Synergistic Antimicrobial Effect of Cold Atmospheric Plasma and Redox-Active Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cold Atmospheric Argon Plasma Source
2.2. Model Organism
2.3. Nanoparticle Synthesis and Characterisation
2.4. Inhibition Zone Test
2.5. Mutation Assay
2.6. RAPD PCR
2.7. Atomic Force Microscopy (AFM) Study of Bacteria after CAP Irradiation
2.8. Statistical Analysis
3. Results
3.1. The Inactivation Effect of Plasma and NPs on E. coli
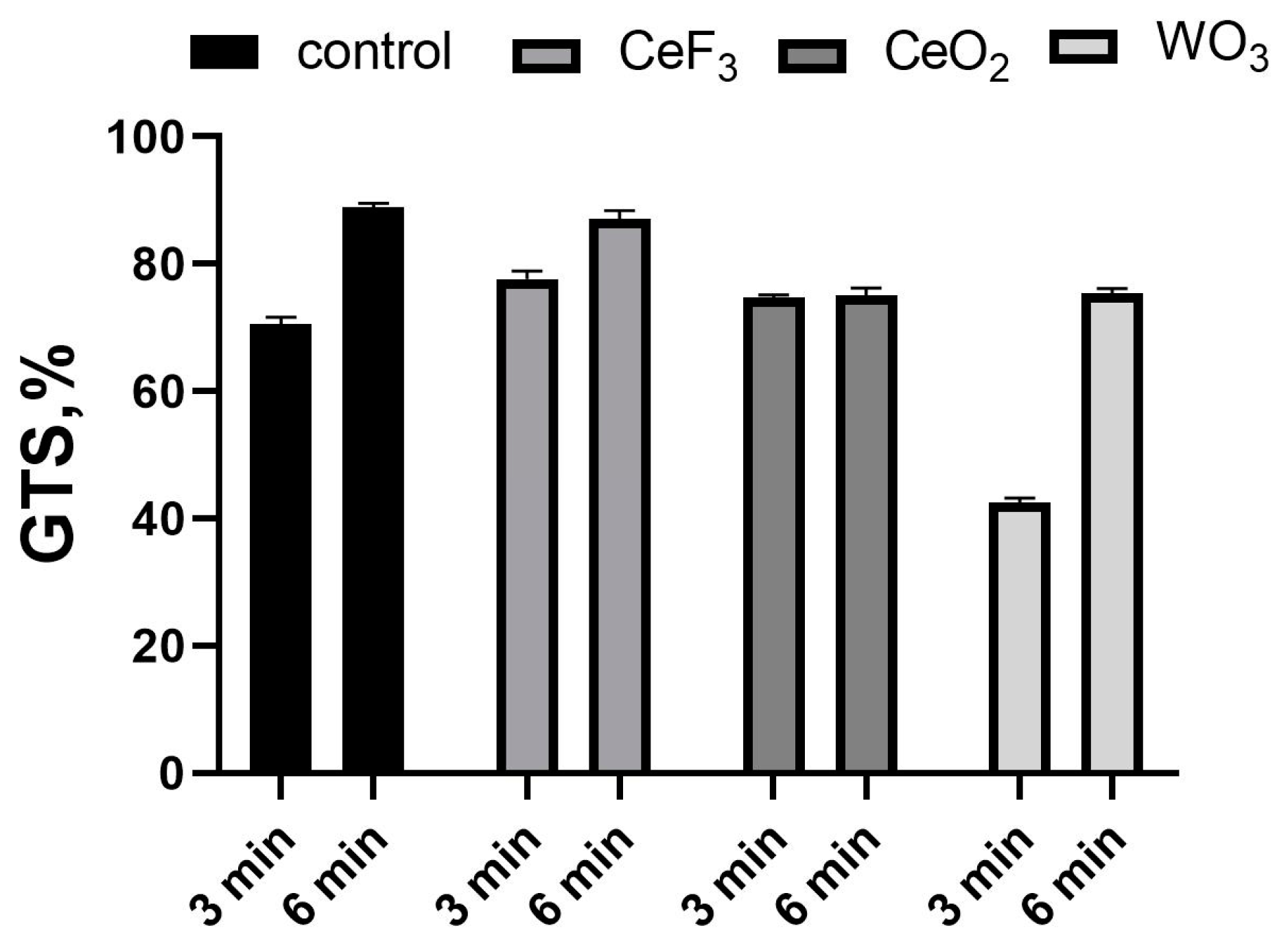
3.2. The Effect of NPs on Mutation in E. coli
3.3. RAPD PCR
3.4. Atomic Force Microscopy
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martusevich, A.K.; Surovegina, A.V.; Bocharin, I.V.; Nazarov, V.V.; Minenko, I.A.; Artamonov, M.Y. Cold Argon Athmospheric Plasma for Biomedicine: Biological Effects, Applications and Possibilities. Antioxidants 2022, 11, 1262. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M. Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Trans. Plasma Sci. 1996, 24, 1188–1191. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Moreau, S.; Pelletier, J.; Tabrizian, M.; Yahia, L.H. Low-temperature sterilization using gas plasmas: A review of the experiments and an analysis of the inactivation mechanisms. Int. J. Pharm. 2001, 226, 1–21. [Google Scholar] [CrossRef]
- Vatansever, F.; de Melo, W.C.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species—Bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar]
- Maisch, T.; Bosserhoff, A.K.; Unger, P.; Heider, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.E.; Landthaler, M.; Karrer, S. Investigation of toxicity and mutagenicity of cold atmospheric argon plasma. Environ. Mol. Mutagen. 2017, 58, 172–177. [Google Scholar] [CrossRef]
- Boeckmann, L.; Schäfer, M.; Bernhardt, T.; Semmler, M.L.; Jung, O.; Ojak, G.; Fischer, T.; Peters, K.; Nebe, B.; Müller-Hilke, B.; et al. Cold Atmospheric Pressure Plasma in Wound Healing and Cancer Treatment. Appl. Sci. 2020, 10, 6898. [Google Scholar] [CrossRef]
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxid. Med. Cell. Longev. 2019, 2019, 3873928. [Google Scholar] [CrossRef]
- Plattfaut, I.; Besser, M.; Severing, A.-L.; Stürmer, E.K.; Opländer, C. Plasma medicine and wound management: Evaluation of the antibacterial efficacy of a medically certified cold atmospheric argon plasma jet. Int. J. Antimicrob. Agents 2021, 57, 106319. [Google Scholar] [CrossRef]
- Corral-Diaz, B.; Peralta-Videa, J.R.; Alvarez-Parrilla, E.; Rodrigo-García, J.; Morales, M.I.; Osuna-Avila, P.; Niu, G.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Cerium oxide nanoparticles alter the antioxidant capacity but do not impact tuber ionome in Raphanus sativus (L). Plant Physiol. Biochem. 2014, 84, 277–285. [Google Scholar] [CrossRef]
- Bellio, P.; Luzi, C.; Mancini, A.; Cracchiolo, S.; Passacantando, M.; Di Pietro, L.; Perilli, M.; Amicosante, G.; Santucci, S.; Celenza, G. Cerium oxide nanoparticles as potential antibiotic adjuvant. Effects of CeO2 nanoparticles on bacterial outer membrane permeability. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2428–2435. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Hou, J.; Wang, P.; You, G.; Miao, L. Effects of cerium oxide nanoparticles on bacterial growth and behaviors: Induction of biofilm formation and stress response. Environ. Sci. Pollut. Res. Int. 2019, 26, 9293–9304. [Google Scholar] [CrossRef]
- Sarif, M.; Jegel, O.; Gazanis, A.; Hartmann, J.; Plana-Ruiz, S.; Hilgert, J.; Frerichs, H.; Viel, M.; Panthöfer, M.; Kolb, U.; et al. High-throughput synthesis of CeO2 nanoparticles for transparent nanocomposites repelling Pseudomonas aeruginosa biofilms. Sci. Rep. 2022, 12, 3935. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Tian, F.; Chang, J.; Bai, X.; Yuan, C.; Wang, C.; Neville, A. Haloperoxidase Mimicry by CeO2−x Nanorods of Different Aspect Ratios for Antibacterial Performance. ACS Sustain. Chem. Eng. 2020, 8, 6744–6752. [Google Scholar]
- Shcherbakov, A.B.; Zholobak, N.M.; Baranchikov, A.E.; Ryabova, A.V.; Ivanov, V.K. Cerium fluoride nanoparticles protect cells against oxidative stress. Mater. Sci. Eng. C 2015, 50, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Filippova, K.O.; Ermakov, A.M.; Popov, A.L.; Ermakova, O.N.; Blagodatsky, A.S.; Chukavin, N.N.; Shcherbakov, A.B.; Baranchikov, A.E.; Ivanov, V.K. Mitogen-like Cerium-Based Nanoparticles Protect Schmidtea mediterranea against Severe Doses of X-rays. Int. J. Mol. Sci. 2023, 24, 1241. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.L.; Zholobak, N.M.; Balko, O.I.; Balko, O.B.; Shcherbakov, A.B.; Popova, N.R.; Ivanova, O.S.; Baranchikov, A.E.; Ivanov, V.K. Photo-induced toxicity of tungsten oxide photochromic nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 178, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Popov, A.; Shekunova, T.; Kozlov, D.; Ivanova, O.; Rumyantsev, A.; Shcherbakov, A.; Popova, N.; Baranchikov, A.Y.; Ivanov, V. Highly crystalline WO3 nanoparticles are non-toxic to stem cells and cancer cells. J. Nanomater. 2019, 2019, 5384132. [Google Scholar] [CrossRef]
- The Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: A collaborative analysis of cohort studies. Lancet HIV 2017, 4, e349–e356. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Chen, S.H.; Lahav, G. Two is better than one; toward a rational design of combinatorial therapy. Curr. Opin. Struct. Biol. 2016, 41, 145–150. [Google Scholar] [CrossRef]
- Tängdén, T. Combination antibiotic therapy for multidrug-resistant Gram-negative bacteria. Upsala J. Med. Sci. 2014, 119, 149–153. [Google Scholar] [CrossRef]
- Ivanova, O.S.; Shekunova, T.O.; Ivanov, V.K.; Shcherbakov, A.B.; Popov, A.L.; Davydova, G.A.; Selezneva, I.I.; Kopitsa, G.P.; Tret’yakov, Y.D. One-stage synthesis of ceria colloid solutions for biomedical use. Dokl. Chem. 2011, 437, 103–106. [Google Scholar] [CrossRef]
- Popov, A.L.; Zholobak, N.M.; Shcherbakov, A.B.; Kozlova, T.O.; Kolmanovich, D.D.; Ermakov, A.M.; Popova, N.R.; Chukavin, N.N.; Bazikyan, E.A.; Ivanov, V.K. The strong protective action of Ce3+/F- combined treatment on tooth enamel and epithelial cells. Nanomaterials 2022, 12, 3034. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.L.; Han, B.; Ermakov, A.M.; Savintseva, I.V.; Ermakova, O.N.; Popova, N.R.; Shcherbakov, A.B.; Shekunova, T.O.; Ivanova, O.S.; Kozlov, D.A.; et al. PVP-stabilized tungsten oxide nanoparticles: pH sensitive anti-cancer platform with high cytotoxicity. Mater. Sci. Eng. C 2020, 108, 110494. [Google Scholar] [CrossRef] [PubMed]
- Atienzar, F.A.; Evenden, A.J.; Jha, A.N.; Depledge, M.H. Use of the random amplified polymorphic DNA (RAPD) assay for the detection of DNA damage and mutations: Possible implications of confounding factors. Biomarkers 2002, 7, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef]
- Duan, G.; Chen, L.; Jing, Z.; De Luna, P.; Wen, L.; Zhang, L.; Zhao, L.; Xu, J.; Li, Z.; Yang, Z.; et al. Robust Antibacterial Activity of Tungsten Oxide (WO3−x) Nanodots. Chem. Res. Toxicol. 2019, 32, 1357–1366. [Google Scholar] [CrossRef]
- Matharu, R.K.; Ciric, L.; Ren, G.; Edirisinghe, M. Comparative Study of the Antimicrobial Effects of Tungsten Nanoparticles and Tungsten Nanocomposite Fibres on Hospital Acquired Bacterial and Viral Pathogens. Nanomaterials 2020, 10, 1017. [Google Scholar] [CrossRef]
- Dar, M.A.; Gul, R.; Karuppiah, P.; Al-Dhabi, N.A.; Alfadda, A.A. Antibacterial Activity of Cerium Oxide Nanoparticles against ESKAPE Pathogens. Crystals 2022, 12, 179. [Google Scholar] [CrossRef]
- Isbary, G.; Heinlin, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.; Schmidt, H.U.; Monetti, R.; Steffes, B.; Bunk, W.; Li, Y.; et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: Results of a randomized controlled trial. Br. J. Dermatol. 2012, 167, 404–410. [Google Scholar] [CrossRef]
- He, X.; Kuang, Y.; Li, Y.; Zhang, H.; Ma, Y.; Bai, W.; Zhang, Z.; Wu, Z.; Zhao, Y.; Chai, Z. Changing exposure media can reverse the cytotoxicity of ceria nanoparticles for Escherichia coli. Nanotoxicology 2012, 6, 233240. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.C.; Qian, Z.; Adhya, S.L. Architecture of the Escherichia coli nucleoid. PLoS Genet. 2019, 15, e1008456, Erratum in PLoS Genet. 2020, 16, e1009148. [Google Scholar] [CrossRef] [PubMed]

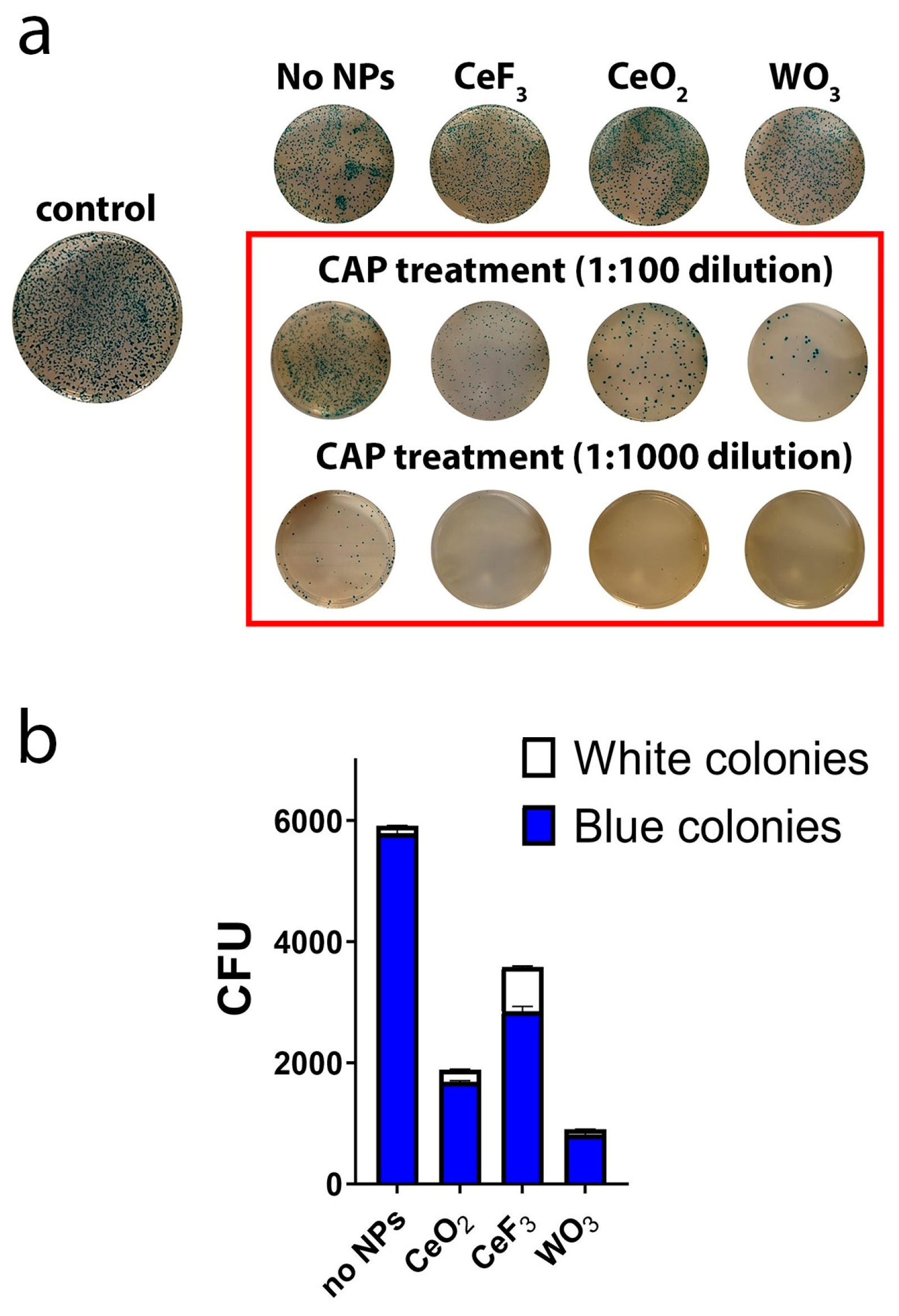
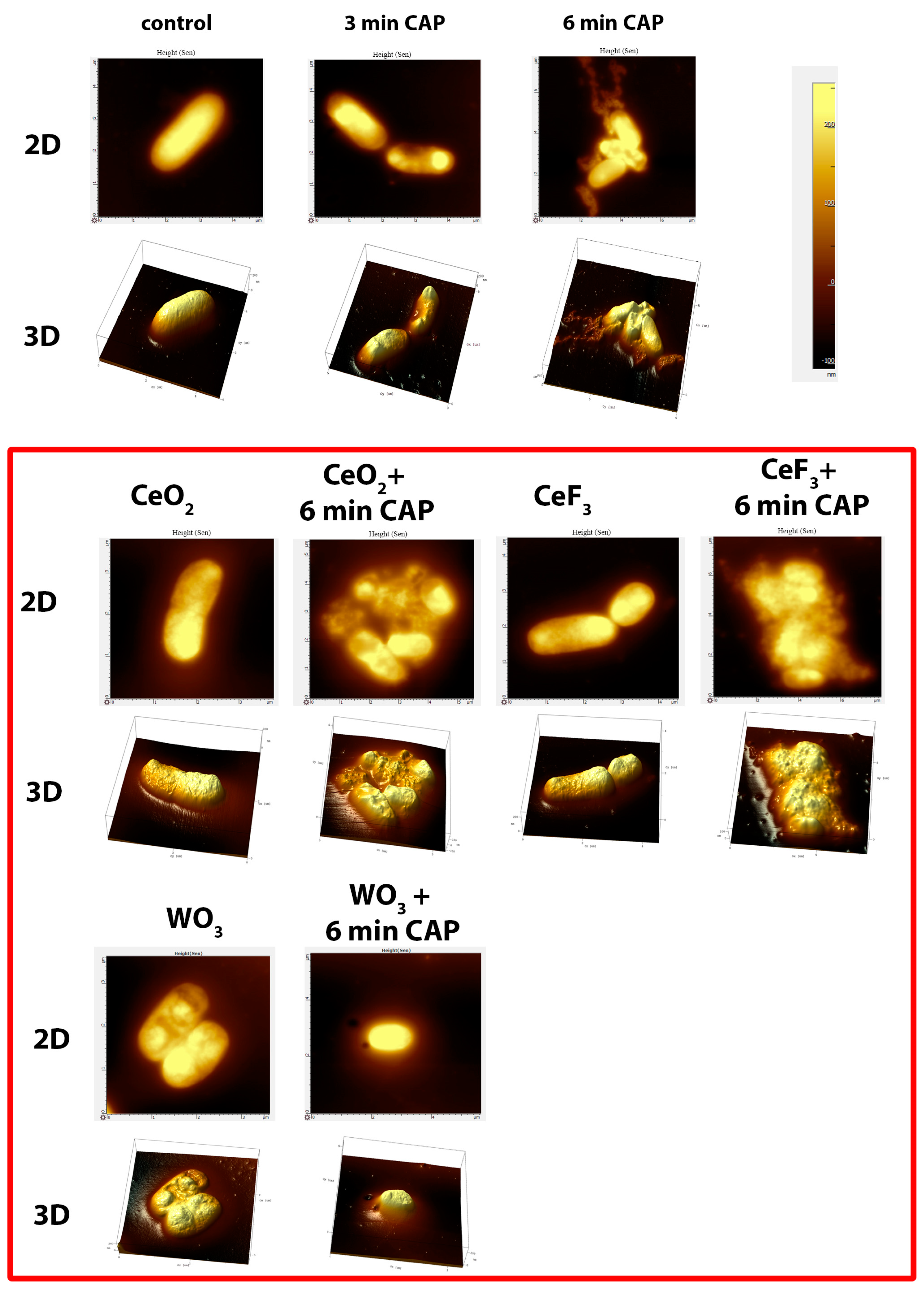
| Primer | Nucleotide Sequence (5′→3′) |
|---|---|
| OPB5 | TGCGCCCTTC |
| OPB7 | GGTGACGCAG |
| OPV14 | GGTCGATCTG |
| OPX7 | GAGCGAGGCT |
| OP-B04 | GATGACCGCC |
| OP-Q18 | GGGAGCGAGT |
| NP Type | Bacterial Growth Suppression with the Combination of CAP and NPs (in Comparison with the Control Treated with CAP Only) | |
|---|---|---|
| 3 min treatment | 6 min treatment | |
| CeF3 | 28.12% ± 1.41 | 17.05% ± 0.85 |
| CeO2 | 24.35% ± 1.12 | 24.73% ± 1.19 |
| WO3 | 22.21% ± 0.91 | 37.04% ± 1.84 |
| Primer | Group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | No NPs | CeF3 | CeO2 | WO3 | ||||||||
| 3 | 6 | n | 3 | 6 | n | 3 | 6 | n | 3 | 6 | ||
| OPB5 | 8 | 0; 4 | 0; 6 | 0; 0 | 0; 3 | 0; 0 | 0; 0 | 0; 4 | 0; 1 | 0; 0 | 0; 6 | 0; 3 |
| OPB7 | 8 | 0; 0 | 0; 0 | 0; 0 | 0; 3 | 0; 2 | 0; 0 | 0; 3 | 0; 2 | 0; 0 | 0; 4 | 0; 3 |
| OPV14 | 7 | 0; 3 | 0; 1 | 0; 0 | 0; 0 | 0; 1 | 0; 0 | 0; 0 | 0; 4 | 0; 0 | 0; 5 | 0; 3 |
| OPX7 | 6 | 0; 5 | 2; 1 | 0; 0 | 1; 0 | 0; 0 | 0; 0 | 1; 0 | 0; 1 | 0; 0 | 0; 2 | 0; 0 |
| OPB4 | 7 | 0; 1 | 0; 0 | 0; 0 | 0; 1 | 0; 1 | 0; 0 | 0; 1 | 0; 1 | 0; 0 | 0; 3 | 0; 0 |
| OPQ18 | 7 | 0; 0 | 0; 0 | 0; 0 | 0; 2 | 0; 2 | 0; 0 | 0; 2 | 0; 2 | 0; 0 | 0; 5 | 0; 2 |
| Total bands | 43 | |||||||||||
| Polymorphic bands | 13 | 5 | 0 | 10 | 6 | 0 | 11 | 11 | 0 | 25 | 11 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermakov, A.M.; Afanasyeva, V.A.; Lazukin, A.V.; Shlyapnikov, Y.M.; Zhdanova, E.S.; Kolotova, A.A.; Blagodatski, A.S.; Ermakova, O.N.; Chukavin, N.N.; Ivanov, V.K.; et al. Synergistic Antimicrobial Effect of Cold Atmospheric Plasma and Redox-Active Nanoparticles. Biomedicines 2023, 11, 2780. https://doi.org/10.3390/biomedicines11102780
Ermakov AM, Afanasyeva VA, Lazukin AV, Shlyapnikov YM, Zhdanova ES, Kolotova AA, Blagodatski AS, Ermakova ON, Chukavin NN, Ivanov VK, et al. Synergistic Antimicrobial Effect of Cold Atmospheric Plasma and Redox-Active Nanoparticles. Biomedicines. 2023; 11(10):2780. https://doi.org/10.3390/biomedicines11102780
Chicago/Turabian StyleErmakov, Artem M., Vera A. Afanasyeva, Alexander V. Lazukin, Yuri M. Shlyapnikov, Elizaveta S. Zhdanova, Anastasia A. Kolotova, Artem S. Blagodatski, Olga N. Ermakova, Nikita N. Chukavin, Vladimir K. Ivanov, and et al. 2023. "Synergistic Antimicrobial Effect of Cold Atmospheric Plasma and Redox-Active Nanoparticles" Biomedicines 11, no. 10: 2780. https://doi.org/10.3390/biomedicines11102780
APA StyleErmakov, A. M., Afanasyeva, V. A., Lazukin, A. V., Shlyapnikov, Y. M., Zhdanova, E. S., Kolotova, A. A., Blagodatski, A. S., Ermakova, O. N., Chukavin, N. N., Ivanov, V. K., & Popov, A. L. (2023). Synergistic Antimicrobial Effect of Cold Atmospheric Plasma and Redox-Active Nanoparticles. Biomedicines, 11(10), 2780. https://doi.org/10.3390/biomedicines11102780






