Response Profiles of BV2 Microglia to IFN-γ and LPS Co-Stimulation and Priming
Abstract
:1. Introduction
2. Materials and Methods
2.1. BV2 Microglia Cell Culture
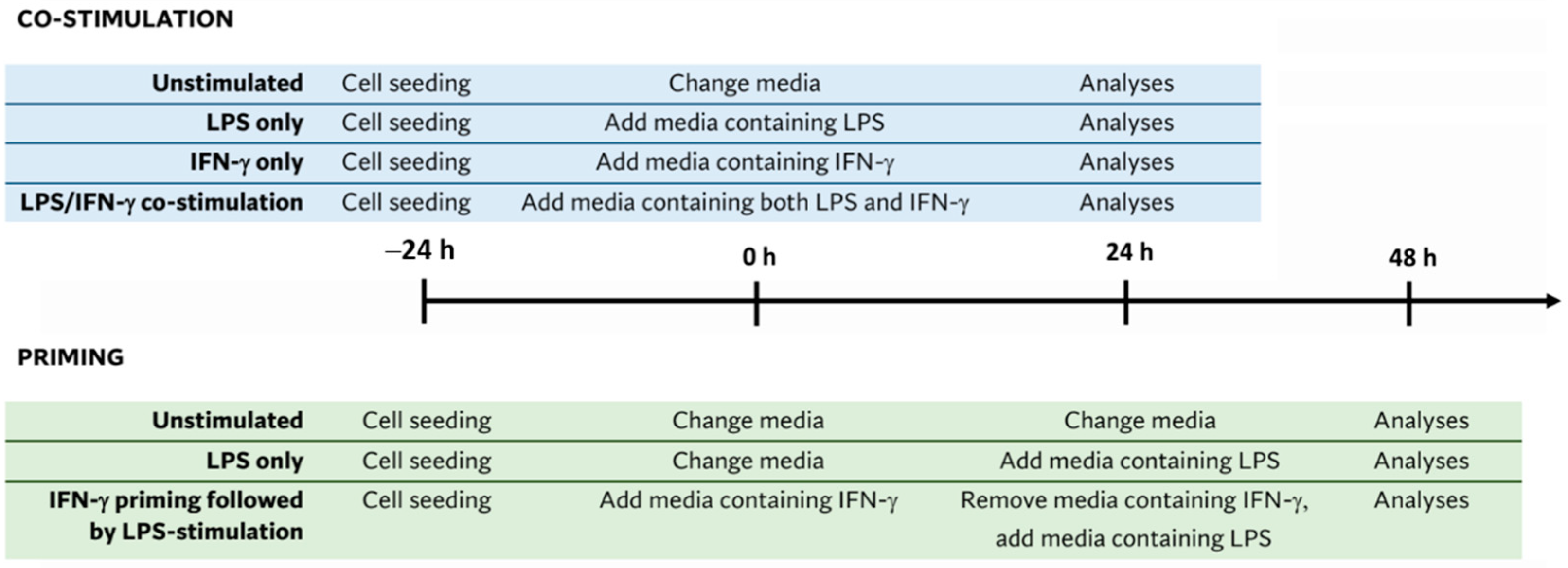
2.2. BV2 Microglia Stimulation Protocols
2.2.1. Single Stimulation with IFN-γ
2.2.2. LPS/IFN-γ Co-Stimulation
2.2.3. IFN-γ Priming Followed by Stimulation with LPS
2.3. Intracellular ROS Measurement
2.4. NO Measurement
2.5. Immunophenotyping
2.6. Transwell Migration Assay
2.7. Statistical Analysis
3. Results
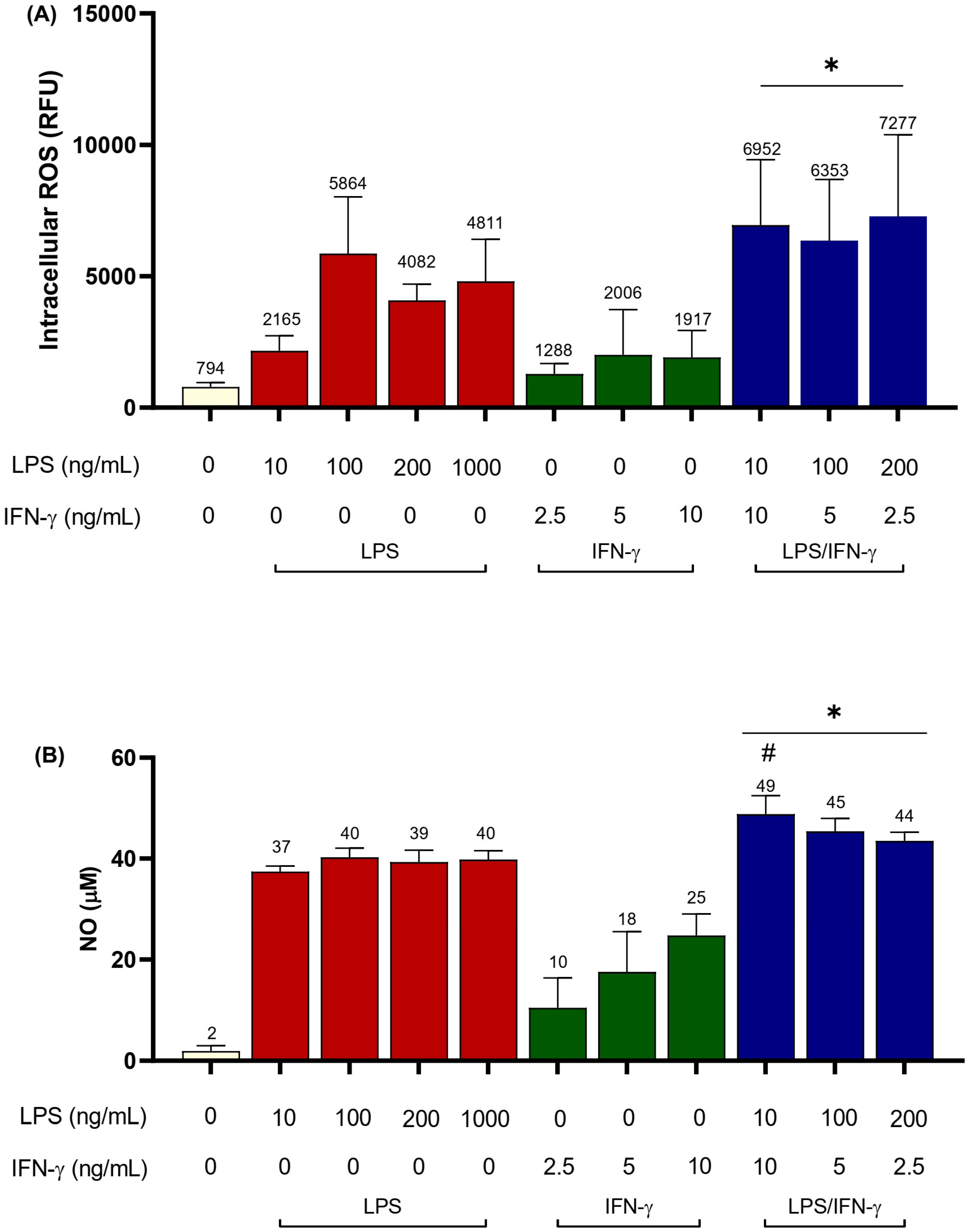
3.1. LPS/IFN-γ Co-Stimulation
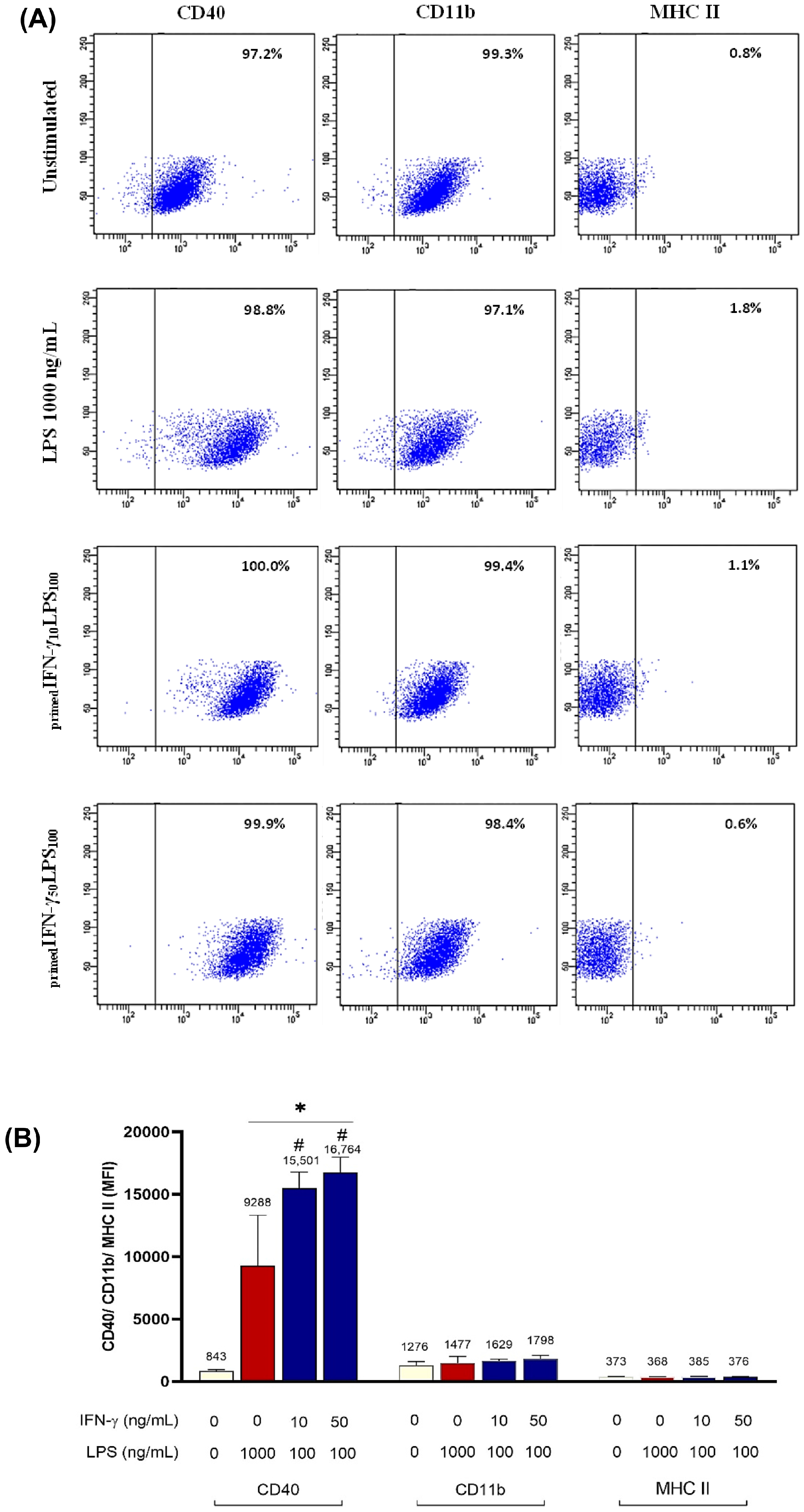
3.2. IFN-γ Priming Followed by LPS Stimulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blasi, E.; Barluzzi, R.; Bocchini, V.; Mazzolla, R.; Bistoni, F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J. Neuroimmunol. 1990, 27, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E.G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Holscher, C.; et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Wakselman, S.; Bechade, C.; Roumier, A.; Bernard, D.; Triller, A.; Bessis, A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J. Neurosci. 2008, 28, 8138–8143. [Google Scholar] [CrossRef]
- Cunningham, C.L.; Martinez-Cerdeno, V.; Noctor, S.C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 2013, 33, 4216–4233. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef]
- Squarzoni, P.; Oller, G.; Hoeffel, G.; Pont-Lezica, L.; Rostaing, P.; Low, D.; Bessis, A.; Ginhoux, F.; Garel, S. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014, 8, 1271–1279. [Google Scholar] [CrossRef]
- Sierra, A.; Encinas, J.M.; Deudero, J.J.; Chancey, J.H.; Enikolopov, G.; Overstreet-Wadiche, L.S.; Tsirka, S.E.; Maletic-Savatic, M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 2010, 7, 483–495. [Google Scholar] [CrossRef]
- Hammond, T.R.; Dufort, C.; Dissing-Olesen, L.; Giera, S.; Young, A.; Wysoker, A.; Walker, A.J.; Gergits, F.; Segel, M.; Nemesh, J.; et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 2019, 50, 253–271.e256. [Google Scholar] [CrossRef]
- Olah, M.; Menon, V.; Habib, N.; Taga, M.F.; Ma, Y.; Yung, C.J.; Cimpean, M.; Khairallah, A.; Coronas-Samano, G.; Sankowski, R.; et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 2020, 11, 6129. [Google Scholar] [CrossRef] [PubMed]
- Kanesan, L.; Zaki, S.S.O.; Vidyadaran, S. A randomised approach for enumerating migrated cells in a transwell migration assay. Malays. J. Med. Health Sci. 2018, 14, 50–53. [Google Scholar]
- Dai, X.J.; Li, N.; Yu, L.; Chen, Z.Y.; Hua, R.; Qin, X.; Zhang, Y.M. Activation of BV2 microglia by lipopolysaccharide triggers an inflammatory reaction in PC12 cell apoptosis through a toll-like receptor 4-dependent pathway. Cell Stress Chaperones 2015, 20, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Jung, J.S.; Lim, S.J.; Kim, J.Y.; Kim, T.H.; Cho, K.H.; Han, I.O. LY294002 inhibits interferon-gamma-stimulated inducible nitric oxide synthase expression in BV2 microglial cells. Biochem. Biophys. Res. Commun. 2004, 318, 691–697. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, D.; Yin, H.; Li, H.; Du, J.; Bao, H. Ganoderic Acid A Attenuates LPS-Induced Neuroinflammation in BV2 Microglia by Activating Farnesoid X Receptor. Neurochem. Res. 2021, 46, 1725–1736. [Google Scholar] [CrossRef]
- Moritz, K.E.; McCormack, N.M.; Abera, M.B.; Viollet, C.; Yauger, Y.J.; Sukumar, G.; Dalgard, C.L.; Burnett, B.G. The role of the immunoproteasome in interferon-gamma-mediated microglial activation. Sci. Rep. 2017, 7, 9365. [Google Scholar] [CrossRef]
- Kook, M.G.; Choi, S.W.; Seo, Y.; Kim, D.W.; Song, B.K.; Son, I.; Kim, S.; Kang, K.S. KCHO-1, a novel herbal anti-inflammatory compound, attenuates oxidative stress in an animal model of amyotrophic lateral sclerosis. J. Vet. Sci. 2017, 18, 487–497. [Google Scholar] [CrossRef]
- Velagapudi, R.; El-Bakoush, A.; Olajide, O.A. Activation of Nrf2 Pathway Contributes to Neuroprotection by the Dietary Flavonoid Tiliroside. Mol. Neurobiol. 2018, 55, 8103–8123. [Google Scholar] [CrossRef]
- Spencer, N.G.; Schilling, T.; Miralles, F.; Eder, C. Mechanisms Underlying Interferon-gamma-Induced Priming of Microglial Reactive Oxygen Species Production. PLoS ONE 2016, 11, e0162497. [Google Scholar] [CrossRef]
- Hayes, M.P.; Freeman, S.L.; Donnelly, R.P. IFN-γ Priming of Monocytes Enhances LPS-Induced TNF Production by Augmenting Both Transcription and MRNA Stability. Cytokine 1995, 7, 427–435. [Google Scholar] [CrossRef]
- Held, T.K.; Weihua, X.; Yuan, L.; Kalvakolanu, D.V.; Cross, A.S. Gamma Interferon Augments Macrophage Activation by Lipopolysaccharide by Two Distinct Mechanisms, at the Signal Transduction Level and via an Autocrine Mechanism Involving Tumor Necrosis Factor Alpha and Interleukin-1. Infect. Immun. 1999, 67, 206–212. [Google Scholar]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An Overview of Signals, Mechanisms and Functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar]
- Papageorgiou, I.E.; Lewen, A.; Galow, L.V.; Cesetti, T.; Scheffel, J.; Regen, T.; Hanisch, U.K.; Kann, O. TLR4-activated microglia require IFN-gamma to induce severe neuronal dysfunction and death in situ. Proc. Natl. Acad. Sci. USA 2016, 113, 212–217. [Google Scholar] [CrossRef]
- Tamai, R.; Sugawara, S.; Takeuchi, O.; Akira, S.; Takada, H. Synergistic effects of lipopolysaccharide and interferon-γ in inducing interleukin-8 production in human monocytic THP-1 cells is accompanied by up-regulation of CD14, toll-like receptor 4, MD-2 and MyD88 expression. J. Endotoxin Res. 2003, 9, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M. Inhibition of interferon (IFN) gamma-induced Jak-STAT1 activation in microglia by vasoactive intestinal peptide: Inhibitory effect on CD40, IFN-induced protein-10, and inducible nitric-oxide synthase expression. J. Biol. Chem. 2003, 278, 27620–27629. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Park-Min, K.H.; Ho, H.H.; Ivashkiv, L.B. IFN-gamma-primed macrophages exhibit increased CCR2-dependent migration and altered IFN-gamma responses mediated by Stat1. J. Immunol. 2005, 175, 3637–3647. [Google Scholar] [CrossRef] [PubMed]
- De Simone, R.; Niturad, C.E.; De Nuccio, C.; Ajmone-Cat, M.A.; Visentin, S.; Minghetti, L. TGF-beta and LPS modulate ADP-induced migration of microglial cells through P2Y1 and P2Y12 receptor expression. J. Neurochem. 2010, 115, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Colton, C.A.; Yao, J.; Keri, J.E.; Gilbert, D. Regulation of microglial function by interferons. J. Neuroimmunol. 1992, 40, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hu, X.; Boumsell, L.; Ivashkiv, L.B. IFN-gamma and STAT1 arrest monocyte migration and modulate RAC/CDC42 pathways. J. Immunol. 2008, 180, 8057–8065. [Google Scholar] [CrossRef]



| Unstimulated | LPS 1000 ng/mL | IFN-γ 2.5 ng/mL | IFN-γ 5 ng/mL | IFN-γ 10 ng/mL | LPS10IFN-γ10 | LPS200IFN-γ2.5 | primedIFN-γ10LPS100 | primedIFN-γ50LPS100 | |
|---|---|---|---|---|---|---|---|---|---|
| iROS (fold increase) | 1 | 3–6 | 1.1 | 0.7 | 1.4 | 8.8 | 9.2 * | 4.3 | 4.0 |
| NO (µM) | 2 | 40–41 | 10 ± 5.9 | 18 ± 7.9 | 25 ± 4.2 | 49 ± 3.7 | 44 ± 1.7 | 52 ± 4.8 | 64 ± 1.4 ** |
| CD40 (MFI) | 800–1000 | 9000–13,000 | N/A | N/A | N/A | 22,418 ± 8846.6 | 28,031 ± 8810.2 | 15,501 ± 1274.9 | 16,764 ± 1210.8 |
| Migration | + | ++ | N/A | N/A | N/A | ++ | +++ | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, M.L.; Ahmad Puzi, N.N.; Ooi, Y.Y.; Ramasamy, R.; Vidyadaran, S. Response Profiles of BV2 Microglia to IFN-γ and LPS Co-Stimulation and Priming. Biomedicines 2023, 11, 2648. https://doi.org/10.3390/biomedicines11102648
Pan ML, Ahmad Puzi NN, Ooi YY, Ramasamy R, Vidyadaran S. Response Profiles of BV2 Microglia to IFN-γ and LPS Co-Stimulation and Priming. Biomedicines. 2023; 11(10):2648. https://doi.org/10.3390/biomedicines11102648
Chicago/Turabian StylePan, Meng Liy, Nur Nabilah Ahmad Puzi, Yin Yin Ooi, Rajesh Ramasamy, and Sharmili Vidyadaran. 2023. "Response Profiles of BV2 Microglia to IFN-γ and LPS Co-Stimulation and Priming" Biomedicines 11, no. 10: 2648. https://doi.org/10.3390/biomedicines11102648
APA StylePan, M. L., Ahmad Puzi, N. N., Ooi, Y. Y., Ramasamy, R., & Vidyadaran, S. (2023). Response Profiles of BV2 Microglia to IFN-γ and LPS Co-Stimulation and Priming. Biomedicines, 11(10), 2648. https://doi.org/10.3390/biomedicines11102648





