Genetic Correlates as a Predictor of Bariatric Surgery Outcomes after 1 Year
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Surgery
2.3. Data Collection
2.4. Psychosocial Questionnaires
2.5. Genetic Addiction Risk Severity (GARS)
2.6. Statistical Analysis
2.7. Ethics
3. Results
3.1. Baseline Demographic Characteristics
3.2. Psychosocial and GARS Data
3.3. Risk Allele Correlates
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Kim, D.; Hou, W.; Wang, F.; Arcan, C. Factors Affecting Obesity and Waist Circumference Among US Adults. Prev. Chronic Dis. 2019, 16, E02. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.S.; Sendra, S.; Lloret, J.; Bosch, I. Systems and WBANs for Controlling Obesity. J. Healthc. Eng. 2018, 2018, 1564748. [Google Scholar] [CrossRef] [PubMed]
- Lemamsha, H.; Randhawa, G.; Papadopoulos, C. Prevalence of Overweight and Obesity among Libyan Men and Women. BioMed Res. Int. 2019, 2019, 8531360. [Google Scholar] [CrossRef] [PubMed]
- Dávila-Torres, J.; González-Izquierdo, J.J.; Barrera-Cruz, A. Obesity in Mexico. Rev. Med. Inst. Mex. Seguro Soc. 2015, 53, 240–249. [Google Scholar]
- Perez-Campos, E.; Mayoral, L.P.-C.; Andrade, G.M.; Mayoral, E.P.-C.; Huerta, T.H.; Canseco, S.P.; Canales, F.J.R.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Santiago, A.D.P.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar] [CrossRef]
- Huse, O.; Hettiarachchi, J.; Gearon, E.; Nichols, M.; Allender, S.; Peeters, A. Obesity in Australia. Obes. Res. Clin. Pract. 2018, 12, 29–39. [Google Scholar] [CrossRef]
- Ferreira, C.M.; dos Reis, N.D.; Castro, A.d.O.; Höfelmann, D.A.; Kodaira, K.; Silva, M.T.; Galvao, T.F. Prevalence of childhood obesity in Brazil: Systematic review and meta-analysis. J. Pediatr. 2021, 97, 490–499. [Google Scholar] [CrossRef]
- Anis, A.H.; Zhang, W.; Bansback, N.; Guh, D.P.; Amarsi, Z.; Birmingham, C.L. Obesity and overweight in Canada: An updated cost-of-illness study. Obes. Rev. 2010, 11, 31–40. [Google Scholar] [CrossRef]
- Jebb, S.A.; Aveyard, P.N.; Hawkes, C. The evolution of policy and actions to tackle obesity in England. Obes. Rev. 2013, 14 (Suppl. S2), 42–59. [Google Scholar] [CrossRef]
- Breen, C.; O’connell, J.; Geoghegan, J.; O’shea, D.; Birney, S.; Tully, L.; Gaynor, K.; O’kelly, M.; O’malley, G.; O’donovan, C.; et al. Obesity in Adults: A 2022 Adapted Clinical Practice Guideline for Ireland. Obes. Facts 2022, 15, 736–752. [Google Scholar] [CrossRef]
- Arroyo-Johnson, C.; Mincey, K.D. Obesity Epidemiology Worldwide. Gastroenterol. Clin. N. Am. 2016, 45, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Carpaij, O.A.; Berge, M.v.D. The asthma–obesity relationship: Underlying mechanisms and treatment implications. Curr. Opin. Pulm. Med. 2018, 24, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Maffetone, P.B.; Rivera-Dominguez, I.; Laursen, P.B. Overfat and Underfat: New Terms and Definitions Long Overdue. Front. Public Health 2016, 4, 279. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.; Yang, W.; Chen, C.-S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef]
- McElroy, S.L.; Keck, P.E., Jr. Obesity in bipolar disorder: An overview. Curr. Psychiatry Rep. 2012, 14, 650–658. [Google Scholar] [CrossRef]
- Copeland, L.A.; Pugh, M.J.; Hicks, P.B.; Noel, P.H. Use of obesity-related care by psychiatric patients. Psychiatr. Serv. 2012, 63, 230–236. [Google Scholar] [CrossRef]
- Stewart, K.E.; Levenson, J.L. Psychological and psychiatric aspects of treatment of obesity and nonalcoholic fatty liver disease. Clin. Liver Dis. 2012, 16, 615–629. [Google Scholar] [CrossRef]
- Stunkard, A.J.; Faith, M.S.; Allison, K.C. Depression and obesity. Biol. Psychiatry 2003, 54, 330–337. [Google Scholar] [CrossRef]
- Tronieri, J.S.; Wurst, C.M.; Pearl, R.L.; Allison, K.C. Sex Differences in Obesity and Mental Health. Curr. Psychiatry Rep. 2017, 19, 29. [Google Scholar] [CrossRef]
- Kalarchian, M.A.; Marcus, M.D. Psychiatric comorbidity of childhood obesity. Int. Rev. Psychiatry 2012, 24, 241–246. [Google Scholar] [CrossRef]
- Berkowitz, R.I.; Fabricatore, A.N. Obesity, psychiatric status, and psychiatric medications. Psychiatr. Clin. N. Am. 2011, 34, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Tessari, L. Attention-Deficit/Hyperactivity Disorder (ADHD) and Obesity: Update 2016. Curr. Psychiatry Rep. 2017, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Bharti, B.; Malhi, P. Psychiatric Comorbidities in Adolescents with Obesity: A Wake-Up Call for Life Course and Multisectoral Interventions. Indian J. Pediatr. 2021, 88, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Da Luz, F.Q.; Hay, P.; Touyz, S.; Sainsbury, A. Obesity with Comorbid Eating Disorders: Associated Health Risks and Treatment Approaches. Nutrients 2018, 10, 829. [Google Scholar] [CrossRef]
- Aguiar, P.V.; Dionisio, W.d.S.; Souza, E.A.d.C.; Vantini, D.; Campanholi, R.; Pinto, T.C.C.; Ximenes, R.C.C. Binge eating, depressive symptoms and suicidal ideation in obese candidates for bariatric surgery. Eat. Weight Disord. 2023, 28, 12. [Google Scholar] [CrossRef]
- Sarwer, D.B.; Allison, K.C.; Wadden, T.A.; Ashare, R.; Spitzer, J.C.; McCuen-Wurst, C.; LaGrotte, C.; Williams, N.N.; Edwards, M.; Tewksbury, C.; et al. Psychopathology, disordered eating, and impulsivity as predictors of outcomes of bariatric surgery. Surg. Obes. Relat. Dis. 2019, 15, 650–655. [Google Scholar] [CrossRef]
- O’brien, P.E.; Hindle, A.; Brennan, L.; Skinner, S.; Burton, P.; Smith, A.; Crosthwaite, G.; Brown, W. Long-Term Outcomes After Bariatric Surgery: A Systematic Review and Meta-analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes After Adjustable Gastric Banding. Obes. Surg. 2019, 29, 3–14. [Google Scholar] [CrossRef]
- Ribeiro, G.; Maia, A.; Cotovio, G.; Oliveira, F.P.M.; Costa, D.C.; Oliveira-Maia, A.J. Striatal dopamine D2-like receptors availability in obesity and its modulation by bariatric surgery: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 49591. [Google Scholar] [CrossRef]
- Wolfe, B.M.; Kvach, E.; Eckel, R.H. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ. Res. 2016, 118, 1844–1855. [Google Scholar] [CrossRef]
- Grönroos, S.; Helmiö, M.; Juuti, A.; Tiusanen, R.; Hurme, S.; Löyttyniemi, E.; Ovaska, J.; Leivonen, M.; Peromaa-Haavisto, P.; Mäklin, S.; et al. Effect of Laparoscopic Sleeve Gastrectomy vs Roux-en-Y Gastric Bypass on Weight Loss and Quality of Life at 7 Years in Patients with Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA Surg. 2021, 156, 137–146. [Google Scholar] [CrossRef]
- Hardman, C.A.; Christiansen, P. Psychological issues and alcohol misuse following bariatric surgery. Nat. Rev. Endocrinol. 2018, 14, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Ivezaj, V.; Benoit, S.C.; Davis, J.; Engel, S.; Lloret-Linares, C.; Mitchell, J.E.; Pepino, M.Y.; Rogers, A.M.; Steffen, K.; Sogg, S. Changes in Alcohol Use after Metabolic and Bariatric Surgery: Predictors and Mechanisms. Curr. Psychiatry Rep. 2019, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Bailey, J.; Gonzalez, A.M.; Oscar-Berman, M.; Liu, Y.; Giordano, J.; Braverman, E.; Gold, M. Neuro-Genetics of Reward Deficiency Syndrome (Rds) as the Root Cause of “Addiction Transfer”: A New Phenomena Common after Bariatric Surgery. J. Genet. Syndr. Gene Ther. 2011, 4. [Google Scholar] [CrossRef] [PubMed]
- Eryılmaz, G.; Noyan, C. Gambling Disorder Following Bariatric Surgery. Curr. Addict. Res. 2018, 2, 62. [Google Scholar] [CrossRef]
- Lannoy, S.; Ohlsson, H.; Stephenson, M.; Kendler, K.S.; Sundquist, J.; Sundquist, K.; Edwards, A.C. Risk of non-fatal suicide attempt in individuals with substance use disorder: The roles of aggregate genetic liability and environmental exposures in a Swedish population-based cohort. Addiction 2022, 117, 2943–2952. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, M.; Lannoy, S.; Edwards, A.C. Shared genetic liability for alcohol consumption, alcohol problems, and suicide attempt: Evaluating the role of impulsivity. Transl. Psychiatry 2023, 13, 87. [Google Scholar] [CrossRef]
- Kimbrel, N.A.; Ashley-Koch, A.E.; Qin, X.J.; Lindquist, J.H.; Garrett, M.E.; Dennis, M.F.; Hair, L.P.; Huffman, J.E.; Jacobson, D.A.; Madduri, R.K.; et al. A genome-wide association study of suicide attempts in the million veterans program identifies evidence of pan-ancestry and ancestry-specific risk loci. Mol. Psychiatry 2022, 27, 2264–2272. [Google Scholar] [CrossRef]
- König, I.R.; Fuchs, O.; Hansen, G.; von Mutius, E.; Kopp, M.V. What is precision medicine? Eur. Respir. J. 2017, 50, 1700391. [Google Scholar] [CrossRef]
- Belligoli, A.; Bettini, S.; Segato, G.; Busetto, L. Predicting Responses to Bariatric and Metabolic Surgery. Curr. Obes. Rep. 2020, 9, 373–379. [Google Scholar] [CrossRef]
- Goodarzi, M.O. Genetics of obesity: What genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018, 6, 223–236. [Google Scholar] [CrossRef]
- Panera, N.; Mandato, C.; Crudele, A.; Bertrando, S.; Vajro, P.; Alisi, A. Genetics, epigenetics and transgenerational transmission of obesity in children. Front. Endocrinol. 2022, 13, 1006008. [Google Scholar] [CrossRef] [PubMed]
- Pigeyre, M.; Yazdi, F.T.; Kaur, Y.; Meyre, D. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin. Sci. 2016, 130, 943–986. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Choi, K.W.; Fried, E.I. Reviewing the genetics of heterogeneity in depression: Operationalizations, manifestations and etiologies. Hum. Mol. Genet. 2020, 29, R10–R18. [Google Scholar] [CrossRef] [PubMed]
- Yau, Y.H.C.M.; Potenza, M.N. Gambling disorder and other behavioral addictions: Recognition and treatment. Harv. Rev. Psychiatry 2015, 23, 134–146. [Google Scholar] [CrossRef]
- Blum, K.; Modestino, E.J.; Gondre-Lewis, M.; Chapman, E.J.; Neary, J.; Siwicki, D.; Baron, D.; Hauser, M.; Smith, D.E.; Roy, A.K.; et al. The Benefits of Genetic Addiction Risk Score (GARS™) Testing in Substance Use Disorder (SUD). Int. J. Genom. Data Min. 2018, 2018, 115. [Google Scholar] [CrossRef]
- Fried, L.; Modestino, E.J.; Siwicki, D.; Lott, L.; Thanos, P.K.; Baron, D.; Badgaiyan, R.D.; Ponce, J.V.; Giordano, J.; Downs, W.B.; et al. Hypodopaminergia and “Precision Behavioral Management” (PBM): It is a Generational Family Affair. Curr. Pharm. Biotechnol. 2020, 21, 528–541. [Google Scholar] [CrossRef]
- Moran, M.; Blum, K.; Ponce, J.V.; Lott, L.; Gondré–Lewis, M.C.; Badgaiyan, S.; Brewer, R.; Downs, B.W.; Fynman, P.; Weingarten, A.; et al. High Genetic Addiction Risk Score (GARS) in Chronically Prescribed Severe Chronic Opioid Probands Attending Multi-pain Clinics: An Open Clinical Pilot Trial. Mol. Neurobiol. 2021, 58, 3335–3346. [Google Scholar] [CrossRef]
- Modestino, E.J.; Blum, K.; Dennen, C.A.; Downs, B.W.; Bagchi, D.; Llanos-Gomez, L.; Elman, I.; Baron, D.; Thanos, P.K.; Badgaiyan, R.D.; et al. Theorizing the Role of Dopaminergic Polymorphic Risk Alleles with Intermittent Explosive Disorder (IED), Violent/Aggressive Behavior and Addiction: Justification of Genetic Addiction Risk Severity (GARS) Testing. J. Pers. Med. 2022, 12, 1946. [Google Scholar] [CrossRef]
- Blum, K.; Oscar-Berman, M.; Demetrovics, Z.; Barh, D.; Gold, M.S. Genetic Addiction Risk Score (GARS): Molecular neurogenetic evidence for predisposition to Reward Deficiency Syndrome (RDS). Mol. Neurobiol. 2014, 50, 765–796. [Google Scholar] [CrossRef]
- Blum, K.; Dennen, C.A.; Elman, I.; Bowirrat, A.; Thanos, P.K.; Badgaiyan, R.D.; Downs, B.W.; Bagchi, D.; Baron, D.; Braverman, E.R.; et al. Should Reward Deficiency Syndrome (RDS) Be Considered an Umbrella Disorder for Mental Illness and Associated Genetic and Epigenetic Induced Dysregulation of Brain Reward Circuitry? J. Pers. Med. 2022, 12, 1719. [Google Scholar] [CrossRef]
- Blum, K.; Han, D.; Gupta, A.; Baron, D.; Braverman, E.R.; Dennen, C.A.; Kazmi, S.; Llanos-Gomez, L.; Badgaiyan, R.D.; Elman, I.; et al. Statistical Validation of Risk Alleles in Genetic Addiction Risk Severity (GARS) Test: Early Identification of Risk for Alcohol Use Disorder (AUD) in 74,566 Case–Control Subjects. J. Pers. Med. 2022, 12, 1385. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Bowirrat, A.; Baron, D.; Lott, L.; Ponce, J.V.; Brewer, R.; Siwicki, D.; Boyett, B.; Gondre-Lewis, M.C.; Smith, D.E.; et al. Biotechnical development of genetic addiction risk score (GARS) and selective evidence for inclusion of polymorphic allelic risk in substance use disorder (SUD). J Syst Integr Neurosci. 2020, 6, 1015761/JSIN1000221. [Google Scholar] [CrossRef]
- Garner, D.M.; Olmsted, M.P.; Bohr, Y.; Garfinkel, P.E. The eating attitudes test: Psychometric features and clinical correlates. Psychol. Med. 1982, 12, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Meule, A.; Hermann, T.; Kübler, A. A short version of the Food Cravings Questionnaire-Trait: The FCQ-T-reduced. Front Psychol. 2014, 5, 190. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons-Craft, E.E.; Keatts, D.A.; Bardone-Cone, A.M. Eating Expectancies in Relation to Eating Disorder Recovery. Cogn. Ther. Res. 2013, 37, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Development of the Yale Food Addiction Scale Version 2.0. Psychol. Addict. Behav. 2016, 30, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Trottier, K.; McFarlane, T.; Olmsted, M.P.; McCabe, R.E. The Weight Influenced Self-Esteem Questionnaire (WISE-Q): Factor structure and psychometric properties. Body Image 2013, 10, 112–120. [Google Scholar] [CrossRef]
- Kaufman, E.A.; Xia, M.; Fosco, G.; Yaptangco, M.; Skidmore, C.R.; Crowell, S.E. The Difficulties in Emotion Regulation Scale Short Form (DERS-SF): Validation and Replication in Adolescent and Adult Samples. J. Psychopathol. Behav. Assess. 2016, 38, 443–455. [Google Scholar] [CrossRef]
- Smarr, K.L.; Keefer, A.L. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Rheum. 2011, 63 (Suppl. S11), S454–S466. [Google Scholar] [CrossRef]
- Schulz, P.; Jansen, L.J.; Schlotz, W. Stressreaktivität: Theoretisches Konzept und Messung. Diagnostica 2005, 51, 124–133. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Thanos, P.K.; Hanna, C.; Mihalkovic, A.; Hoffman, A.B.; Posner, A.R.; Busch, J.; Smith, C.; Badgaiyan, R.D.; Blum, K.; Baron, D.; et al. The First Exploratory Personalized Medicine Approach to Improve Bariatric Surgery Outcomes Utilizing Psychosocial and Genetic Risk Assessments: Encouraging Clinical Research. J. Pers. Med. 2023, 13, 1164. [Google Scholar] [CrossRef]
- Boor, K.; Ronai, Z.; Nemoda, Z.; Gaszner, P.; Sasvari-Szekely, M.; Guttman, A.; Kalasz, H. Noninvasive genotyping of dopamine receptor D4 (DRD4) using nanograms of DNA from substance-dependent patients. Curr. Med. Chem. 2002, 9, 793–797. [Google Scholar] [CrossRef]
- Blum, K.; Thanos, P.K.; Wang, G.-J.; Febo, M.; Demetrovics, Z.; Modestino, E.J.; Braverman, E.R.; Baron, D.; Badgaiyan, R.D.; Gold, M.S. The Food and Drug Addiction Epidemic: Targeting Dopamine Homeostasis. Curr. Pharm. Des. 2018, 23, 6050–6061. [Google Scholar] [CrossRef]
- Toups, M.S.; Myers, A.K.; Wisniewski, S.R.; Kurian, B.M.; Morris, D.W.; Rush, A.J.; Fava, M.; Trivedi, M.H. Relationship between obesity and depression: Characteristics and treatment outcomes with antidepressant medication. Psychosom. Med. 2013, 75, 863–872. [Google Scholar] [CrossRef]
- Schulte, E.M.; Smeal, J.K.; Lewis, J.; Gearhardt, A.N. Development of the Highly Processed Food Withdrawal Scale. Appetite 2018, 131, 148–154. [Google Scholar] [CrossRef]
- Pedram, P.; Wadden, D.; Amini, P.; Gulliver, W.; Randell, E.; Cahill, F.; Vasdev, S.; Goodridge, A.; Carter, J.C.; Zhai, G.; et al. Food addiction: Its prevalence and significant association with obesity in the general population. PLoS ONE 2013, 8, e74832. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Simpatico, T.; Badgaiyan, R.D.; Demetrovics, Z.; Fratantonio, J.; Agan, G.; Febo, M.; Gold, M.S. Coupling Neurogenetics (GARS™) and a Nutrigenomic Based Dopaminergic Agonist to Treat Reward Deficiency Syndrome (RDS): Targeting Polymorphic Reward Genes for Carbohydrate Addiction Algorithms. J. Reward. Defic. Syndr. 2015, 1, 75–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blum, K.; Sheridan, P.J.; Wood, R.C.; Braverman, E.R.; Chen, T.J.; Comings, D.E. Dopamine D2 receptor gene variants: Association and linkage studies in impulsive-addictive-compulsive behaviour. Pharmacogenetics 1995, 5, 121–141. [Google Scholar] [CrossRef]
- Blum, K.; Liu, Y.; Wang, W.; Wang, Y.; Zhang, Y.; Oscar-Berman, M.; Smolen, A.; Febo, M.; Han, D.; Simpatico, T.; et al. rsfMRIeffects of KB220Z™ on neural pathways in reward circuitry of abstinent genotyped heroin addicts. Postgrad. Med. 2015, 127, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Glatt, S.J.; Faraone, S.V.; Lasky-Su, J.A.; Kanazawa, T.; Hwu, H.-G.; Tsuang, M.T. Family-based association testing strongly implicates DRD2 as a risk gene for schizophrenia in Han Chinese from Taiwan. Mol. Psychiatry 2009, 14, 885–893. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carpenter, C.L.; Wong, A.M.; Li, Z.; Noble, E.P.; Heber, D. Association of dopamine D2receptor and leptin receptor genes with clinically severe obesity. Obesity 2012, 21, E467–E473. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.D.; Riou, M.É.; Tesson, F.; Goldfield, G.S.; Rabasa-Lhoret, R.; Brochu, M.; Doucet, É. The TaqIA RFLP is associated with attenuated intervention-induced body weight loss and increased carbohydrate intake in post-menopausal obese women. Appetite 2013, 60, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Crist, R.C.; Reiner, B.C.; Berrettini, W.H. A review of opioid addiction genetics. Curr. Opin. Psychol. 2019, 27, 31–35. [Google Scholar] [CrossRef]
- Sanwald, S.; Montag, C.; Kiefer, M. Cumulative Genetic Score of DRD2 Polymorphisms Is Associated with Impulsivity and Masked Semantic Priming. J. Mol. Neurosci. 2022, 72, 1682–1694. [Google Scholar] [CrossRef]
- Noble, E.P. The DRD2 gene in psychiatric and neurological disorders and its phenotypes. Pharmacogenomics 2000, 1, 309–333. [Google Scholar] [CrossRef]
- Luykx, J.J.; Broersen, J.L.; de Leeuw, M. The DRD2 rs1076560 polymorphism and schizophrenia-related intermediate phenotypes: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2017, 74, 214–224. [Google Scholar] [CrossRef]
- Niu, Y.-M.; Zhang, J.; Tang, H.; Cao, L.-H.; Jiang, T.-Y.; Hu, Y.-Y. Association between DRD2/ANKK1 rs1800497 C > T polymorphism and post-traumatic stress disorder susceptibility: A multivariate meta-analysis. Front. Neurosci. 2023, 17, 1102573. [Google Scholar] [CrossRef]
- Tsou, C.-C.; Chou, H.-W.; Ho, P.-S.; Kuo, S.-C.; Chen, C.-Y.; Huang, C.-C.; Liang, C.-S.; Lu, R.-B.; Huang, S.-Y. DRD2 and ANKK1 genes associate with late-onset heroin dependence in men. World J. Biol. Psychiatry 2019, 20, 605–615. [Google Scholar] [CrossRef]
- Watanabe, Y.; Shibuya, M.; Someya, T. DRD2Ser311Cys polymorphism and risk of schizophrenia. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2015, 168, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Serý, O.; Drtílková, I.; Theiner, P.; Pitelová, R.; Staif, R.; Znojil, V.; Lochman, J.; Didden, W. Polymorphism of DRD2 gene and ADHD. Neuro. Endocrinol. Lett. 2006, 27, 236–240. [Google Scholar] [PubMed]
- Yuan, A.; Su, L.; Yu, S.; Li, C.; Yu, T.; Sun, J. Association between DRD2/ANKK1 TaqIA Polymorphism and Susceptibility with Tourette Syndrome: A Meta-Analysis. PLoS ONE 2015, 10, e0131060. [Google Scholar] [CrossRef] [PubMed]
- D’ambrosio, E.; Pergola, G.; Pardiñas, A.F.; Dahoun, T.; Veronese, M.; Sportelli, L.; Taurisano, P.; Griffiths, K.; Jauhar, S.; Rogdaki, M.; et al. A polygenic score indexing a DRD2-related co-expression network is associated with striatal dopamine function. Sci. Rep. 2022, 12, 12610. [Google Scholar] [CrossRef]
- Gluskin, B.S.; Mickey, B.J. Genetic variation and dopamine D2 receptor availability: A systematic review and meta-analysis of human in vivo molecular imaging studies. Transl. Psychiatry 2016, 6, e747. [Google Scholar] [CrossRef]
- Dang, L.C.; Samanez-Larkin, G.R.; Castrellon, J.J.; Perkins, S.F.; Cowan, R.L.; Zald, D.H. Individual differences in dopamine D2 receptor availability correlate with reward valuation. Cogn. Affect. Behav. Neurosci. 2018, 18, 739–747. [Google Scholar] [CrossRef]
- Suchanecka, A.; Grzywacz, A.; Samochowiec, J. ANKK1 gene in psychiatry. Psychiatr. Polska 2012, 45, 349–356. [Google Scholar]
- Miura, I.; Zhang, J.-P.; Hagi, K.; Lencz, T.; Kane, J.M.; Yabe, H.; Malhotra, A.K.; Correll, C.U. Variants in the DRD2 locus and antipsychotic-related prolactin levels: A meta-analysis. Psychoneuroendocrinology 2016, 72, 1–10. [Google Scholar] [CrossRef]
- Dubertret, C.; Gouya, L.; Hanoun, N.; Deybach, J.C.; Adès, J.; Hamon, M.; Gorwood, P. The 3′ region of the DRD2 gene is involved in genetic susceptibility to schizophrenia. Schizophr. Res. 2004, 67, 75–85. [Google Scholar] [CrossRef]
- Ludmer, J.A.; Levitan, R.; Gonzalez, A.; Kennedy, J.; Villani, V.; Masellis, M.; Basile, V.S.; Atkinson, L. DRD2 and SLC6A3 moderate impact of maternal depressive symptoms on infant cortisol. Psychoneuroendocrinology 2015, 62, 243–251. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, J.-W.; Lee, J.-M.; Yun, H.J.; Sohn, C.-H.; Shin, M.-S.; Kim, B.; Chae, J.; Roh, J.; Kim, B.-N. Interaction between DRD2 and lead exposure on the cortical thickness of the frontal lobe in youth with attention-deficit/hyperactivity disorder. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2018, 82, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Lee, S.-Y.; Chen, S.-L.; Chang, Y.-H.; Wang, T.-Y.; Lin, S.-H.; Wang, C.-L.; Huang, S.-Y.; Lee, I.; Chen, P.; et al. Role of DRD2 and ALDH2 genes in bipolar II disorder with and without comorbid anxiety disorder. Eur. Psychiatry 2014, 29, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Comings, D.; Flanagan, S.; Dietz, G.; Muhleman, D.; Knell, E.; Gysin, R. The dopamine D2 receptor (DRD2) as a major gene in obesity and height. Biochem. Med. Metab. Biol. 1993, 50, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Kroemer, N.B.; Veldhuizen, M.G.; Babbs, A.E.; de Araujo, I.E.; Gitelman, D.R.; Sherwin, R.S.; Sinha, R.; Small, D.M. Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J. Neurosci. 2015, 35, 7964–7976. [Google Scholar] [CrossRef]
- Arinami, T.; Itokawa, M.; Aoki, J.; Shibuya, H.; Ookubo, Y.; Iwawaki, A.; Ota, K.; Shimizu, H.; Hamaguchi, H.; Toru, M. Further association study on dopamine D2 receptor variant S311C in schizophrenia and affective disorders. Am. J. Med. Genet. 1996, 67, 133–138. [Google Scholar] [CrossRef]
- Noble, E.P.; Blum, K.; Ritchie, T.; Montgomery, A.; Sheridan, P.J. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism or gene ism. Arch. Gen. Psychiatry 1991, 48, 648–654. [Google Scholar] [CrossRef]
- Blum, K.; Chen, T.J.; Downs, B.W.; Bowirrat, A.; Waite, R.L.; Braverman, E.R.; Madigan, M.; Oscar-Berman, M.; DiNubile, N.; Stice, E.; et al. Neurogenetics of dopaminergic receptor supersensitivity in activation of brain reward circuitry and relapse: Proposing “Deprivation-Amplification Relapse Therapy” (DART). Postgrad. Med. 2009, 121, 176–196. [Google Scholar] [CrossRef]
- Noble, E.P. The D2 dopamine receptor gene: A review of association studies in alcoholism. Behav. Genet. 1993, 23, 119–129. [Google Scholar] [CrossRef]
- Uhl, G.; Blum, K.; Noble, E.; Smith, S. Substance abuse vulnerability and D2 receptor genes. Trends Neurosci. 1993, 16, 83–88. [Google Scholar] [CrossRef]
- Chen, A.L.C.; Blum, K.; Chen, T.J.H.; Giordano, J.; Downs, B.W.; Han, D.; Barh, D.; Braverman, E.R. Correlation of the Taq1 dopamine D2 receptor gene and percent body fat in obese and screened control subjects: A preliminary report. Food Funct. 2012, 3, 40–48. [Google Scholar] [CrossRef]
- Bouchard, C.; Perusse, L.; Leblanc, C.; Tremblay, A.; Thériault, G. Inheritance of the amount and distribution of human body fat. Int. J. Obes. 1988, 12, 205–215. [Google Scholar] [PubMed]
- Lawford, B.R.; Young, R.M.; Rowell, J.A.; Qualichefski, J.; Fletcher, B.H.; Syndulko, K.; Ritchie, T.; Noble, E.P. Bromocriptine in the treatment of alcoholics with the D2 dopamine receptor A1 allele. Nat. Med. 1995, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-F.; Chen, L.-H.; Yuan, K.; Liang, L.; Wang, C.-L. Dopamine receptor D2 polymorphism is associated with alleviation of obesity after 8-year follow-up: A retrospective cohort study in obese Chinese children and adolescents. J. Zhejiang Univ. Sci. B 2018, 19, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wise, R.A. How can drug addiction help us understand obesity? Nat. Neurosci. 2005, 8, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.-J.; Fowler, J.S.; Telang, F. Overlapping neuronal circuits in addiction and obesity: Evidence of systems pathology. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 3191–3200. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef]
- Steele, K.E.; Prokopowicz, G.P.; Schweitzer, M.A.; Magunsuon, T.H.; Lidor, A.O.; Kuwabawa, H.; Kumar, A.; Brasic, J.; Wong, D.F. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes. Surg. 2010, 20, 369–374. [Google Scholar] [CrossRef]
- van der Zwaal, E.M.; de Weijer, B.A.; van de Giessen, E.M.; Janssen, I.; Berends, F.J.; van de Laar, A.; Ackermans, M.T.; Fliers, E.; la Fleur, S.E.; Booij, J.; et al. Striatal dopamine D2/3 receptor availability increases after long-term bariatric surgery-induced weight loss. Eur. Neuropsychopharmacol. 2016, 26, 1190–1200. [Google Scholar] [CrossRef]
- Hamilton, J.; Swenson, S.; Hajnal, A.; Thanos, P.K. Roux-en-Y gastric bypass surgery normalizes dopamine D1, D2, and DAT levels. Synapse 2018, 72, e22058. [Google Scholar] [CrossRef]
- de Weijer, B.A.; van de Giessen, E.; Janssen, I.; Berends, F.J.; van de Laar, A.; Ackermans, M.T.; Fliers, E.; la Fleur, S.E.; Booij, J.; Serlie, M.J. Striatal dopamine receptor binding in morbidly obese women before and after gastric bypass surgery and its relationship with insulin sensitivity. Diabetologia 2014, 57, 1078–1080. [Google Scholar] [CrossRef]
- Nummenmaa, L.; Saanijoki, T.; Tuominen, L.; Hirvonen, J.; Tuulari, J.J.; Nuutila, P.; Kalliokoski, K. μ-opioid receptor system mediates reward processing in humans. Nat. Commun. 2018, 9, 1500. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, A.; Melka, M.G.; Bernard, M.; Abrahamowicz, M.; Leonard, G.T.; Richer, L.; Perron, M.; Veillette, S.; Xu, C.J.; Greenwood, C.M.T.; et al. Opioid receptor mu 1 gene, fat intake and obesity in adolescence. Mol. Psychiatry 2014, 19, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Joutsa, J.; Karlsson, H.K.; Majuri, J.; Nuutila, P.; Helin, S.; Kaasinen, V.; Nummenmaa, L. Binge eating disorder and morbid obesity are associated with lowered mu-opioid receptor availability in the brain. Psychiatry Res. Neuroimaging 2018, 276, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Kantonen, T.; Karjalainen, T.; Pekkarinen, L.; Isojärvi, J.; Kalliokoski, K.; Kaasinen, V.; Hirvonen, J.; Nuutila, P.; Nummenmaa, L. Cerebral μ-opioid and CB1 receptor systems have distinct roles in human feeding behavior. Transl. Psychiatry 2021, 11, 442. [Google Scholar] [CrossRef]
- Kantonen, T.; Pekkarinen, L.; Karjalainen, T.; Bucci, M.; Kalliokoski, K.; Haaparanta-Solin, M.; Aarnio, R.; Dickens, A.M.; von Eyken, A.; Laitinen, K.; et al. Obesity risk is associated with altered cerebral glucose metabolism and decreased μ-opioid and CB1 receptor availability. Int. J. Obes. 2022, 46, 400–407. [Google Scholar] [CrossRef]
- Karlsson, H.K.; Tuominen, L.; Tuulari, J.J.; Hirvonen, J.; Parkkola, R.; Helin, S.; Salminen, P.; Nuutila, P.; Nummenmaa, L. Obesity is associated with decreased μ-opioid but unaltered dopamine D2Receptor availability in the brain. J. Neurosci. 2015, 35, 3959–3965. [Google Scholar] [CrossRef]
- Brunner, H.G. MAOA deficiency and abnormal behaviour: Perspectives on an assocation. Ciba Found Symp. 2007, 194, 155–164, discussion 164–157. [Google Scholar] [CrossRef]
- Need, A.C.; Ahmadi, K.R.; Spector, T.D.; Goldstein, D.B. Obesity is associated with genetic variants that alter dopamine availability. Ann. Hum. Genet. 2006, 70, 293–303. [Google Scholar] [CrossRef]
- Ziegler, C.; Domschke, K. Epigenetic signature of MAOA and MAOB genes in mental disorders. J. Neural Transm. 2018, 125, 1581–1588. [Google Scholar] [CrossRef]
- Kanarik, M.; Grimm, O.; Mota, N.R.; Reif, A.; Harro, J. ADHD co-morbidities: A review of implication of gene × environment effects with dopamine-related genes. Neurosci. Biobehav. Rev. 2022, 139, 104757. [Google Scholar] [CrossRef]
- Avsar, O.; Kuskucu, A.; Sancak, S.; Genc, E. Are dopaminergic genotypes risk factors for eating behavior and obesity in adults? Neurosci. Lett. 2017, 654, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Dias, H.; Muc, M.; Padez, C.; Manco, L. Association of polymorphisms in 5-HTT (SLC6A4) and MAOA genes with measures of obesity in young adults of Portuguese origin. Arch. Physiol. Biochem. 2016, 122, 8–13. [Google Scholar] [CrossRef] [PubMed]

| Gene | Polymorphism | Location | Risk Allele(s) |
|---|---|---|---|
| Dopamine D1 Receptor DRD1 | rs4532 SNP | Chr5 | A |
| Dopamine D2 Receptor DRD2 | rs1800497 SNP | Chr11 | A |
| Dopamine D3 Receptor DRD3 | rs6280 SNP | Chr3 | C |
| Dopamine D4 Receptor DRD4 | rs1800955 SNP | Chr11 | C |
| 48 bases Repeat VNTR | Chr11, Exon 3 | 7R, 8R, 9R, 10R, 11R | |
| Catechol-O-Methyltransferase COMT | rs4680 SNP | Chr22 | G |
| Mu-Opioid Receptor OPRM1 | rs1799971 SNP | Chr6 | G |
| Dopamine Active Transporter DAT 1 | 40 bases Repeat VNTR | Chr5, Exon 15 | 3R, 4R, 5R, 6R, 7R, 8R |
| Monoamine Oxidase A MAOA | 30 bases Repeat VNTR | Chr X, Promoter | 3.5R, 4R |
| Serotonin Transporter SLC6A4 (5HTTLPR) | 43 bases Repeat INDEL/VNTR plus rs25531 SNP | Chr 17 | LG, S |
| GABA(A) Receptor, Alpha-3 GABRB3 | CA-Repeat DNR | Chr 15 (downstream) | 181 |
| 6 Months | 12 Months | |
|---|---|---|
| ∆BMI and a mean % excess weight loss | (56 ± 13.8%) | % EWL (p < 0.05), ∆Weight (p < 0.05), and ∆BMI (p < 0.05). |
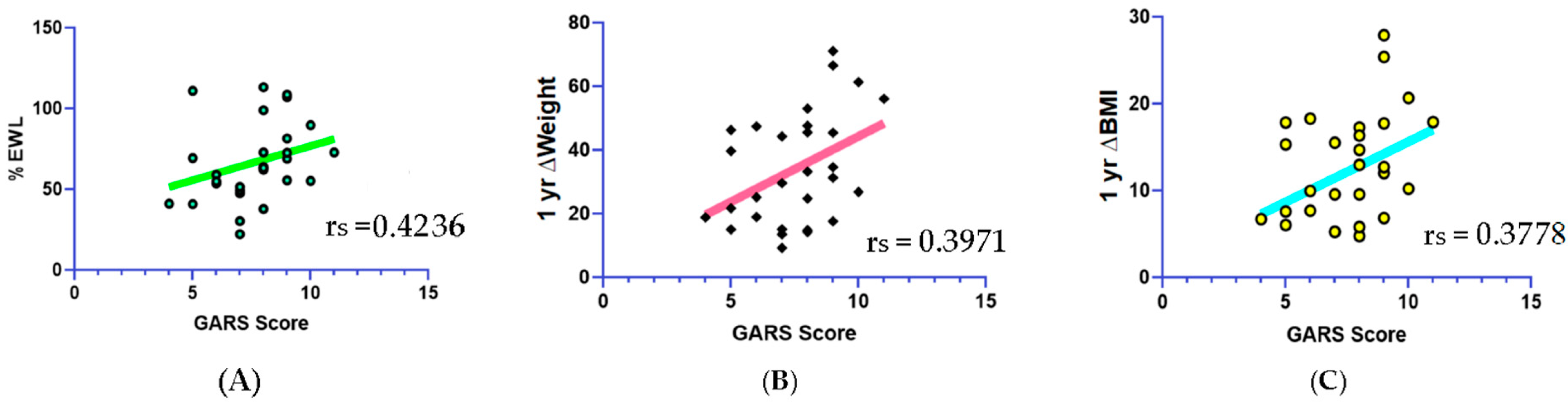
| GARS scores above 7 | 76% of subjects GARS significantly correlated (increases) with ∆ weight and ∆ BMI | 76% of subjects correlated with ∆ weight and ∆ BMI. |
| GARS scores | significantly correlated (increases) with ∆ weight and ∆ BMI | |
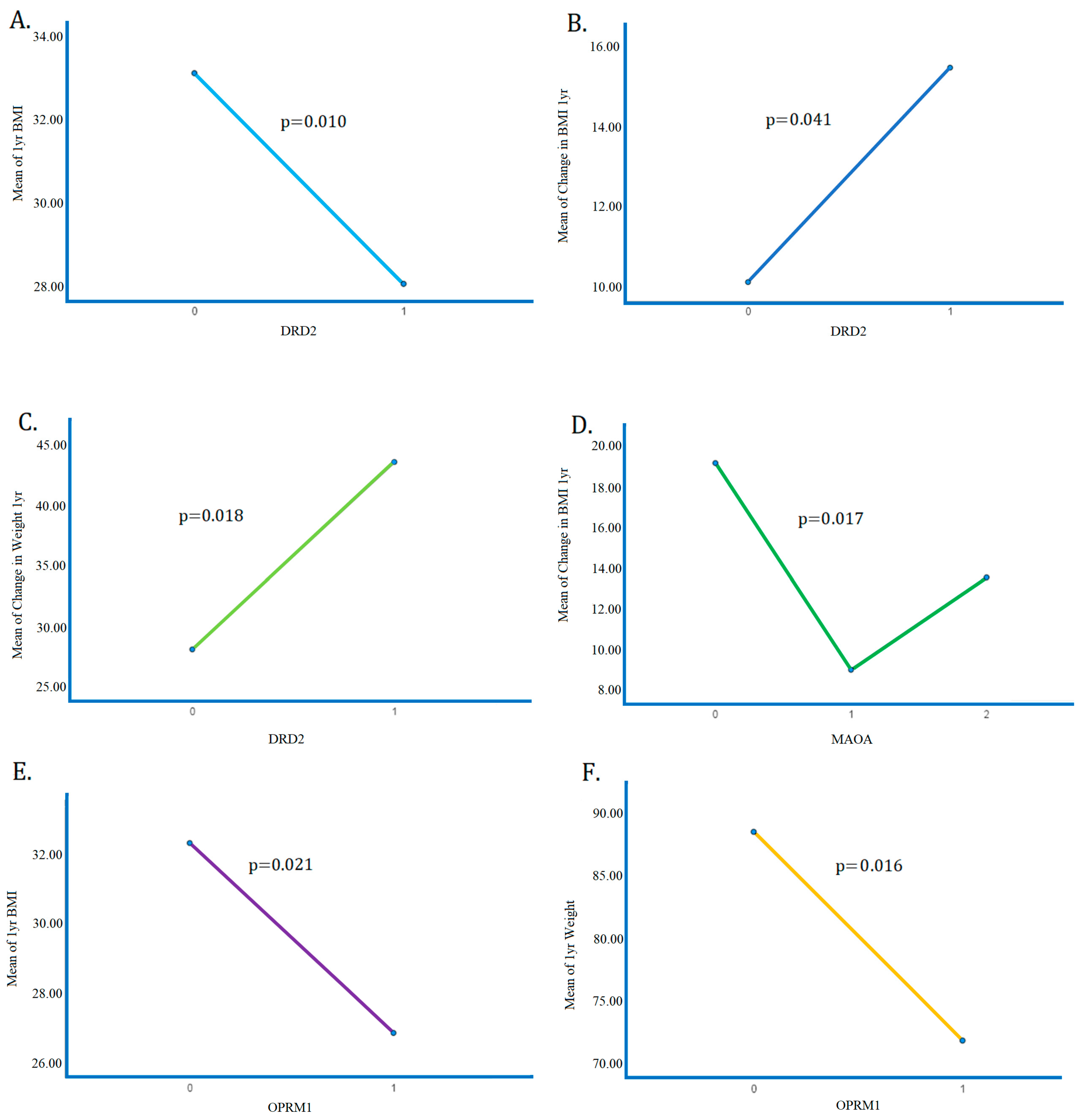
| The DRD2 risk allele | Positively correlated (increases) with ∆Weight (p < 0.05), and positively correlated (increases) with % Expected Weight Loss (EWL) (p < 0.05)-negatively correlated (decreases) with BMI at 1 year (p < 0.05). -one copy of the risk allele was associated with lower BMI. | |
| The COMT risk allele | negative correlation (decreases) with EEI scores p < 0.05) and PSQI scores (p < 0.05) | |
| GABRB3 risk allele | correlated positively (increases) with EEI (p < 0.01) and FCQ scores p < 0.01) | |
| OPRM1 risk allele | positive correlation (increases) with the DERS score (p < 0.05) | Spearman’s correlations showed a significant negative correlation (decreases) with 1-year weight (p < 0.01) and BMI (p < 0.05) |
| The DRD2 risk allele | -negatively correlated (decreases) with BMI at 1 year (p < 0.05). -one copy of the risk allele was associated with lower BMI. -positively correlated (increases) with ∆Weight (p < 0.05), and positively correlated (increases) with % EWL (p < 0.05) | |
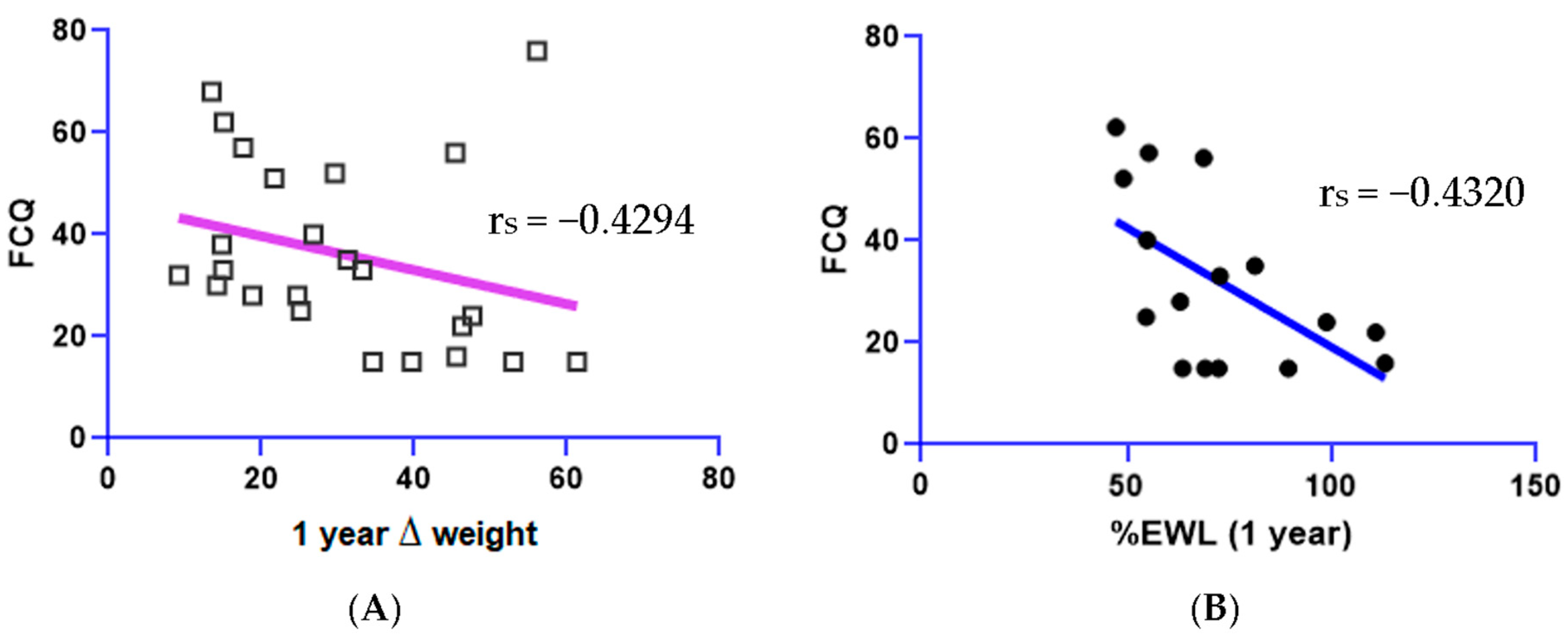
| Food Cravings Questionnaire (FCQ) scores | negatively correlated (decreases) with %EWL (p < 0.05) and ∆Weight (p < 0.05). | |
| CONCLUSIONS These data support the potential benefit of a personalized medicine approach, including genetic testing and psychosocial trait questionnaires when counseling patients with obesity considering bariatric surgery. | CONCLUSIONS Based on previous work, carriers of the DRD2 risk allele (rs1800497) are significantly more compliant with pharmacological treatment, and spearmen correlations had the highest compliance to behavioral therapeutics, thus lower BMI compared to non-carriers. |
| Eating Attitudes Test-26 | Total: 14.9 (8.1) |
|---|---|
| Food Cravings Questionnaire—Trait Reduced (FCQ-T) |
|
| Eating Expectancies Inventory |
|
| Modified Yale Food Addiction Scale 2.0 | Mean Symptom Count (SD): 1.32 (1.23) No Food Addiction (%): 61 Mild (%): 31 Moderate (%): 4 Severe (%): 4 |
| Weight-Influenced Self Esteem Questionnaire | M (SD): 1.6 (1.3) |
| Difficulties in Emotion Regulation Scale—Short Form | Total Mean (SD): 33.81 (10.96)
|
| Center for Epidemiological Studies Depression Scale | Total Score (Mean, range): 12.7, 0–35 No Depression (%): 69 Mild Depression (%): 8 Probable Depression (%): 23 |
| Chronic Stress Index | Perceived Everyday Unfair Treatment (Mean Score): 1.8 Major Negative Life Events in Past Year: 1.13 |
| Quality of Life Enjoyment and Satisfaction Questionnaire | M (SD): 3.24 (0.89) |
| Pittsburgh Sleep Quality Index | M (SD): 8.0 (3.74) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanos, P.K.; Hanna, C.; Mihalkovic, A.; Hoffman, A.; Posner, A.; Butsch, J.; Blum, K.; Georger, L.; Mastrandrea, L.D.; Quattrin, T. Genetic Correlates as a Predictor of Bariatric Surgery Outcomes after 1 Year. Biomedicines 2023, 11, 2644. https://doi.org/10.3390/biomedicines11102644
Thanos PK, Hanna C, Mihalkovic A, Hoffman A, Posner A, Butsch J, Blum K, Georger L, Mastrandrea LD, Quattrin T. Genetic Correlates as a Predictor of Bariatric Surgery Outcomes after 1 Year. Biomedicines. 2023; 11(10):2644. https://doi.org/10.3390/biomedicines11102644
Chicago/Turabian StyleThanos, Panayotis K., Colin Hanna, Abrianna Mihalkovic, Aaron Hoffman, Alan Posner, John Butsch, Kenneth Blum, Lesley Georger, Lucy D. Mastrandrea, and Teresa Quattrin. 2023. "Genetic Correlates as a Predictor of Bariatric Surgery Outcomes after 1 Year" Biomedicines 11, no. 10: 2644. https://doi.org/10.3390/biomedicines11102644
APA StyleThanos, P. K., Hanna, C., Mihalkovic, A., Hoffman, A., Posner, A., Butsch, J., Blum, K., Georger, L., Mastrandrea, L. D., & Quattrin, T. (2023). Genetic Correlates as a Predictor of Bariatric Surgery Outcomes after 1 Year. Biomedicines, 11(10), 2644. https://doi.org/10.3390/biomedicines11102644








