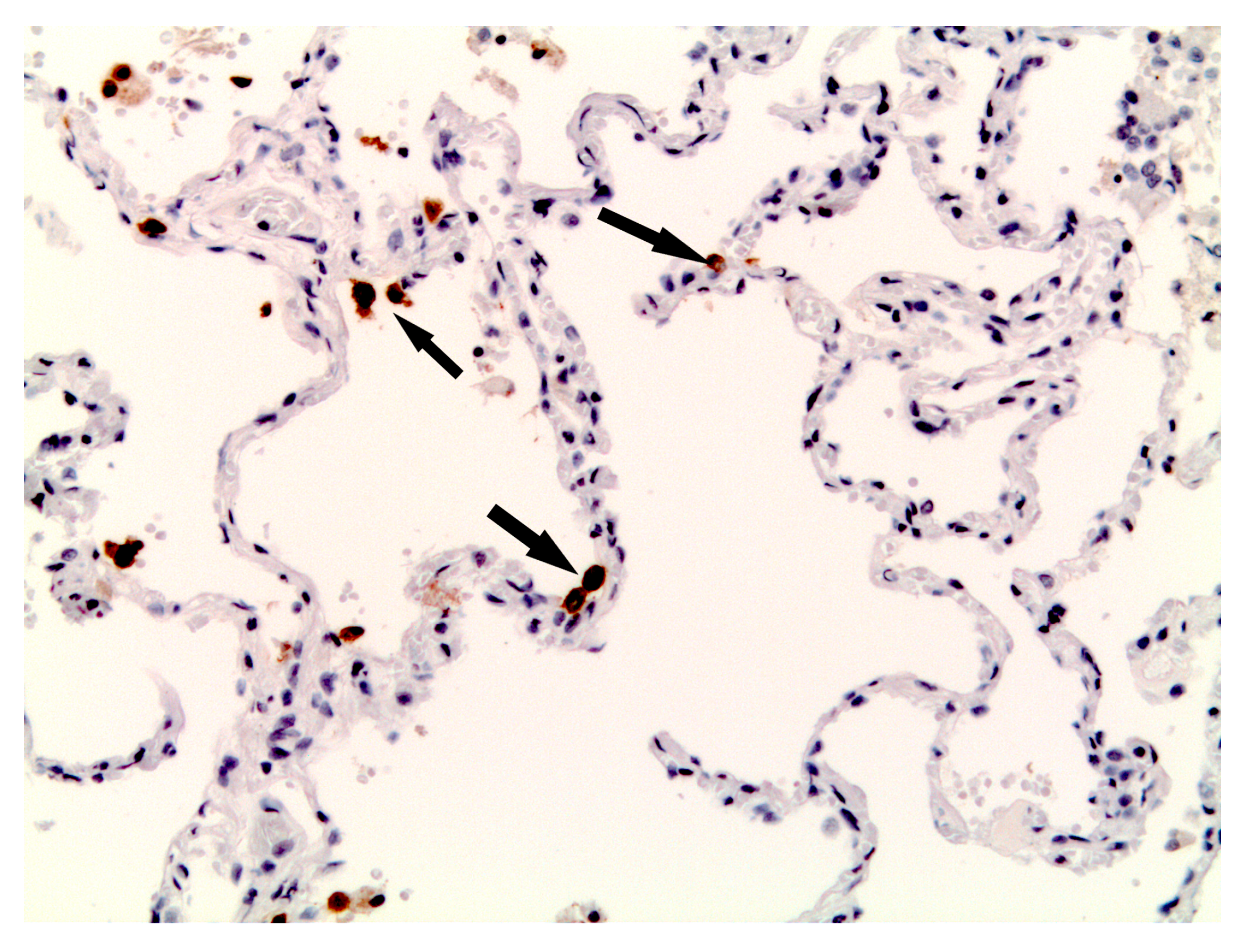
3.6. Immunohistochemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, V.; Chrysovergis, A.; Ragos, V.; Tsiambas, E.; Katsinis, S.; Manoli, A.; Papouliakos, S.; Roukas, D.; Mastronikolis, S.; Peschos, D.; et al. From delta to Omicron: S1-RBD/S2 mutation/deletion equilibrium in SARS-CoV-2 defined variants. Gene 2022, 814, 146134. [Google Scholar] [CrossRef]
- Verma, J.; Subbarao, N. A comparative study of human betacoronavirus spike proteins: Structure, function and therapeutics. Arch. Virol. 2021, 166, 697–714. [Google Scholar] [CrossRef] [PubMed]
- Villalba, J.A.; Hilburn, C.F.; Garlin, M.A.; Elliott, G.A.; Li, Y.; Kunitoki, K.; Poli, S.; Alba, G.A.; Madrigal, E.; Taso, M.; et al. Vasculopathy and Increased Vascular Congestion in Fatal COVID-19 and Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2022, 206, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Ochani, R.; Asad, A.; Yasmin, F.; Shaikh, S.; Khalid, H.; Batra, S.; Sohail, M.R.; Mahmood, S.F.; Ochani, R.; Arshad, M.H.; et al. COVID-19 pandemic: From origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez. Med. 2021, 29, 20–36. [Google Scholar] [PubMed]
- Castiello, T.; Georgiopoulos, G.; Finocchiaro, G.; Claudia, M.; Gianatti, A.; Delialis, D.; Aimo, A.; Prasad, S. COVID-19 and myocarditis: A systematic review and overview of current challenges. Heart Fail. Rev. 2022, 27, 251–261. [Google Scholar] [CrossRef]
- Becker, R.C. COVID-19-associated vasculitis and vasculopathy. J. Thromb. Thrombolysis 2020, 50, 499–511. [Google Scholar] [CrossRef]
- Tiwari, N.R.; Phatak, S.; Sharma, V.R.; Agarwal, S.K. COVID-19 and thrombotic microangiopathies. Thromb. Res. 2021, 202, 191–198. [Google Scholar] [CrossRef]
- Müller-Wieland, D.; Marx, N.; Dreher, M.; Fritzen, K.; Schnell, O. COVID-19 and Cardiovascular Comorbidities. Exp. Clin. Endocrinol. Diabetes 2022, 130, 178–189. [Google Scholar] [CrossRef]
- Djaharuddin, I.; Munawwarah, S.; Nurulita, A.; Ilyas, M.; Tabri, N.A.; Lihawa, N. Comorbidities and mortality in COVID-19 patients. Gac. Sanit. 2021, 35 (Suppl. S2), S530–S532. [Google Scholar] [CrossRef]
- Buxbaum, J.N.; Dispenzieri, A.; Eisenberg, D.S.; Fändrich, M.; Merlini, G.; Saraiva, M.J.M.; Sekijima, Y.; Westermark, P. Amyloid nomenclature 2022: Update, novel proteins, and recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid 2022, 29, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Muchtar, E.; Dispenzieri, A.; Magen, H.; Grogan, M.; Mauermann, M.; McPhail, E.D.; Kurtin, P.J.; Leung, N.; Buadi, F.K.; Dingli, D.; et al. Systemic amyloidosis from A (AA) to T (ATTR): A review. J. Intern. Med. 2021, 289, 268–292. [Google Scholar] [CrossRef] [PubMed]
- Picken, M.M. The Pathology of Amyloidosis in Classification: A Review. Acta Haematol. 2020, 143, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A.; Dispenzieri, A. Systemic Amyloidosis Recognition, Prognosis, and Therapy: A Systematic Review. JAMA 2020, 324, 79–89. [Google Scholar] [CrossRef]
- Kastritis, E.; Wechalekar, A.; Schönland, S.; Sanchorawala, V.; Merlini, G.; Palladini, G.; Minnema, M.; Roussel, M.; Jaccard, A.; Hegenbart, U.; et al. Challenges in the management of patients with systemic light chain (AL) amyloidosis during the COVID-19 pandemic. Br. J. Haematol. 2020, 190, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.-Y.; Wu, H.H.L.; Floyd, L.; Ponnusamy, A.; Chinnadurai, R. COVID-19 Infection and Vaccination and Its Relation to Amyloidosis: What Do We Know Currently? Vaccines 2023, 11, 1139. [Google Scholar] [CrossRef]
- Frank, G.A.; Kovalev, A.V.; Gribunov, Y.P.; Zaslavskij, G.I.; Kil’dyushov, E.M.; Yagmurov, O.D.; Tuchik, E.S.; Timerzyanov, M.I.; Putincev, V.A.; Minaeva, P.V. Issledovaniye Umershikh s Podozreniyem na Koronavirusnuyu Infektsiyu (COVID-19). In Vremennyye Metodicheskiye Rekomendatsii, M.Z.R.F.; Version 15 (30 April 2020); Ministry of Health of the Russian Federation: Moscow, Russia, 2020; 256p. (In Russian) [Google Scholar]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausi ello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Scorza, F.A.; Scorza, C.A.; Fiorini, A.C. Extrapulmonary onset manifestations of COVID-19. Clinics 2021, 76, e2900. [Google Scholar] [CrossRef]
- Elrobaa, I.H.; New, K.J. COVID-19: Pulmonary and Extra Pulmonary Manifestations. Front. Public Health 2021, 9, 711616. [Google Scholar] [CrossRef]
- Ihne, S.; Morbach, C.; Sommer, C.; Geier, A.; Knop, S.; Störk, S. Amyloidosis—The Diagnosis and Treatment of an Underdiagnosed Disease. Dtsch. Aerzteblatt Online 2020, 117, 159–166. [Google Scholar] [CrossRef]
- Qatanani, A.; Dahab, S.; Douedi, S.; Hamid, Y.; Girgis, S.; Daniels, S.; Lamba, G.; Zafar, M. Delayed Diagnosis of Cardiac Amyloidosis Secondary to Multiple Myeloma. J. Med. Cases 2021, 12, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Chatzileontiadou, S.; Zegkos, T.; Frouzaki, C.; Apsemidou, A.; Efthimiadis, G.; Parcharidou, D.; Papaioannou, M. Real world data on light chain cardiac amyloidosis: Still a delayed diagnosis. Front. Oncol. 2022, 12, 944503. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Jin, Z.; Gan, T.J.; Wang, E. Silent Hypoxemia in Patients with COVID-19 Pneumonia: A Review. Experiment 2021, 27, e930776. [Google Scholar] [CrossRef] [PubMed]
- Dhont, S.; Derom, E.; Van Braeckel, E.; Depuydt, P.; Lambrecht, B.N. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir. Res. 2020, 21, 198. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Wi, Y.M.; Park, H.; Lee, J.E.; Kim, S.-H.; Lee, K.S. Current and Emerging Knowledge in COVID-19. Radiology 2023, 306, e222462. [Google Scholar] [CrossRef]
- Tian, S.; Xiong, Y.; Liu, H.; Niu, L.; Guo, J.; Liao, M.; Xiao, S.-Y. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. 2020, 33, 1007–1014. [Google Scholar] [CrossRef]
- Barton, L.M.; Duval, E.J.; Stroberg, E.; Ghosh, S.; Mukhopadhyay, S. COVID-19 Autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020, 153, 725–733. [Google Scholar] [CrossRef]
- Grosse, C.; Grosse, A.; Salzer, H.J.; Dünser, M.W.; Motz, R.; Langer, R. Analysis of cardiopulmonary findings in COVID-19 fatalities: High incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc. Pathol. 2020, 49, 107263. [Google Scholar] [CrossRef]
- Peiris, S.; Mesa, H.; Aysola, A.; Manivel, J.; Toledo, J.; Borges-Sa, M.; Aldighieri, S.; Reveiz, L. Pathological findings in organs and tissues of patients with COVID-19: A systematic review. PLoS ONE 2021, 16, e0250708. [Google Scholar] [CrossRef]
- Mikhaleva, L.M.; Cherniaev, A.L.; Samsonova, M.V.; Zayratyants, O.V.; Kakturskiy, L.V.; Vasyukova, O.A.; Birukov, A.E.; Kontorshchikov, A.S.; Sorokina, A.V.; Sinelnikov, M.Y. Pathological Features in 100 Deceased Patients with COVID-19 in Correlation with Clinical and Laboratory Data. Pathol. Oncol. Res. 2021, 27, 1609900. [Google Scholar] [CrossRef]
- Sofizan, N.M.F.B.N.; Rahman, A.F.B.A.; Soon, L.P.; Ly, C.K.; Abdullah, N.Z.B. Autopsy findings in COVID-19 infection-related death: A systematic review. Egypt. J. Forensic Sci. 2022, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Milani, P.; Basset, M.; Russo, F.; Foli, A.; Palladini, G.; Merlini, G. The lung in amyloidosis. Eur. Respir. Rev. 2017, 26, 170046. [Google Scholar] [CrossRef] [PubMed]
- Brannagan, T.H., 3rd; Auer-Grumbach, M.; Berk, J.L.; Briani, C.; Bril, V.; Coelho, T.; Damy, T.; Dispenzieri, A.; Drachman, B.M.; Fine, N.; et al. ATTR amyloidosis during the COVID-19 pandemic: Insights from a global medical roundtable. Orphanet J. Rare Dis. 2021, 16, 204. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, F.; Wang, L. COVID-19 and cardiovascular diseases. J. Mol. Cell Biol. 2021, 13, 161–167. [Google Scholar] [CrossRef]
- Khalil, F.; Oleszak, F.; Stys, T.; Stys, A. COVID-19 and Cardiovascular Disease: A Comprehensive Review. S. Dak. Med. 2022, 75, 54–60. [Google Scholar]
- Xanthopoulos, A.; Bourazana, A.; Giamouzis, G.; Skoularigki, E.; Dimos, A.; Zagouras, A.; Papamichalis, M.; Leventis, I.; Magouliotis, D.E.; Triposkiadis, F.; et al. COVID-19 and the heart. World J. Clin. Cases 2022, 10, 9970–9984. [Google Scholar] [CrossRef]
- Vosko, I.; Zirlik, A.; Bugger, H. Impact of COVID-19 on Cardiovascular Disease. Viruses 2023, 15, 508. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Yan, Y.; Zhang, Z.; Gong, W.; Nie, S. Clinical Characterization and Possible Pathological Mechanism of Acute Myocardial Injury in COVID-19. Front. Cardiovasc. Med. 2022, 9, 862571. [Google Scholar] [CrossRef]
- Grassi, S.; Arena, V.; Zedda, M.; Cazzato, F.; Cianci, R.; Gambassi, G.; Oliva, A. What can autopsy say about COVID-19? A case series of 60 autopsies. Leg. Med. 2023, 62, 102241. [Google Scholar] [CrossRef]
- Basu-Ray, I.; Almaddah, N.K.; Adeboye, A.; Soos, M.P. Cardiac Manifestations of Coronavirus (COVID-19). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Naeem, A.; Tabassum, S.; Gill, S.; Khan, M.Z.; Mumtaz, N.; Qaiser, Q.; Karamat, M.; Arif, M.; Naeem, F.; Afifi, A.; et al. COVID-19 and Cardiovascular Diseases: A Literature Review From Pathogenesis to Diagnosis. Cureus 2023, 15, e35658. [Google Scholar] [CrossRef]
- Kovrigina, A.; Shalamova, E.; Berezovskiy, Y.; Kalinin, D.; Gretsov, E.; Bagdasaryan, T.; Semenova, L.S.; Chebotarev, D.; Samsonova, M.; Chernyaev, A.; et al. Pathomorphological and immunohistochemical features of lymph nodes in COVID-19 patients (autopsy study). Clin. Exp. Morphol. 2020, 9, 12–23. (In Russian) [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Sun, H.; Su, X.; Huang, L.; Mu, D.; Qu, Y. Research Progress on the Cardiac Injury from ACE2 Targeting in SARS-CoV-2 Infection. Biomolecules 2021, 11, 196. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506, Erratum in Lancet 2020, 395, 496. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. China Medical Treatment Expert Group for COVID-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-C.; Wu, W.; Zhang, S.-Y. Manifestations and Mechanism of SARS-CoV2 Mediated Cardiac Injury. Int. J. Biol. Sci. 2022, 18, 2703–2713. [Google Scholar] [CrossRef]
- Crees, Z.D.; Stockerl-Goldstein, K. COVID-19 and Light Chain Amyloidosis, Adding Insult to Injury. Am. J. Med. 2022, 135 (Suppl. S1), S49–S52. [Google Scholar] [CrossRef]
- Driggin, E.; Helmke, S.; Santos, J.D.L.; Teruya, S.; Guadalupe, S.; Goldsmith, J.; Maurer, M.S. Markers of nutritional status and inflammation in transthyretin cardiac amyloidosis: Association with outcomes and the clinical phenotype. Amyloid 2020, 27, 73–80. [Google Scholar] [CrossRef]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. Post-mortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef]
- Gurung, R.; Li, T. Renal Amyloidosis: Presentation, Diagnosis, and Management. Am. J. Med. 2022, 135 (Suppl. S1), S38–S43. [Google Scholar] [CrossRef]
- Adrogue, H.E. Amyloidosis of the Heart and Kidney. Methodist DeBakey Cardiovasc. J. 2022, 18, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Reis, T.; Husain-Syed, F. Management of acute kidney injury in patients with COVID-19. Lancet Respir. Med. 2020, 8, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Benigni, A.; Remuzzi, G. Should COVID-19 Concern Nephrologists? Why and to What Extent? The Emerging Impasse of Angiotensin Blockade. Nephron 2020, 144, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Jonigk, D.; Werlein, C.; Acker, T.; Aepfelbacher, M.; Amann, K.U.; Baretton, G.; Barth, P.; Bohle, R.M.; Büttner, A.; Büttner, R.; et al. Organ manifestations of COVID-19: What have we learned so far (not only) from autopsies? Virchows Arch. 2022, 481, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.; Fine, N.; Miller, R.J.H.; Hahn, C.; Chhibber, S.; Mahe, E.; Tay, J.; Duggan, P.; McCulloch, S.; Bahlis, N.; et al. Amyloidosis and COVID-19: Experience from an amyloid program in Canada. Ann. Hematol. 2022, 101, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.A.; Neuberg, D.S.; Thompson, J.C.; Tallman, M.S.; Sekeres, M.A.; Sehn, L.H.; Anderson, K.C.; Goldberg, A.D.; Pennell, N.A.; Niemeyer, C.M.; et al. Outcomes of patients with hematologic malignancies and COVID-19: A report from the ASH Research Collaborative Data Hub. Blood Adv. 2020, 4, 5966–5975. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Zanwar, S.; Buadi, F.K.; Ailawadhi, S.; Larsen, J.; Bergsagel, L.; Binder, M.; Chanan-Khan, A.; Dingli, D.; Dispenzieri, A.; et al. Risk factors for severe infection and mortality In patients with COVID-19 in patients with multiple myeloma and AL amyloidosis. Am. J. Hematol. 2023, 98, 49–55. [Google Scholar] [CrossRef]
| Finding | Number of Cases | % |
|---|---|---|
| Pulmonary capillary congestion | 22/22 | 100 |
| Hyaline membranes | 17/22 | 77 |
| Fibrin in alveoli | 18/22 | 81 |
| Interstitial edema | 9/22 | 41 |
| Metaplasia of alveolar epithelium | 6/22 | 27 |
| Desquamation of alveolar epithelium | 19/22 | 86 |
| Pulmonary infarction | 2/22 | 9 |
| Microvascular thrombosis | 16/22 | 72 |
| Giant cells | 8/22 | 36 |
| Hyperplasia of Type II alveolocytes | 10/22 | 45 |
| Pleuritis with fibrin | 2/22 | 9 |
| Amyloid deposits | 8/22 | 36 |
| Patient No. | Gender | Age | Amyloid Type | Heart | Lung | Kidney | Liver | Spleen | Pancreas | Gastrointestinal Tract | Thyroid Gland | Adrenal Gland |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | m | 56 | AA | * | * | * | * | |||||
| 2 | f | 63 | AA | * | * | * | ||||||
| 3 | m | 53 | AA | * | * | * | * | * | * | * | * | |
| 4 | m | 71 | AA | * | * | * | * | * | * | |||
| 5 | f | 80 | AA | * | * | * | * | * | ||||
| 6 | m | 84 | AL-lambda | * | * | * | * | |||||
| 7 | f | 73 | AL-lambda | * | * | * | ||||||
| 8 | m | 70 | AL-lambda | * | * | * | * | * | ||||
| 9 | f | 69 | AL-lambda | * | * | * | * | * | ||||
| 10 | f | 87 | AL-lambda | * | * | * | * | |||||
| 11 | m | 59 | AL-lambda | * | * | * | * | |||||
| 12 | f | 60 | AL-lambda | * | * | * | ||||||
| 13 | f | 75 | AL-kappa | * | * | * | * | * | * | * | * | |
| 14 | m | 88 | AL-kappa | * | * | * | * | |||||
| 15 | f | 89 | ATTR | * | * | * | ||||||
| 16 | f | 80 | ATTR | * | * | * | ||||||
| 17 | m | 88 | ATTR | * | * | * | * | |||||
| 18 | f | 92 | ATTR | * | * | * | ||||||
| 19 | m | 95 | ATTR | * | * | * | ||||||
| 20 | m | 91 | ATTR | * | * | * | * | |||||
| 21 | m | 95 | ATTR | * | * | * | * | |||||
| 22 | f | 93 | ATTR | * | * | * | * | |||||
| Organ involvement in amyloidosis (%) revealed by 22 autopsies | 86 | 36 | 73 | 45 | 59 | 23 | 73 | 14 | 18 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhaleva, L.; Gioeva, Z.; Varyasin, V.; Berezhnaja, E.; Vandysheva, R.; Gutyrchik, N.; Pechnikova, V.; Kontorshchikov, A.; Midiber, K.; Kakturskij, L. Pathomorphological Features of the Novel Coronavirus Disease in Patients with Systemic Amyloidosis. Biomedicines 2023, 11, 2811. https://doi.org/10.3390/biomedicines11102811
Mikhaleva L, Gioeva Z, Varyasin V, Berezhnaja E, Vandysheva R, Gutyrchik N, Pechnikova V, Kontorshchikov A, Midiber K, Kakturskij L. Pathomorphological Features of the Novel Coronavirus Disease in Patients with Systemic Amyloidosis. Biomedicines. 2023; 11(10):2811. https://doi.org/10.3390/biomedicines11102811
Chicago/Turabian StyleMikhaleva, Liudmila, Zarina Gioeva, Valery Varyasin, Elvira Berezhnaja, Rositsa Vandysheva, Nikita Gutyrchik, Valentina Pechnikova, Andrej Kontorshchikov, Konstantin Midiber, and Lev Kakturskij. 2023. "Pathomorphological Features of the Novel Coronavirus Disease in Patients with Systemic Amyloidosis" Biomedicines 11, no. 10: 2811. https://doi.org/10.3390/biomedicines11102811
APA StyleMikhaleva, L., Gioeva, Z., Varyasin, V., Berezhnaja, E., Vandysheva, R., Gutyrchik, N., Pechnikova, V., Kontorshchikov, A., Midiber, K., & Kakturskij, L. (2023). Pathomorphological Features of the Novel Coronavirus Disease in Patients with Systemic Amyloidosis. Biomedicines, 11(10), 2811. https://doi.org/10.3390/biomedicines11102811






