An Overview of Potential Natural Photosensitizers in Cancer Photodynamic Therapy
Abstract
1. Introduction
- (1)
- Pathways that directly kill the cancer cell;
- (2)
- Those that damage the vasculature to stop the oxygen supply to cells;
- (3)
- Those that activate/stimulate the systemic immunity response.
2. Basics of Photodynamic Therapy (PDT)
- (1)
- The specific wavelength of light;
- (2)
- Cellular oxygen; and
- (3)
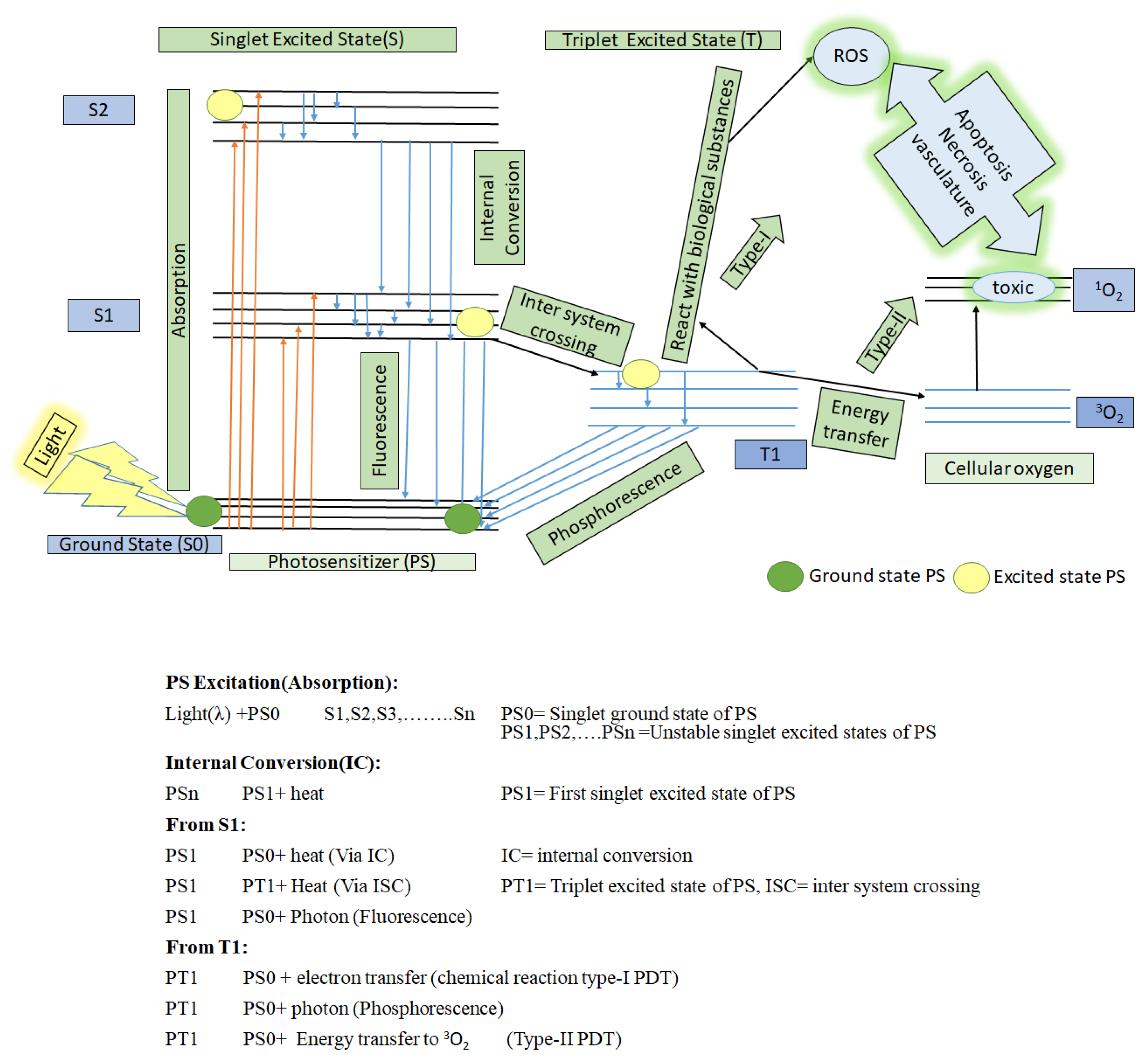
- (1)
- It can react with nearby bio-substrate such as fatty acid and DNA via electron transfer to generate free radicals (anion/cation). These free radicals further interact quickly with biological molecules such as lipids, proteins, and nucleic acids to generate ROX (hydroxyl/superoxide radicals), which eventually cause cancer cell death. This is type-I PDT [9,25].
- (2)
- It can react with surrounding cellular oxygen (3O2, which has a triplet ground state) via direct energy transfer. 3O2 quickly jumps to a singlet excited state to become 1O2 (highly reactive oxygen). This is type II PDT. This singlet state toxic oxygen (1O2) can oxidize the amino acids in lipids, proteins, sugar linkages or bases in DNA and induce changes in the lipid and calcium metabolism, upregulation of stress proteins and cytokines, and ultimately, induce cell death to occur via necrosis and apoptosis [26,27,28].
3. Photosensitizer (PS), a Light-Activable Drug
3.1. Features of Ideal PS
- (1)
- Should be a pure chemical compound;
- (2)
- Must have a high quantum yield (ΦΔ) of singlet oxygen;
- (3)
- Non-toxic effect for normal healthy cell;
- (4)
- Selective long localization period in malignant cells and fast excretion rate from healthy cells;
- (5)
3.2. Conventionally Approved Photosensitizers for Cancer PDT
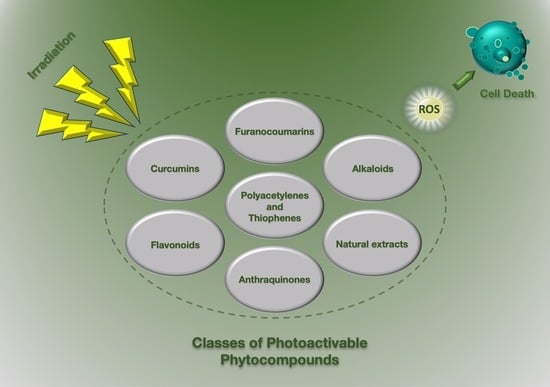
4. Herbal Photo-Activable Compounds from Natural Reservoirs
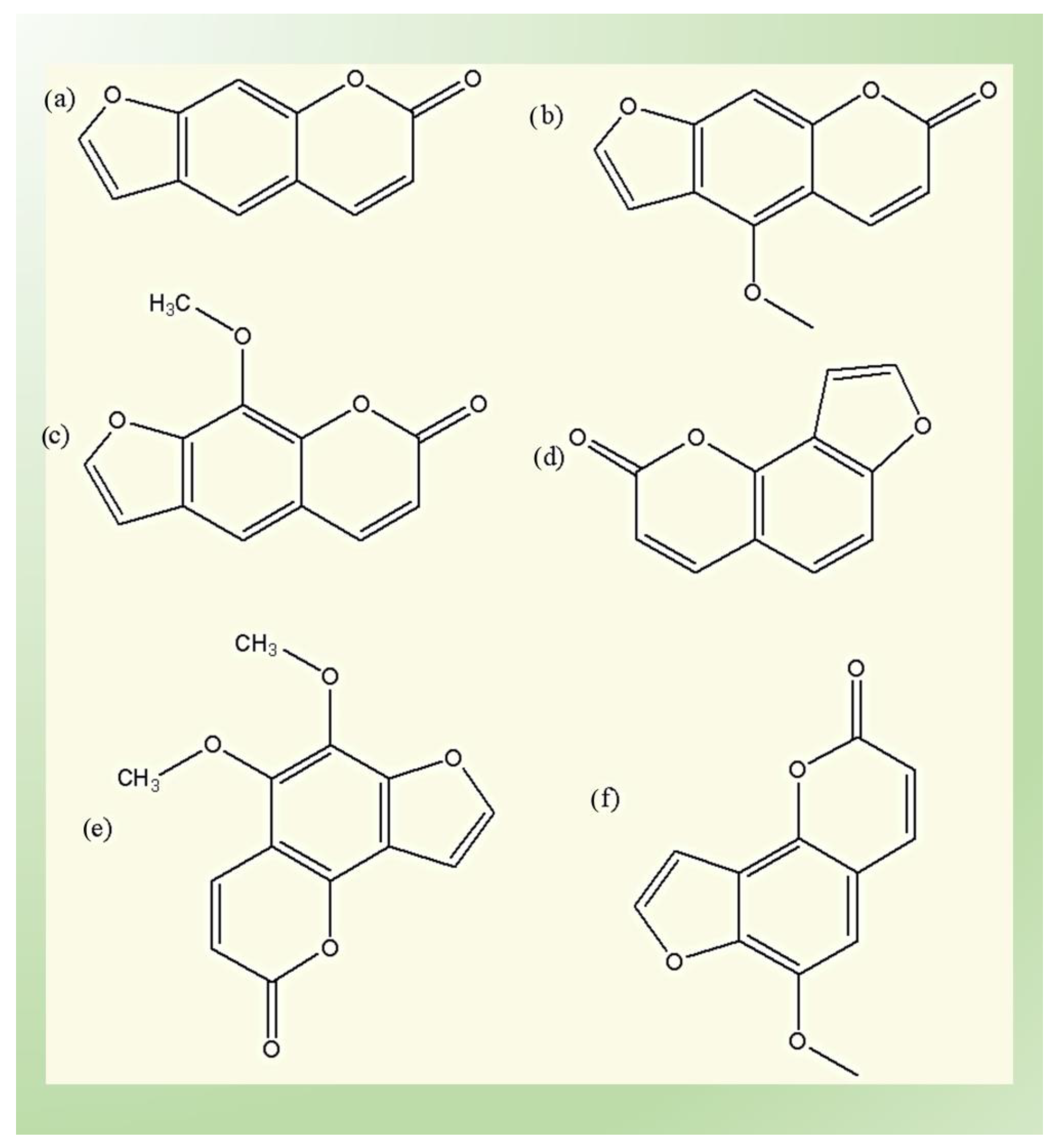
4.1. Furanocoumarins
- (1)
- The linear (psoralens) group, which includes psoralen, bergapten, and xanthotoxin;and
- (2)
- The angular (angelicins) group, which includes angelicin, pimpinellin and sphondin.
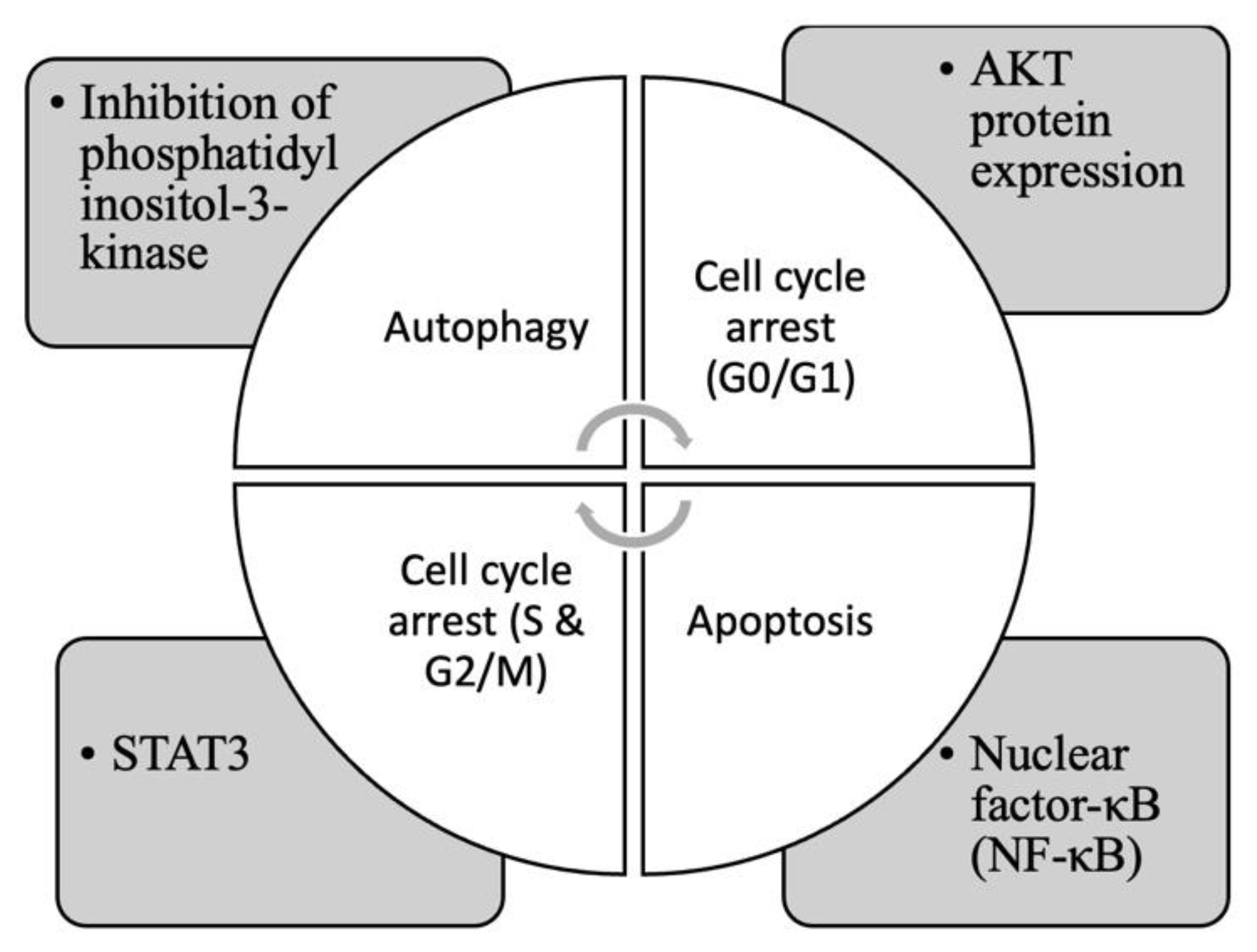
4.2. Alkaloids
4.3. Poly-Acetylenes and Thiophenes Derivatives
- (1)
- Straight-chain aliphatic acetylenes;
- (2)
- Partly cyclized; and
- (3)
- Thiophene derivatives (addition of sulfur into polyacetylene) [87].
4.4. Curcumins
4.5. Flavonoids
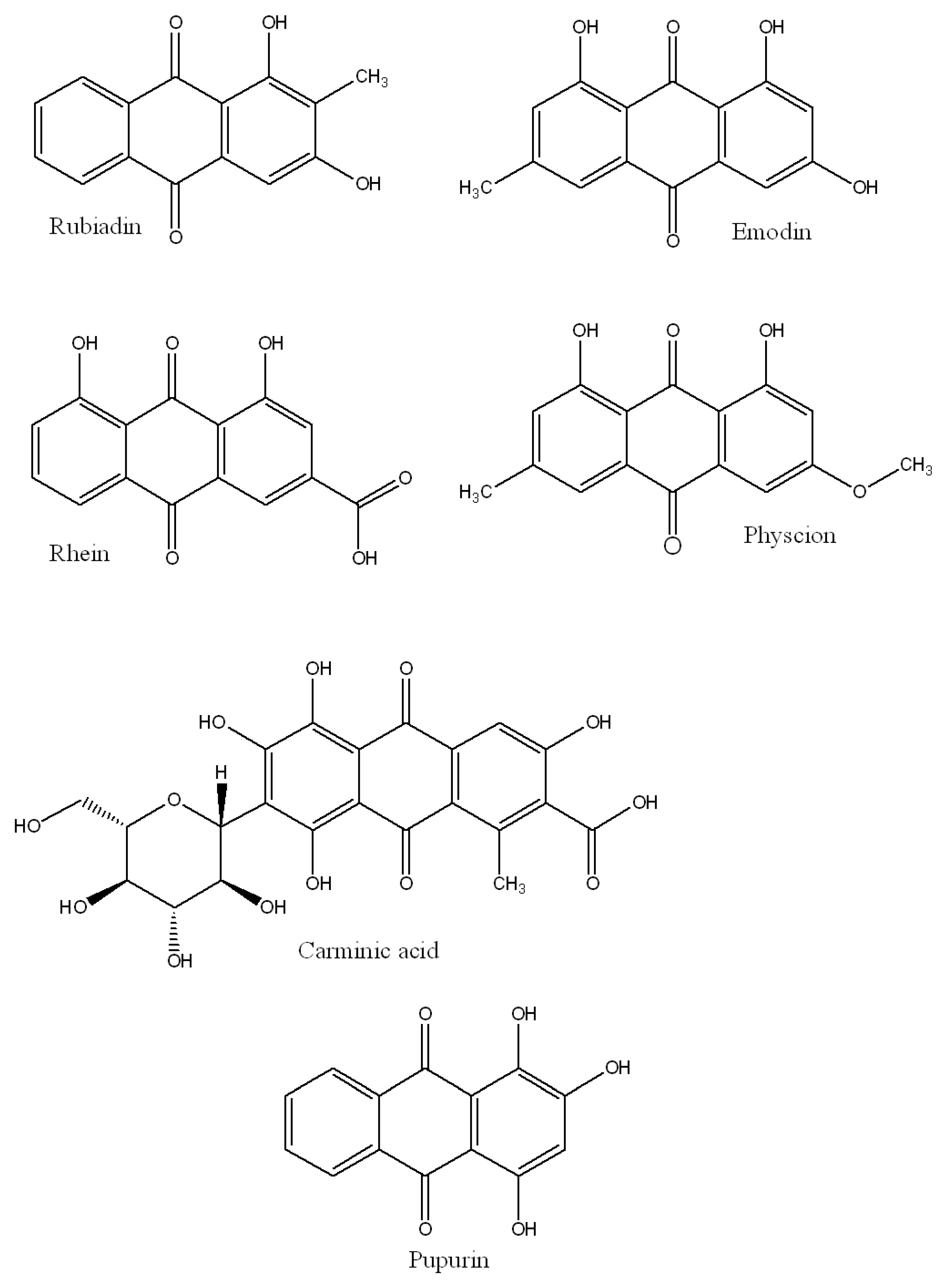
4.6. Anthraquinones
4.7. Natural Extracts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Horn, G.; Moulton, K.; Oza, A.; Byler, S.; Kokolus, S.; Longacre, M. Cancer Development, Progression, and Therapy: An Epigenetic Overview. Int. J. Mol. Sci. 2013, 14, 21087–21113. [Google Scholar] [CrossRef] [PubMed]
- Polomano, R.C.; Farrar, J.T. Pain and Neuropathy in Cancer Survivors: Surgery, radiation, and chemotherapy can cause pain; research could improve its detection and treatment. AJN Am. J. Nurs. 2006, 106. Available online: https://journals.lww.com/ajnonline/Fulltext/2006/03003/Pain_and_Neuropathy_in_Cancer_Survivors__Surgery,.15.aspx (accessed on 22 November 2022).
- Abdel-Kader, M.H. Photodynamic Therapy; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Luksiene, Z. Photodynamic therapy: Mechanism of action and ways to improve the efficiency of treatment. Medicina 2003, 39, 1137–1150. [Google Scholar]
- Oniszczuk, A.; Wojtunik-Kulesza, K.A.; Oniszczuk, T.; Kasprzak, K. The potential of photodynamic therapy (PDT)—Experimental investigations and clinical use. Biomed. Pharmacother. 2016, 83, 912–929. [Google Scholar] [CrossRef]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell Death Pathways in Photodynamic Therapy of Cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z. Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer. Biomedicines 2021, 9, 689. [Google Scholar] [CrossRef]
- Zhao, Y.; Dunmall, L.S.C.; Cheng, Z.; Wang, Y.; Si, L. Natural products targeting glycolysis in cancer. Front. Pharmacol. 2022, 13, 4610. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, N.; Yang, L.; Song, X.-Q. Bioactive natural products in COVID-19 therapy. Front. Pharmacol. 2022, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S. Natural Products That Changed Society. Biomedicines 2021, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Pezzani, R.; Salehi, B.; Vitalini, S.; Iriti, M.; Zuñiga, F.; Sharifi-Rad, J.; Martorell, M.; Martins, N. Synergistic Effects of Plant Derivatives and Conventional Chemotherapeutic Agents: An Update on the Cancer Perspective. Medicina 2019, 55, 110. [Google Scholar] [CrossRef] [PubMed]
- Mangla, B.; Kohli, K. Combination of Natural Agent with Synthetic Drug for the Breast Cancer Therapy. Int. J. Drug Dev. Res. 2018, 10, 22–26. [Google Scholar]
- Mansoori, B.; Mohammadi, A.; Doustvandi, M.A.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic therapy for cancer: Role of natural products. Photodiagnosis Photodyn. Ther. 2019, 26, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Shukla, Y. MINI-REVIEW Herbal Medicine: Current Status and the Future. Asian Pac. J. Cancer Prev. 2003, 4, 281–288. [Google Scholar]
- Hemaiswarya, S.; Doble, M. Potential synergism of natural products in the treatment of cancer. Phytother. Res. 2006, 20, 239–249. [Google Scholar] [CrossRef]
- Alade, P.; Irobi, O. Antimicrobial activities of crude leaf extracts of Acalypha wilkesiana. J. Ethnopharmacol. 1993, 39, 171–174. [Google Scholar] [CrossRef]
- Sengupta, S.; Eavarone, D.; Capila, I.; Zhao, G.; Watson, N.; Kiziltepe, T.; Sasisekharan, R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature 2005, 436, 568–572. [Google Scholar] [CrossRef]
- Moraes, C.D.G.D.O.; Godoi, B.H.; Carvalho, I.C.S.; Pinto, J.C.; Rossato, R.C.; da Silva, N.S.; Soares, C.P. Genotoxic effects of photodynamic therapy in laryngeal cancer cells—An in vitro study. Exp. Biol. Med. 2019, 244, 262–271. [Google Scholar] [CrossRef]
- Villacorta, R.B.; Roque, K.F.J.; Tapang, G.A.; Jacinto, S.D. Plant extracts as natural photosensitizers in photodynamic therapy: In vitro activity against human mammary adenocarcinoma MCF-7 cells. Asian Pac. J. Trop. Biomed. 2017, 7, 358–366. [Google Scholar] [CrossRef]
- Chaturvedi, D.; Singh, K.; Singh, V.K. Therapeutic and pharmacological aspects of photodynamic product chlorophyllin. Eur. J. Biol. Res. 2019, 9, 64–76. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Goradel, N.H.; Farhood, B.; Salehi, E.; Solhjoo, S.; Toolee, H.; Kharazinejad, E.; Mortezaee, K. Tumor microenvironment: Interactions and therapy. J. Cell Physiol. 2018, 234, 5700–5721. [Google Scholar] [CrossRef]
- Lim, C.K.; Heo, J.; Shin, S.; Jeong, K.; Seo, Y.H.; Jang, W.-D.; Park, C.R.; Park, S.Y.; Kim, S.; Kwon, I.C. Nanophotosensitizers toward advanced photodynamic therapy of Cancer. Cancer Lett. 2013, 334, 176–187. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part three—Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis Photodyn. Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two—Cellular signaling, cell metabolism and modes of cell death. Photodiagnosis Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.H.; Childs, C.J.; Sibata, C.H. Photosensitizers in clinical PDT. Photodiagnosis Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photodynamic Therapy for Cancer: What’s Past is Prologue. Photochem. Photobiol. 2019, 96, 506–516. [Google Scholar] [CrossRef]
- Huang, Z. A Review of Progress in Clinical Photodynamic Therapy. Technol. Cancer Res. Treat. 2005, 4, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Fargnoli, M.C.; Peris, K. Photodynamic therapy for basal cell carcinoma. Futur. Oncol. 2015, 11, 2991–2996. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Goa, K.L. Verteporfin. Drugs Aging 2000, 16, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Tamiaki, H.; Machida, S.; Mizutani, K. Modification of 3-substituents in (bacterio) chlorophyll derivatives to prepare 3-ethylated, methylated, and unsubstituted (nickel) pyropheophorbides and their optical properties. J. Org. Chem. 2012, 77, 4751–4758. [Google Scholar] [CrossRef]
- Kelleher, D.K.; Thews, O.; Scherz, A.; Salomon, Y.; Vaupel, P. Perfusion, oxygenation status and growth of experimental tumors upon photodynamic therapy with Pd-bacteriopheophorbide. Int. J. Oncol. 2004, 24, 1505–1511. [Google Scholar] [CrossRef]
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef]
- Alali, F.Q.; Tawaha, K. Dereplication of bioactive constituents of the genus hypericum using LC-(+,−)-ESI-MS and LC-PDA techniques: Hypericum triquterifolium as a case study. Saudi Pharm. J. 2009, 17, 269–274. [Google Scholar] [CrossRef]
- Karg, C.A.; Wang, P.; Vollmar, A.M.; Moser, S. Re-opening the stage for Echinacea research—Characterization of phylloxanthobilins as a novel anti-oxidative compound class in Echinacea purpurea. Phytomedicine 2019, 60, 152969. [Google Scholar] [CrossRef]
- Tan, P.J.; Appleton, D.R.; Mustafa, M.R.; Lee, H.B. Rapid Identification of Cyclic Tetrapyrrolic Photosensitisers for Photodynamic Therapy Using On-line Hyphenated LC-PDA-MS Coupled with Photo-cytotoxicity Assay. Phytochem. Anal. 2011, 23, 52–59. [Google Scholar] [CrossRef]
- Andreazza, N.L.; Vevert-Bizet, C.; Bourg-Heckly, G.; Sureau, F.; Salvador, M.J.; Bonneau, S. Berberine as a photosensitizing agent for antitumoral photodynamic therapy: Insights into its association to low density lipoproteins. Int. J. Pharm. 2016, 510, 240–249. [Google Scholar] [CrossRef]
- Mittermair, E.; Krenn, L.; Marian, B. Prenylated xanthones from Metaxya rostrata suppress FoxM1 and induce active cell death by distinct mechanisms. Phytomedicine 2019, 60, 152912. [Google Scholar] [CrossRef] [PubMed]
- Senapathy, G.J.; George, B.P.; Abrahamse, H. Exploring the role of phytochemicals as potent natural photosensitizers in photodynamic therapy. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2020, 20, 1831–1844. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.C. Photodynamic Therapy Based on Arrabidaea chica (Crajiru) Extract Nanoemulsion: In vitro Activity against Monolayers and Spheroids of Human Mammary Adenocarcinoma MCF-7 Cells. J. Nanomed. Nanotechnol. 2015, 6, 3. [Google Scholar] [CrossRef]
- Ptushenko, O.S.; Ptushenko, V.V.; Solovchenko, A.E. Spectrum of Light as a Determinant of Plant Functioning: A Historical Perspective. Life 2020, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.-G.; Okajima, K. Diverse photoreceptors and light responses in plants. J. Plant Res. 2016, 129, 111–114. [Google Scholar] [CrossRef]
- Rajesh, S.; Anvita, D.; Vikas, P.; Dilip, G.; Alok, P.J. Chromophore- An Utility in UV Spectrophotometer. Inven. J. 2017, 2012, 4. [Google Scholar]
- Christensen, L.P. Polyphenols and Polyphenol-Derived Compounds from Plants and Contact Dermatitis, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Hung, W.-L.; Suh, J.H.; Wang, Y. Chemistry and health effects of furanocoumarins in grapefruit. J. Food Drug Anal. 2016, 25, 71–83. [Google Scholar] [CrossRef]
- Sumorek-Wiadro, J.; Zając, A.; Maciejczyk, A.; Jakubowicz-Gil, J. Furanocoumarins in anticancer therapy—For and against. Fitoterapia 2020, 142, 104492. [Google Scholar] [CrossRef]
- Pynam, H.; Dharmesh, S.M. Antioxidant and anti-inflammatory properties of marmelosin from Bael (Aegle marmelos L.); Inhibition of TNF-α mediated inflammatory/tumor markers. Biomed. Pharmacother. 2018, 106, 98–108. [Google Scholar] [CrossRef]
- Niu, C.; Lu, X.; Aisa, H.A. Preparation of novel 1,2,3-triazole furocoumarin derivatives via click chemistry and their anti-vitiligo activity. RSC Adv. 2019, 9, 1671–1678. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, H.; Aschner, M.; Mirzae, H.; Akkol, E.K.; Capasso, R. Anticancer Potential of Furanocoumarins: Mechanistic and Therapeutic Aspects. Int. J. Mol. Sci. 2020, 21, 5622. [Google Scholar] [CrossRef] [PubMed]
- George, B.P.; Abrahamse, H. Light-Activated Phytochemicals in Photodynamic Therapy for Cancer: A Mini Review. Photobiomodulation Photomed. Laser Surg. 2022, 40, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Melough, M.M.; Cho, E.; Chun, O.K. Furocoumarins: A review of biochemical activities, dietary sources and intake, and potential health risks. Food Chem. Toxicol. 2018, 113, 99–107. [Google Scholar] [CrossRef]
- Luo, W.; Song, Z.; Sun, H.; Liang, J.; Zhao, S. Bergamottin, a natural furanocoumarin abundantly present in grapefruit juice, suppresses the invasiveness of human glioma cells via inactivation of Rac1 signaling. Oncol. Lett. 2017, 15, 3259–3266. [Google Scholar] [CrossRef]
- Conforti, F.; Menichini, G.; Zanfini, L.; Tundis, R.; Statti, G.A.; Provenzano, E.; Somma, F.; Alfano, C. Evaluation of phototoxic potential of aerial components of the fig tree against human melanoma. Cell Prolif. 2012, 45, 279–285. [Google Scholar] [CrossRef]
- Ibbotson, S.H. A Perspective on the Use of NB-UVB Phototherapy vs. PUVA Photochemotherapy. Front. Med. 2018, 5, 184. [Google Scholar] [CrossRef] [PubMed]
- Panno, M.L.; Giordano, F.; Rizza, P.; Pellegrino, M.; Zito, D.; Mauro, L.; Catalano, S.; Aquila, S.; Sisci, D.; De Amicis, F.; et al. Bergapten induces ER depletion in breast cancer cells through SMAD4-mediated ubiquitination. Breast Cancer Res. Treat. 2012, 136, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Panno, M.; Giordano, F.; Palma, M.; Bartella, V.; Rago, V.; Maggiolini, M.; Sisci, D.; Lanzino, M.; De Amicis, F.; Ando, S. Evidence that Bergapten, Independently of its Photoactivation, Enhances p53 Gene Expression and Induces Apoptosis in Human Breast Cancer Cells. Curr. Cancer Drug Targets 2009, 9, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Korman, N.J.; Elmets, C.A.; Feldman, S.R.; Gelfand, J.M.; Gordon, K.B.; Gottlieb, A.; Koo, J.Y.; Lebwohl, M.; Lim, H.W.; et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J. Am. Acad. Dermatol. 2010, 62, 114–135. [Google Scholar] [CrossRef]
- Holtick, U.; Wang, X.; Marshall, S.; von Bergwelt-Baildon, M.; Scheid, C.; Dickinson, A.M. In Vitro PUVA Treatment Preferentially Induces Apoptosis in Alloactivated T Cells. Transplantation 2012, 94. Available online: https://journals.lww.com/transplantjournal/Fulltext/2012/09150/In_Vitro_PUVA_Treatment_Preferentially_Induces.25.aspx (accessed on 20 November 2022). [CrossRef]
- Kitamura, N.; Kohtani, S.; Nakagaki, R. Molecular aspects of furocoumarin reactions: Photophysics, photochemistry, photobiology, and structural analysis. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 168–185. [Google Scholar] [CrossRef]
- El-Domyati, M.; Moftah, N.; Nasif, G.A.; Abdel-Wahab, H.; Barakat, M.T.; Abdel-Aziz, R.T. Evaluation of apoptosis regulatory proteins in response to PUVA therapy for psoriasis. Photodermatol. Photoimmunol. Photomed. 2013, 29, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Gooden, D.; Liu, L.; Zhao, S.; Soderblom, E.J.; Toone, E.J.; Jr, W.F.B.; Walder, H.; Spector, N.L. Photo-Activated Psoralen Binds the ErbB2 Catalytic Kinase Domain, Blocking ErbB2 Signaling and Triggering Tumor Cell Apoptosis. PLoS ONE 2014, 9, e88983. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Lee, J.H.; Sethi, G.; Kim, C.; Baek, S.H.; Nam, D.; Chung, W.-S.; Shim, B.S.; Ahn, K.S. Bergamottin, a natural furanocoumarin obtained from grapefruit juice induces chemosensitization and apoptosis through the inhibition of STAT3 signaling pathway in tumor cells. Cancer Lett. 2014, 354, 153–163. [Google Scholar] [CrossRef]
- Liszewski, W.; Naym, D.G.; Biskup, E.; Gniadecki, R. Psoralen with ultraviolet A-induced apoptosis of cutaneous lymphoma cell lines is augmented by type I interferons via the JAK1-STAT1 pathway. Photodermatol. Photoimmunol. Photomed. 2017, 33, 164–171. [Google Scholar] [CrossRef]
- Kim, S.-M.; Lee, E.-J.; Lee, J.H.; Yang, W.M.; Nam, D.; Lee, J.-H.; Lee, S.-G.; Um, J.-Y.; Shim, B.S.; Ahn, K.S. Simvastatin in combination with bergamottin potentiates TNF-induced apoptosis through modulation of NF-κB signalling pathway in human chronic myelogenous leukaemia. Pharm. Biol. 2016, 54, 2050–2060. [Google Scholar] [CrossRef]
- Van Aelst, B.; Devloo, R.; Zachée, P.; T’Kindt, R.; Sandra, K.; Vandekerckhove, P.; Compernolle, V.; Feys, H.B. Psoralen and Ultraviolet A Light Treatment Directly Affects Phosphatidylinositol 3-Kinase Signal Transduction by Altering Plasma Membrane Packing. J. Biol. Chem. 2016, 291, 24364–24376. [Google Scholar] [CrossRef]
- Ge, Z.-C.; Qu, X.; Yu, H.-F.; Zhang, H.-M.; Wang, Z.-H.; Zhang, Z.-T. Antitumor and apoptotic effects of bergaptol are mediated via mitochondrial death pathway and cell cycle arrest in human breast carcinoma cells. Bangladesh J. Pharmacol. 2016, 11, 489. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, F.; Lin, X.; Shen, L.; Feng, Y. Developments in drug delivery of bioactive alkaloids derived from traditional Chinese medicine. Drug Deliv. 2018, 25, 398–416. [Google Scholar] [CrossRef]
- Andreazza, N.L.; de Lourenço, C.C.; Hernandez-Tasco, J.; Pinheiro, M.L.B.; Stefanello, M.A.; Costa, E.V.; Salvador, M.J. Antimicrobial photodynamic effect of extracts and oxoaporphine alkaloid isomoschatoline from Guatteria blepharophylla. J. Photochem. Photobiol. B Biol. 2016, 160, 154–162. [Google Scholar] [CrossRef]
- Desgagné-Penix, I. Biosynthesis of alkaloids in Amaryllidaceae plants: A review. Phytochem. Rev. 2021, 20, 409–431. [Google Scholar] [CrossRef]
- Taha, H.S.; El-Bahr, M.; Seif-El-Nasr, M.M. In vitro studies on Egyptian Catharanthus roseus (L.). Ii. Effect of biotic and abiotic stress on indole alkaloids production. J. Appl. Sci. Res. 2009, 5, 1826–1831. [Google Scholar]
- Peng, J.; Zheng, T.-T.; Li, X.; Liang, Y.; Wang, L.-J.; Huang, Y.-C.; Xiao, H.-T. Plant-Derived Alkaloids: The Promising Disease-Modifying Agents for Inflammatory Bowel Disease. Front. Pharmacol. 2019, 10, 351. [Google Scholar] [CrossRef]
- Lu, J.-J.; Bao, J.-L.; Chen, X.-P.; Huang, M.; Wang, Y.-T. Alkaloids Isolated from Natural Herbs as the Anticancer Agents. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Roy, A. A Review on the Alkaloids an Important Therapeutic Compound from Plants. Int. J. Plant Biotechnol. 2017, 3, 1–9. [Google Scholar]
- Aniszewski, T. Applied Potential and Current Applications of Alkaloids, 2nd ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Kurek, J. Chemistry Department, Adam Mickiewicz University, Poznań, Introductory Chapter: Alkaloids—Their Importance in Nature and for Human Life; Intech Open: London, UK, 2019. [Google Scholar]
- Eom, K.S.; Kim, H.-J.; So, H.-S.; Park, R.; Kim, T.Y. Berberine-Induced Apoptosis in Human Glioblastoma T98G Cells Is Mediated by Endoplasmic Reticulum Stress Accompanying Reactive Oxygen Species and Mitochondrial Dysfunction. Biol. Pharm. Bull. 2010, 33, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xun, K.; Wang, Y.; Chen, X. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anticancer. Drugs 2009, 20. Available online: https://journals.lww.com/anti-cancerdrugs/Fulltext/2009/10000/A_systematic_review_of_the_anticancer_properties.1.aspx (accessed on 22 November 2022). [CrossRef]
- Görner, H.; Miskolczy, Z.; Megyesi, M.; Biczók, L. Photoreduction and Ketone-sensitized Reduction of Alkaloids. Photochem. Photobiol. 2011, 87, 284–291. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Gupta, P.; Bandyopadhyay, S.K.; Patro, B.S.; Chattopadhyay, S. Coralyne, a protoberberine alkaloid, causes robust photosenstization of cancer cells through ATR-p38 MAPK-BAX and JAK2-STAT1-BAX pathways. Chem. Interact. 2018, 285, 27–39. [Google Scholar] [CrossRef]
- Lopes, T.Z.; de Moraes, F.R.; Tedesco, A.C.; Arni, R.K.; Rahal, P.; Calmon, M.F. Berberine associated photodynamic therapy promotes autophagy and apoptosis via ROS generation in renal carcinoma cells. Biomed. Pharmacother. 2019, 123, 109794. [Google Scholar] [CrossRef]
- Oliveira, P.; Lopes, T.; Tedesco, A.; Rahal, P.; Calmon, M. Effect of berberine associated with photodynamic therapy in cell lines. Photodiagnosis Photodyn. Ther. 2020, 32, 102045. [Google Scholar] [CrossRef] [PubMed]
- Arnason, T.; Towers, G.H.N.; Philogène, B.J.R.; Lambert, J.D.H. The Role of Natural Photosensitizers in Plant Resistance to Insects. In Plant Resistance to Insects; American Chemical Society: New York, NY, USA, 1983; Volume 208, pp. 139–151, SE–8. [Google Scholar]
- Abivardi, C. Polyacetylenes (And Their Thiophene Derivatives). In Encyclopedia of Entomology; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar] [CrossRef]
- Simpson, D.; Amos, S. Other Plant Metabolites; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Minto, R.E.; Blacklock, B.J. Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res. 2008, 47, 233–306. [Google Scholar] [CrossRef] [PubMed]
- Postigo, A.; Funes, M.; Petenatti, E.; Bottai, H.; Pacciaroni, A.; Sortino, M. Antifungal photosensitive activity of Porophyllum obscurum (Spreng.) DC.: Correlation of the chemical composition of the hexane extract with the bioactivity. Photodiagnosis Photodyn. Ther. 2017, 20, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Marchant, Y.Y.; Cooper, G.K. Structure and Function Relationships in Polyacetylene Photoactivity. In Light-Activated Pesticides; American Chemical Society: New York, NY, USA, 1987; Volume 339, pp. 17–241. [Google Scholar]
- Ibrahim, S.R.M.; Abdallah, H.M.; El-Halawany, A.M.; Mohamed, G.A. Naturally occurring thiophenes: Isolation, purification, structural elucidation, and evaluation of bioactivities. Phytochem. Rev. 2015, 15, 197–220. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Li, L.-H.; Meng, D.-L.; Li, Z.-L.; Li, N. Two New Thiophenes from Echinops latifolius and their Phototoxic Activities. Planta Med. 2007, 73, 696–698. [Google Scholar] [CrossRef]
- Xu, D.G.; Lv, W.; Dai, C.Y.; Zhu, F.F.; Xu, G.H.; Ma, Z.J.; Chen, Z. 2-(Pro-1-ynyl)-5-(5,6-dihydroxypenta-1,3-diynyl) Thiophene Induces Apoptosis Through Reactive Oxygen Species-Mediated JNK Activation in Human Colon Cancer SW620 Cells. Anat. Rec. 2014, 298, 376–385. [Google Scholar] [CrossRef]
- Zhang, P.; Liang, D.; Jin, W.; Qu, H.; Cheng, Y.; Li, X.; Ma, Z. Cytotoxic Thiophenes from the Root of Echinops grijisii Hance. Z. Für Nat. C 2009, 64, 193–196. [Google Scholar] [CrossRef]
- Dos Santos, F.A.; Pereira, M.C.; de Oliveira, T.B.; Junior, F.J.B.M.; de Lima, M.D.C.A.; Pitta, M.G.D.R.; Pitta, I.D.R.; Rêgo, M.J.B.D.M. Anticancer properties of thiophene derivatives in breast cancer MCF-7 cells. Anti-Cancer Drugs 2018, 29, 157–166. [Google Scholar] [CrossRef]
- Jin, W.; Shi, Q.; Hong, C.; Cheng, Y.; Ma, Z.; Qu, H. Cytotoxic properties of thiophenes from Echinops grijissi Hance. Phytomedicine 2008, 15, 768–774. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; El Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photossensitizersfot photodynamic therapy. Biochem. J. 2017, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Chaturvedi, M.M.; Aggarwal, B.B. Role of Curcumin in Cancer Therapy. Curr. Probl. Cancer 2007, 31, 243–305. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Mbese, Z.; Khwaza, V.; Aderibigbe, B.A. Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules 2019, 24, 4386. [Google Scholar] [CrossRef]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef]
- Qadir, M.I.; Naqvi, S.T.Q.; Muhammad, S.A. Curcumin: A Polyphenol with Molecular Targets for Cancer Control. Asian Pac. J. Cancer Prev. 2016, 17, 2735–2739. [Google Scholar]
- Park, K.; Lee, J.-H. Photosensitizer effect of curcumin on UVB-irradiated HaCaT cells through activation of caspase pathways. Oncol. Rep. 2007, 17, 537–540. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Lin, J.-N.; Ma, J.-W.; Yang, N.-S.; Ho, C.-T.; Kuo, S.-C.; Way, T.-D. Demethoxycurcumin induces autophagic and apoptotic responses on breast cancer cells in photodynamic therapy. J. Funct. Foods 2015, 12, 439–449. [Google Scholar] [CrossRef]
- Bernd, A. Visible light and/or UVA offer a strong amplification of the anti-tumor effect of curcumin. Phytochem. Rev. 2013, 13, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Wang, D.; Zhang, F. In vitro antimicrobial effect of curcumin-based photodynamic therapy on Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. Photodiagnosis Photodyn. Ther. 2020, 32, 102055. [Google Scholar] [CrossRef]
- Huang, J.; Chen, B.; Li, H.; Zeng, Q.-H.; Wang, J.J.; Liu, H.; Pan, Y.; Zhao, Y. Enhanced antibacterial and antibiofilm functions of the curcumin-mediated photodynamic inactivation against Listeria monocytogenes. Food Control. 2020, 108, 106886. [Google Scholar] [CrossRef]
- Gao, J.; Matthews, K.R. Effects of the photosensitizer curcumin in inactivating foodborne pathogens on chicken skin. Food Control. 2019, 109, 106959. [Google Scholar] [CrossRef]
- Abdulrahman, H.; Misba, L.; Ahmad, S.; Khan, A.U. Curcumin induced photodynamic therapy mediated suppression of quorum sensing pathway of Pseudomonas aeruginosa: An approach to inhibit biofilm in vitro. Photodiagnosis Photodyn. Ther. 2019, 30, 101645. [Google Scholar] [CrossRef] [PubMed]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Latest Innovations and Nanotechnologies with Curcumin as a Nature-Inspired Photosensitizer Applied in the Photodynamic Therapy of Cancer. Pharmaceutics 2021, 13, 1562. [Google Scholar] [CrossRef]
- Maciel, L.T.R.; Marcolino, L.M.C.; Maciel, F.B.S.P.; Pinto, J.G.; Ferreira-Strixino, J. Effect of serial photodynamic therapy with curcumin on Leishmania braziliensis and Leishmania amazonensis promastigotes. Res. Soc. Dev. 2021, 10, e219101119544. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Blázquez, M.A.; López-Gresa, M.P.; Mena, P.; García-Viguera, C. Editorial: Flavonoids: From Biosynthesis and Metabolism to Health Benefits. Front. Plant Sci. 2021, 12, 10–12. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 1–13. [Google Scholar] [CrossRef]
- Ghidoli, M.; Colombo, F.; Sangiorgio, S.; Landoni, M.; Giupponi, L.; Nielsen, E.; Pilu, R. Food Containing Bioactive Flavonoids and Other Phenolic or Sulfur Phytochemicals With Antiviral Effect: Can We Design a Promising Diet Against COVID-19? Front. Nutr. 2021, 8, 661331. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.D.P.; Tini, I.R.P.; Soares, C.P.; da Silva, N.S. Effect of photodynamic therapy supplemented with quercetin in HEp-2 cells. Cell Biol. Int. 2014, 38, 716–722. [Google Scholar] [CrossRef]
- Ahn, J.-C.; Biswas, R.; Chung, P.-S. Combination with genistein enhances the efficacy of photodynamic therapy against human anaplastic thyroid cancer cells. Lasers Surg. Med. 2012, 44, 840–849. [Google Scholar] [CrossRef]
- Ferenc, P.; Solár, P.; Kleban, J.; Mikeš, J.; Fedoročko, P. Down-regulation of Bcl-2 and Akt induced by combination of photoactivated hypericin and genistein in human breast cancer cells. J. Photochem. Photobiol. B Biol. 2010, 98, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-J.; Sun, D.; Hao, J.-B.; Wei, Y.-F.; Yin, L.-F.; Liu, X. The effect of dietary soyabean isoflavones on photodynamic therapy in K562 leukemia cells. J. Photochem. Photobiol. B Biol. 2012, 110, 28–33. [Google Scholar] [CrossRef]
- Teerakapong, A.; Damrongrungruang, T.; Sattayut, S.; Morales, N.P.; Tantananugool, S. Efficacy of erythrosine and cyanidin-3-glucoside mediated photodynamic therapy on Porphyromonas gingivalis biofilms using green light laser. Photodiagnosis Photodyn. Ther. 2017, 20, 154–158. [Google Scholar] [CrossRef]
- Josewin, S.W.; Ghate, V.; Kim, M.-J.; Yuk, H.-G. Antibacterial effect of 460 nm light-emitting diode in combination with riboflavin against Listeria monocytogenes on smoked salmon. Food Control. 2018, 84, 354–361. [Google Scholar] [CrossRef]
- Siewert, B.; Stuppner, H. The photoactivity of natural products—An overlooked potential of phytomedicines? Phytomedicine 2019, 60, 152985. [Google Scholar] [CrossRef]
- Comini, L.R.; Fernandez, I.M.; Vittar, N.B.R.; Nunez Montoya, S.C.; Cabrera, J.L.; Rivarola, V.A. Photodynamic activity of anthraquinones isolated from Heterophyllaea pustulata Hook f. (Rubiaceae) on MCF-7c3 breast cancer cells. Phytomedicine 2011, 18, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Mugas, M.L.; Calvo, G.; Marioni, J.; Céspedes, M.; Martinez, F.; Vanzulli, S.; Sáenz, D.; Di Venosa, G.; Montoya, S.N.; Casas, A. Photosensitization of a subcutaneous tumour by the natural anthraquinone parietin and blue light. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Vittar, N.B.R.; Awruch, J.; Azizuddin, K.; Rivarola, V. Caspase-independent apoptosis, in human MCF-7c3 breast cancer cells, following photodynamic therapy, with a novel water-soluble phthalocyanine. Int. J. Biochem. Cell Biol. 2010, 42, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- George, B.P.; Abrahamse, H.; Hemmaragala, N.M. Anticancer effects elicited by combination of Rubus extract with phthalocyanine photosensitiser on MCF-7 human breast cancer cells. Photodiagnosis Photodyn. Ther. 2017, 19, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Bark, K.-M.; Heo, E.P.; Han, K.D.; Kim, M.-B.; Lee, S.-T.; Gil, E.-M.; Kim, T.H. Evaluation of the phototoxic potential of plants used in oriental medicine. J. Ethnopharmacol. 2010, 127, 11–18. [Google Scholar] [CrossRef]
- Gonçalves, M.L.L.; da Mota, A.C.C.; Deana, A.M.; Cavalcante, L.A.D.S.; Horliana, A.C.R.T.; Pavani, C.; Motta, L.J.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; da Silva, D.F.T.; et al. Antimicrobial photodynamic therapy with Bixa orellana extract and blue LED in the reduction of halitosis—A randomized, controlled clinical trial. Photodiagnosis Photodyn. Ther. 2020, 30, 101751. [Google Scholar] [CrossRef] [PubMed]
- Kubrak, T.P.; Kołodziej, P.; Sawicki, J.; Mazur, A.; Koziorowska, K.; Aebisher, D. Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy. Molecules 2022, 27, 1192. [Google Scholar] [CrossRef]
- Makhadmeh, G.N.; Abuelsamen, A.; Al-Akhras, M.-A.H.; Aziz, A.A. Silica Nanoparticles Encapsulated Cichorium Pumilum as a Promising Photosensitizer for Osteosarcoma Photodynamic Therapy: In-vitro study. Photodiagnosis Photodyn. Ther. 2022, 38, 102801. [Google Scholar] [CrossRef]
| Photosensitizer | Generic Name | λ (nm) Max. | Chemical Structure | Drug Light Interval | Approved for | Ref. |
|---|---|---|---|---|---|---|
| 5-Aminolevulinic acid (ALA) | Luvalan | 635 | 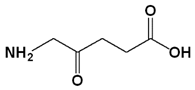 | 6 h | Actinic keratosis (USA 1999) | [32,33] |
| Hematoporphyrin derivatives (HPD) | Photofrin (Porfmer sodium) | 630 |  | 48 h | (i) Bladder cancer (Canada 1993) (ii) Early-stage lung cancer (Japan 1994) (iii) Esophageal cancer (FDA USA 1995), Early-stage non-small-cell lung cancer (FDA USA 1998) | [29,31] |
| Meta-tetra(hydroxyphenyl) chlorin (mTHPC) | Foscan (temoporfin) | 652 |  | 96 h | (i) Head and neck squamous cell carcinoma (Europe 2001) | [29,31] |
| Benzoporphyrin derivative monoacid ring A | Verteporfin or Visudyne | 690 | 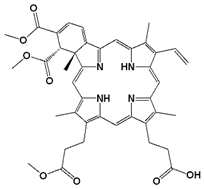 | 30 min | Choroidal neovascularization (age-related macular degeneration (AMD) (FDA 2000)) | [34] |
| Palladium (Pd)—substituted bacteriochlorophyll derivative | Tookad (WST09 (padoporfin) WST11 (padeliporfin or TOOKAD Soluble)) | 763 | 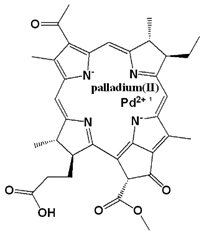 | Short interval mins | Clinical trial for prostate cancer | [29,35,36] |
| N-aspartyl chlorine e6 (NPe6) | Talaporfin sodium (Laserphyrin®) | 664 |  | 0.25–4 h | (i) Early-stage lung cancer (Japan 2003) | [12,29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, B.; Aziz, I.; Khurshid, A.; Raoufi, E.; Esfahani, F.N.; Jalilian, Z.; Mozafari, M.R.; Taghavi, E.; Ikram, M. An Overview of Potential Natural Photosensitizers in Cancer Photodynamic Therapy. Biomedicines 2023, 11, 224. https://doi.org/10.3390/biomedicines11010224
Aziz B, Aziz I, Khurshid A, Raoufi E, Esfahani FN, Jalilian Z, Mozafari MR, Taghavi E, Ikram M. An Overview of Potential Natural Photosensitizers in Cancer Photodynamic Therapy. Biomedicines. 2023; 11(1):224. https://doi.org/10.3390/biomedicines11010224
Chicago/Turabian StyleAziz, Bushra, Iffat Aziz, Ahmat Khurshid, Ehsan Raoufi, Fahime Nasr Esfahani, Zahra Jalilian, M. R. Mozafari, Elham Taghavi, and Masroor Ikram. 2023. "An Overview of Potential Natural Photosensitizers in Cancer Photodynamic Therapy" Biomedicines 11, no. 1: 224. https://doi.org/10.3390/biomedicines11010224
APA StyleAziz, B., Aziz, I., Khurshid, A., Raoufi, E., Esfahani, F. N., Jalilian, Z., Mozafari, M. R., Taghavi, E., & Ikram, M. (2023). An Overview of Potential Natural Photosensitizers in Cancer Photodynamic Therapy. Biomedicines, 11(1), 224. https://doi.org/10.3390/biomedicines11010224