Extracellular Vesicles Derived from Osteogenic-Differentiated Human Bone Marrow-Derived Mesenchymal Cells Rescue Osteogenic Ability of Bone Marrow-Derived Mesenchymal Cells Impaired by Hypoxia
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Isolation, Culture and Characterization of Immunophenotype of Human BMSCs
2.3. Generation of EVs-Depleted FCS
2.4. Osteogenic Differentiation of Human BMSCs
2.5. Induction of Hypoxia
2.6. Collection of Conditioned Medium for EV Isolation
2.7. EV Isolation
2.8. Conditioned Medium pH under Hypoxia and Normoxia
2.9. Subcellular Protein Extraction
2.10. Western Blotting Analysis
2.10.1. Detection of EV Markers
2.10.2. Detection of HIF-1α and RUNX2
2.11. EV Uptake Test
2.12. Nanoparticle Tracking Analysis (NTA)
2.13. Alkaline Phosphatase Assay
2.14. Alizarin Red Staining
2.15. RNA Isolation and Real-Time -qPCR
2.16. Measurement of Reactive Oxygen Species (ROS) Level
2.17. Proteomic Analysis
2.17.1. Sample Preparation of EVs for Proteomics Analysis
2.17.2. Quantitative Proteomic Analysis by LC-MS/MS
2.17.3. Database Search and Bioinformatics Analysis
2.18. Statistical Analysis
3. Results
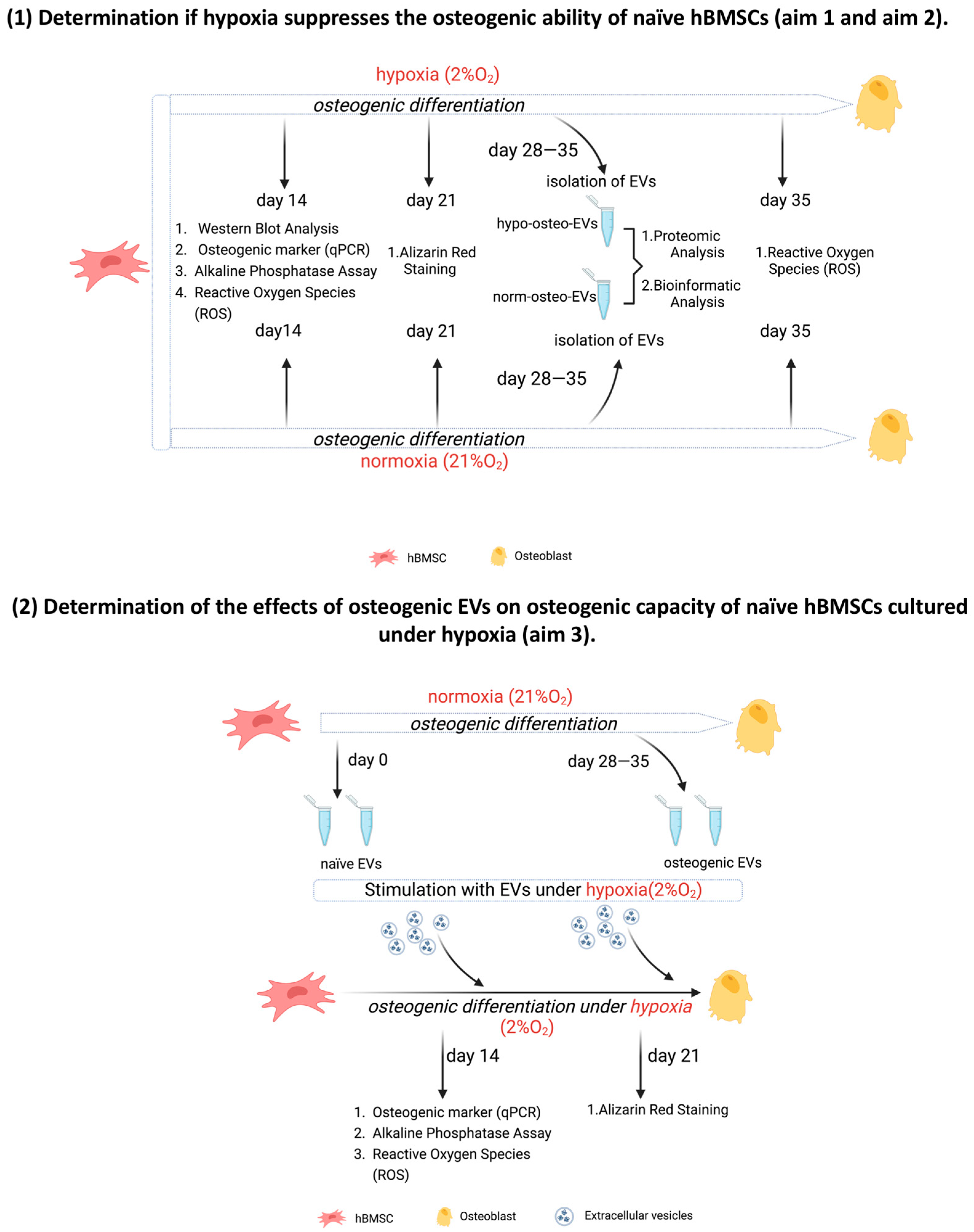
3.1. Generating Conditioned Medium for Preparation of Osteogenic EVs from a Hypoxic (Hypo-Osteo EVs) Environment and Osteogenic EVs from a Normoxic (Norm-Osteo EVs) Environment
3.2. Characterization of EVs
3.2.1. NTA Evaluation of EVs
3.2.2. Uptake of EVs by Naïve hBMSCs
3.2.3. Surface Markers of EVs
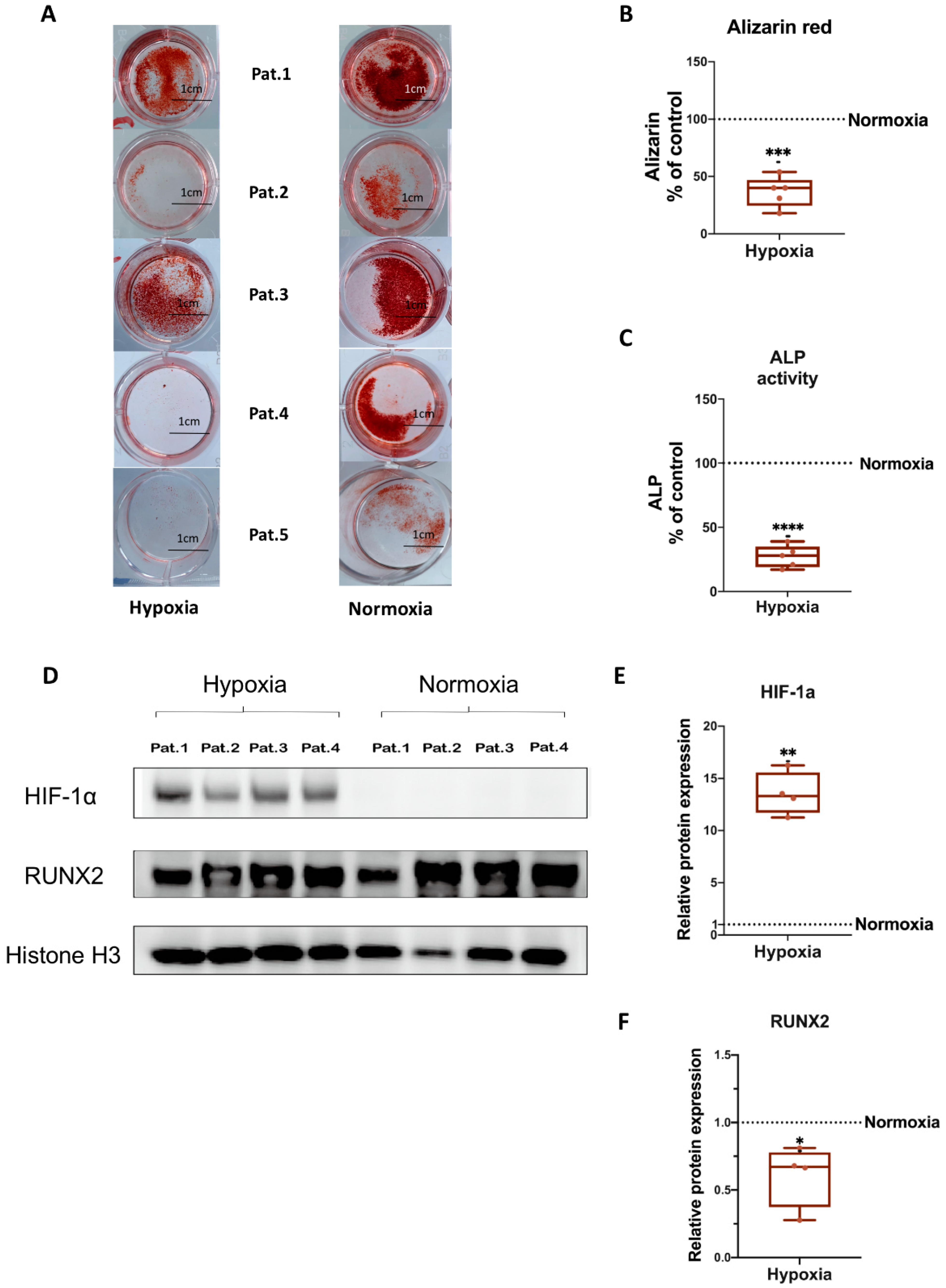
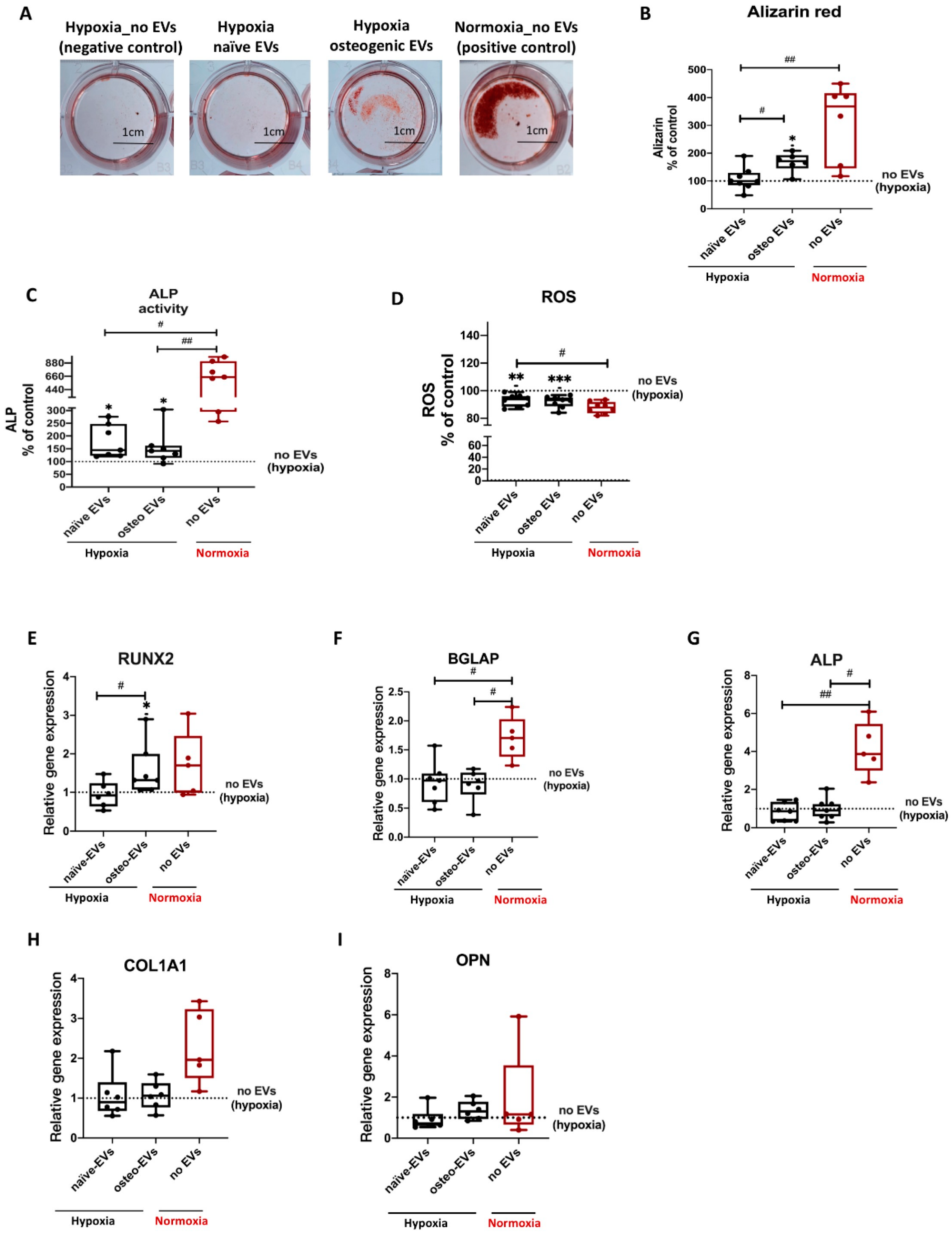
3.3. Osteogenic Differentiation of hBMSCs under Hypoxia
3.4. HIF-1α and RUNX2 Protein Expression in hBMSCs Undergoing Osteogenic Differentiation under Hypoxia
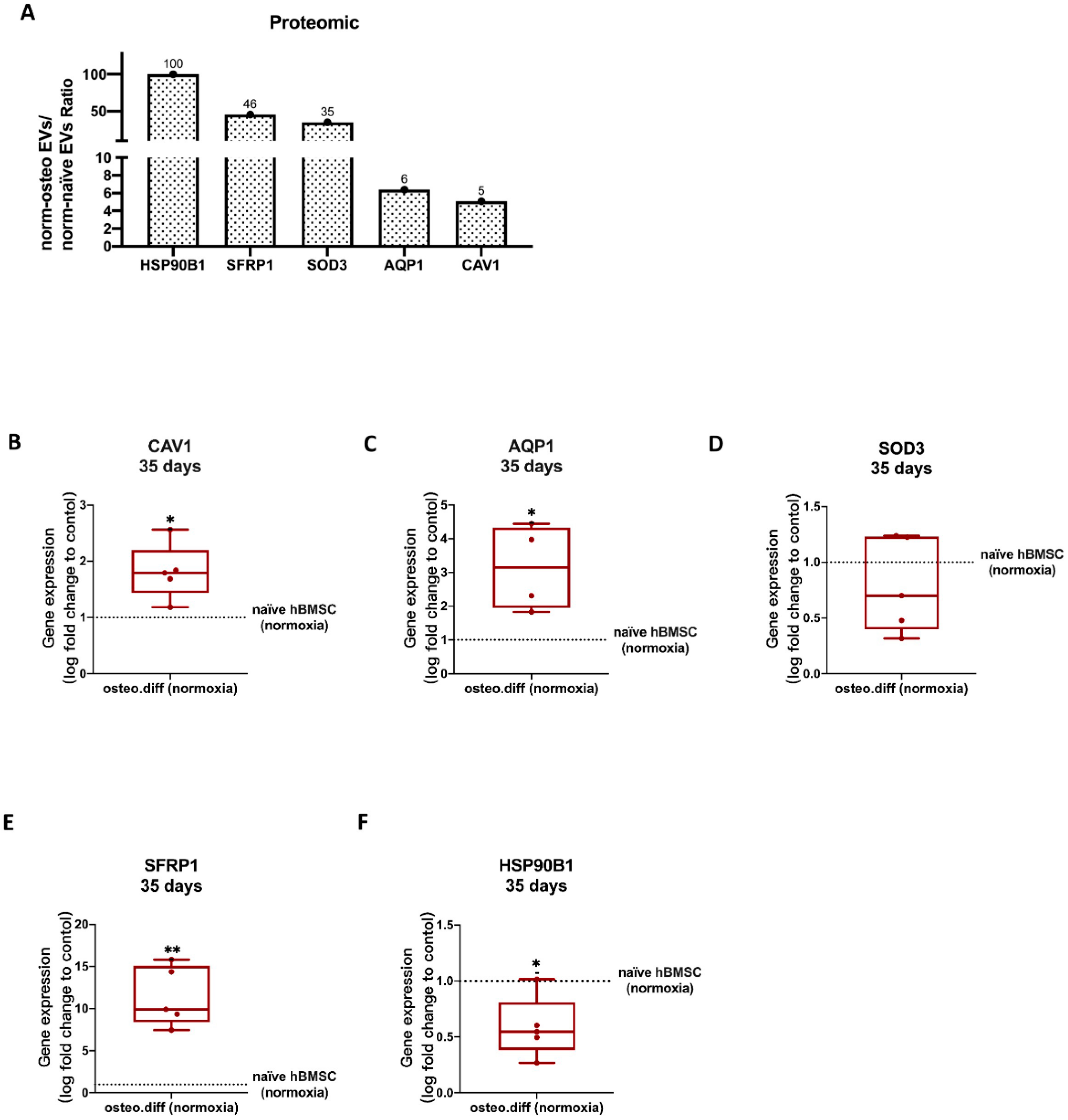
3.5. Proteomic Analysis of Osteogenic EVs Produced under Hypoxia and Normoxia
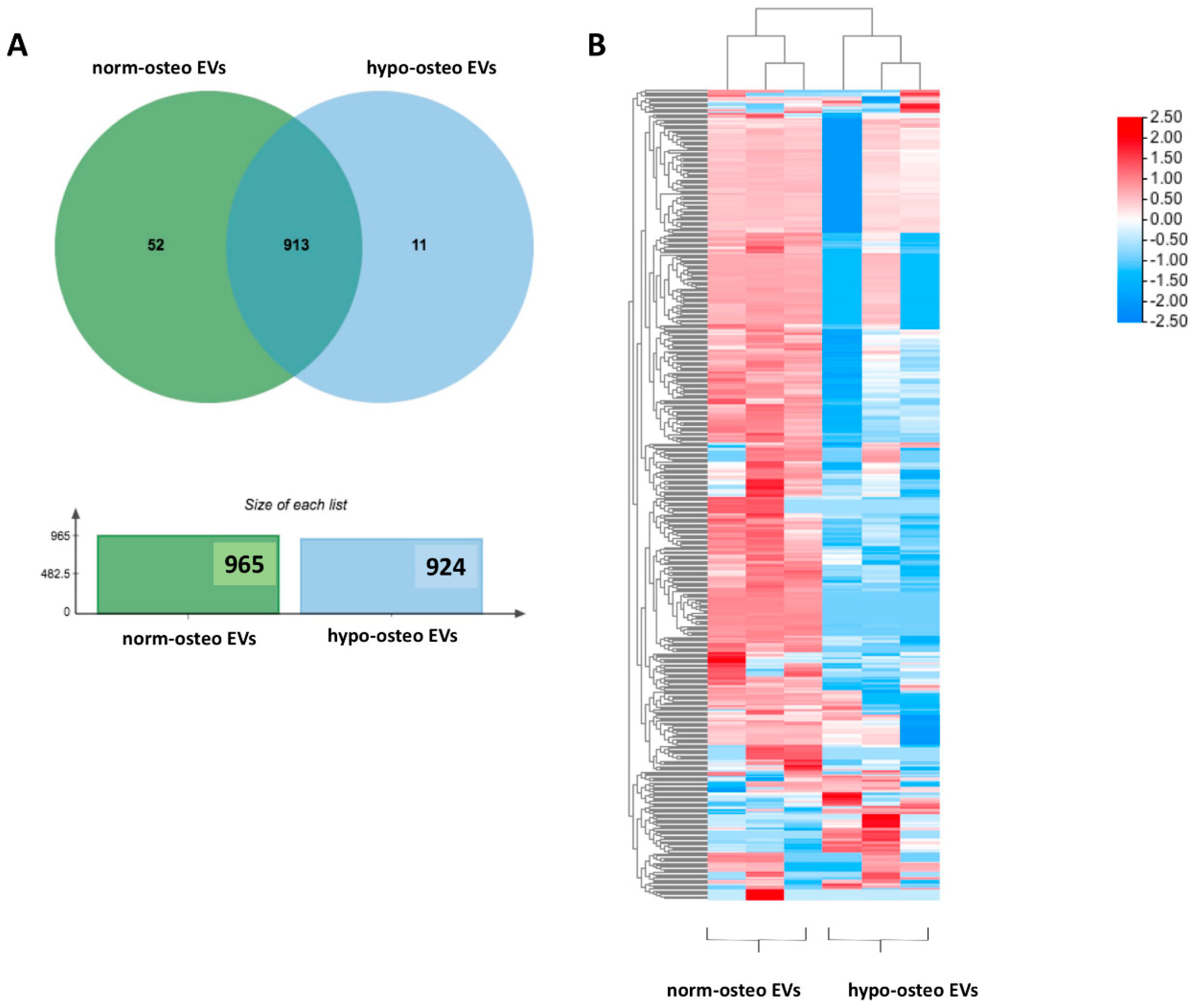
3.5.1. Summary of the Proteomic Profiles
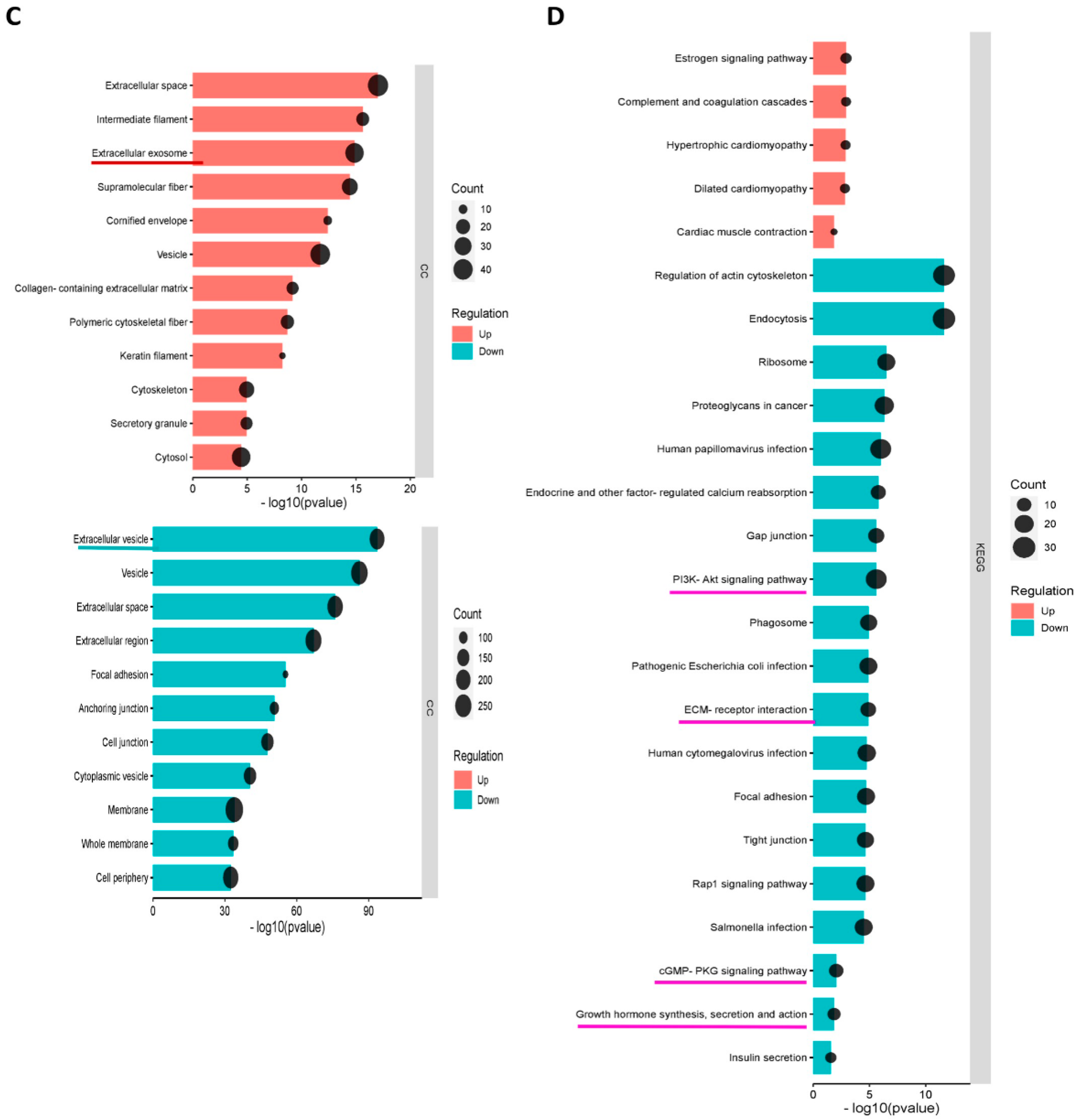
3.5.2. GO and KEGG Enrichment Analyses
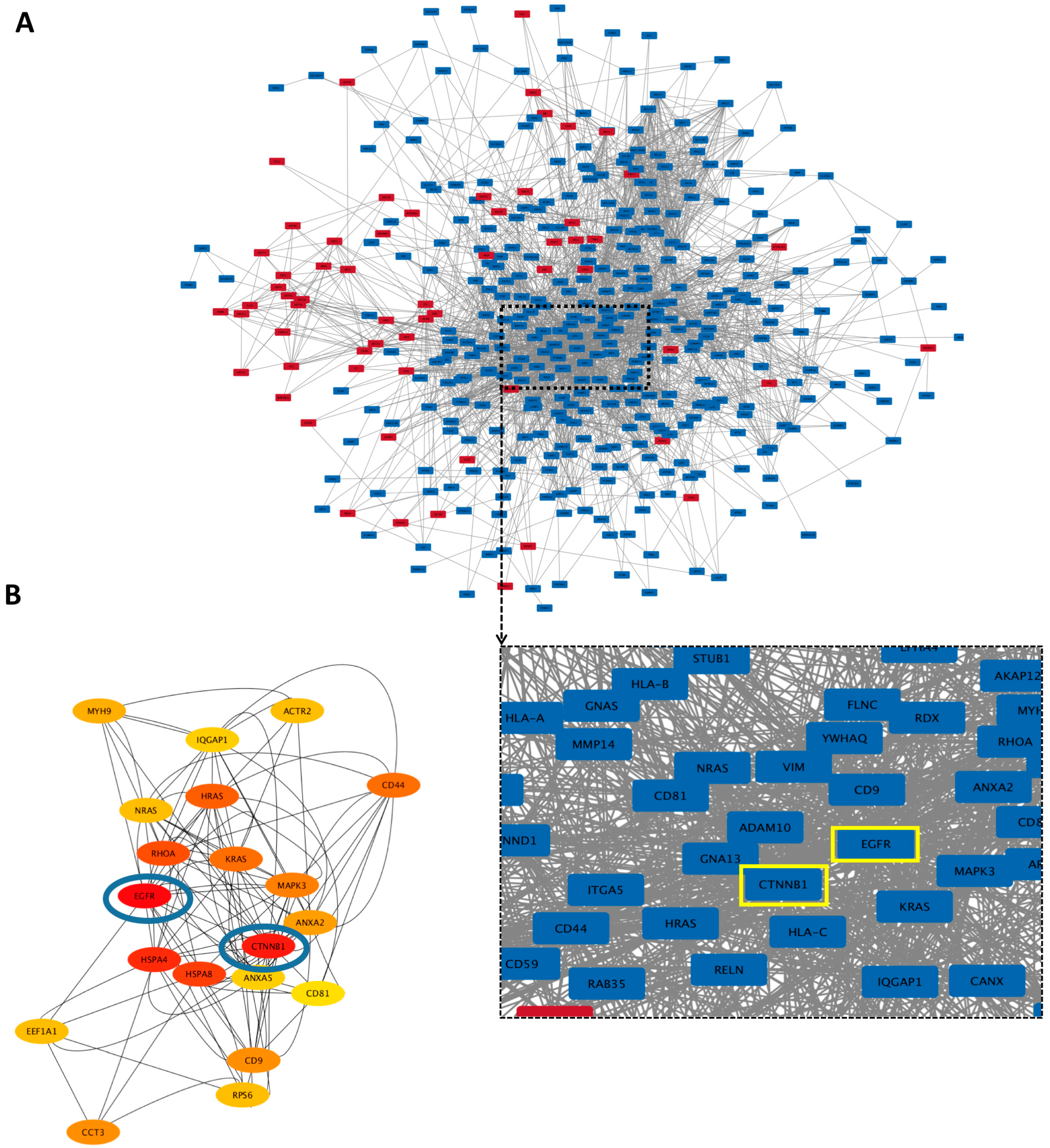
3.5.3. Hub Proteins
3.6. Effects of EVs Derived from Late Stage Osteogenic-Differentiated hBMSCs under Normoxia on Osteogenic Ability of hBMSCs Cultured under Hypoxia
3.6.1. GO Enrichment Analyses and Protein–Protein Interaction (PPI) Network Analyses of Norm-Naïve EVs and norm-Osteo EVs
3.6.2. Hub Proteins
3.6.3. Anti-Hypoxic Proteins
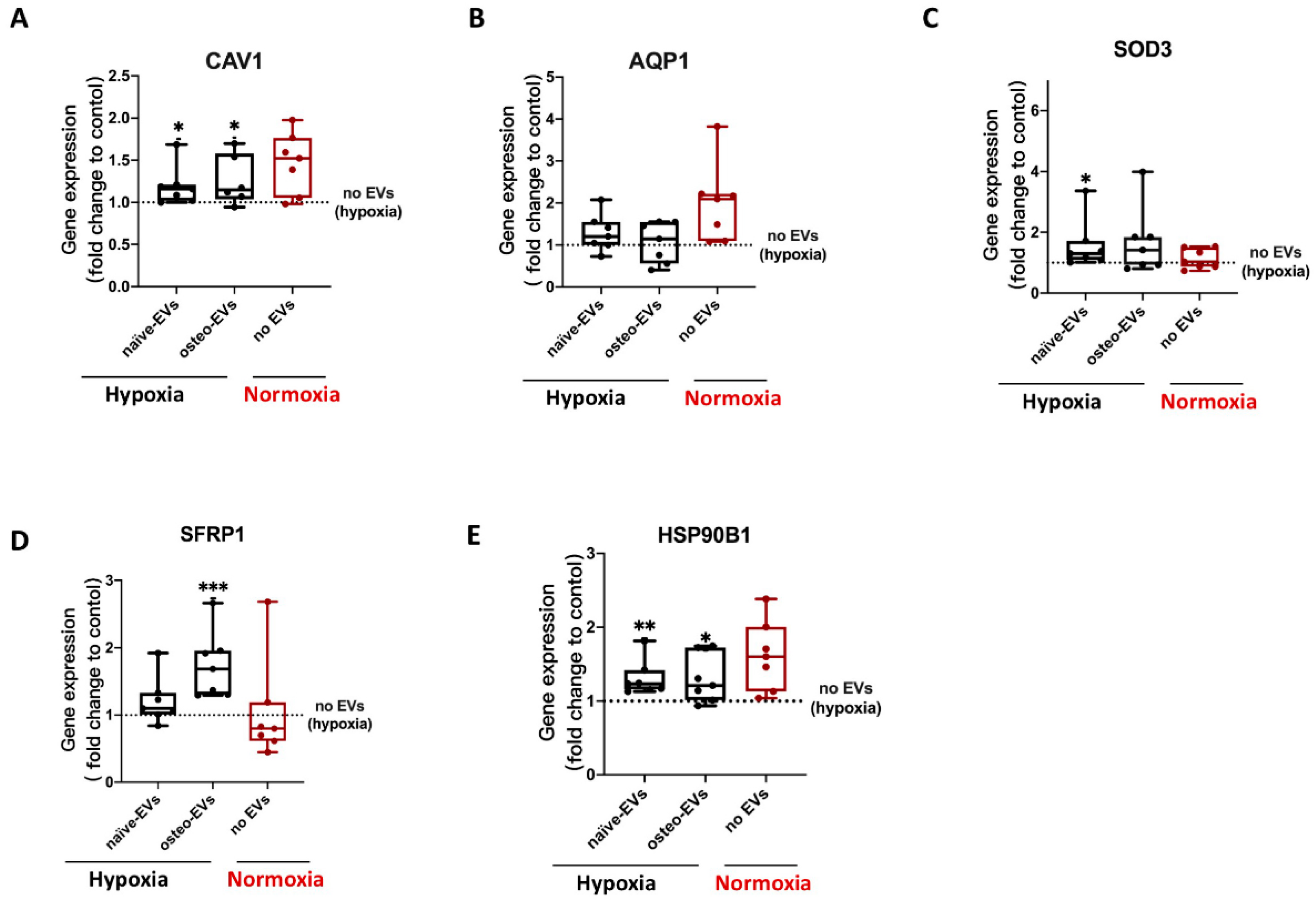
3.7. Validation of Proteomics Data
3.7.1. Anti-Hypoxic Gene Expression Levels in the EVs’ Parent Cells
3.7.2. Anti-Hypoxic Gene Expression Levels in hBMSCs Undergoing Osteogenic Differentiation under Hypoxia after EV Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bister, N.; Pistono, C.; Huremagic, B.; Jolkkonen, J.; Giugno, R.; Malm, T. Hypoxia and extracellular vesicles: A review on methods, vesicular cargo and functions. J. Extracell. Vesicles 2020, 10, e12002. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Ko, J.; Ju, C.; Eltzschig, H.K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Laroche, M.; Moulinier, L.; Leger, P.; Lefebvre, D.; Mazieres, B.; Boccalon, H. Bone mineral decrease in the leg with unilateral chronic occlusive arterial disease. Clin. Exp. Rheumatol. 2003, 21, 103–106. [Google Scholar]
- Salim, A.; Nacamuli, R.P.; Morgan, E.F.; Giaccia, A.J.; Longaker, M.T. Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. J. Biol. Chem. 2004, 279, 40007–40016. [Google Scholar] [CrossRef] [PubMed]
- Li, L.J.; Jiang, D.M. Hypoxia-responsive miRNA-21-5p inhibits Runx2 suppression by targeting SMAD7 in MC3T3-E1 cells. J. Cell. Biochem. 2019, 120, 16867–16875. [Google Scholar] [CrossRef]
- Qin, Q.Y.; Liu, Y.P.; Yang, Z.; Aimaijiang, M.; Ma, R.; Yang, Y.X.; Zhang, Y.D.; Zhou, Y.M. Hypoxia-Inducible Factors Signaling in Osteogenesis and Skeletal Repair. Int. J. Mol. Sci. 2022, 23, 11201. [Google Scholar] [CrossRef]
- Wagegg, M.; Gaber, T.; Lohanatha, F.L.; Hahne, M.; Strehl, C.; Fangradt, M.; Tran, C.L.; Schonbeck, K.; Hoff, P.; Ode, A.; et al. Hypoxia Promotes Osteogenesis but Suppresses Adipogenesis of Human Mesenchymal Stromal Cells in a Hypoxia-Inducible Factor-1 Dependent Manner. PLoS ONE 2012, 7, e46483. [Google Scholar] [CrossRef]
- Ivan, M.; Kondo, K.; Yang, H.F.; Kim, W.; Valiando, J.; Ohh, M.; Salic, A.; Asara, J.M.; Lane, W.S.; Kaelin, W.G. HIF alpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O-2 sensing. Science 2001, 292, 464–468. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Gorlach, A.; Dimova, E.Y.; Petry, A.; Martinez-Ruiz, A.; Hernansanz-Agustin, P.; Rolo, A.P.; Palmeira, C.M.; Kietzmann, T. Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox Biol. 2015, 6, 372–385. [Google Scholar] [CrossRef]
- Hannah, S.S.; McFadden, S.; McNeilly, A.; McClean, C. “Take My Bone Away?” Hypoxia and bone: A narrative review. J. Cell. Physiol. 2021, 236, 721–740. [Google Scholar] [CrossRef] [PubMed]
- Badila, A.E.; Radulescu, D.M.; Ilie, A.; Niculescu, A.G.; Grumezescu, A.M.; Radulescu, A.R. Bone Regeneration and Oxidative Stress: An Updated Overview. Antioxidants 2022, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.; Toth, A.; Mehes, G.; Trencsenyi, G.; Paragh, G.; Jeney, V. Hypoxia Triggers Osteochondrogenic Differentiation of Vascular Smooth Muscle Cells in an HIF-1 (Hypoxia-Inducible Factor 1)-Dependent and Reactive Oxygen Species-Dependent Manner. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.P.; Ma, X.N.; Ge, B.F.; Zhen, P.; Zhou, J.; Gao, Y.H.; Xian, C.J.; Chen, K.M. Icariin attenuates hypoxia-induced oxidative stress and apoptosis in osteoblasts and preserves their osteogenic differentiation potential in vitro. Cell Prolif. 2014, 47, 527–539. [Google Scholar] [CrossRef]
- Matic, S.; Dia, V.P. Bovine milk exosomes affected proliferation of macrophages under hypoxia. Curr. Res. Food Sci. 2022, 5, 2108–2113. [Google Scholar] [CrossRef]
- Nicolaije, C.; Koedam, M.; van Leeuwen, J. Decreased oxygen tension lowers reactive oxygen species and apoptosis and inhibits osteoblast matrix mineralization through changes in early osteoblast differentiation. J. Cell. Physiol. 2012, 227, 1309–1318. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Edgar, J.R. Q&A: What are exosomes, exactly? BMC Biol. 2016, 14, 46. [Google Scholar] [CrossRef]
- Herrmann, M.; Diederichs, S.; Melnik, S.; Riegger, J.; Trivanovic, D.; Li, S.; Jenei-Lanzl, Z.; Brenner, R.E.; Huber-Lang, M.; Zaucke, F.; et al. Extracellular Vesicles in Musculoskeletal Pathologies and Regeneration. Front. Bioeng. Biotechnol. 2021, 8, 624096. [Google Scholar] [CrossRef]
- Wang, X.Q.; Omar, O.; Vazirisani, F.; Thomsen, P.; Ekstrom, K. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PLoS ONE 2018, 13, e0193059. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Qin, S.; Xu, Y.; Tang, Y.; Huang, Y.; Situ, B.; Inal, J.M.; Zheng, L. Exosomes serve as tumour markers for personalized diagnostics owing to their important role in cancer metastasis. J. Extracell. Vesicles 2015, 4, 27522. [Google Scholar] [CrossRef] [PubMed]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, S.; Najminejad, H.; Dabaghian, M.; Karimi, M.H.; Abdollahpour-Alitappeh, M.; Rad, F.; Mahi-Birjand, M.; Mohammadi, S.; Mohseni, F.; Lari, M.S.; et al. How hypoxia regulate exosomes in ischemic diseases and cancer microenvironment? IUBMB Life 2020, 72, 1286–1305. [Google Scholar] [CrossRef]
- Dorayappan, K.D.P.; Wanner, R.; Wallbillich, J.J.; Saini, U.; Zingarelli, R.; Suarez, A.A.; Cohn, D.E.; Selvendiran, K. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: A novel mechanism linking STAT3/Rab proteins. Oncogene 2018, 37, 3806–3821. [Google Scholar] [CrossRef]
- Ramteke, A.; Ting, H.; Agarwal, C.; Mateen, S.; Somasagara, R.; Hussain, A.; Graner, M.; Frederick, B.; Agarwal, R.; Deep, G. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol. Carcinog. 2015, 54, 554–565. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.L.; Sun, M.Y.; Yang, P.; Hu, X.Q.; Ao, Y.F.; Cheng, J. The tissue origin effect of extracellular vesicles on cartilage and bone regeneration. Acta Biomater. 2021, 125, 253–266. [Google Scholar] [CrossRef]
- Zhang, L.; Jiao, G.; Ren, S.; Zhang, X.; Li, C.; Wu, W.; Wang, H.; Liu, H.; Zhou, H.; Chen, Y. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 2020, 11, 38. [Google Scholar] [CrossRef]
- Wang, C.L.; Stockl, S.; Li, S.S.; Herrmann, M.; Lukas, C.; Reinders, Y.; Sickmann, A.; Grässel, S. Effects of Extracellular Vesicles from Osteogenic Differentiated Human BMSCs on Osteogenic and Adipogenic Differentiation Capacity of Naive Human BMSCs. Cells 2022, 11, 2491. [Google Scholar] [CrossRef]
- Leyh, M.; Seitz, A.; Durselen, L.; Springorum, H.R.; Angele, P.; Ignatius, A.; Grifka, J.; Grässel, S. Osteoarthritic cartilage explants affect extracellular matrix production and composition in cocultured bone marrow-derived mesenchymal stem cells and articular chondrocytes. Stem Cell Res. Ther. 2014, 5, 77. [Google Scholar] [CrossRef]
- Leyh, M.; Seitz, A.; Duerselen, L.; Schaumburger, J.; Ignatius, A.; Grifka, J.; Grässel, S. Subchondral bone influences chondrogenic differentiation and collagen production of human bone marrow-derived mesenchymal stem cells and articular chondrocytes. Arthritis Res. Ther. 2014, 16, 453. [Google Scholar] [CrossRef] [PubMed]
- Grässel, S.; Stockl, S.; Jenei-Lanzl, Z. Isolation, culture, and osteogenic/chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Methods Mol. Biol. 2012, 879, 203–267. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Stöckl, S.; Lukas, C.; Goetz, J.; Herrmann, M.; Federlin, M.; Grässel, S. hBMSC-Derived Extracellular Vesicles Attenuate IL-1 beta-Induced Catabolic Effects on OA-Chondrocytes by Regulating Pro-inflammatory Signaling Pathways. Front. Bioeng. Biotechnol. 2020, 8, 603598. [Google Scholar] [CrossRef] [PubMed]
- Niedermair, T.; Lukas, C.; Li, S.; Stoeckl, S.; Craiovan, B.; Brochhausen, C.; Federlin, M.; Herrmann, M.; Grässel, S. Influence of Extracellular Vesicles Isolated from Osteoblasts of Patients with Cox-Arthrosis and/or Osteoporosis on Metabolism and Osteogenic Differentiation of BMSCs. Front. Bioeng. Biotechnol. 2020, 8, 615520. [Google Scholar] [CrossRef] [PubMed]
- Pattappa, G.; Schewior, R.; Hofmeister, I.; Seja, J.; Zellner, J.; Johnstone, B.; Docheva, D.; Angele, P. Physioxia Has a Beneficial Effect on Cartilage Matrix Production in Interleukin-1 Beta-Inhibited Mesenchymal Stem Cell Chondrogenesis. Cells 2019, 8, 936. [Google Scholar] [CrossRef]
- Pattappa, G.; Reischl, F.; Jahns, J.; Schewior, R.; Lang, S.G.; Zellner, J.; Johnstone, B.; Docheva, D.; Angele, P. Fibronectin Adherent Cell Populations Derived from Avascular and Vascular Regions of the Meniscus Have Enhanced Clonogenicity and Differentiation Potential Under Physioxia. Front. Bioeng. Biotechnol. 2022, 9, 789621. [Google Scholar] [CrossRef]
- Li, S.S.; Stockl, S.; Lukas, C.; Herrmann, M.; Brochhausen, C.; Konig, M.A.; Johnstone, B.; Grässel, S. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1 beta-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p. Stem Cell Res. Ther. 2021, 12, 252. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudie, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Vanstraalen, J.P.; Sanders, E.; Prummel, M.F.; Sanders, G.T.B. Bone-alkaline phosphatase as indicator of bone-formation. Clin. Chim. Acta 1991, 201, 27–34. [Google Scholar] [CrossRef]
- Bando, Y.; Katayama, T.; Kasai, K.; Taniguchi, M.; Tamatani, M.; Tohyama, M. GRP94 (94 kDa glucose-regulated protein) suppresses ischemic neuronal cell death against ischemia/reperfusion injury. Eur. J. Neurosci. 2003, 18, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Abudoukelimu, M.; Ma, Y.T.; Yang, Y.N.; Li, X.M.; Chen, B.D.; Liu, F.; He, C.H.; Li, H.Y. Secreted frizzled related protein 1 protects H9C2 cells from hypoxia/re-oxygenation injury by blocking the Wnt signaling pathway. Lipids Health Dis. 2016, 15, 72. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sibenaller, Z.A.; Welsh, J.L.; Du, C.B.; Witmer, J.R.; Schrock, H.E.; Du, J.; Buettner, G.R.; Goswami, P.C.; Cieslak, J.A.; Cullen, J.J. Extracellular superoxide dismutase suppresses hypoxia-inducible factor-1 alpha in pancreatic cancer. Free. Radic. Biol. Med. 2014, 69, 357–366. [Google Scholar] [CrossRef]
- Sada, K.; Nishikawa, T.; Kukidome, D.; Yoshinaga, T.; Kajihara, N.; Sonoda, K.; Senokuchi, T.; Motoshima, H.; Matsumura, T.; Araki, E. Hyperglycemia Induces Cellular Hypoxia through Production of Mitochondrial ROS Followed by Suppression of Aquaporin-1. PLoS ONE 2016, 11, e0158619. [Google Scholar] [CrossRef]
- Sanhueza, C.; Bennett, J.C.; Valenzuela-Valderrama, M.; Contreras, P.; Lobos-Gonzalez, L.; Campos, A.; Wehinger, S.; Lladser, A.; Kiessling, R.; Leyton, L.; et al. Caveolin-1-Mediated Tumor Suppression Is Linked to Reduced HIF1 alpha S-Nitrosylation and Transcriptional Activity in Hypoxia. Cancers 2020, 12, 2349. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Balliet, R.M.; Rivadeneira, D.B.; Chiavarina, B.; Pavlides, S.; Wang, C.G.; Whitaker-Menezes, D.; Daumer, K.M.; Lin, Z.; Witkiewicz, A.K.; et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle 2010, 9, 3256–3276. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Pizzo, P.; Scapin, C.; Vitadello, M.; Florean, C.; Gorza, L. Grp94 acts as a mediator of curcumin-induced antioxidant defence in myogenic cells. J. Cell. Mol. Med. 2010, 14, 970–981. [Google Scholar] [CrossRef]
- Pattappa, G.; Thorpe, S.D.; Jegard, N.C.; Heywood, H.K.; de Bruijn, J.D.; Lee, D.A. Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng. Methods 2013, 19, 68–79. [Google Scholar] [CrossRef]
- Yang, T.; Grafe, I.; Bae, Y.J.; Chen, S.; Chen, Y.Q.; Bertin, T.K.; Jiang, M.M.; Ambrose, C.G.; Lee, B. E-selectin ligand 1 regulates bone remodeling by limiting bioactive TGF-beta in the bone microenvironment. Proc. Natl. Acad. Sci. USA 2013, 110, 7336–7341. [Google Scholar] [CrossRef]
- Mizuhashi, K.; Kanamoto, T.; Ito, M.; Moriishi, T.; Muranishi, Y.; Omori, Y.; Terada, K.; Komori, T.; Furukawa, T. OBIF, an osteoblast induction factor, plays an essential role in bone formation in association with osteoblastogenesis. Dev. Growth Differ. 2012, 54, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.; Hecking, M.; Glitzner, E.; Zwerina, K.; Holcmann, M.; Bakiri, L.; Ruocco, M.G.; Tuckermann, J.; Schett, G.; Wagner, E.F.; et al. EGFR controls bone development by negatively regulating mTOR-signaling during osteoblast differentiation. Cell Death Differ. 2018, 25, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.S.; Zheng, J.M.; Zhang, M.D.; Ai, T.T.; Cai, B. FOXQ1 promotes the osteogenic differentiation of bone mesenchymal stem cells via Wnt/beta-catenin signaling by binding with ANXA2. Stem Cell Res. Ther. 2020, 11, 403. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A.R. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Volloch, V.; Olsen, B.R. Why cellular stress suppresses adipogenesis in skeletal tissue, but is ineffective in adipose tissue: Control of mesenchymal cell differentiation via integrin binding sites in extracellular matrices. Matrix Biol. 2013, 32, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Docheva, D.; Popov, C.; Alberton, P.; Aszodi, A. Integrin signaling in skeletal development and function. Birth Defects Res.-Embryo Today-Rev. 2014, 102, 13–36. [Google Scholar] [CrossRef]
- Hirano, T.; Higuchi, T.; Ueda, M.; Inoue, T.; Kataoka, N.; Maeda, M.; Fujiwara, H.; Fujii, S. CD9 is expressed in extravillous trophoblasts in association with integrin alpha(3) and integrin alpha(5). Mol. Hum. Reprod. 1999, 5, 162–167. [Google Scholar] [CrossRef][Green Version]
- Justo, B.L.; Jasiulionis, M.G. Characteristics of TIMP1, CD63, and beta 1-Integrin and the Functional Impact of Their Interaction in Cancer. Int. J. Mol. Sci. 2021, 22, 9319. [Google Scholar] [CrossRef]
- Stipp, C.S.; Hemler, M.E. Transmembrane-4-superfamily proteins CD151 and CD81 associate with alpha 3 beta 1 integrin, and selectively contribute to alpha 3 beta 1-dependent neurite outgrowth. J. Cell Sci. 2000, 113, 1871–1882. [Google Scholar] [CrossRef]
- Horvath, G.; Serru, V.; Clay, D.; Billard, M.; Boucheix, C.; Rubinstein, E. CD19 is linked to the integrin-associated tetraspans CD9, CD81, and CD82. J. Biol. Chem. 1998, 273, 30537–30543. [Google Scholar] [CrossRef]
- Singh, A.; Lester, C.; Drapp, R.; Hu, D.Z.; Glimcher, L.H.; Jones, D. Tetraspanin CD9 and ectonucleotidase CD73 identify an osteochondroprogenitor population with elevated osteogenic properties. Development 2015, 142, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Torres, M. Reactive oxygen species and cell signaling—Respiratory burst in macrophage signaling. Am. J. Respir. Crit. Care Med. 2002, 166, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.P.; Foretz, M.; Xie, Z.L.; Zhang, M.; Zhu, Z.R.; Xing, J.J.; Leclerc, J.; Gaudry, M.; Viollet, B.; Zou, M.H. PRKAA1/AMPK alpha 1 is required for autophagy-dependent mitochondrial clearance during erythrocyte maturation. Autophagy 2014, 10, 1522–1534. [Google Scholar] [CrossRef]
- Siegel, D.; Gustafson, D.L.; Dehn, D.L.; Han, J.Y.; Boonchoong, P.; Berliner, L.J.; Ross, D. NAD(P)H: Quinone oxidoreductase 1: Role as a superoxide scavenger. Mol. Pharmacol. 2004, 65, 1238–1247. [Google Scholar] [CrossRef]
- Kiani-Esfahani, A.; Sheykhshabani, S.K.; Peymani, M.; Hashemi, M.S.; Ghaedi, K.; Nasr-Esfahani, M.H. Overexpression of Annexin A1 Suppresses Pro-Inflammatory Factors in PC12 Cells Induced by 1-Methyl-4-Phenylpyridinium. Cell J. 2016, 18, 197. [Google Scholar]
- Ahn, E.H.; Kim, D.W.; Shin, M.J.; Kim, Y.N.; Kim, H.R.; Woo, S.J.; Kim, S.M.; Kim, D.S.; Kim, J.; Park, J.; et al. PEP-1-ribosomal protein S3 protects dopaminergic neurons in an MPTP-induced Parkinson’s disease mouse model. Free. Radic. Biol. Med. 2013, 55, 36–45. [Google Scholar] [CrossRef]
- Svensson, K.J.; Kucharzewska, P.; Christianson, H.C.; Skold, S.; Lofstedt, T.; Johansson, M.C.; Morgelin, M.; Bengzon, J.; Ruf, W.; Belting, M. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 13147–13152. [Google Scholar] [CrossRef]
- Narayanan, R.; Huang, C.-C.; Ravindran, S. Hijacking the Cellular Mail: Exosome Mediated Differentiation of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 3808674. [Google Scholar] [CrossRef]
- Varadaraj, K.; Kumari, S.S. Lens aquaporins function as peroxiporins to facilitate membrane transport of hydrogen peroxide. Biochem. Biophys. Res. Commun. 2020, 524, 1025–1029. [Google Scholar] [CrossRef]
- Khanh, V.C.; Yamashita, T.; Ohneda, K.; Tokunaga, C.; Kato, H.; Osaka, M.; Hiramatsu, Y.; Ohneda, O. Rejuvenation of mesenchymal stem cells by extracellular vesicles inhibits the elevation of reactive oxygen species. Sci. Rep. 2020, 10, 17315. [Google Scholar] [CrossRef] [PubMed]


| UniProt | Protein Names | Gene Names | Hypo-Osteo EVs/Norm-Osteo EVs Ratio (Fold Change) |
|---|---|---|---|
| Q92896 | Golgi apparatus protein 1 | GLG1 | 0.01 |
| Q4V9L6 | Transmembrane protein 119 | TMEM119 | 0.106 |
| P05186 | Alkaline phosphatase | ALPL | 0.154 |
| P17813 | Endoglin | ENG | 0.245 |
| Q04771 | Activin receptor type-1 | ACVR1 | 0.265 |
| Q9ULC3 | Ras-related protein Rab-23 | RAB23 | 0.287 |
| P26373 | 60S ribosomal protein L13 | RPL13 | 0.292 |
| P20020 | Plasma membrane calcium-transporting ATPase 1 | ATP2B1 | 0.303 |
| P35222 | Catenin beta-1 | CTNNB1 | 0.304 |
| P00533 | Epidermal growth factor receptor | EGFR | 0.305 |
| Q16610 | Extracellular matrix protein 1 | ECM1 | 0.326 |
| P50281 | Matrix metalloproteinase-14 | MMP14 | 0.331 |
| P08133 | Annexin A6 | ANXA6 | 0.356 |
| P07355 | Annexin A2 | ANXA2 | 0.362 |
| P13797 | Plastin-3 | PLS3 | 0.387 |
| Q16832 | Discoidin domain-containing receptor 2 | DDR2 | 0.401 |
| P22413 | Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 | ENPP1 | 0.438 |
| Q5JWF2 | Guanine nucleotide-binding protein G(s) subunit alpha isoforms XLas | GNAS | 0.448 |
| O00299 | Chloride intracellular channel protein 1 | CLIC1 | 0.458 |
| Q13491 | Neuronal membrane glycoprotein M6-b | GPM6B | 0.494 |
| P61586 | Transforming protein RhoA | RHOA | 0.498 |
| UniProt | Protein Names | Gene Names | Hypo-Osteo EVs /Norm-Osteo EVs Ratio (Fold Change) |
|---|---|---|---|
| Q12965 | Unconventional myosin-Ie | MYO1E | 0.01 |
| P02458 | Collagen alpha-1(II) chain | COL2A1 | 0.01 |
| Q01955 | Collagen alpha-3(IV) chain | COL4A3 | 0.01 |
| Q12965 | Unconventional myosin-Ie | MYO1E | 0.01 |
| Q16610 | Extracellular matrix protein 1 | ECM1 | 0.326 |
| O75578 | Integrin alpha-10 | ITGA10 | 0.189 |
| P17813 | Endoglin | ENG | 0.245 |
| P26006 | Integrin alpha-3 | ITGA3 | 0.256 |
| P06756 | Integrin alpha-V | ITGAV | 0.323 |
| Q13683 | Integrin alpha-7 | ITGA7 | 0.326 |
| P50281 | Matrix metalloproteinase-14 | MMP14 | 0.331 |
| P18084 | Integrin beta-5 | ITGB5 | 0.347 |
| P98095 | Fibulin-2 | FBLN2 | 0.347 |
| Q08722 | Leukocyte surface antigen CD47 | CD47 | 0.352 |
| P07355 | Annexin A2 | ANXA2 | 0.362 |
| Q14112 | Nidogen-2 | NID2 | 0.379 |
| P24821 | Tenascin | TNC | 0.396 |
| Q16832 | Discoidin domain-containing receptor 2 | DDR2 | 0.401 |
| P16070 | CD44 antigen | CD44 | 0.412 |
| O14672 | Disintegrin and metalloproteinase domain-containing protein 10 | ADAM10 | 0.428 |
| P08648 | Integrin alpha-5 | ITGA5 | 0.498 |
| UniProt | Protein Names | Gene Names | Hypo-Osteo EVs /Norm-Osteo EVs Ratio (Fold Change) |
|---|---|---|---|
| Q13131 | 5’-AMP-activated protein kinase catalytic subunit alpha-1 | PRKAA1 | 0.143 |
| P15559 | NAD(P)H dehydrogenase [quinone] 1 | NQO1 | 0.298 |
| P04083 | Annexin A1 | ANXA1 | 0.343 |
| P23396 | 40S ribosomal protein S3 | RPS3 | 0.438 |
| Q06830 | Peroxiredoxin-1 | PRDX1 | 0.485 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Stöckl, S.; Pattappa, G.; Schulz, D.; Hofmann, K.; Ilic, J.; Reinders, Y.; Bauer, R.J.; Sickmann, A.; Grässel, S. Extracellular Vesicles Derived from Osteogenic-Differentiated Human Bone Marrow-Derived Mesenchymal Cells Rescue Osteogenic Ability of Bone Marrow-Derived Mesenchymal Cells Impaired by Hypoxia. Biomedicines 2023, 11, 2804. https://doi.org/10.3390/biomedicines11102804
Wang C, Stöckl S, Pattappa G, Schulz D, Hofmann K, Ilic J, Reinders Y, Bauer RJ, Sickmann A, Grässel S. Extracellular Vesicles Derived from Osteogenic-Differentiated Human Bone Marrow-Derived Mesenchymal Cells Rescue Osteogenic Ability of Bone Marrow-Derived Mesenchymal Cells Impaired by Hypoxia. Biomedicines. 2023; 11(10):2804. https://doi.org/10.3390/biomedicines11102804
Chicago/Turabian StyleWang, Chenglong, Sabine Stöckl, Girish Pattappa, Daniela Schulz, Korbinian Hofmann, Jovana Ilic, Yvonne Reinders, Richard J. Bauer, Albert Sickmann, and Susanne Grässel. 2023. "Extracellular Vesicles Derived from Osteogenic-Differentiated Human Bone Marrow-Derived Mesenchymal Cells Rescue Osteogenic Ability of Bone Marrow-Derived Mesenchymal Cells Impaired by Hypoxia" Biomedicines 11, no. 10: 2804. https://doi.org/10.3390/biomedicines11102804
APA StyleWang, C., Stöckl, S., Pattappa, G., Schulz, D., Hofmann, K., Ilic, J., Reinders, Y., Bauer, R. J., Sickmann, A., & Grässel, S. (2023). Extracellular Vesicles Derived from Osteogenic-Differentiated Human Bone Marrow-Derived Mesenchymal Cells Rescue Osteogenic Ability of Bone Marrow-Derived Mesenchymal Cells Impaired by Hypoxia. Biomedicines, 11(10), 2804. https://doi.org/10.3390/biomedicines11102804





