Bacterial Tyrosinase Inhibition, Hemolytic and Thrombolytic Screening, and In Silico Modeling of Rationally Designed Tosyl Piperazine-Engrafted Dithiocarbamate Derivatives
Abstract
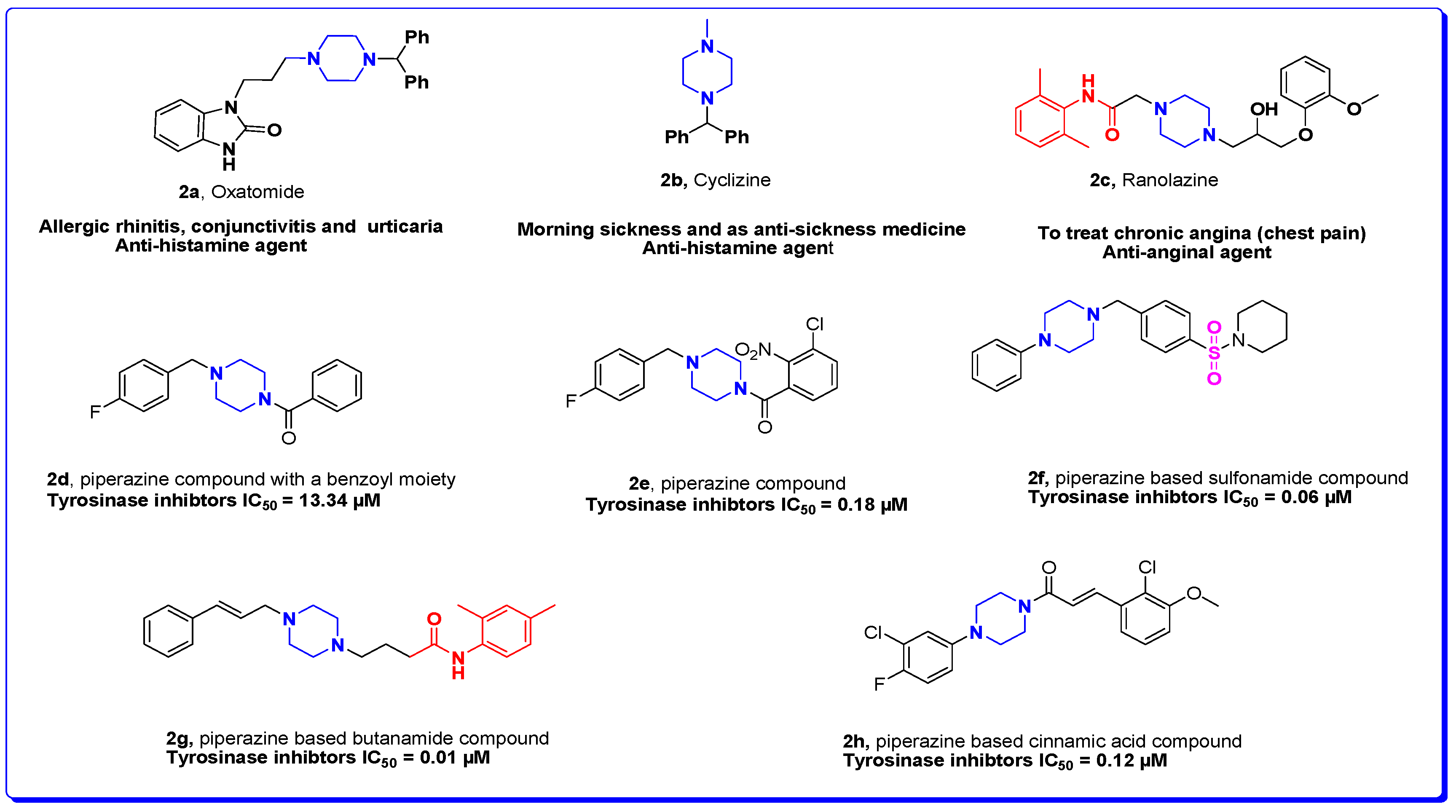
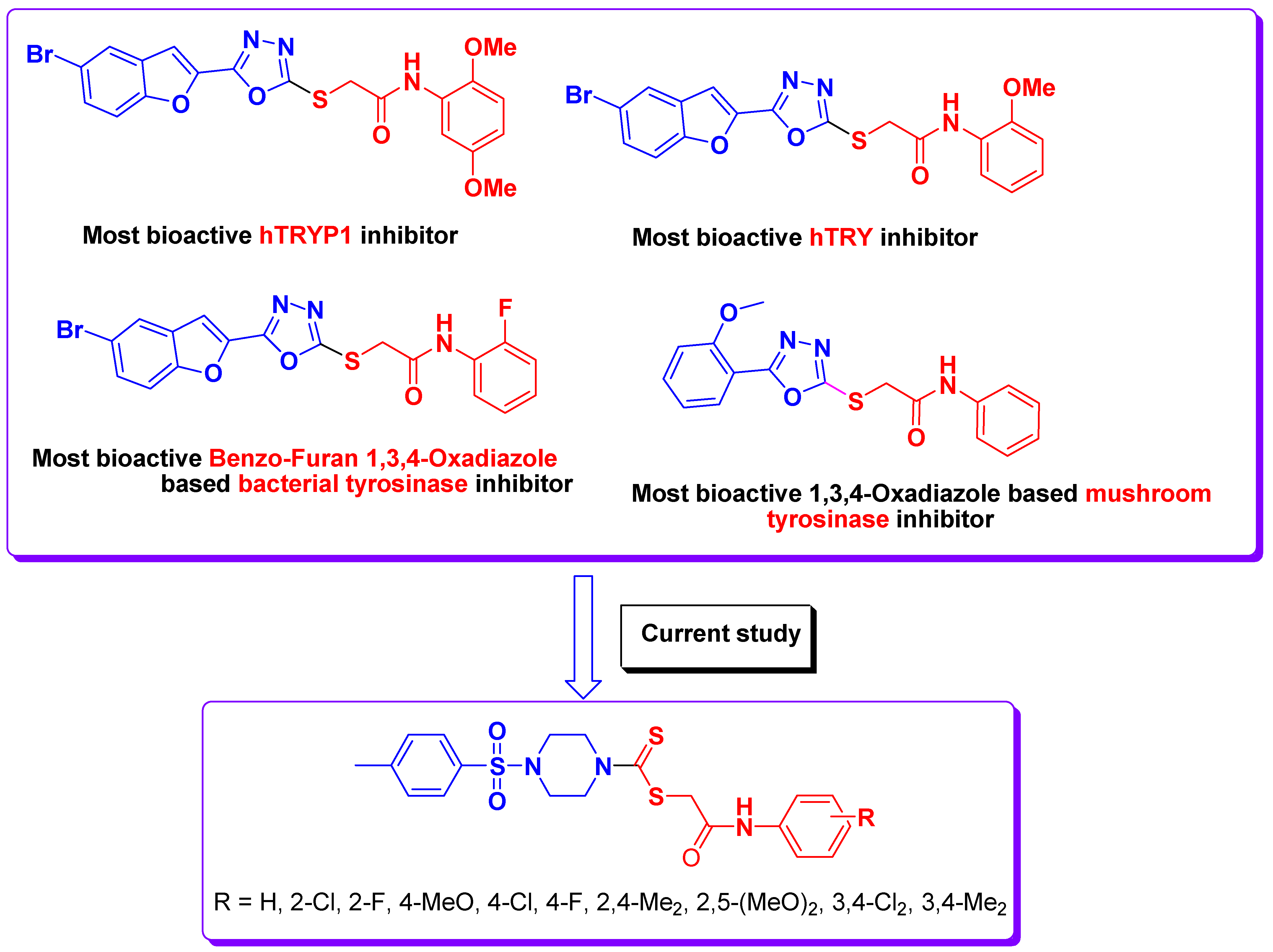
:1. Introduction
2. Materials and Methods
2.1. Materials
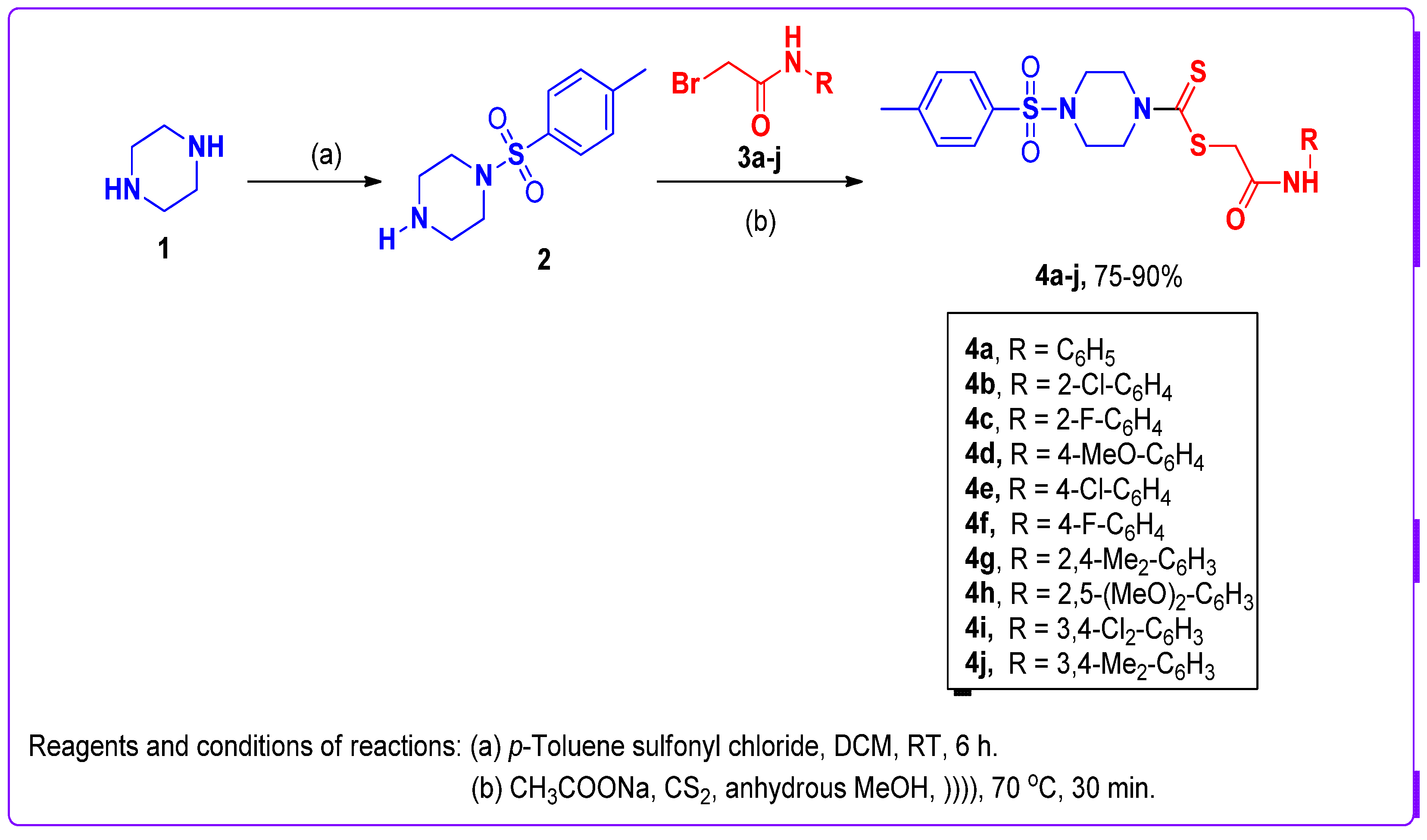
2.2. General Synthetic Procedure for Compounds
2.3. Biological Screening of 1-Tosyl piperazine-dithiocarbamate Hybrid Derivatives 4a–j
2.3.1. Tyrosinase Inhibition Evaluation of 1-Tosyl piperazine-dithiocarbamate Hybrid Derivatives 4a–j
2.3.2. Hemolytic Activity of 1-Tosyl piperazine-dithiocarbamate Hybrid Derivatives 4a–j
2.3.3. Thrombolytic Activity of 1-Tosyl piperazine-dithiocarbamate Hybrid Derivatives 4a–j
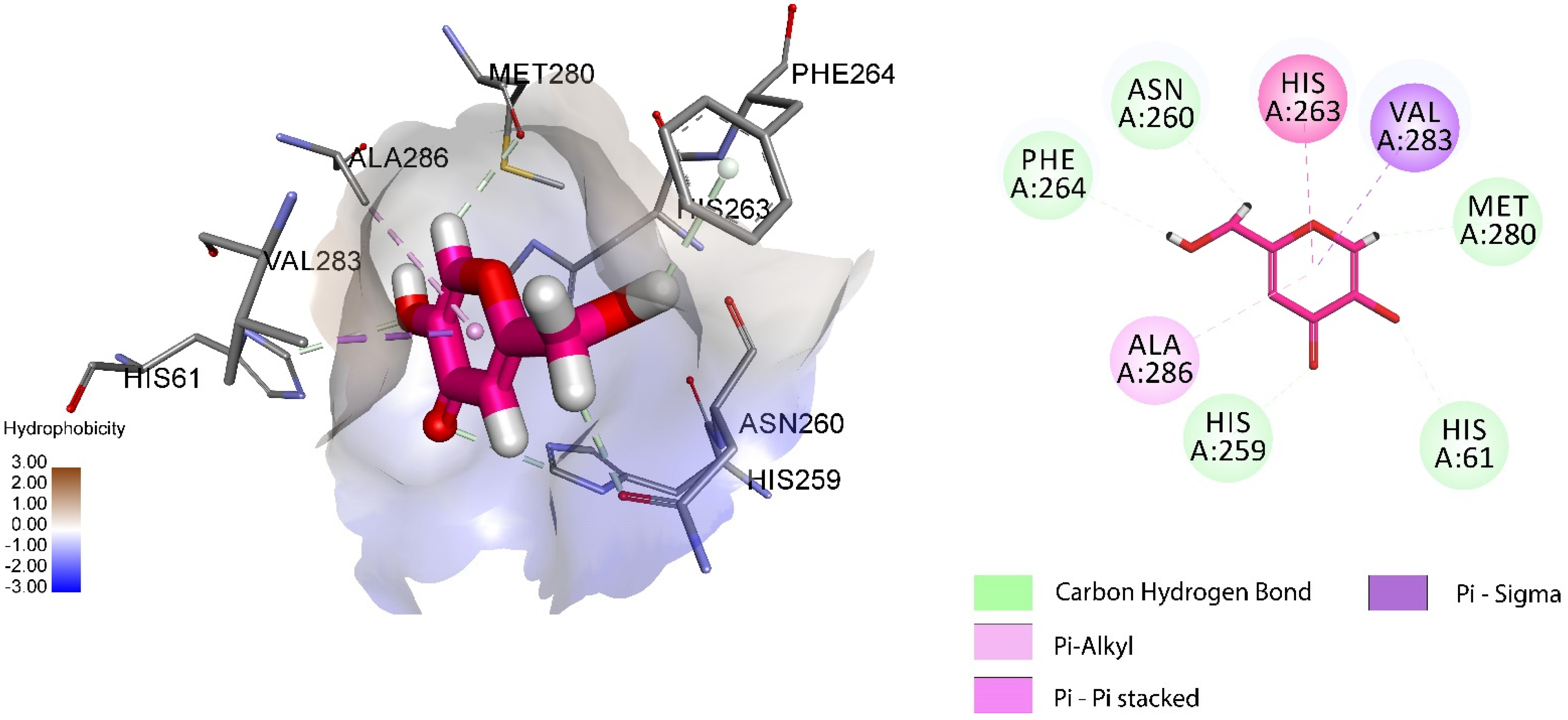
2.3.4. Molecular Docking Studies of 1-Tosyl piperazine-dithiocarbamate Hybrid Derivative 4d
3. Results and Discussion
3.1. Synthetic Chemistry of 1-Tosyl piperazine-dithiocarbamate Hybrid Derivatives 4a–j
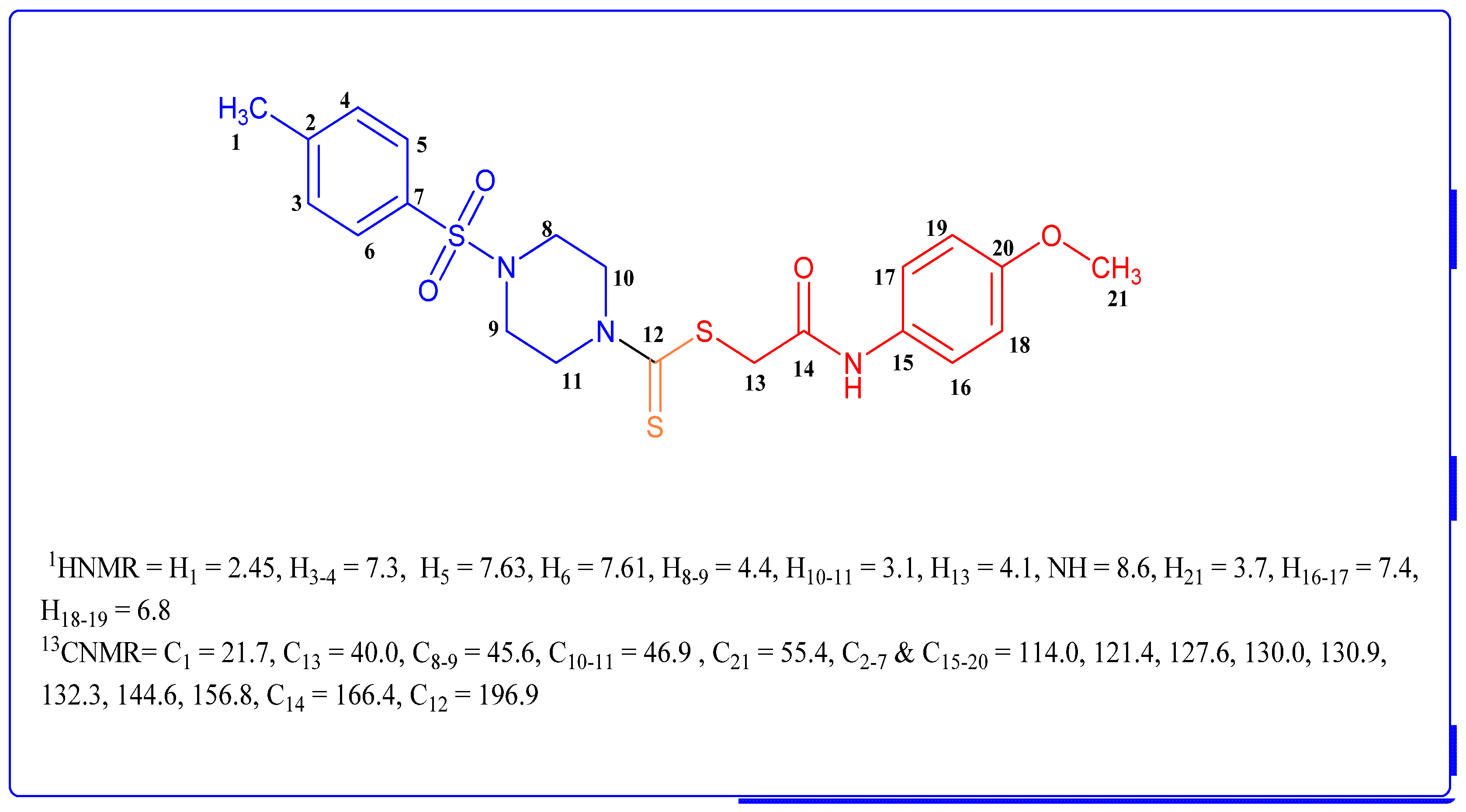
3.2. Spectral Characterization of the Representative and Most Bioactive Derivative 4d
3.3. Biological Screening of 1-Tosyl piperazine-dithiocarbamate Hybrid Derivatives 4a–j
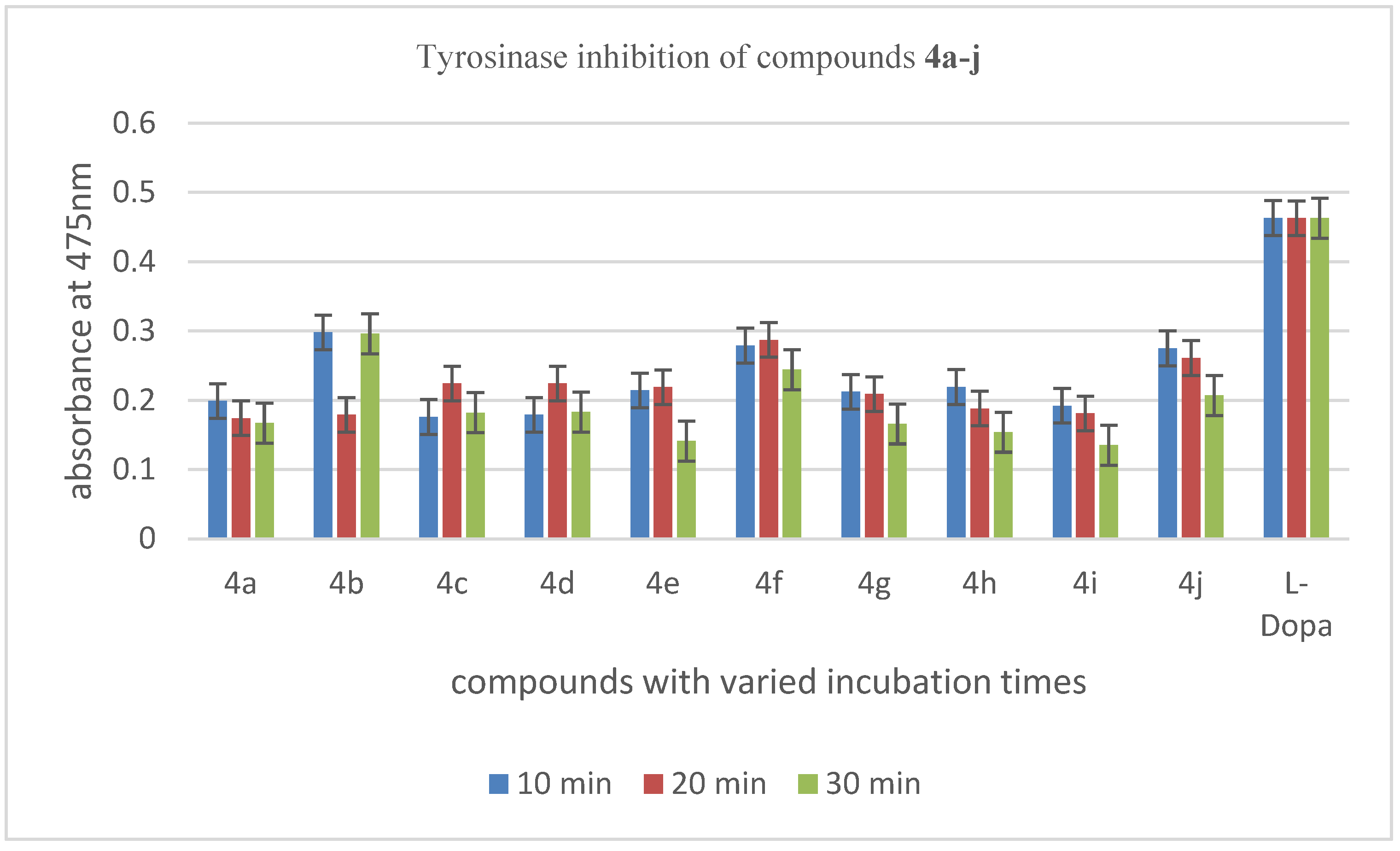
3.3.1. Tyrosinase Inhibition Evaluation of 1-Tosyl piperazine-dithiocarbamate Hybrid Derivatives 4a–j
3.3.2. Hemolytic Activity of 1-Tosyl piperazine-dithiocarbamate Hybrid Derivatives 4a–j
3.3.3. Thrombolytic Activity of 1-Tosyl piperazine-dithiocarbamate Hybrid Derivatives 4a–j
3.3.4. SAR (Structure Activity Relationship) Studies of 1-Tosyl piperazine-dithiocarbamate Derivatives 4a–j
3.3.5. Molecular Docking Studies of 1-Tosyl piperazine-dithiocarbamate Derivative 4d
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fairhead, M.; Thöny-Meyer, L. Role of the C-terminal extension in a bacterial tyrosinase. FEBS J. 2010, 277, 2083–2095. [Google Scholar] [CrossRef]
- Zaidi, K.U.; Ali, A.S.; Ali, S.A.; Naaz, I. Microbial tyrosinases: Promising enzymes for pharmaceutical, food bioprocessing, and environmental industry. Biochem. Res. Int. 2014, 2014, 854687. [Google Scholar] [CrossRef]
- Vittorio, S.; Dank, C.; Ielo, L. Heterocyclic Compounds as Synthetic Tyrosinase Inhibitors: Recent Advances. Int. J. Mol. Sci. 2023, 24, 9097. [Google Scholar] [CrossRef]
- Irfan, A.; Zahoor, A.F.; Kamal, S.; Hassan, M.; Kloczkowski, A. Ultrasonic-Assisted Synthesis of Benzofuran Appended Oxadiazole Molecules as Tyrosinase Inhibitors: Mechanistic Approach through Enzyme Inhibition, Molecular Docking, Chemoinformatics, ADMET and Drug-Likeness Studies. Int. J. Mol. Sci. 2022, 23, 10979. [Google Scholar] [CrossRef] [PubMed]
- Rolff, M.; Schottenheim, J.; Decker, H.; Tuczek, F. Copper-O2 reactivity of tyrosinase models towards external monophenolic substrates: Molecular mechanism and comparison with the enzyme. Chem. Soc. Rev. 2011, 40, 4077–4098. [Google Scholar] [CrossRef] [PubMed]
- Cabezudo, I.; Ramallo, I.A.; Alonso, V.L.; Furlan, R.L. Effect directed synthesis of a new tyrosinase inhibitor with anti-browning activity. Food Chem. 2021, 341, 128232. [Google Scholar] [CrossRef]
- Andersen, S.O. Insect cuticular sclerotization: A review. Insect Biochem. Mol. Biol. 2010, 40, 166–178. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B.; Seiji, M.; McGugan, A.D. Melanin pigmentation. N. Engl. J. Med. 1961, 265, 328–332. [Google Scholar] [CrossRef]
- Chen, L.-H.; Hu, Y.-H.; Song, W.; Song, K.-K.; Liu, X.; Jia, Y.-L.; Zhuang, J.-X.; Chen, Q.-X. Synthesis and antityrosinase mechanism of benzaldehyde thiosemicarbazones: Novel tyrosinase inhibitors. J. Agric. Food Chem. 2012, 60, 1542–1547. [Google Scholar] [CrossRef]
- Banik, B.K.; Sahoo, B.M.; Kumar, B.V.; Panda, K.C.; Jena, J.; Mahaputra, M.K.; Borah, P. Green Synthetic Approach: An Efficient eco-friendly tool for synthesis of biologically active oxadiazole derivatives. Molecules 2021, 26, 1163. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Imran, S.; Rokie, M.Q.; Saad, S.M.; Khan, K.M. Synthesis of new oxadiazole derivatives as α-glucosidase inhibitors. Bioorg. Med. Chem. 2015, 23, 4155–4162. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, R.S.; Sarda, S.R.; Ardhapure, S.S.; Jadhav, W.N.; Bhusare, S.R.; Pawar, R.P. An efficient protocol for the synthesis of quinoxaline derivatives at room temperature using molecular iodine as the catalyst. Tetrahedron Lett. 2005, 46, 7183–7186. [Google Scholar] [CrossRef]
- Shahzadi, I.; Zahoor, A.F.; Tüzün, B.; Mansha, A.; Anjum, M.N.; Rasul, A.; Irfan, A.; Kotwica-Mojzych, K.; Mojzych, M. Repositioning of acefylline as anti-cancer drug: Synthesis, anticancer and computational studies of azomethines derived from acefylline tethered 4-amino-3-mercapto-1,2,4-triazole. PLoS ONE 2022, 17, e0278027. [Google Scholar] [CrossRef] [PubMed]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-Aizari, F.A.; Ansar, M.H. Synthesis and pharmacological activities of pyrazole derivatives: A review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef]
- Barea, C.; Pabón, A.; Castillo, D.; Zimic, M.; Quiliano, M.; Galiano, S.; Pérez-Silanes, S.; Monge, A.; Deharo, E.; Aldana, I. New salicylamide and sulfonamide derivatives of quinoxaline 1, 4-di-N-oxide with antileishmanial and antimalarial activities. Bioorg. Med. Chem. Lett. 2011, 21, 4498–4502. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Lefebvre, I.; Puy, J.Y.; Périgaud, C. A facile and effective synthesis of lamivudine 5′-diphosphate. Tetrahedron Lett. 2011, 52, 1250–1252. [Google Scholar] [CrossRef]
- Yan, S.L.; Yang, M.Y.; Sun, Z.H.; Min, L.J.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Liu, X.H. Synthesis and antifungal activity of 1, 2, 3-thiadiazole derivatives containing 1, 3, 4-thiadiazole moiety. Lett. Drug Des. Discov. 2014, 11, 940–943. [Google Scholar] [CrossRef]
- Zhan, P.; Liu, X.; Li, Z.; Fang, Z.; Li, Z.; Wang, D.; Pannecouque, C.; De Clercq, E. Novel 1, 2, 3-thiadiazole derivatives as HIV-1 NNRTIs with improved potency: Synthesis and preliminary SAR studies. Bioorg. Med Chem. 2009, 17, 5920–5927. [Google Scholar] [CrossRef]
- Mohammed, H.H.; Abbas, S.H.; Abdelhafez, E.S.; Berger, J.M.; Mitarai, S.; Arai, M.; Abuo-Rahma, G.E. Synthesis, molecular docking, antimicrobial evaluation, and DNA cleavage assay of new thiadiazole/oxadiazole ciprofloxacin derivatives. Monatshefte Für Chem. Chem. Mon. 2019, 150, 1809–1824. [Google Scholar] [CrossRef]
- Irfan, A.; Faisal, S.; Ahmad, S.; Al-Hussain, S.A.; Javed, S.; Zahoor, A.F.; Parveen, B.; Zaki, M.E.A. Structure-Based Virtual Screening of Furan-1,3,4-Oxadiazole Tethered N-phenylacetamide Derivatives as Novel Class of hTYR and hTYRP1 Inhibitors. Pharmaceuticals 2023, 16, 344. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, S.; Wang, Y.; Tu, H.; Yu, L.; Zhao, Z.; Wang, Z.; Wu, J. Novel trifluoromethylpyridine piperazine derivatives as potential plant activators. Front. Plant Sci. 2022, 13, 1086057. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, F.; Zahoor, A.F.; Rasul, A.; Mansha, A.; Noreen, R.; Raza, Z.; Ali, K.G.; Irfan, A.; El-Hiti, G.A. Ultrasound-Assisted Synthesis and In Silico Modeling of Methanesulfonyl-Piperazine-Based Dithiocarbamates as Potential Anticancer, Thrombolytic, and Hemolytic Structural Motifs. Molecules 2022, 27, 4776. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.F.; Moreira, L.K.S.; Menegatti, R.; Costa, E.A. Piperazine derivatives with central pharmacological activity used as therapeutic tools. Fundam. Clin. Pharmacol. 2019, 33, 13–24. [Google Scholar] [CrossRef]
- Gupta, S.S.; Kumari, S.; Kumar, I.; Sharma, U. Eco-friendly and sustainable synthetic approaches to biologically significant fused N-heterocycles. Chem. Heterocycl. Compd. 2020, 56, 433–444. [Google Scholar] [CrossRef]
- Dayalan, A.; Gurumurthy, P.; Babu, C.S.M.; Visweswaran, V. Synthesis, Characterization and Antimicrobial Activity Studies of 1-and 2-[4-{(4-Chloro phenyl) phenyl methyl}-1-piperazinyl] sulphonyl naphthalenes. Asian J. Chem. 2007, 19, 5041. [Google Scholar]
- Kharb, R.; Bansal, K.; Sharma, A.K. A valuable insight into recent advances on antimicrobial activity of piperazine derivatives. Der Pharma Chem. 2012, 4, 2470–2488. [Google Scholar]
- Hessein, S.A.; El-Sharief, M.A.S.; Abbas, S.Y.; Thabet, H.K.; Ammar, Y.A. Synthesis and antimicrobial activity of furochromone, benzofuran and furocoumarin derivatives bearing sulfonyl moiety. Croat. Chem. Acta 2016, 89, 91–100. [Google Scholar] [CrossRef]
- Ryckebusch, A.; Debreu-Fontaine, M.-A.; Mouray, E.; Grellier, P.; Sergheraert, C.; Melnyk, P. Synthesis and antimalarial evaluation of new N1-(7-chloro-4-quinolyl)-1, 4-bis (3-aminopropyl) piperazine derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Masuda, T.; Yamada, K.; Kawakatsu, N.; Kubota, N.; Mitani, M.; Kishii, K.; Inazu, M.; Kiuchi, Y.; Oguchi, K. Antioxidative activities of novel diphenylalkyl piperazine derivatives with high affinities for the dopamine transporter. Bioorg. Med. Chem. Lett. 2004, 14, 4287–4290. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Chaudhary, J.; Khaira, H.; Chopra, B.; Dhingra, A. Piperazine: A promising scaffold with analgesic and anti-inflammatory potential. Drug Res. 2021, 71, 62–72. [Google Scholar] [CrossRef]
- Hatnapure, G.D.; Keche, A.P.; Rodge, A.H.; Birajdar, S.S.; Tale, R.H.; Kamble, V.M. Synthesis and biological evaluation of novel piperazine derivatives of flavone as potent anti-inflammatory and antimicrobial agent. Bioorg. Med Chem Lett. 2012, 22, 6385–6390. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghorbani, M.; Gouda, M.A.; Baashen, M.; Alharbi, O.; Almalki, F.A.; Ranganatha, L.V. Piperazine heterocycles as potential anticancer agents: A review. Pharm. Chem. J. 2022, 56, 29–37. [Google Scholar] [CrossRef]
- Asar, F.J.; Soleymani, F.; Hooshmand, S.E.; Halimehjani, A.Z. Direct synthesis of piperazines containing dithiocarbamate derivatives via DABCO bond cleavage. Tetrahedron Lett. 2020, 61, 152610. [Google Scholar] [CrossRef]
- Fu, D.-J.; Li, J.-H.; Yang, J.-J.; Li, P.; Zhang, Y.-B.; Liu, S.; Li, Z.-R.; Zhang, S.-Y. Discovery of novel chalcone-dithiocarbamates as ROS-mediated apoptosis inducers by inhibiting catalase. Bioorg. Chem. 2019, 86, 375–385. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Ajiboye, T.T.; Marzouki, R.; Onwudiwe, D.C. The Versatility in the Applications of Dithiocarbamates. Int. J. Mol. Sci. 2022, 23, 1317. [Google Scholar] [CrossRef] [PubMed]
- Altıntop, M.D.; Sever, B.; Çiftçi, G.A.; Kucukoglu, K.; Özdemir, A.; Soleimani, S.S.; Nadaroglu, H.; Kaplancıklı, Z.A. Synthesis and evaluation of new benzodioxole-based dithiocarbamate derivatives as potential anticancer agents and hCA-I and hCA-II inhibitors. Eur. J. Med. Chem. 2017, 125, 190–196. [Google Scholar] [CrossRef]
- Shiino, M.; Watanabe, Y.; Umezawa, K. Synthesis of N-substituted N-nitrosohydroxylamines as inhibitors of mushroom tyrosinase. Bioorg. Med. Chem. 2001, 9, 1233–1240. [Google Scholar] [CrossRef]
- Habib, A.; Iqbal, M.A.; Bhatti, H.N.; Kamal, A.; Kamal, S. Synthesis of Alkyl/aryl linked binuclear silver(I)-N-Heterocyclic carbene complexes and evaluation of their antimicrobial, hemolytic and thrombolytic potential. Inorg. Chem. Commun. 2020, 111, 107670. [Google Scholar] [CrossRef]
- Zhong, H.; Tran, L.M.; Stang, J.L. Induced-fit docking studies of the active and inactive states of protein tyrosine kinases. J. Mol. Graph. Model. 2009, 28, 336–346. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef]
- Shahzadi, I.; Zahoor, A.F.; Rasul, A.; Mansha, A.; Ahmad, S.; Raza, Z. Synthesis, Hemolytic Studies, and In Silico Modeling of Novel Acefylline–1, 2, 4-Triazole Hybrids as Potential Anti-cancer Agents against MCF-7 and A549. ACS Omega 2021, 6, 11943–11953. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.R.; Silva, J.R.A.; De Tássia Carvalho Cardoso, É.; Silva, E.O.; Lameira, J.; Do Nascimento, J.L.M.; Do Socorro Barros Brasil, D.; Alves, C.N. Combined kinetic studies and computational analysis on kojic acid analogs as tyrosinase inhibitors. Molecules 2014, 19, 9591–9605. [Google Scholar] [CrossRef] [PubMed]






| Compounds | –R | IC50 (µM) Tyrosinase Inhibition | % Hemolysis | % Thrombolysis |
|---|---|---|---|---|
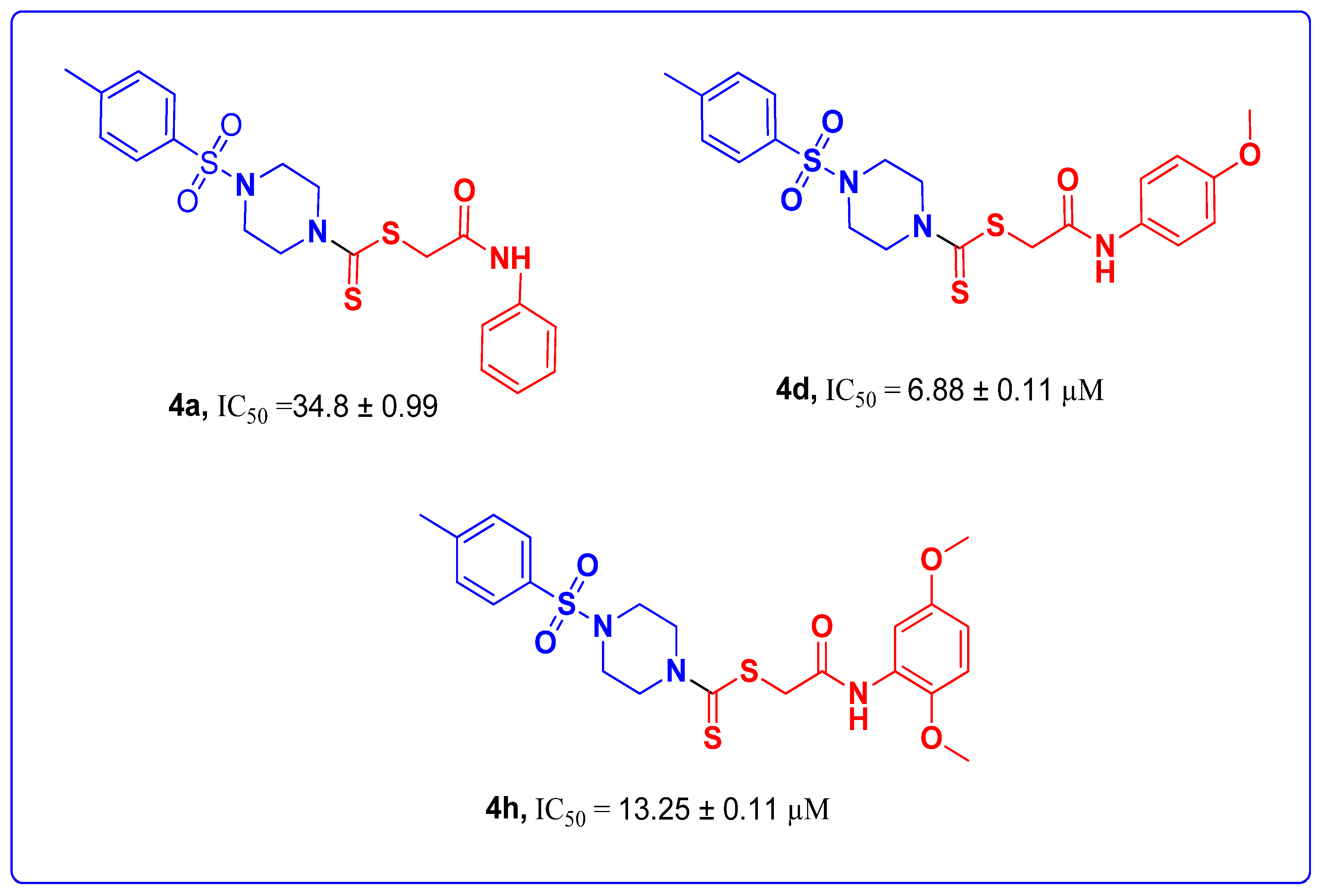
| 4a |  | 34.8 ± 0.99 | 0.55 ± 0.03 | 62.3 ± 0.41 |
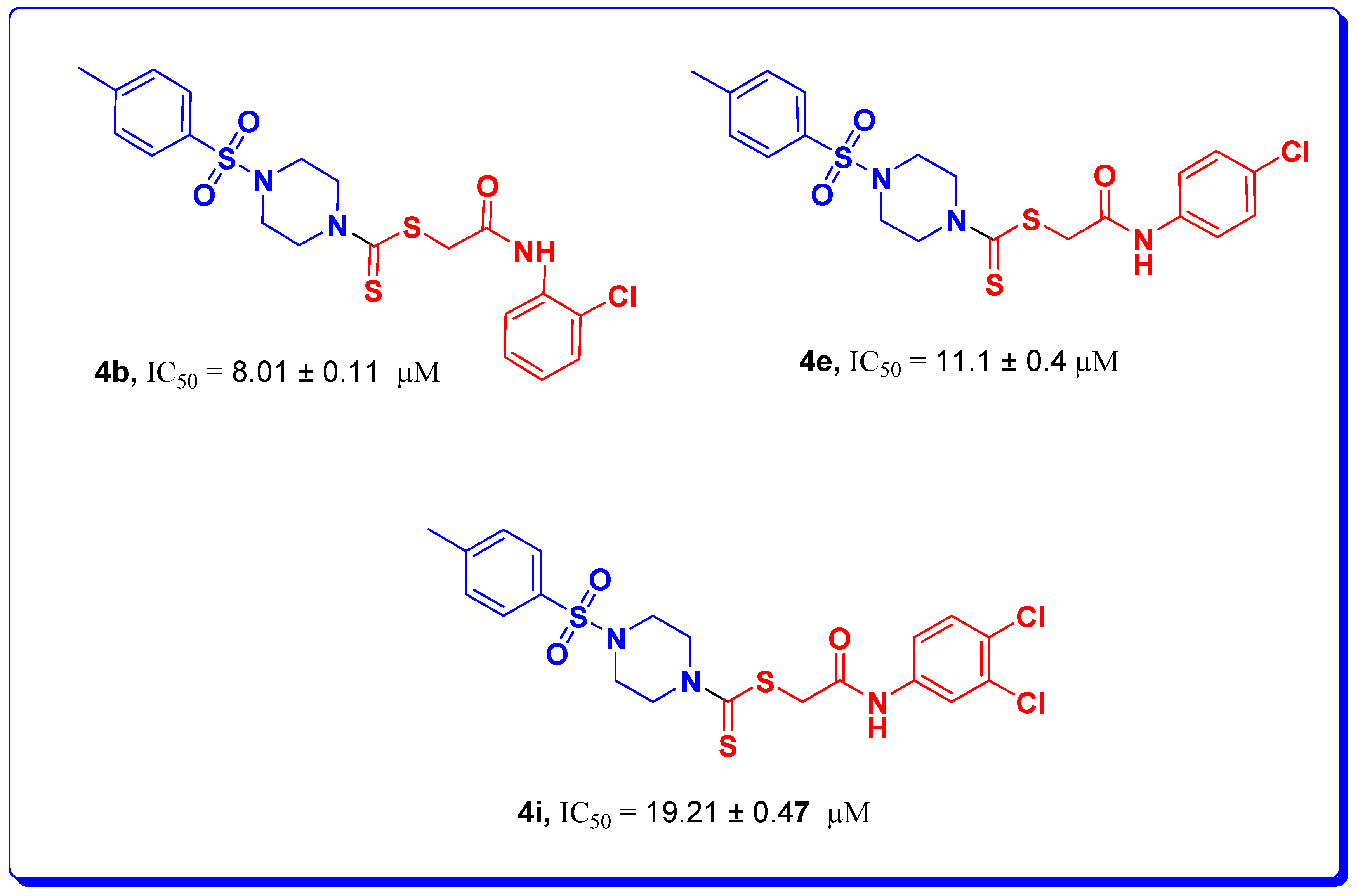
| 4b |  | 8.01 ± 0.11 | 0.29 ± 0.01 | 54.43 ± 0.23 |
| 4c | 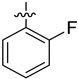 | 8.1 ± 0.30 | 6.6 ± 0.37 | 56.76 ± 0.15 |
| 4d | 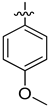 | 6.88 ± 0.11 | 15.6 ± 0.5 | 52.4 ± 0.20 |
| 4e | 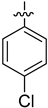 | 11.1 ± 0.4 | 7.5 ± 0.4 | 67.3 ± 0.2 |
| 4f | 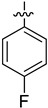 | 10.3 ± 0.26 | 4.53 ± 0.35 | 56.53 ± 0.55 |
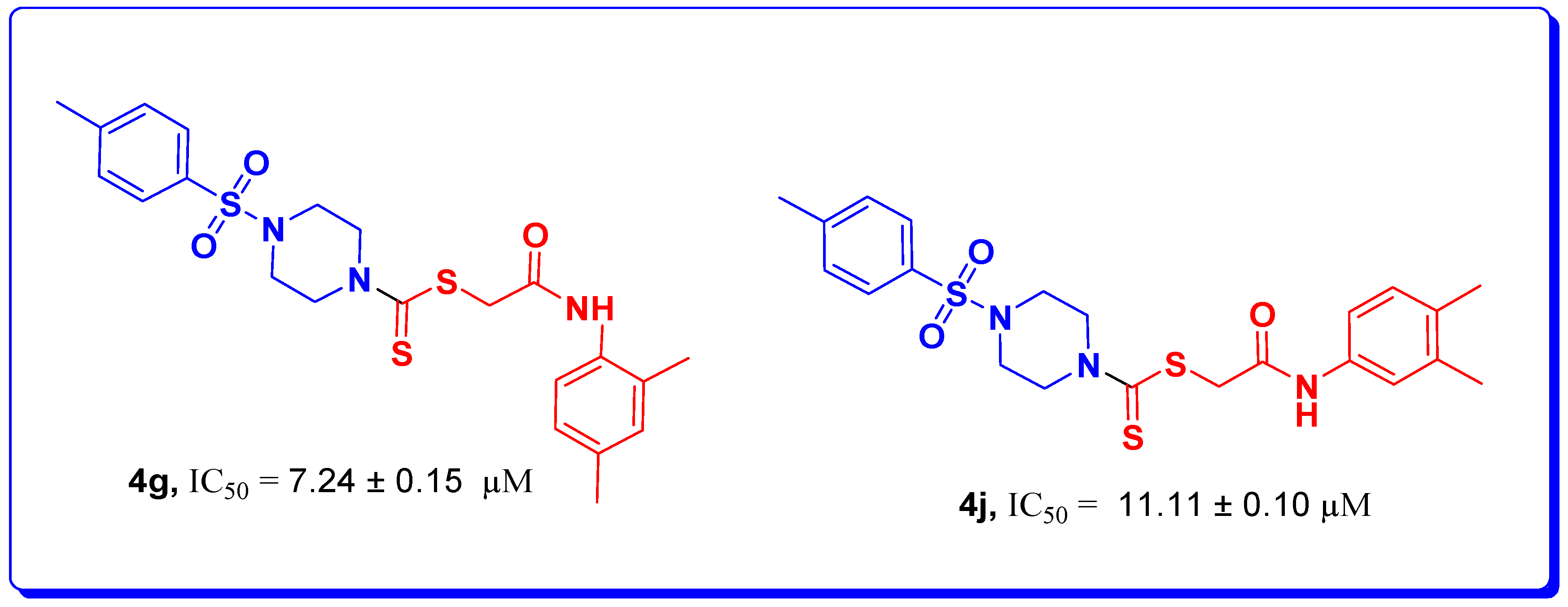
| 4g | 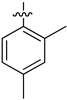 | 7.24 ± 0.15 | 1.53 ± 0.35 | 55.26 ± 0.15 |
| 4h | 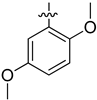 | 13.25 ± 0.11 | 11.32 ± 0.22 | 60.83 ± 0.14 |
| 4i | 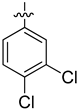 | 19.21 ± 0.47 | 12.8 ± 0.35 | 54.38 ± 0.53 |
| 4j |  | 11.11 ± 0.10 | 2.56 ± 0.30 | 56.6 ± 0.23 |
| Kojic Acid | 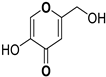 | 30.34 ± 0.75 | -- | -- |
| Ascorbic Acid [4] | 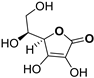 | 11.5 ± 1.00 | ||
| DMSO | -- | 0.0 | 0.57 | |
| ABTS | -- | 95.9 | 80 |
| Compounds | Binding Score (S) Kcal/mol | Interacting Residues | Interaction Type |
|---|---|---|---|
| Kojic acid | −4.84 | MET280, PHE264, ALA283, HIS263, ASN260, VAL283, HIS259 | H-bonding, π- π stacked, π-alkyl, π-σ |
| 4d | −7.3 | GLU322, VAL248, HIS244, SER282, PRO277, ARG268 | H-bonding, π-π T-Shaped, π-alkyl, π-cation, π-anion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahoor, A.F.; Hafeez, F.; Mansha, A.; Kamal, S.; Anjum, M.N.; Raza, Z.; Khan, S.G.; Javid, J.; Irfan, A.; Bhat, M.A. Bacterial Tyrosinase Inhibition, Hemolytic and Thrombolytic Screening, and In Silico Modeling of Rationally Designed Tosyl Piperazine-Engrafted Dithiocarbamate Derivatives. Biomedicines 2023, 11, 2739. https://doi.org/10.3390/biomedicines11102739
Zahoor AF, Hafeez F, Mansha A, Kamal S, Anjum MN, Raza Z, Khan SG, Javid J, Irfan A, Bhat MA. Bacterial Tyrosinase Inhibition, Hemolytic and Thrombolytic Screening, and In Silico Modeling of Rationally Designed Tosyl Piperazine-Engrafted Dithiocarbamate Derivatives. Biomedicines. 2023; 11(10):2739. https://doi.org/10.3390/biomedicines11102739
Chicago/Turabian StyleZahoor, Ameer Fawad, Freeha Hafeez, Asim Mansha, Shagufta Kamal, Muhammad Naveed Anjum, Zohaib Raza, Samreen Gul Khan, Jamila Javid, Ali Irfan, and Mashooq Ahmad Bhat. 2023. "Bacterial Tyrosinase Inhibition, Hemolytic and Thrombolytic Screening, and In Silico Modeling of Rationally Designed Tosyl Piperazine-Engrafted Dithiocarbamate Derivatives" Biomedicines 11, no. 10: 2739. https://doi.org/10.3390/biomedicines11102739
APA StyleZahoor, A. F., Hafeez, F., Mansha, A., Kamal, S., Anjum, M. N., Raza, Z., Khan, S. G., Javid, J., Irfan, A., & Bhat, M. A. (2023). Bacterial Tyrosinase Inhibition, Hemolytic and Thrombolytic Screening, and In Silico Modeling of Rationally Designed Tosyl Piperazine-Engrafted Dithiocarbamate Derivatives. Biomedicines, 11(10), 2739. https://doi.org/10.3390/biomedicines11102739







