Incorporation of Perillyl Alcohol into Lipid-Based Nanocarriers Enhances the Antiproliferative Activity in Malignant Glioma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
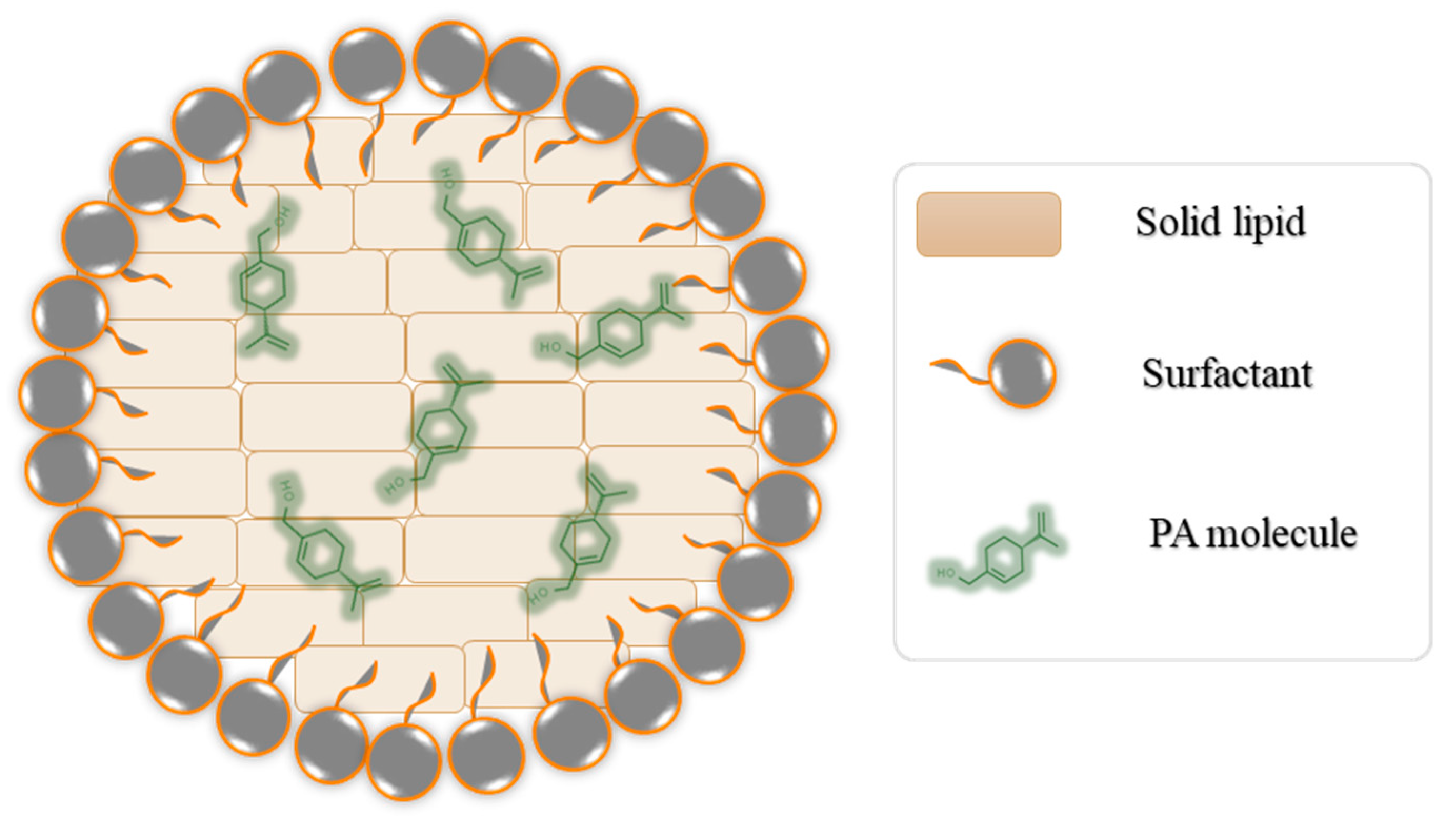
2.2. Preparation of Nanocarriers
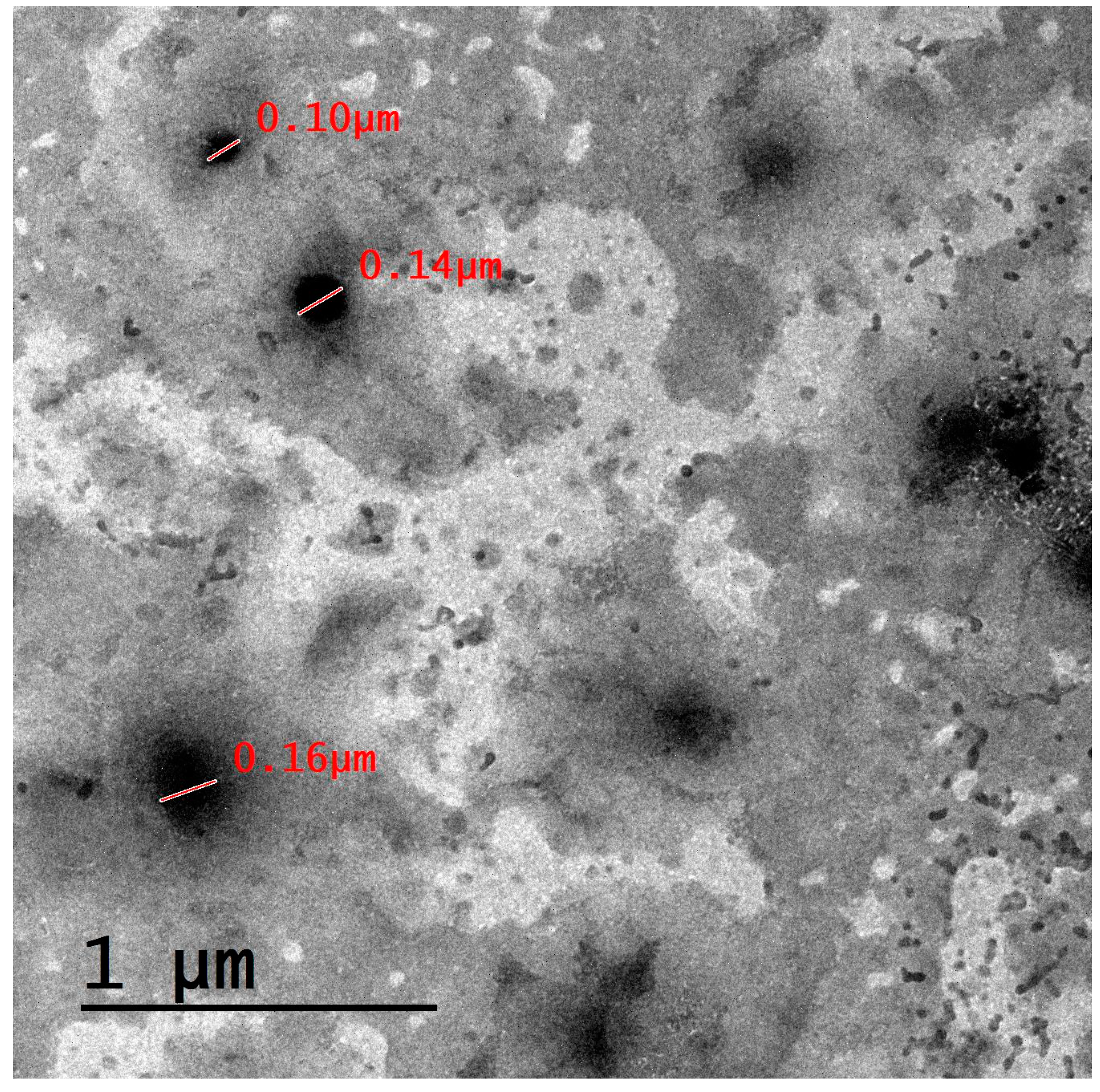
2.3. Characterization of the Prepared Nanocarriers
2.4. Molecular Docking Study
2.4.1. Preparation of Protein
2.4.2. Ligand Preparation
2.4.3. Grid Generation and Molecular Docking
2.5. Determination of the Half Maximal Inhibitory Concentration “IC50”
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Prepared Nanocarriers
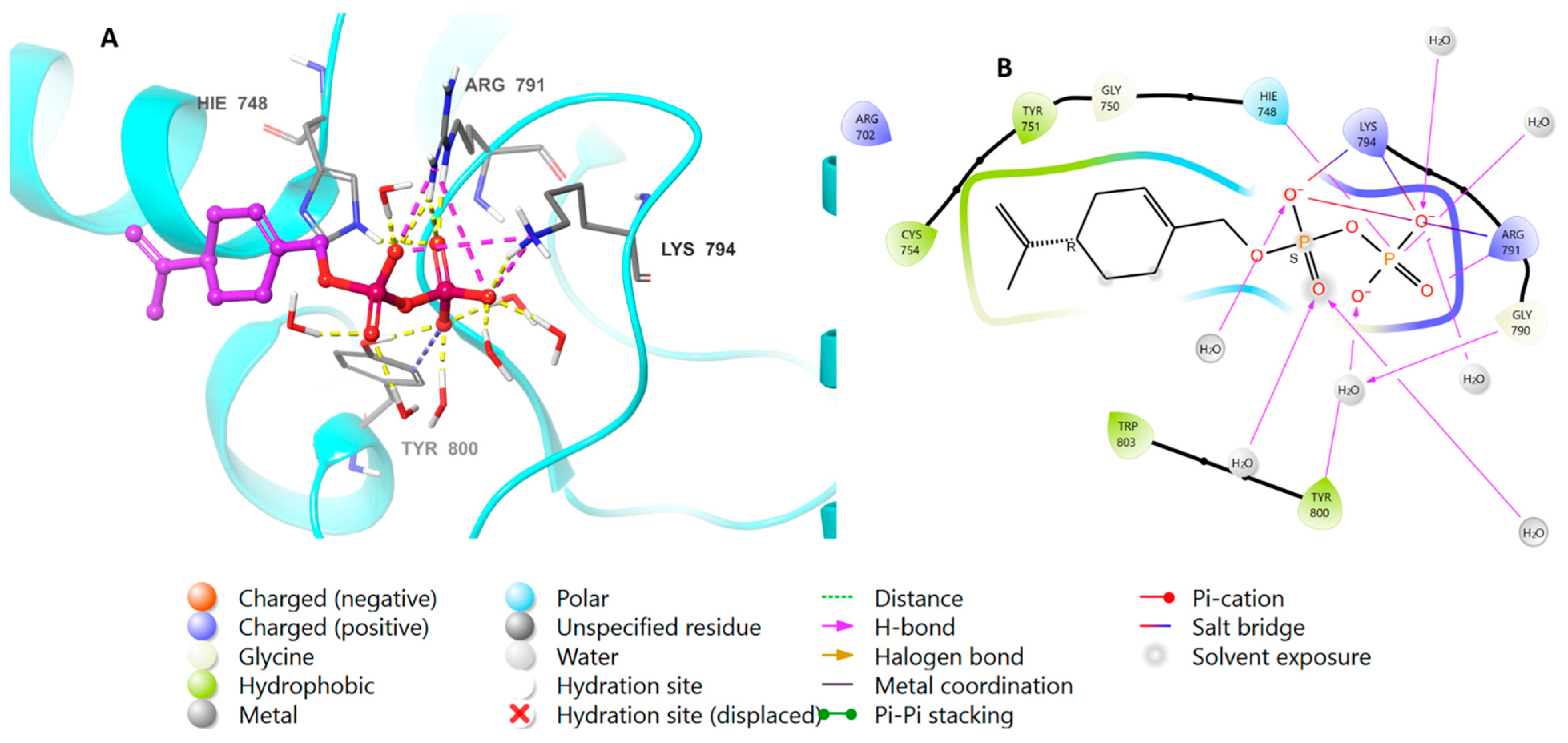
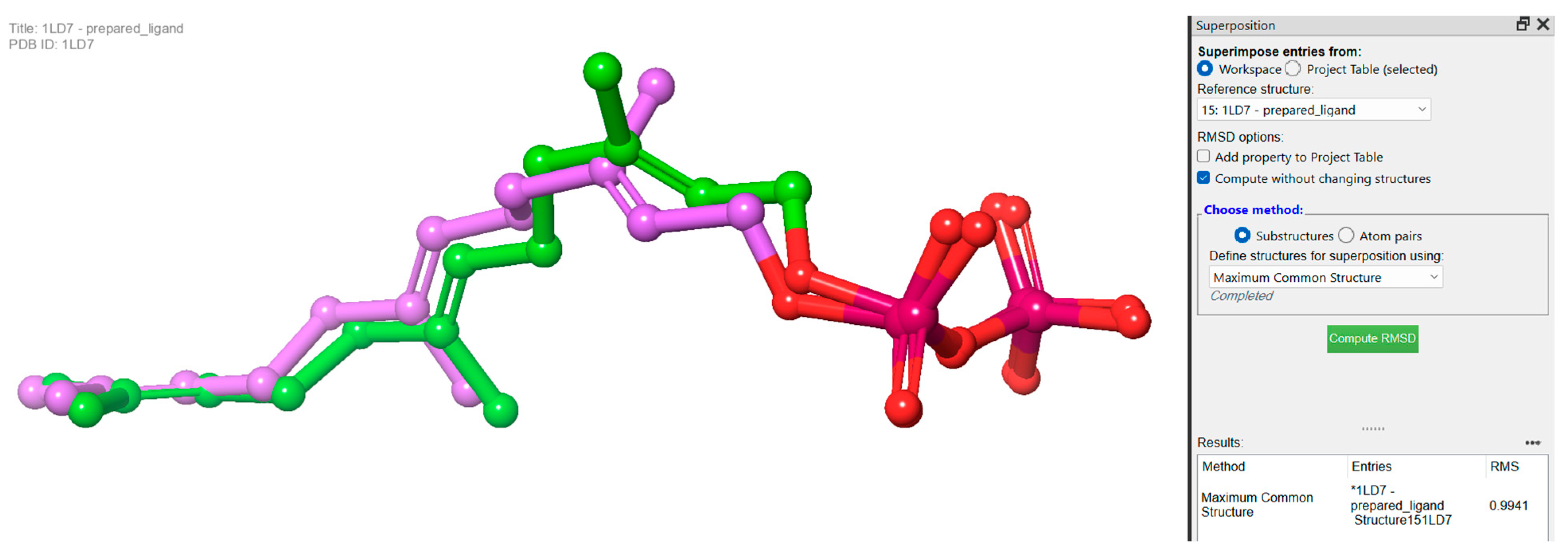
3.2. Molecular Docking Studies Analysis
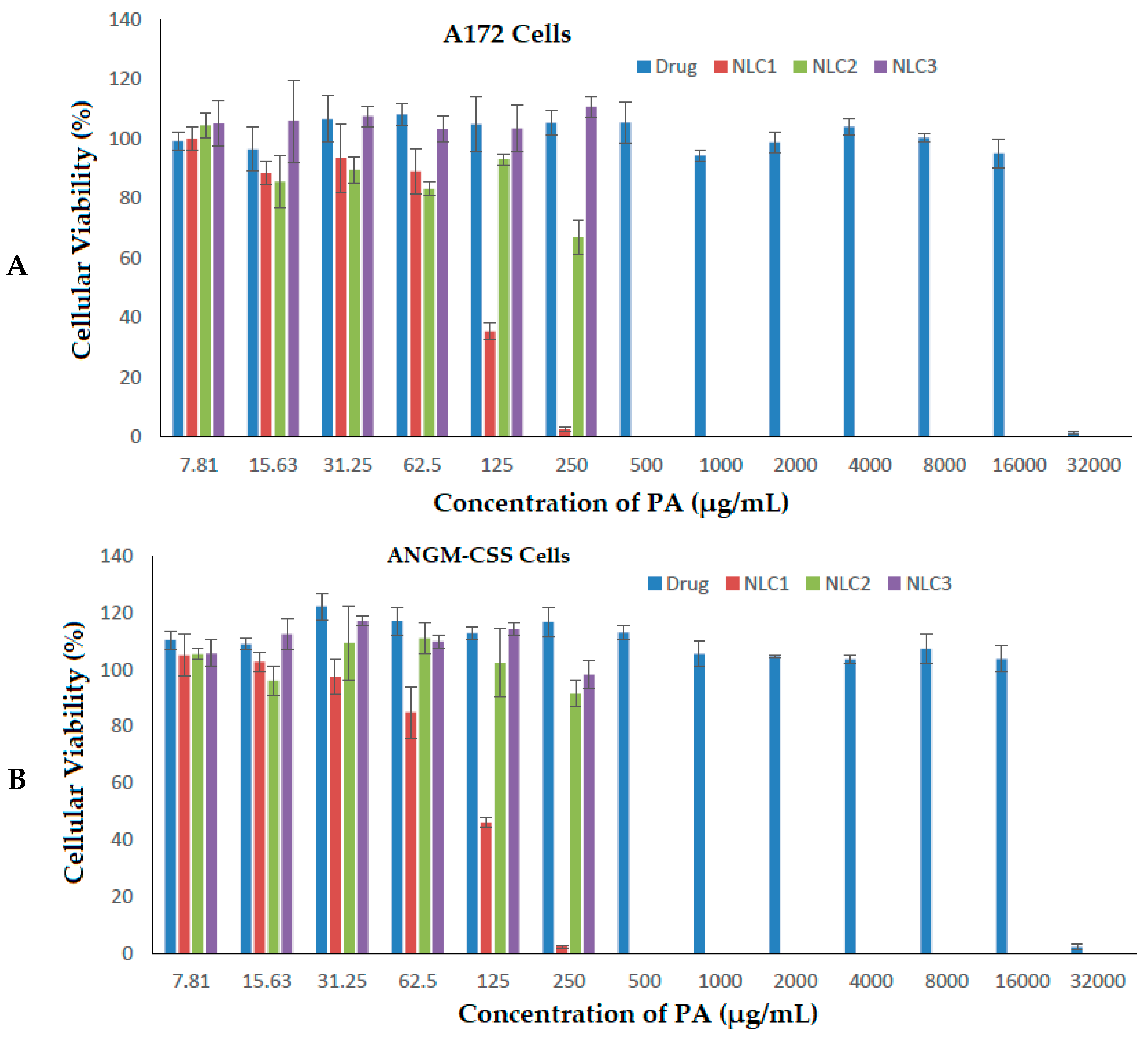
3.3. Antiproliferative Activity against ANGM-CSS and A172 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef]
- Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; De la Luz Cádiz-Gurrea, M.; Pimentel-Moral, S.; Segura-Carretero, A. The Role of High-Resolution Analytical Techniques in the Development of Functional Foods. Int. J. Mol. Sci. 2021, 22, 3220. [Google Scholar] [CrossRef]
- Chen, T.C.; da Fonseca, C.O.; Levin, D.; Schönthal, A.H. The monoterpenoid perillyl alcohol: Anticancer agent and medium to overcome biological barriers. Pharmaceutics 2021, 13, 2167. [Google Scholar] [CrossRef]
- Crowell, P.L.; Lin, S.; Vedejs, E.; Gould, M.N. Identification of metabolites of the antitumor agent d-limonene capable of inhibiting protein isoprenylation and cell growth. Cancer Chemother. Pharmacol. 1992, 31, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Crowell, P.L.; Ren, Z.; Lin, S.; Vedejs, E.; Gould, M.N. Structure-activity relationships among monoterpene inhibitors of protein isoprenylation and cell proliferation. Biochem. Pharmacol. 1994, 47, 1405–1415. [Google Scholar] [CrossRef]
- Gelb, M.H.; Tamanoi, F.; Yokoyama, K.; Ghomashchi, F.; Esson, K.; Gould, M.N. The inhibition of protein prenyltransferases by oxygenated metabolites of limonene and perillyl alcohol. Cancer Lett. 1995, 91, 169–175. [Google Scholar] [CrossRef]
- Crowell, P.L.; Chang, R.R.; Ren, Z.; Elson, C.E.; Gould, M.N. Selective inhibition of isoprenylation of 21-26-kDa proteins by the anticarcinogen d-limonene and its metabolites. J. Biol. Chem. 1991, 266, 17679–17685. [Google Scholar] [CrossRef]
- Ariazi, E.A.; Satomi, Y.; Ellis, M.J.; Haag, J.D.; Shi, W.; Sattler, C.A.; Gould, M.N. Activation of the transforming growth factor β signaling pathway and induction of cytostasis and apoptosis in mammary carcinomas treated with the anticancer agent perillyl alcohol. Cancer Res. 1999, 59, 1917–1928. [Google Scholar]
- Koyama, M.; Sowa, Y.; Hitomi, T.; Iizumi, Y.; Watanabe, M.; Taniguchi, T.; Ichikawa, M.; Sakai, T. Perillyl alcohol causes G1 arrest through p15INK4b and p21 WAF1/Cip1 induction. Oncol. Rep. 2013, 29, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, D.A.; Werner, S.R.; Crowell, P.L. Cell cycle arrest by the isoprenoids perillyl alcohol, geraniol, and farnesol is mediated by p21Cip1 and p27Kip1 in human pancreatic adenocarcinoma cells. J. Pharmacol. Exp. Ther. 2007, 320, 1163–1170. [Google Scholar] [CrossRef]
- Yuri, T.; Danbara, N.; Tsujita-Kyutoku, M.; Kiyozuka, Y.; Senzaki, H.; Shikata, N.; Kanzaki, H.; Tsubura, A. Perillyl alcohol inhibits human breast cancer cell growth in vitro and in vivo. Breast Cancer Res. Treat. 2004, 84, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Satomi, Y.; Miyamoto, S.; Gould, M.N. Induction of AP-1 activity by perillyl alcohol in breast cancer cells. Carcinogenesis 1999, 20, 1957–1961. [Google Scholar] [CrossRef] [PubMed]
- Sundin, T.; Peffley, D.M.; Hentosh, P. Disruption of an hTERT-mTOR-RAPTOR protein complex by a phytochemical perillyl alcohol and rapamycin. Mol. Cell. Biochem. 2013, 375, 97–104. [Google Scholar] [CrossRef]
- Sundin, T.; Peffley, D.M.; Gauthier, D.; Hentosh, P. The isoprenoid perillyl alcohol inhibits telomerase activity in prostate cancer cells. Biochimie 2012, 94, 2639–2648. [Google Scholar] [CrossRef]
- Garcia, D.G.; Amorim, L.M.F.; Faria, M.V.d.C.; Freire, A.S.; Santelli, R.E.; Da Fonseca, C.O.; Quirico-Santos, T.; Burth, P. The anticancer drug perillyl alcohol is a Na/K-ATPase inhibitor. Mol. Cell. Biochem. 2010, 345, 29–34. [Google Scholar] [CrossRef]
- Garcia, D.G.; de Castro-Faria-Neto, H.C.; da Silva, C.I.; de Souza e Souza, K.F.; Gonçalves-de-Albuquerque, C.F.; Silva, A.R.; de Amorim, L.M.; Freire, A.S.; Santelli, R.E.; Diniz, L.P.; et al. Na/K-ATPase as a target for anticancer drugs: Studies with perillyl alcohol. Mol. Cancer. 2015, 14, 105. [Google Scholar] [CrossRef]
- Tabassum, R.; Vaibhav, K.; Shrivastava, P.; Khan, A.; Ahmed, M.E.; Ashafaq, M.; Khan, M.B.; Islam, F.; Safhi, M.M.; Islam, F. Perillyl alcohol improves functional and histological outcomes against ischemia-reperfusion injury by attenuation of oxidative stress and repression of COX-2, NOS-2 and NF-κB in middle cerebral artery occlusion rats. Eur. J. Pharmacol. 2015, 747, 190–199. [Google Scholar] [CrossRef]
- Khan, A.Q.; Nafees, S.; Sultana, S. Perillyl alcohol protects against ethanol induced acute liver injury in Wistar rats by inhibiting oxidative stress, NFκ-B activation and proinflammatory cytokine production. Toxicology 2011, 279, 108–114. [Google Scholar] [CrossRef]
- Berchtold, C.M.; Chen, K.S.; Miyamoto, S.; Gould, M.N. Perillyl alcohol inhibits a calcium-dependent constitutive nuclear factor-κB pathway. Cancer Res. 2005, 65, 8558–8566. [Google Scholar] [CrossRef]
- Ma, Y.; Bian, J.; Zhang, F. Inhibition of perillyl alcohol on cell invasion and migration depends on the Notch signaling pathway in hepatoma cells. Mol. Cell. Biochem. 2016, 411, 307–315. [Google Scholar] [CrossRef]
- Ma, J.; Li, J.; Wang, K.S.; Mi, C.; Piao, L.X.; Xu, G.H.; Li, X.; Lee, J.J.; Jin, X. Perillyl alcohol efficiently scavenges activity of cellular ROS and inhibits the translational expression of hypoxia-inducible factor-1α via mTOR/4E-BP1 signaling pathways. Int. Immunopharmacol. 2016, 39, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y.; Wang, W.; Jhaveri, N.; Torres, S.; Tseng, J.; Leong, M.N.; Lee, D.J.; Goldkorn, A.; Xu, T.; Petasis, N.A.; et al. Perillyl alcohol for the treatment of temozolomide-resistant gliomas. Mol. Cancer Ther. 2012, 11, 2462–2472. [Google Scholar] [CrossRef] [PubMed]
- Bailey, H.H.; Levy, D.; Harris, L.S.; Schink, J.C.; Foss, F.; Beatty, P.; Wadler, S. A phase II trial of daily perillyl alcohol in patients with advanced ovarian cancer: Eastern cooperative oncology group study E2E96. Gynecol. Oncol. 2002, 85, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Bailey, H.H.; Attia, S.; Love, R.R.; Fass, T.; Chappell, R.; Tutsch, K.; Harris, L.; Jumonville, A.; Hansen, R.; Shapiro, G.R.; et al. Phase II trial of daily oral perillyl alcohol (NSC 641066) in treatment-refractory metastatic breast cancer. Cancer Chemother. Pharmacol. 2008, 62, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Oettel, K.; Bailey, H.; Van Ummersen, L.; Tutsch, K.; Staab, M.J.; Horvath, D.; Alberti, D.; Arzoomanian, R.; Rezazadeh, H.; et al. Phase II trial of perillyl alcohol (NSC 641066) administered daily in patients with metastatic androgen independent prostate cancer. Investig. New Drugs 2003, 21, 367–372. [Google Scholar] [CrossRef]
- Matos, J.M.; Schmidt, C.M.; Thomas, H.J.; Cummings, O.W.; Wiebke, E.A.; Madura, J.A.; Patrick, L.J.; Crowell, P. A Pilot Study of Perillyl Alcohol in Pancreatic Cancer. J. Surg. Res. 2008, 147, 194–199. [Google Scholar] [CrossRef]
- Meadows, S.M.; Mulkerin, D.; Berlin, J.; Bailey, H.; Kolesar, J.; Warren, D.; Thomas, J.P. Phase II trial of perillyl alcohol in patients with metastatic colorectal cancer. Int. J. Gastrointest. Cancer 2002, 32, 125–128. [Google Scholar] [CrossRef]
- Miastkowska, M.; Konieczna, M.; Lason, E.; Tabaszewska, M.; Sikora, E.; Ogonowski, J. The Release of Perillyl Alcohol from the Different Kind of Vehicles. Curr. Pharm. Biotechnol. 2018, 19, 573–580. [Google Scholar] [CrossRef]
- Peczek, S.H.; Tartari, A.P.S.; Zittlau, I.C.; Diedrich, C.; Machado, C.S.; Mainardes, R.M. Enhancing Oral Bioavailability and Brain Biodistribution of Perillyl Alcohol Using Nanostructured Lipid Carriers. Pharmaceuticals 2023, 16, 1055. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Aldawsari, H. Intranasal optimized solid lipid nanoparticles loaded in situ gel for enhancing trans-mucosal delivery of simvastatin. J. Drug Deliv. Sci. Technol. 2018, 48, 499–508. [Google Scholar] [CrossRef]
- Ahmad, M.; Sahabjada; Akhtar, J.; Hussain, A.; Badaruddeen; Arshad, H.; Mishra, A. Development of a new rutin nanoemulsion and its application on prostate carcinoma PC3 cell line. EXCLI J. 2017, 16, 810–823. [Google Scholar]
- Jamal, A.; Asseri, A.H.; Ali, E.M.M.; El-Gowily, A.H.; Khan, M.I.; Hosawi, S.; Alsolami, R.; Ahmed, T.A. Preparation of 6-Mercaptopurine Loaded Liposomal Formulation for Enhanced Cytotoxic Response in Cancer Cells. Nanomaterials 2022, 12, 4029. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, C.O.; Linden, R.; Futuro, D.; Gattass, C.R.; Quirico-Santos, T. Ras pathway activation in gliomas: A strategic target for intranasal administration of perillyl alcohol. Arch. Immunol. Ther. Exp. 2008, 56, 267. [Google Scholar] [CrossRef] [PubMed]
- Holstein, S.A.; Hohl, R.J. Monoterpene regulation of Ras and Ras-related protein expression. J. Lipid Res. 2003, 44, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Bell, I.M.; Gallicchio, S.N.; Abrams, M.; Beese, L.S.; Beshore, D.C.; Bhimnathwala, H.; Bogusky, M.J.; Buser, C.A.; Culberson, J.C.; Davide, J.; et al. 3-Aminopyrrolidinone farnesyltransferase inhibitors: Design of macrocyclic compounds with improved pharmacokinetics and excellent cell potency. J. Med. Chem. 2002, 45, 2388–2409. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.H.M.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical pKa Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef]
- LigPrep; Schrödinger LLC.: New York, NY, USA, 2021; Available online: https://www.schrodinger.com/products/ligprep (accessed on 12 February 2023).
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Glide; Schrödinger LLC.: New York, NY, USA, 2021; Available online: https://www.schrodinger.com/products/glide (accessed on 12 February 2023).
- Herrmann, F.C.; Sivakumar, N.; Jose, J.; Costi, M.P.; Pozzi, C.; Schmidt, T.J. In silico identification and in vitro evaluation of natural inhibitors of leishmania major pteridine reductase I. Molecules 2017, 22, 2166. [Google Scholar] [CrossRef]
- Alzahrani, N.M.; Booq, R.Y.; Aldossary, A.M.; Bakr, A.A.; Almughem, F.A.; Alfahad, A.J.; Alsharif, W.K.; Jarallah, S.J.; Alharbi, W.S.; Alsudir, S.A.; et al. Liposome-Encapsulated Tobramycin and IDR-1018 Peptide Mediated Biofilm Disruption and Enhanced Antimicrobial Activity against Pseudomonas aeruginosa. Pharmaceutics 2022, 14, 960. [Google Scholar] [CrossRef] [PubMed]
- Bopp, S.K.; Lettieri, T. Comparison of four different colorimetric and fluorometric cytotoxicity assays in a zebrafish liver cell line. BMC Pharmacol. 2008, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Kalaycioglu, G.D.; Aydogan, N. Preparation and investigation of solid lipid nanoparticles for drug delivery. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 510, 77–86. [Google Scholar] [CrossRef]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Kurakula, M.; Ahmed, O.A.A.; Fahmy, U.A.; Ahmed, T.A. Solid lipid nanoparticles for transdermal delivery of avanafil: Optimization, formulation, in-vitro and ex-vivo studies. J. Liposome Res. 2016, 26, 288–296. [Google Scholar] [CrossRef]
- Aditya, N.; Macedo, A.S.; Doktorovova, S.; Souto, E.B.; Kim, S.; Chang, P.-S.; Ko, S. Development and evaluation of lipid nanocarriers for quercetin delivery: A comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nanoemulsions (LNE). LWT-Food Sci. Technol. 2014, 59, 115–121. [Google Scholar] [CrossRef]
- Müller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy: Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Zimmermann, E.; Müller, R.H. Electrolyte- and pH-stabilities of aqueous solid lipid nanoparticle (SLNTM) dispersions in artificial gastrointestinal media. Eur. J. Pharm. Biopharm. 2001, 52, 203–210. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C.W. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Kikuchi, E.; Ishijima, A.; Azuma, T.; Sakuma, I.; Ito, T. Investigating the optimum size of nanoparticles for their delivery into the brain assisted by focused ultrasound-induced blood–brain barrier opening. Sci. Rep. 2020, 10, 18220. [Google Scholar] [CrossRef] [PubMed]
- Folkesson, H.G.; Westrom, B.R.; Karlsson, B.W. Permeability of the respiratory tract to different-sized macromolecules after intratracheal instillation in young and adult rats. Acta Physiol. Scand. 1990, 139, 347–354. [Google Scholar] [CrossRef]
- Khan, M.S.; Mohapatra, S.; Gupta, V.; Ali, A.; Naseef, P.P.; Kurunian, M.S.; Alshadidi, A.A.F.; Alam, S.; Mirza, M.A.; Iqbal, Z. Potential of Lipid-Based Nanocarriers against Two Major Barriers to Drug Delivery—Skin and Blood–Brain Barrier. Membranes 2023, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, J.; Guillama Barroso, G.; Masoudi Asil, S.; Alvarado, M.; Armendariz, I.; Bernal, J.; Carabaza, X.; Chavez, S.; Cruz, P.; Escalante, V.; et al. Nanocarriers as Potential Drug Delivery Candidatesfor Overcoming the Blood–Brain Barrier: Challenges and Possibilities. ACS Omega 2020, 5, 12583. [Google Scholar] [CrossRef]
- Ahmed, T.A.; El-Say, K.M.; Ahmed, O.A.; Aljaeid, B.M. Superiority of TPGS-loaded micelles in the brain delivery of vinpocetine via administration of thermosensitive intranasal gel. Int. J. Nanomed. 2019, 14, 5555–5567. [Google Scholar] [CrossRef]
- Ahmed, T.A. Preparation of finasteride capsules-loaded drug nanoparticles: Formulation, optimization, in vitro, and pharmacokinetic evaluation. Int. J. Nanomed. 2016, 11, 515–527. [Google Scholar] [CrossRef]
- Palsuledesai, C.C.; Distefano, M.D. Protein Prenylation: Enzymes, Therapeutics, and BiotechnologyApplications. ACS Chem. Biol. 2015, 10, 51. [Google Scholar] [CrossRef]
- Joo, J.H.; Jetten, A.M. Molecular Mechanisms involved in Farnesol-Induced Apoptosis. Cancer Lett. 2010, 287, 123. [Google Scholar] [CrossRef]
- Fernandes, J.; Da Fonseca, C.O.; Teixeira, A.; Gattass, C.R. Perillyl alcohol induces apoptosis in human glioblastoma multiforme cells. Oncol. Rep. 2005, 13, 943–947. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]

| Formulation | NLC1 | NLC2 | NLC3 |
|---|---|---|---|
| Composition | |||
| % of Stearic acid (w/v) | 1.272 | 1.272 | 1.272 |
| % of Cholesterol (w/v) | 0.16 | 0.16 | 0.16 |
| Sefsol (mg/mL) | - | 5 | - |
| PA (mg/mL) | 5 | - | - |
| % of Tween 80 in aqueous phase (w/v) | 1 | 1 | 1 |
| Characterization | |||
| Size (nm) | 330.47 ± 22.11 | 248.67 ± 12.42 | 1124.21 ± 12.77 |
| PDI | 0.509 ± 0.064 | 0.504 ± 0.065 | 0.418 ± 0.043 |
| Zeta potential (mV) | −15.20 ± 0.96 | −17.76 ± 1.12 | −36.91 ± 1.31 |
| Compounds | Docking Score | XP Gscore | Glide Gscore | Glide Emodel |
|---|---|---|---|---|
| Farnesyl diphosphate | −11.963 | −11.963 | −11.963 | −142.926 |
| Perillyl diphosphate | −9.54 | −9.628 | −9.628 | −124.045 |
| Formulations | IC50 (µg/mL) | |
|---|---|---|
| A172 Cells | ANGM-CSS Cells | |
| Free drug | 21,449.449 | 22,036.925 |
| NLC1 | 109.717 | 110.576 |
| NLC2 | 274.671 | 287.126 |
| NLC3 | 353.244 | 335.586 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, T.A.; Almehmady, A.M.; Alharbi, W.S.; Alshehri, A.A.; Almughem, F.A.; Altamimi, R.M.; Alshabibi, M.A.; Omar, A.M.; El-Say, K.M. Incorporation of Perillyl Alcohol into Lipid-Based Nanocarriers Enhances the Antiproliferative Activity in Malignant Glioma Cells. Biomedicines 2023, 11, 2771. https://doi.org/10.3390/biomedicines11102771
Ahmed TA, Almehmady AM, Alharbi WS, Alshehri AA, Almughem FA, Altamimi RM, Alshabibi MA, Omar AM, El-Say KM. Incorporation of Perillyl Alcohol into Lipid-Based Nanocarriers Enhances the Antiproliferative Activity in Malignant Glioma Cells. Biomedicines. 2023; 11(10):2771. https://doi.org/10.3390/biomedicines11102771
Chicago/Turabian StyleAhmed, Tarek A., Alshaimaa M. Almehmady, Waleed S. Alharbi, Abdullah A. Alshehri, Fahad A. Almughem, Reem M. Altamimi, Manal A. Alshabibi, Abdelsattar M. Omar, and Khalid M. El-Say. 2023. "Incorporation of Perillyl Alcohol into Lipid-Based Nanocarriers Enhances the Antiproliferative Activity in Malignant Glioma Cells" Biomedicines 11, no. 10: 2771. https://doi.org/10.3390/biomedicines11102771
APA StyleAhmed, T. A., Almehmady, A. M., Alharbi, W. S., Alshehri, A. A., Almughem, F. A., Altamimi, R. M., Alshabibi, M. A., Omar, A. M., & El-Say, K. M. (2023). Incorporation of Perillyl Alcohol into Lipid-Based Nanocarriers Enhances the Antiproliferative Activity in Malignant Glioma Cells. Biomedicines, 11(10), 2771. https://doi.org/10.3390/biomedicines11102771








