Acute Spontaneous Lobar Cerebral Hemorrhages Present a Different Clinical Profile and a More Severe Early Prognosis than Deep Subcortical Intracerebral Hemorrhages—A Hospital-Based Stroke Registry Study
Abstract
1. Introduction
Hypothesis and Objectives
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
3.1. General Data
3.2. Differences between Lobar Cerebral Hemorrhages and Deep Subcortical Intracerebral Hemorrhages
3.3. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tasso, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart disease and stroke statistics-2022 Update: A report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Mencl, S.; Garz, C.; Niklass, S.; Braun, H.; Göb, E.; Homola, G.; Heinze, H.J.; Reymann, K.G.; Kleinschnitz, C.; Schreiber, S. Early Microvascular Dysfunction in Cerebral Small Vessel Disease Is Not Detectable on 3.0 Tesla Magnetic Resonance Imaging: A Longitudinal Study in Spontaneously Hypertensive Stroke-Prone Rats. Exp. Transl. Stroke Med. 2013, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Lawes, C.M.; Bennet, D.A.; Barker-Collo, S.L.; Parag, V. World-wide stroke incidence and early case fatality reported in 55 population-based studies: A systematic review. Lancet Neurol. 2009, 8, 355–369. [Google Scholar] [CrossRef]
- Wang, W.X.; Springer, J.E.; Hatton, K.W. MicroRNAs as Biomarkers for Predicting Complications Following Aneurysmal Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2021, 22, 9492. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Zong, N.; Hu, Y.; Chen, Y.; Xu, Y. Neuronal Death Mechanisms and Therapeutic Strategy in Ischemic Stroke. Neurosci. Bull. 2022, 38, 1229–1247. [Google Scholar] [CrossRef]
- Cullell, N.; Gallego-Fábrega, C.; Cárcel-Márquez, J.; Muiño, E.; Llucià-Carol, L.; Lledós, M.; Martín-Campos, J.M.; Molina, J.; Casas, L.; Almería, M.; et al. ICA1L is associated with small vessel disease: A proteome-wide association study in small vessel stroke and intracerebral haemorrhage. Int. J. Mol. Sci. 2022, 23, 3161. [Google Scholar] [CrossRef]
- Giralt-Steinhauer, E.; Jiménez-Baladó, J.; Fernández-Pérez, I.; Rey, L.A.; Rodríguez-Campello, A.; Ois, A.; Cuadrado-Godia, E.; Jiménez-Conde, J.; Roquer, J. Genetics and epigenetics of spontaneous intracerebral hemorrhage. Int. J. Mol. Sci. 2022, 23, 6479. [Google Scholar] [CrossRef]
- Mustanoja, S.; Putaala, J.; Koivunen, R.J.; Surakka, I.; Tatlisumak, T. Blood pressure levels in the acute phase after intracerebral hemorrhage are associated with mortality in young adults. Eur. J. Neurol. 2018, 8, 1034–1040. [Google Scholar] [CrossRef]
- Martínez-Domeño, A.; Martí-Fabregas, J. Hemorragia cerebral. Medicine 2015, 11, 4242–4251. [Google Scholar] [CrossRef]
- Wilkinson, D.A.; Pandey, A.S.; Thompson, B.G.; Keep, R.F.; Hua, Y.; Xi, G. Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology 2018, 134, 240–248. [Google Scholar] [CrossRef]
- Sorimachi, T.; Fujii, Y. Early neurological change in patients with spontaneous supratentorial intracerebral hemorrhage. J. Clin. Neurosci. 2010, 17, 1367–1371. [Google Scholar] [CrossRef]
- Kim, K.Y.; Shin, K.Y.; Chang, K.A. Potential Biomarkers for Post-Stroke Cognitive Impairment: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 602. [Google Scholar] [CrossRef]
- Pérez-Núñez, A.; Lagares, A.; Pascual, B.; Rivas, J.J.; Alday, R.; González, P.; Cabrera, A.; Lobato, R.D. Tratamiento quirúrgico de la hemorragia intracerebral espontánea. Parte I: Hemorragia supratentorial. Neurocirugía 2008, 19, 12–24. [Google Scholar] [CrossRef]
- Falcone, G.J.; Biffi, A.; Brouwers, B.; Anderson, C.D.; Battey, T.W.; Ayres, A.M.; Vashkevich, A.; Schwab, K.; Rost, N.S.; Goldstein, J.N.; et al. Predictors of hematoma volume in deep and supratentorial intracerebral hemorrhage. JAMA Neurol. 2014, 70, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.C.; Roebken, A.; Singh, V. Primary intraventricular hemorrhage: Yeld of diagnostic angiography and clinical outcome. Neurocrit. Care 2008, 8, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Thabet, A.M.; Kottapally, M.; Hemphill, J.C. Management of intracerebral hemorrhage. Handb. Clin. Neurol. 2017, 140, 177–194. [Google Scholar] [PubMed]
- Qureshi, A.I.; Mendelow, A.D.; Hanley, D.F. Intracerebral hemorrhage. Lancet 2009, 373, 1632–1644. [Google Scholar] [CrossRef] [PubMed]
- Morotti, A.; Goldstein, J.N. Diagnosis and management of acute intracerebral hemorrhage. Emerg. Med. Clin. N. Am. 2016, 34, 883–899. [Google Scholar] [CrossRef]
- Morotti, A.; Nawabi, J.; Schlunk, F.; Poli, L.; Costa, P.; Mazzacane, F.; Busto, G.; Scola, E.; Arba, F.; Brancaleoni, L.; et al. Characteristics of early presenters after intracerebral hemorrhage. J. Stroke 2022, 24, 423–428. [Google Scholar] [CrossRef]
- Raposo, N.; Zotin, M.C.Z.; Seiffge, D.J.; Li, Q.; Goeldlin, M.B.; Charidimou, A.; Shoamanesh, A.; Jäger, H.R.; Greenberg, S.M.; Werring, D.J.; et al. A causal classification system for intracerebral hemorrhages subtypes (CLASS-ICH). Ann. Neurol. 2023, 93, 16–18. [Google Scholar] [CrossRef]
- Hafeez, S.; Behrouz, R. The safety and feasibility of admitting patients with intracerebral hemorrhage to the step-down unit. J. Intensive Care Med. 2016, 31, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Bai, Q.; Liu, Y.; Zhang, Y.; Sheng, Z.; Xue, M.; Yong, W. Intracerebral hemorrhage in translational research. Brain Hemorrhages 2020, 1, 13–18. [Google Scholar] [CrossRef]
- Syed, B.; Nirwane, A.; Yao, Y. In vitro models of intracerebral hemorrhage. Brain Hemorrhages 2022, 3, 105–107. [Google Scholar] [CrossRef]
- Lv, Y.; Wei, W. Clinical treatment progress of small amounts thalamus hemorrhage. Brain Hemorrhages 2021, 2, 84–87. [Google Scholar] [CrossRef]
- Marti-Fabregas, J.; Prats-Sanchez, L.; Martinez-Domeno, A.; Camps-Renom, P.; Marín, R.; Jiménez-Xarrié, E.; Fuentes, B.; Dorado, L.; Purroy, F.; Arias-Rivas, S. The H-ATOMIC Criteria for the Etiologic Classification of Patients with intracerebral Hemorrhage. PLoS ONE 2016, 11, e0156992. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Tuhrim, S.; Broderick, J.P.; Batjer, H.H.; Hondo, H.; Hanley, D.F. Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 2001, 344, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, C.; Demchuk, A.; Ziai, W.; Anderson, C.S. Intracerebral hemorrhage; current approaches to acute management. Lancet 2018, 392, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Roquer, J.; Rodríguez-Campello, A.; Jiménez-Conde, J.; Cuadrado-Godia, E.; Giralt-Steinhauer, E.; Vivanco Hidalgo, R.M.; Soriano, C.; Ois, A. Sex-related differences in primary intracerebral hemorrhage. Neurology 2016, 87, 257–262. [Google Scholar] [CrossRef]
- Morotti, A.; Goldstein, J.N. Anticoagulant-associated intracerebral hemorrhage. Brain Hemorrhages 2020, 1, 89–94. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Ziai, W.Z.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C., III; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients with Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar] [CrossRef]
- Cho, S.; Rehni, A.K.; Dave, K.R. Tobacco Use: A Major Risk Factor of Intracerebral Hemorrhage. J. Stroke 2021, 23, 37–50. [Google Scholar] [CrossRef]
- Sheth, K.N. Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 2022, 387, 1589–1596. [Google Scholar] [CrossRef]
- Meissner, A. Hypertension and the brain: A risk factor for more than heart disease. Cerebrovasc. Dis. 2016, 42, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Gąsecki, D.; Kwarciany, M.; Nyka, W.; Narkiewicz, K. Hypertension, brain damage and cognitive decline. Curr. Hypertens. Rep. 2013, 15, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Zonneveld, T.P.; Richard, E.; Vergouwen, M.D.I.; Nederkoorn, P.J.; de Haan, R.; Roos, Y.B.; Kruyt, N.D. Blood Pressure-Lowering Treatment for Preventing Recurrent Stroke, Major Vascular Events, and Dementia in Patients with a History of Stroke or Transient Ischaemic Attack. Cochrane. Database Syst. Rev. 2018, 7, CD007858. [Google Scholar] [CrossRef] [PubMed]
- Woo, D.; Comeau, M.E.; Venema, S.U.; Anderson, C.D.; Flaherty, M.; Testal, F.; Kittner, S.; Frankel, M.; James, M.J.; Sung, G.; et al. Risk Factors Associated With Mortality and Neurologic Disability After Intracerebral Hemorrhage in a Racially and Ethnically Diverse Cohort. JAMA Netw. Open 2022, 5, e221103. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Khan, S.; Liu, Y.; Wei, R.; Yong, V.W.; Xue, M. Therapeutic strategies for intracerebral hemorrhage. Front. Neurol. 2022, 13, 10323343. [Google Scholar] [CrossRef] [PubMed]
- Palomeras Soler, E.; Fossas Felip, P.; Casado Ruiz, V.; Cano Orgaz, A.; Sanz Cartagena, P.; Muriana Batiste, D. The Mataró Stroke Registry: A 10-year registry in a community hospital. Neurologia 2015, 30, 283–289. [Google Scholar] [CrossRef]
- Arboix, A.; Massons, J.; Oliveres, M.; García, L.; Titus, F. An analysis of 1000 consecutive patients with acute cerebrovascular disease. The registry of cerebrovascular disease of La Alianza-Hospital Central of Barcelona. Med. Clin. 1993, 101, 281–285. [Google Scholar]
- Law, Z.K.; Desborough, M.; Rakkar, K.; Bath, P.M.; Bayraktutan, U.; Sprigg, N. Elevated plasminogen activators are associated with hematoma progression in spontaneous intracerebral hemorrhage. Brain Hemorrhages 2020, 1, 75–79. [Google Scholar] [CrossRef]
- Alkhacroum, A.M.; Bentho, O.; Chari, N.; Kulhari, A.; Xiong, W. Neurosciences step-dwon unit admission criteria for patients with intracerebral hemorrhage. Clin. Neurol. Neurosurg. 2017, 162, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A.; Martínez-Rebollar, M.; Oliveres, M.; Massons, J.; Garcia-Eroles, L.; Targa, C. Acute isolated capsular stroke: A clinical study of 148 cases. Clin. Neurol. Neurosurg. 2005, 107, 88–94. [Google Scholar] [PubMed]
- Arboix, A.; Alvarez-Sabin, J.; Soler, L. Stroke. [Classification and diagnostic criteria. Ad hoc Editorial Committee of the Task Force on Cerebrovascular Diseases of SEN]. Neurologia 1998, 13 (Suppl 3), S3–S10. [Google Scholar]
- Special Report from the National Institute of Neurological Disorders and Stroke: Classification of cerebrovascular diseases III. Stroke 1990, 21, 637–676. [CrossRef] [PubMed]
- Societat Catalana de Neurologia. Guies mèdiques oficials de diagnòstic i tractament. In Malalties Vasculars Cerebrals, 2nd ed.; Societat Catalana de Neurologia: Barcelona, Spain, 2011; pp. 159–240. [Google Scholar]
- Arboix, A.; García-Plata, C.; García-Eroles, L.; Massons, J.; Comes, E. Clinical study of 99 patients with pure sensory stroke. J. Neurol. 2005, 252, 156–162. [Google Scholar] [CrossRef]
- Rudilosso, S.; Rodríguez-Vázquez, A.; Urra, X.; Arboix, A. The Potential Impact of Neuroimaging and Translational Research on the Clinical Management of Lacunar Stroke. Int. J. Mol. Sci. 2022, 23, 1497. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.L.; Norris, J.W.; Lewis, A.J.; Hachinski, V.C. Early mortality following stroke: A prospective review. Stroke 1984, 15, 492–496. [Google Scholar] [CrossRef]
- Kumar, S.; Selim, M.; Caplan, L. Medical complications after stroke. Lancet Neurol. 2010, 9, 105–118. [Google Scholar] [CrossRef]
- Bamford, J.M.; Sandercock, P.A.G.; Warlow, C.P.; Slattery, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1989, 20, 828. [Google Scholar] [CrossRef]
- Armitage, P. Statistical Methods in Medical Research; Wiley: New York, NY, USA, 1977. [Google Scholar]
- Dixon, W.J. BMDP Statistical Software Manual; University of California Press: Berkeley, CA, USA, 1990; pp. 300–344. [Google Scholar]
- d’Annunzio, A.; Arboix, A.; García-Eroles, L.; Sánchez-López, M.J. Vertigo in acute stroke is a predictor of brain location but is not related to early outcome: The experience of Sagrat Cor Hospital of Barcelona Stroke Registry. Biomedicines 2022, 10, 2830. [Google Scholar] [CrossRef]
- Orzuza, G.; Zurrú, M.G. Epidemiological aspects of stroke in very old patients. Cardiovasc. Hematol. Disord. Drug Targets 2011, 11, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Luy, M.; Gast, K. Do women live longer or do men die earlier? Reflections on the causes of sex differences in life expectancy. Gerontology 2014, 60, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, L.C.; Beeken, R.J.; Meisel, S.F. Biopsychosocial predictors of perceived life expectancy in a national sample of older men and women. PLoS ONE 2017, 12, e0189245. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.Y.; Dell, C.A.; Sparks, M.J.; Watson, T.D.; Langefeld, C.D.; Comeau, M.E.; Rosand, J.; Battey, T.W.; Koch, S.; Perez, M.L.; et al. Clinician judgment vs formal scales for predicting intracerebral hemorrhage outcomes. Neurology 2016, 86, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Hoya, K.; Tanaka, Y.; Uchida, T.; Takano, I.; Nagaishi, M.; Kowata, K.; Hyodo, A. Intracerebral hemorrhage in patients with chronic liver disease. Neurol. Med. Chir. 2012, 52, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.S.; Kamel, H.; Navi, B.B.; Iadecola, C.; Merkler, A.E.; Jesudian, A.; Dawson, J.; Falcone, G.J.; Sheth, K.N.; Roh, D.J.; et al. Liver Fibrosis Indices and Outcomes After Primary Intracerebral Hemorrhage. Stroke 2020, 51, 830–837. [Google Scholar] [CrossRef]
- Faught, E.; Peters, D.; Bartolucci, A.; Moore, L.; Miller, P.C. Seizures after primary intracerebral hemorrhage. Neurology 1989, 39, 1089. [Google Scholar] [CrossRef]
- Jamieson, D.G.; Cheng, N.T.; Skliut, M. Headache and Acute Stroke. Curr. Pain Headache Rep. 2014, 18, 444. [Google Scholar] [CrossRef]
- Arboix, A.; Garcia-Eroles, L.; Massons, J.; Oliveres, M.; Targa, C. Hemorrhagic lacunar stroke. Cerebrovasc. Dis. 2000, 10, 229–234. [Google Scholar] [CrossRef]
- Passero, S.; Rocchi, R.; Rossi, S.; Ulivelli, M.; Vatti, G. Seizures after Spontaneous Supratentorial Intracerebral Hemorrhage. Epilepsia 2002, 43, 1175–1180. [Google Scholar] [CrossRef]
- Derex, L.; Rheims, S.; Peter-Derex, L. Seizures and epilepsy after intracerebral hemorrhage: An update. J. Neurol. 2021, 268, 2605–2615. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.Y.; Obeidat, A.Z.; Sekar, P.; Moomaw, C.J.; Osborne, J.; Testai, F.D.; Koch, S.; Lowe, M.R.; Demel, S.; Coleman, E.R.; et al. Risk factors for seizures after intracerebral hemorrhage: Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) Study. Clin. Neurol. Neurosurg. 2020, 192, 105731. [Google Scholar] [CrossRef]
- Voll, C.L.; Auer, R.N. Postischemic seizures and necrotizing ischemic brain damage: Neuroprotective effect of postischemic diazepan and insulin. Neurology 1991, 41, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.K.M.; Williams, C.E.; Gunn, A.J.; Mallard, C.E.; Gluckman, P.D. Supression of postischemic epileptiform activity with MK-801 improves neural outcome in fetal sleep. Ann. Neurol. 1992, 32, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.W.; Rutecki, P.A.; Sutula, T.P. The effects of seizures on the brain. Curr. Opin. Neurol. 1996, 9, 97–102. [Google Scholar] [CrossRef]
- Gilmore, E.J.; Maciel, C.B.; Hirsch, L.J.; Sheth, K.N. Review of the Utility of Prophylactic Anticonvulsant Use in Critically Ill Patients with Intracerebral Hemorrhage. Stroke 2016, 47, 2666–2672. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Moran, A.E.; Whelton, P.K. Treatment of Hypertension: A Review. JAMA 2022, 328, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Sturiale, C.L.; Puca, A.; Calandrelli, R.; D’Arrigo, S.; Albanese, A.; Marchese, E.; Alexandre, A.; Colosimo, C.; Maira, G. Relevance of bleeding pattern on clinical appearance and outcome in patients with hemorrhagic brain arteriovenous malformations. J. Neurol. Sci. 2013, 324, 118–123. [Google Scholar] [CrossRef]
- Kalaria, R.N.; Akinyemi, R.; Ihara, M. Stroke injury, cognitive impairment and vascular dementia. Biochim. Biophys. Acta 2016, 1862, 915–925. [Google Scholar] [CrossRef]
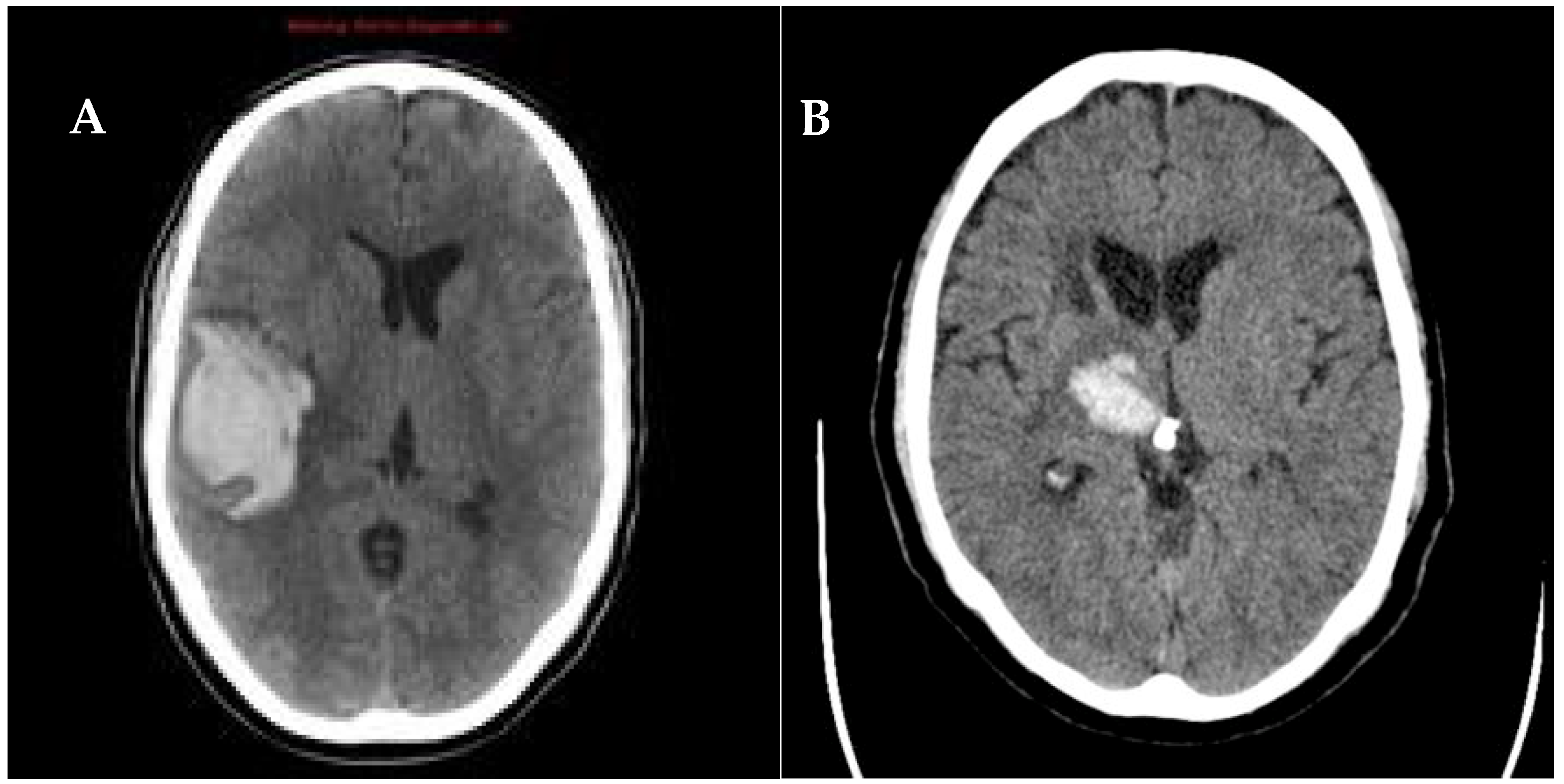



| Arterial Hypertension (Deep Perforating Vasculopathy) | Cerebral Amyloid Angiopathy |
|---|---|
Acute arterial hypertension
| Brain vascular malformations
|
Intracranial venous thrombosis (CVT)
| Hemorrhagic transformation of cerebral infarction
|
Cerebral tumors
| Hemostatic and hematologic disorders
|
Vasculitis and related vasculopathies
| Changes in cerebral blood flow
|
Toxic
| Other
|
| Variables | Lobar Intracerebral Hemorrhages (n = 165) | Deep Subcortical Intracerebral Hemorrhages (n = 133) | p |
|---|---|---|---|
| Female sex | 89 (53.9) | 51 (38.3) | 0.007 |
| Age (years), mean (SD) | 72.48 (13.01) | 72.23 (11.91) | 0.867 |
| Age ≥ 85 years | 27 (16.4) | 19 (14.3) | 0.622 |
| Variables | Lobar Intracerebral Hemorrhages (n = 165) | Subcortical Intracerebral Hemorrhages (n = 133) | p |
|---|---|---|---|
| Cerebrovascular risk factors (%) | |||
| Hypertension | 80 (48.5) | 94 (70.7) | 0.000 |
| Diabetes mellitus | 22 (13.3) | 19 (14.3) | 0.812 |
| Valvular heart disease | 7 (4.2) | 3 (2.3) | 0.344 |
| Coronary heart disease | 14 (8.5) | 9 (6.8) | 0.581 |
| Atrial fibrillation | 25 (15.2) | 16 (12.0) | 0.437 |
| Congestive heart failure | 3 (1.8) | 4 (3.0) | 0.500 |
| History of transient ischemic attack (TIA) | 11 (6.7) | 7 (5.3) | 0.613 |
| Previous cerebral infarction | 13 (7.9) | 14 (10.5) | 0.429 |
| Previous intracerebral hemorrhage | 19 (11.5) | 5 (3.8) | 0.014 |
| Headache | 8 (4.8) | 2 (1.5) | 0.111 |
| Chronic obstructive pulmonary disease | 13 (7.9) | 13 (9.8) | 0.564 |
| Chronic renal disease | 1 (0.6) | 3 (2.3) | 0.219 |
| Peripheral vascular disease | 8 (4.8) | 9 (6.8) | 0.478 |
| Obesity | 3 (1.8) | 10 (7.5) | 0.017 |
| Alcohol abuse (>80 gr/day) | 3 (1.8) | 12 (9.0) | 0.005 |
| Anticoagulants | 11 (6.7) | 5 (3.8) | 0.268 |
| Chronic liver disease | 16 (9.7) | 5 (3.8) | 0.046 |
| Heavy smoking (>20 cigarettes/day) | 16 (9.7) | 11 (8.3) | 0.670 |
| Hyperlipidemia | 19 (11.5) | 18 (13.5) | 0.599 |
| Clinical features (%) | |||
| Sudden onset | 87 (52.7) | 85 (63.9) | 0.052 |
| Acute onset (hours) | 44 (26.7) | 27 (20.3) | 0.200 |
| Subacute onset (days) | 8 (4.8) | 6 (4.5) | 0.891 |
| Headache | 67 (40.6) | 33 (24.8) | 0.004 |
| Dizziness/vertigo | 7 (4.2) | 2 (1.5) | 0.170 |
| Early seizures | 15 (9.1) | 2 (1.5) | 0.005 |
| Nausea/vomiting | 34 (20.6) | 23 (17.3) | 0.470 |
| Altered consciousness | 71 (43.0) | 39 (29.3) | 0.015 |
| Limb weakness | 107 (64.8) | 112 (84.2) | 0 |
| Sensory deficit | 55 (33.3) | 74 (55.6) | 0 |
| Hemianopia | 52 (31.5) | 20 (15.0) | 0.001 |
| Speech disturbances | 55 (33.3) | 49 (36.8) | 0.528 |
| Ataxia | 9 (5.5) | 5 (3.8) | 0.492 |
| Cranial nerve palsy | 1 (0.6) | 5 (3.8) | 0.054 |
| Extrapyramidal disorders | 3 (1.8) | 2 (1.5) | 0.834 |
| In-hospital outcomes | |||
| Neurological complications | 43 (26.1) | 16 (12.0) | 0.003 |
| Respiratory complications | 18 (10.9) | 17 (12.8) | 0.618 |
| Digestive complications | 5 (3.0) | 3 (2.3) | 0.681 |
| Renal complications | 1 (0.6) | 6 (4.5) | 0.027 |
| Urinary complications | 17 (10.3) | 15 (11.3) | 0.787 |
| Cardiac events | 5 (3.0) | 6 (4.5) | 0.500 |
| Vascular complications | 2 (1.2) | 1 (0.8) | 0.692 |
| Hemorrhagic events | 2 (1.2) | 0 (0.0) | 0.203 |
| Infectious complications | 25 (15.2) | 34 (25.6) | 0.025 |
| Symptom free at discharge | 10 (6.1) | 11 (8.3) | 0.459 |
| In-hospital death | 44 (26.7) | 22 (16.5) | 0.036 |
| Length of stay, days, median (interquartile range) | 15.00 (8–26) | 18 (8–27) | 0.938 |
| Prolonged hospital stay > 12 days | 96 (58.2) | 86(58.2) | 0.254 |
| Variables | β | SE (β) | OR (CI 95%) | p |
|---|---|---|---|---|
| Demographic data and cerebrovascular risk factors 1 | ||||
| Chronic liver disease | 1.655 | 0.699 | 5.233 (1.328–20.610) | 0.018 |
| Previous intracerebral hemorrhage | 1.117 | 0.538 | 3.056 (1.064–8.775) | 0.038 |
| Female sex | 0.563 | 0.253 | 1.755 (1.070–2.879) | 0.026 |
| Hypertension | −0.916 | 0.259 | 0.400 (0.241–0.664) | 0.000 |
| Alcohol abuse (>80 gr/day) | −2.237 | 0.801 | 0.107 (0.022–0.513) | 0.005 |
| Demographic data, cerebrovascular risk factors, and clinical features 2 | ||||
| Early seizures | 1.776 | 0.858 | 5.906 (1.099–31.749) | 0.038 |
| Chronic liver disease | 1.584 | 0.763 | 4.875 (1.093–21.745) | 0.038 |
| Previous intracerebral hemorrhage | 1.176 | 0.595 | 3.240 (1.009–10.399) | 0.048 |
| Hemianopia | 0.868 | 0.346 | 2.383 (1.210–4.693) | 0.012 |
| Headache | 0.654 | 0.301 | 1.922 (1.066–3.467) | 0.030 |
| Altered consciousness | 0.608 | 0.284 | 1.837 (1.053–3.206) | 0.032 |
| Limb weakness | −0.747 | 0.346 | 0.474 (0.241–0.933) | 0.031 |
| Sensory deficit | −0.814 | 0.285 | 0.443 (0.253–0.775) | 0.004 |
| Hypertension | −0.902 | 0.283 | 0.385 (0.221–0.670) | 0.001 |
| Alcohol abuse (>80 gr/day) | −2.171 | 0.849 | 0.114 (0.022–0.603) | 0.011 |
| Demographic data, cerebrovascular risk factors, clinical features, and outcomes 3 | ||||
| Early seizures | 1.918 | 0.855 | 6.806 (1.273–36.397) | 0.025 |
| Chronic liver disease | 1.515 | 0.759 | 4.551 (1.027–20.154) | 0.046 |
| Hemianopia | 0.937 | 0.358 | 2.551 (1.264–5.149) | 0.009 |
| Headache | 0.644 | 0.298 | 1.904 (1.062–3.414) | 0.031 |
| Limb weakness | −0.744 | 0.342 | 0.475 (0.243–0.928) | 0.029 |
| Sensory deficit | −0.834 | 0.286 | 0.434 (0.248–0.761) | 0.004 |
| Hypertension | −0.902 | 0.281 | 0.406 (0.234–0.704) | 0.001 |
| Alcohol abuse (>80 gr/day) | −2.318 | 0.857 | 0.098 (0.018–0.528) | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendiola, J.M.F.-P.d.; Arboix, A.; García-Eroles, L.; Sánchez-López, M.J. Acute Spontaneous Lobar Cerebral Hemorrhages Present a Different Clinical Profile and a More Severe Early Prognosis than Deep Subcortical Intracerebral Hemorrhages—A Hospital-Based Stroke Registry Study. Biomedicines 2023, 11, 223. https://doi.org/10.3390/biomedicines11010223
Mendiola JMF-Pd, Arboix A, García-Eroles L, Sánchez-López MJ. Acute Spontaneous Lobar Cerebral Hemorrhages Present a Different Clinical Profile and a More Severe Early Prognosis than Deep Subcortical Intracerebral Hemorrhages—A Hospital-Based Stroke Registry Study. Biomedicines. 2023; 11(1):223. https://doi.org/10.3390/biomedicines11010223
Chicago/Turabian StyleMendiola, Joana Maria Flaquer-Pérez de, Adrià Arboix, Luís García-Eroles, and Maria José Sánchez-López. 2023. "Acute Spontaneous Lobar Cerebral Hemorrhages Present a Different Clinical Profile and a More Severe Early Prognosis than Deep Subcortical Intracerebral Hemorrhages—A Hospital-Based Stroke Registry Study" Biomedicines 11, no. 1: 223. https://doi.org/10.3390/biomedicines11010223
APA StyleMendiola, J. M. F.-P. d., Arboix, A., García-Eroles, L., & Sánchez-López, M. J. (2023). Acute Spontaneous Lobar Cerebral Hemorrhages Present a Different Clinical Profile and a More Severe Early Prognosis than Deep Subcortical Intracerebral Hemorrhages—A Hospital-Based Stroke Registry Study. Biomedicines, 11(1), 223. https://doi.org/10.3390/biomedicines11010223







