Exploring the Use of Cold Atmospheric Plasma to Overcome Drug Resistance in Cancer
Abstract
1. Introduction
1.1. An Overview of Drug Resistance in Cancer
- Changes in the drug target: cancer cells can acquire mutations at the target site of the drug that make them less sensitive to the drug.
- Activation of protective signaling pathways: upregulating pathways that promote cell survival or inhibiting those that promote cell death.
- Expression of drug efflux pumps in cancer cells: proteins that actively transport drugs out of the cell and prevent them from reaching their targets.
- The presence of cells with stem cell-like properties: cancer stem cells (CSC) are a subset of cells that are less sensitive to the current drugs, most likely because they usually present lower levels of drug targets or better mechanisms of DNA damage repair.
- The tumor microenvironment: which includes the surrounding stroma and immune system and can provide a protective environment for cancer cells making them less sensitive to xenobiotic compounds. The stroma may produce growth factors that support the survival of cancer cells.
1.2. Drug Resistance: Challenges and Limitations of Current Strategies
- Combination therapy: the use of multiple drugs to simultaneously attack different pathways or targets in cancer cells, which may help prevent the development of resistance to a single drug.
- Targeting drug resistance mechanisms: using specific drugs or agents targeted to resistance mechanisms such as drug efflux pumps or signaling pathways.
- Repurposing existing drugs: identifying new uses for existing drugs that were originally developed for other indications, such as repurposing antibiotics to treat cancer.
- Novel therapeutic strategies: developing new therapeutic approaches, such as immunotherapy or gene therapy, to attack tumor cells in ways that may be less susceptible to resistance.
- Personalized medicine: deciphering genetic or molecular profiling of cancer cells to identify individualized treatment strategies that are tailored to the specific characteristics of a patient’s cancer and are less likely to be affected by resistance.
- Novel drug delivery systems: exploring advanced drug delivery systems, such as nanoparticles or targeted carriers, to improve the delivery of anti-tumor compounds and reduce the potential for resistance.
- Novel drug combinations: testing new combinations of existing drugs or novel agents that may show synergistic effects, thus solving drug resistance to a single agent.
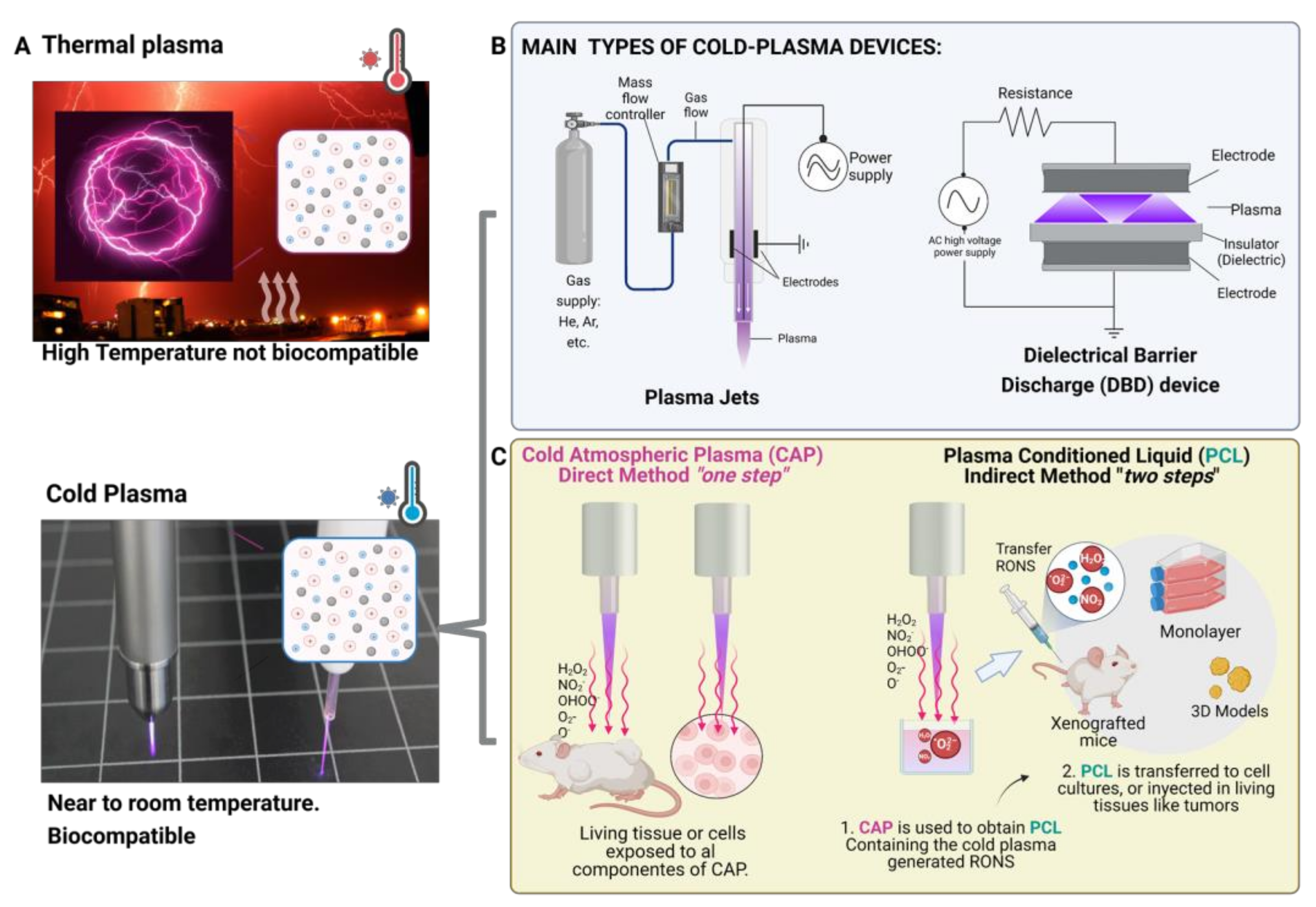
2. Plasma, the Fourth State of Matter
2.1. Cold-Plasma for Medicine
2.1.1. Cold-Plasma Devices
- Sample location: In a DBD device, the sample acts as an electrode and is typically located within the plasma discharge region, whereas in a plasma jet, the sample is typically located outside the plasma discharge region, downstream from the nozzle or orifices.
- Plasma production: DBD devices use high-voltage electric fields to ionize gas and produce plasma within an enclosed chamber. Plasma jets use a similar principle, but the plasma is generated within a nozzle or a series of orifices and is expelled as a jet or beam.
- Plasma characteristics: The plasma produced by a DBD device is typically more homogeneous and uniform than the plasma produced by a plasma jet, which tends to be more directional and focused.
- Limitations: DBD devices are generally limited to the production of plasma at low pressures (atmospheric or below), whereas plasma jets can be used to produce plasma at higher pressures. DBD devices are also limited in the types of gases that can be used, as some gases may not be stable under the high-voltage conditions required for plasma production. Plasma jets may be less efficient at producing plasma than DBD devices, as a significant portion of the plasma is lost through the nozzle or orifices.
- Advantages: Plasma jets offer several advantages for in vitro and in vivo research, including their small size, ease of handling, and ability to treat small volumes of liquid. Based on these properties and features, plasma jet devices may be more suitable for gentle treatment of a small area of a sample, whereas DBD devices are relatively simple and compact and can be used to produce a wide range of plasma species. Plasma jets are highly directional and can be used to produce a focused plasma beam that can be used for other many applications.
2.1.2. Plasma Medicine
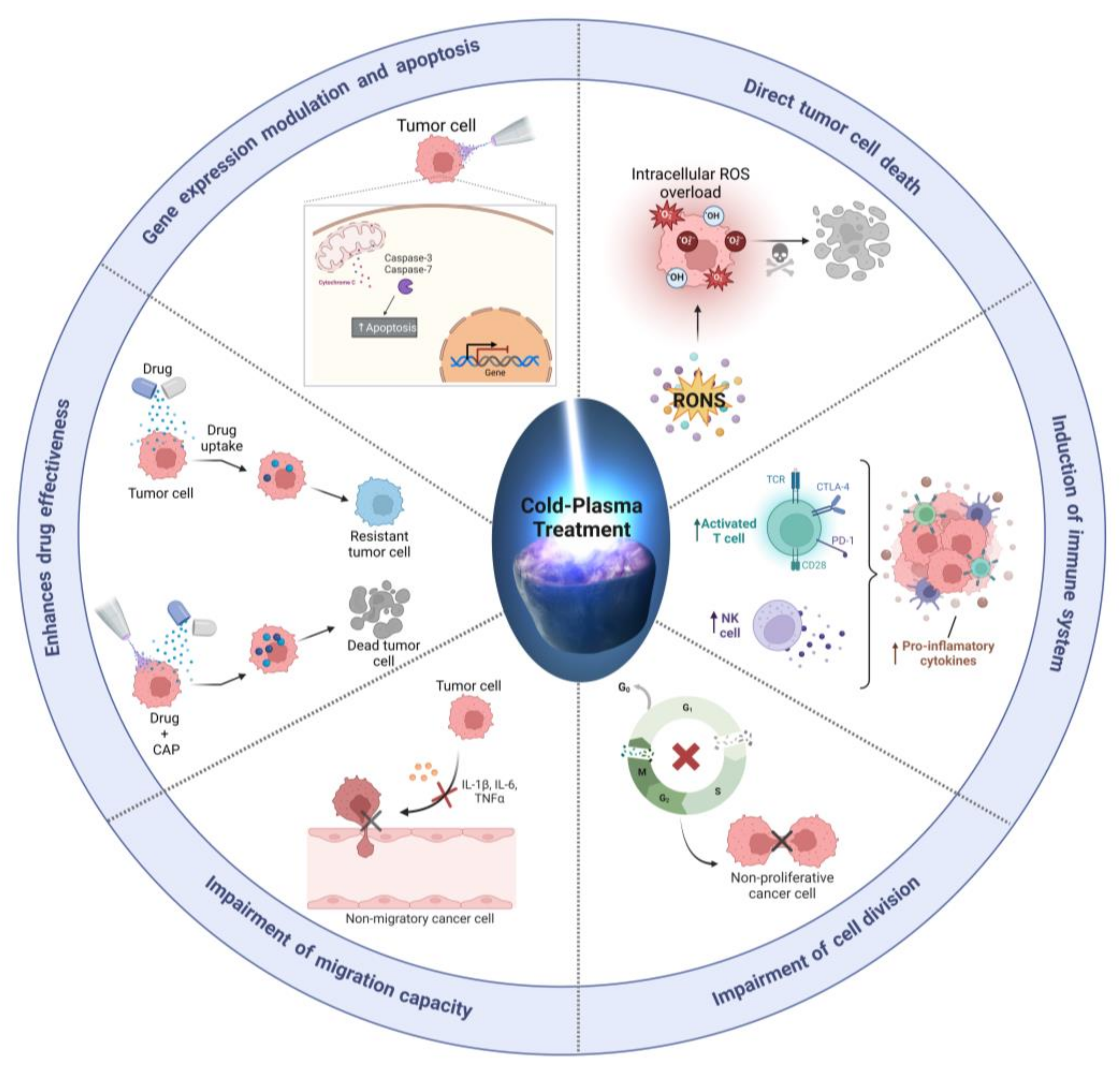
2.2. Cold Plasma-Induced Oxidative Stress as a Cancer Therapy
2.3. Plasma Oncology: Direct and Indirect Approaches
- Ringers: a solution that is regularly used to maintain the physiological conditions of cells in culture. It contains ions and macromolecules that are similar to those found in the human body, which may be advantageous for in vitro applications in osteosarcoma [40,52] and for in vivo applications in prostate cancer [53].
- Phosphate-buffered saline (PBS): a liquid frequently used in biological and medical research. PBS is a buffer solution designed to maintain a constant pH and, as it contains ions and macromolecules that are similar to those found in the human body, it can support the survival and proliferation of cells while they are exposed to plasma-generated reactive oxygen and nitrogen species (RONS). Cytotoxicity of PCL using PBS as a liquid has been reported in glioblastoma [54] and breast cancer in vivo [55].
- Cell culture medium: To date, cell culture medium has been the most commonly employed liquid for studying the effects of plasma-generated RONS on cancer cells and in vivo tumors [11,44]. Noting that most of these studies have been conducted in monolayer cell cultures, with only a few studies conducted in vivo [44], it has been observed that the amount of RONS generated is greatly influenced by the biochemical composition of the cell culture medium sample, and slight differences in the composition, such as the inclusion of sodium pyruvate, can significantly alter the amount of RONS and the resulting cytotoxic effects [38]. While cell culture medium contains a variety of nutrients, hormones, and growth factors that can support the survival and proliferation of cancer cells, it is important to consider the effect of the biochemical composition of the medium on the generation of RONS [16] and their cytotoxic effects in order to accurately interpret the results of these studies.
2.4. Cold Plasma for Cancer Treatment
- Non-toxic: direct CAP is a non-toxic treatment that does not generate harmful by-products or cause systemic side effects, unlike chemotherapeutic agents and other anticancer drugs, which can cause a wide range of side effects, including nausea, vomiting, hair loss, and immune suppression.
- Selectivity: CAP can selectively kill cancer cells while sparing normal cells. CAP generates reactive oxygen and nitrogen species (RONS) that preferentially damage DNA, proteins, and other biomolecules in cancer cells, leading to cell death. In contrast, many anti-tumor drugs are non-selective, killing both tumor and non-tumor cells, thus leading to the toxic side effects observed in patients.
- Versatile: Cold plasma can be directly applied to the tumor site, making it a potentially effective local treatment for solid tumors. Additionally, CAP can be delivered through numerous routes, including topical and intravenous administration, depending on the specific clinical setting. This versatility makes CAP a promising option for treating a wide range of cancer types and stages.
- Combinatorial: CAP can be used in combination with other anticancer drugs to enhance their efficacy and overcome drug resistance. Most likely because CAP can reverse the mechanisms that allow cancer cells to become resistant to drugs, thereby making them more sensitive to medication. In addition, CAP can stimulate the immune system to recognize and attack cancer cells, increasing the effectiveness of immunotherapies.
3. Cold Plasma and Chemotherapy for Enhanced Cancer Treatment
3.1. Temozolomide
3.2. Doxorubicin
3.3. Epirubicin
3.4. Cisplatin
3.5. Paclitaxel
3.6. Tamoxifen
3.7. 5-Fluorouracil
4. Challenges in Proposing CAP Therapy for Cancer
4.1. A Closer Look at CAP Selectivity in Targeting Cancer Cells
4.2. CSC: A Pending Subject of Study
- Antioxidant enzymes: high levels of antioxidant enzymes observed in CSCs protect them from ROS-induced DNA damage. These enzymes catalyze the conversion of ROS into less harmful molecules such as hydrogen peroxide and water, which reduces their cytotoxic effects. Enhanced expression of scavenging genes, including superoxide dismutase, glutathione peroxidase, and catalase, helps maintain intracellular ROS at nearly identical levels to those of normal stem cells. Besides, the activation of oncogenic transcription factors, such as c-Myc, can increase ROS levels and activate NRF2, a transcription factor that upregulates genes involved in detoxification and antioxidant activity. NRF2 activates the expression of efflux transporters and anti-apoptotic proteins, and contributes to iron homeostasis, making it a potential target for CSCs therapy [156].
- Upregulation of antioxidant genes: CSCs can upregulate the expression of genes that encode antioxidant enzymes, such as GPXs, in response to ROS. In addition, CSCs are characterized by the elevated expression of the cellular markers CD44 and ALDH, which are associated with enhanced GSH synthesis and stronger protection against ROS [157]. The ALDH enzyme, highly expressed in CSCs [158], has a detoxifying function by reducing ROS and generating antioxidant compounds, therefore protecting against alkylating agents and increasing the activation of DNA repair mechanisms.
- Activation of survival pathways: ROS stimulate the expression of anti-apoptotic proteins, such as Bcl-2, which inhibits the initiation of programmed cell death, and promote the activation of survival pathways such as PI3K/AKT/mTOR or STAT3 [57]. For instance, it has been reported that activation of DNA damage responses increases the number of CSCs by approximately 2–4 fold [8]. In glioma, activation of DNA damage checkpoints was found to be more effective in CD133+ cells after radiation exposure than in their CD133- counterparts [15]. Enhanced DNA repair mechanisms present in glioblastoma stem cells, compared to progenitor cells [128], make these cells highly sensitive to the inhibition of PARP and ATR [129]. Furthermore, overexpression of polymerase η confers resistance to cisplatin in ovarian cancer cells, whereas activation of mir-93, which regulates polymerase η expression, increases the sensitivity of CSCs to cisplatin [130]. Nevertheless, it should be noted that the response to DNA damage can be a double-edged sword and have opposite effects. Whereas in non-tumor stem cells, this process promotes optimal functioning of healthy tissues, in cancer stem cells it leads to survival and resistance. CSCs can tolerate high levels of replication stress through this mechanism. In fact, CSCs can resist chemotherapeutics specifically designed to damage DNA [129].
5. Conclusions and Future Perspectives
- Plasma mechanism of action: It is important to understand how CAP exerts its effects on cancer cells and its underlying mechanisms of action. This involves studying the role of reactive oxygen and nitrogen species (RONS) and other plasma-generated species as well as the impact of CAP on signaling pathways, gene expression, and cell cycle regulation.
- Effect of CAP on cancer stem cells: Cancer stem cells (CSCs) are a subset of cancer cells resistant to chemotherapy and radiotherapy, and they are thought to play a key role in the development and progression of cancer. It is important to study the effect of CAP on CSCs to determine whether this technology can be proposed as a potential therapeutic agent against drug resistance.
- Existence of the tumor microenvironment: It is also significant to study the impact of CAP on the tumor microenvironment, which includes the surrounding cells, extracellular matrix, and soluble factors, and plays a crucial role in the growth and survival of cancer cells and CSCs. The contribution of plasma to eliminating tumor surroundings could support its proposal as a therapeutic strategy against drug resistance.
- Preclinical studies: Prior to considering CAP as an anticancer therapy, it is essential to conduct preclinical studies using appropriate model systems, such as three-dimensional (3D) organoids, engineered models, or spheroids, to better mimic the in vivo situation and understand the effectiveness of CAP.
- Clinical trials: The effectiveness of CAP as a therapy against drug resistance in cancer will have to be evaluated in clinical trials involving human subjects. In this regard, carefully designing and conducting these trials will accurately assess the safety and effectiveness of CAP in this setting.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OH: Hydroxyl |
| 5-FU: 5-fluorouracil |
| CAP: Cold atmospheric plasma |
| CHF: Congestive heart failure |
| CIS: Cisplatin |
| CNS: Central nervous system |
| CSC: Cancer stem cells |
| DBDs: Dielectric barrier discharge devices |
| DOX: Doxorubicin |
| DPD: Dihydropyrimidine dehydrogenase |
| EPI: Epirubicin |
| ER: Estrogen receptor |
| GBM: Glioblastoma multiforme |
| GSH: Gluthatione |
| H2O2: Hydrogen peroxide |
| HCC: Hepatocellular carcinoma |
| LV: Left ventricle |
| MGMT: Methylguanine-DNA methyl transferase |
| N: Nitrogen |
| NO: Nitric oxide |
| O: Oxygen |
| O2: Singlet-delta oxygen |
| O2−: Superoxide |
| OHOO: Hydroxyl peroxide |
| ONOO−: peroxynitrite |
| PCL: Plasma-conditioned liquid |
| PTX: Paclitaxel |
| RNS: Reactive nitrogen species |
| RONS: Reactive oxygen and nitrogen species |
| TMZ: Temozolomide |
| TOP2A: Topoisomerase II |
| TS: Thymidylate synthase |
References
- Ward, R.A.; Fawell, S.; Floc’h, N.; Flemington, V.; McKerrecher, D.; Smith, P.D. Challenges and Opportunities in Cancer Drug Resistance. Chem. Rev. 2021, 121, 3297–3351. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A View on Drug Resistance in Cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [PubMed]
- Labrie, M.; Brugge, J.S.; Mills, G.B.; Zervantonakis, I.K. Therapy Resistance: Opportunities Created by Adaptive Responses to Targeted Therapies in Cancer. Nat. Rev. Cancer 2022, 22, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Chitcholtan, K.; Asselin, E.; Parent, S.; Sykes, P.H.; Evans, J.J. Differences in Growth Properties of Endometrial Cancer in Three Dimensional (3d) Culture and 2d Cell Monolayer. Exp. Cell Res. 2013, 319, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Van der Worp, H.B.; Howells, D.W.; Sena, E.S.; Porritt, M.J.; Rewell, S.; O’Collins, V.; Macleod, M.R. Can Animal Models of Disease Reliably Inform Human Studies? PLoS Med. 2010, 7, e1000245. [Google Scholar] [CrossRef]
- Kimlin, L.C.; Casagrande, G.; Virador, V.M. In Vitro Three-Dimensional (3d) Models in Cancer Research: An Update. Mol. Carcinog. 2013, 52, 167–182. [Google Scholar] [CrossRef]
- De Luca, A.; Raimondi, L.; Salamanna, F.; Carina, V.; Costa, V.; Bellavia, D.; Alessandro, R.; Fini, M.; Giavaresi, G. Relevance of 3d Culture Systems to Study Osteosarcoma Environment. J. Exp. Clin. Cancer Res. 2018, 37, 2. [Google Scholar] [CrossRef]
- Tornin, J.; Labay, C.; Tampieri, F.; Ginebra, M.-P.; Canal, C. Evaluation of the Effects of Cold Atmospheric Plasma and Plasma-Treated Liquids in Cancer Cell Cultures. Nat. Protoc. 2021, 16, 2826–2850. [Google Scholar] [CrossRef]
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxid. Med. Cell Longev 2019, 2019, 3873928. [Google Scholar] [CrossRef]
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.-P.; Laurencin-Dalicieux, S.; Cousty, S. Use of Cold-Atmospheric Plasma in Oncology: A Concise Systematic Review. Adv. Med. Oncol. 2018, 10, 1758835918786475. [Google Scholar] [CrossRef] [PubMed]
- Izadjoo, M.; Zack, S.; Kim, H.; Skiba, J. Medical Applications of Cold Atmospheric Plasma: State of the Science. J. Wound Care 2018, 27, S4–S10. [Google Scholar] [CrossRef] [PubMed]
- Attri, P.; Yusupov, M.; Park, J.H.; Lingamdinne, L.P.; Koduru, J.R.; Shiratani, M.; Choi, E.H.; Bogaerts, A. Mechanism and Comparison of Needle-Type Non-Thermal Direct and Indirect Atmospheric Pressure Plasma Jets on the Degradation of Dyes. Sci. Rep. 2016, 6, 34419. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive Species in Non-Equilibrium Atmospheric-Pressure Plasmas: Generation, Transport, and Biological Effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Rakesh Ruchel, K.; Bailung, H. Cold Atmospheric Pressure Plasma Technology for Biomedical Application. In Plasma Science and Technology; Shahzad, A., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 1. [Google Scholar]
- Khlyustova, A.; Labay, C.; Machala, Z.; Ginebra, M.-P.; Canal, C. Important Parameters in Plasma Jets for the Production of Rons in Liquids for Plasma Medicine: A Brief Review. Front. Chem. Sci. Eng. 2019, 13, 238–252. [Google Scholar] [CrossRef]
- Lis, K.A.; Kehrenberg, C.; Boulaaba, A.; von Köckritz-Blickwede, M.; Binder, S.; Li, Y.; Zimmermann, J.L.; Pfeifer, Y.; Ahlfeld, B. Inactivation of Multidrug-Resistant Pathogens and Y. Enterocolitica with Cold Atmospheric Pressure Plasma on Stainless Steel Surfaces. Int. J. Antimicrob. Agents 2018, 52, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Ercan, U.K.; Ibiş, F.; Dikyol, C.; Horzum, N.; Karaman, O.; Yıldırım, Ç.; Çukur, E.; Demirci, E.A. Prevention of Bacterial Colonization on Non-Thermal Atmospheric Plasma Treated Surgical Sutures for Control and Prevention of Surgical Site Infections. PLoS ONE 2018, 13, e0202703. [Google Scholar] [CrossRef]
- Guo, L.; Xu, R.; Gou, L.; Liu, Z.; Zhao, Y.; Liu, D.; Zhang, L.; Chen, H.; Kong, M.G. Mechanism of Virus Inactivation by Cold Atmospheric-Pressure Plasma and Plasma-Activated Water. Appl. Env. Microbiol. 2018, 84, e00726-18. [Google Scholar] [CrossRef]
- Chen, Z.; Garcia, G., Jr.; Arumugaswami, V.; Wirz, R.E. Cold Atmospheric Plasma for SARS-CoV-2 Inactivation. Phys. Fluids 2020, 32, 111702. [Google Scholar] [CrossRef]
- Borges, A.C.; de Morais Gouvêa Lima, G.; Nishime, T.M.C.; Gontijo, A.V.L.; Kostov, K.G.; Koga-Ito, C.Y. Amplitude-Modulated Cold Atmospheric Pressure Plasma Jet for Treatment of Oral Candidiasis: In Vivo Study. PLoS ONE 2018, 13, e199832. [Google Scholar] [CrossRef]
- Tan, F.; Rui, X.; Xiang, X.; Yu, Z.; Al-Rubeai, M. Multimodal Treatment Combining Cold Atmospheric Plasma and Acidic Fibroblast Growth Factor for Multi-Tissue Regeneration. FASEB J. 2021, 35, e21442. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.R.; Hosseini, M.S.; Fatollah, S.; Mirpour, S.; Ghoranneviss, M.; Larijani, B.; Mohajeri-Tehrani, M.R.; Khorramizadeh, M.R. Beneficial Effects of Cold Atmospheric Plasma on Inflammatory Phase of Diabetic Foot Ulcers; a Randomized Clinical Trial. J. Diabetes Metab. Disord. 2020, 19, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, C.; Banzet, S.; Lataillade, J.; Rousseau, A.; Frescaline, N. Cold Atmospheric Plasma Modulates Endothelial Nitric Oxide Synthase Signalling and Enhances Burn Wound Neovascularisation. J. Pathol. 2019, 249, 368–380. [Google Scholar] [CrossRef]
- Borchardt, T.; Ernst, J.; Helmke, A.; Tanyeli, M.; Schilling, A.F.; Felmerer, G.; Viöl, W. Effect of Direct Cold Atmospheric Plasma (Dicap) on Microcirculation of Intact Skin in a Controlled Mechanical Environment. Microcirculation 2017, 24, e12399. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Takamatsu, T.; Kawano, H.; Miyahara, H.; Okino, A.; Yoshida, M.; Azuma, T. Investigation of Blood Coagulation Effect of Nonthermal Multigas Plasma Jet in Vitro and in Vivo. J. Surg. Res. 2017, 219, 302–309. [Google Scholar] [CrossRef]
- Haertel, B.; von Woedtke, T.; Weltmann, K.D.; Lindequist, U. Non-Thermal Atmospheric-Pressure Plasma Possible Application in Wound Healing. Biomol. Ther. 2014, 22, 477–490. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jeong, W.-S.; Seo, S.-J.; Kim, H.-W.; Kim, K.-N.; Choi, E.-H.; Kim, K.-M. Non-Thermal Atmospheric Pressure Plasma Functionalized Dental Implant for Enhancement of Bacterial Resistance and Osseointegration. Dent. Mater. 2017, 33, 257–270. [Google Scholar] [CrossRef]
- Duske, K.; Jablonowski, L.; Koban, I.; Matthes, R.; Holtfreter, B.; Sckell, A.; Nebe, J.B.; von Woedtke, T.; Weltmann, K.D.; Kocher, T. Cold Atmospheric Plasma in Combination with Mechanical Treatment Improves Osteoblast Growth on Biofilm Covered Titanium Discs. Biomaterials 2015, 52, 327–334. [Google Scholar] [CrossRef]
- Omori, M.; Tsuchiya, S.; Hara, K.; Kuroda, K.; Hibi, H.; Okido, M.; Ueda, M. A New Application of Cell-Free Bone Regeneration: Immobilizing Stem Cells from Human Exfoliated Deciduous Teeth-Conditioned Medium onto Titanium Implants Using Atmospheric Pressure Plasma Treatment. Stem. Cell Res. Ther. 2015, 6, 124. [Google Scholar] [CrossRef]
- Won, H.-R.; Kang, S.U.; Kim, H.J.; Jang, J.Y.; Shin, Y.S.; Kim, C.-H. Non-Thermal Plasma Treated Solution with Potential as a Novel Therapeutic Agent for Nasal Mucosa Regeneration. Sci. Rep. 2018, 8, 13754. [Google Scholar] [CrossRef]
- Eisenhauer, P.; Chernets, N.; Song, Y.; Dobrynin, D.; Pleshko, N.; Steinbeck, M.J.; Freeman, T.A. Chemical Modification of Extracellular Matrix by Cold Atmospheric Plasma-Generated Reactive Species Affects Chondrogenesis and Bone Formation. J. Tissue Eng. Regen. Med. 2016, 10, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhao, S.; Yan, X. Nerve Stem Cell Differentiation by a One-Step Cold Atmospheric Plasma Treatment in Vitro. J. Vis. Exp. 2019, 143, e58663. [Google Scholar] [CrossRef]
- Tan, F.; Fang, Y.; Zhu, L.; Al-Rubeai, M. Controlling Stem Cell Fate Using Cold Atmospheric Plasma. Stem. Cell Res. Ther. 2020, 11, 368. [Google Scholar] [CrossRef]
- Canady Helios Cold Plasma Scalpel Treatment at the Surgical Margin and Macroscopic Tumor Sites. Available online: https://ClinicalTrials.gov/show/NCT04267575 (accessed on 17 October 2022).
- Mateu-Sanz, M.; Tornin, J.; Ginebra, M.P.; Canal, C. Cold Atmospheric Plasma: A New Strategy Based Primarily on Oxidative Stress for Osteosarcoma Therapy. J. Clin. Med. 2021, 10, 893. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G.; Sersenová, D.; Graves, D.B.; Machala, Z. Cold Atmospheric Plasma and Plasma-Activated Medium Trigger Rons-Based Tumor Cell Apoptosis. Sci. Rep. 2019, 9, 14210. [Google Scholar] [CrossRef] [PubMed]
- Tornin, J.; Mateu-Sanz, M.; Rodríguez, A.; Labay, C.; Rodríguez, R.; Canal, C. Pyruvate Plays a Main Role in the Antitumoral Selectivity of Cold Atmospheric Plasma in Osteosarcoma. Sci. Rep. 2019, 9, 10681. [Google Scholar] [CrossRef]
- Mateu-Sanz, M.; Ginebra, M.-P.; Tornín, J.; Canal, C. Cold Atmospheric Plasma Enhances Doxorubicin Selectivity in Metastasic Bone Cancer. Free. Radic. Biol. Med. 2022, 189, 32–41. [Google Scholar] [CrossRef]
- Mateu-Sanz, M.; Tornín, J.; Brulin, B.; Khlyustova, A.; Ginebra, M.-P.; Layrolle, P.; Canal, C. Cold Plasma-Treated Ringer’s Saline: A Weapon to Target Osteosarcoma. Cancers 2020, 12, 227. [Google Scholar] [CrossRef]
- Freund, E.; Liedtke, K.R.; van der Linde, J.; Metelmann, H.R.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Physical Plasma-Treated Saline Promotes an Immunogenic Phenotype in Ct26 Colon Cancer Cells in Vitro and in Vivo. Sci. Rep. 2019, 9, 634. [Google Scholar] [CrossRef]
- Bauer, G. The Synergistic Effect between Hydrogen Peroxide and Nitrite, Two Long-Lived Molecular Species from Cold Atmospheric Plasma, Triggers Tumor Cells to Induce Their Own Cell Death. Redox Biol. 2019, 26, 101291. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-Phase Chemistry and Bactericidal Effects from an Air Discharge Plasma in Contact with Water: Evidence for the Formation of Peroxynitrite through a Pseudo-Second-Order Post-Discharge Reaction of H2o 2 and Hno2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Solé-Martí, X.; Espona-Noguera, A.; Ginebra, M.-P.; Canal, C. Plasma-Conditioned Liquids as Anticancer Therapies in Vivo: Current State and Future Directions. Cancers 2021, 13, 452. [Google Scholar] [CrossRef]
- Girard, P.-M.; Arbabian, A.; Fleury, M.; Bauville, G.; Puech, V.; Dutreix, M.; Sousa, J.S. Synergistic Effect of H2O2 and No2 in Cell Death Induced by Cold Atmospheric He Plasma. Sci. Rep. 2016, 6, 29098. [Google Scholar] [CrossRef]
- Bauer, G. Cold Atmospheric Plasma and Plasma-Activated Medium: Antitumor Cell Effects with Inherent Synergistic Potential. Plasma Med. 2019, 9, 57–88. [Google Scholar] [CrossRef]
- Bekeschus, S.; Liebelt, G.; Menz, J.; Berner, J.; Sagwal, S.K.; Wende, K.; Weltmann, K.-D.; Boeckmann, L.; von Woedtke, T.; Metelmann, H.-R.; et al. Tumor Cell Metabolism Correlates with Resistance to Gas Plasma Treatment: The Evaluation of Three Dogmas. Free. Radic. Biol. Med. 2021, 167, 12–28. [Google Scholar] [CrossRef] [PubMed]
- de Sa Junior, P.L.; Câmara, D.A.D.; Porcacchia, A.S.; Fonseca, P.M.M.; Jorge, S.D.; Araldi, R.P.; Ferreira, A.K. The Roles of Ros in Cancer Heterogeneity and Therapy. Oxid. Med. Cell. Longev. 2017, 2017, 2467940. [Google Scholar] [CrossRef]
- Kurake, N.; Tanaka, H.; Ishikawa, K.; Kondo, T.; Sekine, M.; Nakamura, K.; Kajiyama, H.; Kikkawa, F.; Mizuno, M.; Hori, M. Cell Survival of Glioblastoma Grown in Medium Containing Hydrogen Peroxide and/or Nitrite, or in Plasma-Activated Medium. Arch. Biochem. Biophys. 2016, 605, 102–108. [Google Scholar] [CrossRef]
- Mahdikia, H.; Shokri, B.; Majidzadeh, K.A. The Feasibility Study of Plasma-Activated Water as a Physical Therapy to Induce Apoptosis in Melanoma Cancer Cells in-Vitro. Iran. J. Pharm. Res. 2021, 20, 337–350. [Google Scholar] [CrossRef]
- Raud, S.; Raud, J.; Jõgi, I.; Piller, C.-T.; Plank, T.; Talviste, R.; Teesalu, T.; Vasar, E. The Production of Plasma Activated Water in Controlled Ambient Gases and Its Impact on Cancer Cell Viability. Plasma Chem. Plasma Process. 2021, 41, 1381–1395. [Google Scholar] [CrossRef]
- Tornín, J.; Villasante, A.; Solé-Martí, X.; Ginebra, M.-P.; Canal, C. Osteosarcoma Tissue-Engineered Model Challenges Oxidative Stress Therapy Revealing Promoted Cancer Stem Cell Properties. Free. Radic. Biol. Med. 2021, 164, 107–118. [Google Scholar] [CrossRef]
- Sato, Y.; Yamada, S.; Takeda, S.; Hattori, N.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Hori, M.; Kodera, Y. Effect of Plasma-Activated Lactated Ringer’s Solution on Pancreatic Cancer Cells in Vitro and in Vivo. Ann. Surg. Oncol. 2018, 25, 299–307. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Gorbanev, Y.; Dewilde, S.; Smits, E.; Bogaerts, A. Reduction of Human Glioblastoma Spheroids Using Cold Atmospheric Plasma: The Combined Effect of Short- and Long-Lived Reactive Species. Cancers 2018, 10, 394. [Google Scholar] [CrossRef]
- Zhou, X.; Cai, D.; Xiao, S.; Ning, M.; Zhou, R.; Zhang, S.; Chen, X.; Ostrikov, K.; Dai, X. Invivopen: A Novel Plasma Source for in Vivo Cancer Treatment. J. Cancer 2020, 11, 2273–2282. [Google Scholar] [CrossRef]
- Motaln, H.; Recek, N.; Rogelj, B. Intracellular Responses Triggered by Cold Atmospheric Plasma and Plasma-Activated Media in Cancer Cells. Molecules 2021, 26, 1336. [Google Scholar] [CrossRef]
- Kumari, S.; Badana, A.K.; Murali, M.G.; Shailender, G.; Malla, R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark Insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef]
- Köritzer, J.; Boxhammer, V.; Schäfer, A.; Shimizu, T.; Klämpfl, T.G.; Li, Y.-F.; Welz, C.; Schwenk-Zieger, S.; Morfill, G.E.; Zimmermann, J.L.; et al. Restoration of Sensitivity in Chemo-Resistant Glioma Cells by Cold Atmospheric Plasma. PLoS ONE 2013, 8, e64498. [Google Scholar] [CrossRef]
- Gjika, E.; Pal-Ghosh, S.; Kirschner, M.E.; Lin, L.; Sherman, J.H.; Stepp, M.A.; Keidar, M. Combination Therapy of Cold Atmospheric Plasma (Cap) with Temozolomide in the Treatment of U87mg Glioblastoma Cells. Sci. Rep. 2020, 10, 16495. [Google Scholar] [CrossRef]
- Soni, V.; Adhikari, M.; Simonyan, H.; Lin, L.; Sherman, J.H.; Young, C.N.; Keidar, M. In Vitro and in Vivo Enhancement of Temozolomide Effect in Human Glioblastoma by Non-Invasive Application of Cold Atmospheric Plasma. Cancers 2021, 13, 4485. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Privat-Maldonado, A.; Smits, E.; Bogaerts, A. Cold Atmospheric Plasma Increases Temozolomide Sensitivity of Three-Dimensional Glioblastoma Spheroids Via Oxidative Stress-Mediated DNA Damage. Cancers 2021, 13, 1780. [Google Scholar] [CrossRef]
- Sagwal, S.K.; Pasqual-Melo, G.; Bodnar, Y.; Gandhirajan, R.K.; Bekeschus, S. Combination of Chemotherapy and Physical Plasma Elicits Melanoma Cell Death Via Upregulation of Slc22a16. Cell Death Dis. 2018, 9, 1179. [Google Scholar] [CrossRef]
- Pefani-Antimisiari, K.; Athanasopoulos, D.K.; Marazioti, A.; Sklias, K.; Rodi, M.; de Lastic, A.-L.; Mouzaki, A.; Svarnas, P.; Antimisiaris, S.G. Synergistic Effect of Cold Atmospheric Pressure Plasma and Free or Liposomal Doxorubicin on Melanoma Cells. Sci. Rep. 2021, 11, 14788. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, S.; Zhang, J.; Li, B.; Liu, D.; Guo, L.; Liu, Z.; Xu, D. Synergistic Anticancer Effects of Different Combinations of He+O2 Plasma Jet and Doxorubicin on A375 Melanoma Cells. Plasma Process. Polym. 2021, 18, 2000239. [Google Scholar] [CrossRef]
- Zahedian, S.; Hekmat, A.; Tackallou, S.H.; Ghoranneviss, M. The Impacts of Prepared Plasma-Activated Medium (Pam) Combined with Doxorubicin on the Viability of Mcf-7 Breast Cancer Cells: A New Cancer Treatment Strategy. Rep. Biochem. Mol. Biol. 2022, 10, 640–652. [Google Scholar] [CrossRef]
- Brunner, T.F.; Probst, F.A.; Troeltzsch, M.; Schwenk-Zieger, S.; Zimmermann, J.L.; Morfill, G.; Becker, S.; Harréus, U.; Welz, C. Primary Cold Atmospheric Plasma Combined with Low Dose Cisplatin as a Possible Adjuvant Combination Therapy for Hnscc Cells-an in-Vitro Study. Head Face Med. 2022, 18, 21. [Google Scholar] [CrossRef]
- Lee, C.-M.; Jeong, Y.-I.; Kook, M.-S.; Kim, B.-H. Combinatorial Effect of Cold Atmosphere Plasma (Cap) and the Anticancer Drug Cisplatin on Oral Squamous Cell Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 7646. [Google Scholar] [CrossRef]
- Li, Y.; Tang, T.; Lee, H.; Song, K. Selective Anti-Cancer Effects of Plasma-Activated Medium and Its High Efficacy with Cisplatin on Hepatocellular Carcinoma with Cancer Stem Cell Characteristics. Int. J. Mol. Sci. 2021, 22, 3956. [Google Scholar] [CrossRef]
- Mihai, C.-T.; Mihaila, I.; Pasare, M.A.; Pintilie, R.M.; Ciorpac, M.; Topala, I. Cold Atmospheric Plasma-Activated Media Improve Paclitaxel Efficacy on Breast Cancer Cells in a Combined Treatment Model. Curr. Issues Mol. Biol. 2022, 44, 1995–2014. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Ji, H.W.; Kim, H.W.; Yun, S.H.; Choi, E.H.; Kim, S.J. Cold Atmospheric Plasma Restores Paclitaxel Sensitivity to Paclitaxel-Resistant Breast Cancer Cells by Reversing Expression of Resistance-Related Genes. Cancers 2019, 11, 2011. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Jeong, D.; Ham, J.; Park, S.; Choi, E.H.; Kim, S.J. Cold atmospheric plasma restores tamoxifen sensitivity in resistant MCF-7 breast cancer cell. Free. Radic. Biol. Med. 2017, 110, 280–290. [Google Scholar] [CrossRef]
- Yang, H.; Lu, R.; Xian, Y.; Gan, L.; Lu, X.; Yang, X. Effects of Atmospheric Pressure Cold Plasma on Human Hepatocarcinoma Cell and Its 5-Fluorouracil Resistant Cell Line. Phys. Plasmas 2015, 22, 122006. [Google Scholar] [CrossRef]
- Jones, O.; Cheng, X.; Murthy, S.R.K.; Ly, L.; Zhuang, T.; Basadonna, G.; Keidar, M.; Canady, J. The synergistic effect of Canady Helios cold atmospheric plasma and a FOLFIRINOX regimen for the treatment of cholangiocarcinoma in vitro. Sci. Rep. 2021, 11, 8967. [Google Scholar] [CrossRef]
- Moody, C.L.; Wheelhouse, R.T. The Medicinal Chemistry of Imidazotetrazine Prodrugs. Pharmaceuticals 2014, 7, 797–838. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide Resistance in Glioblastoma Multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef]
- Glioblastoma; De Vleeschouwer, S., Ed.; Springer: Brisbane, Astralia, 2017. [Google Scholar]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of Its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Haar, C.P.; Hebbar, P.; Wallace, G.C.; Das, A.; Vandergrift, W.A.; Smith, J.A.; Giglio, P.; Patel, S.J.; Ray, S.K.; Banik, N.L. Drug Resistance in Glioblastoma: A Mini Review. Neurochem. Res. 2012, 37, 1192–1200. [Google Scholar] [CrossRef]
- Danson, S.J.; Middleton, M.R. Temozolomide: A Novel Oral Alkylating Agent. Expert Rev. Anticancer. 2001, 1, 13–19. [Google Scholar] [CrossRef]
- Zhang, J.; Stevens, M.F.; Laughton, C.A.; Madhusudan, S.; Bradshaw, T.D. Acquired Resistance to Temozolomide in Glioma Cell Lines: Molecular Mechanisms and Potential Translational Applications. Oncology 2010, 78, 103–114. [Google Scholar] [CrossRef]
- Alonso, M.M.; Gomez-Manzano, C.; Bekele, B.N.; Yung, W.K.; Fueyo, J. Adenovirus-Based Strategies Overcome Temozolomide Resistance by Silencing the O6-Methylguanine-DNA Methyltransferase Promoter. Cancer Res. 2007, 67, 11499–11504. [Google Scholar] [CrossRef]
- Hermisson, M.; Klumpp, A.; Wick, W.; Wischhusen, J.; Nagel, G.; Roos, W.; Kaina, B.; Weller, M. O6-Methylguanine DNA Methyltransferase and P53 Status Predict Temozolomide Sensitivity in Human Malignant Glioma Cells. J. Neurochem. 2006, 96, 766–776. [Google Scholar] [CrossRef]
- Kohsaka, S.; Wang, L.; Yachi, K.; Mahabir, R.; Narita, T.; Itoh, T.; Tanino, M.; Kimura, T.; Nishihara, H.; Tanaka, S. Stat3 Inhibition Overcomes Temozolomide Resistance in Glioblastoma by Downregulating Mgmt Expression. Mol. Cancer 2012, 11, 1289–1299. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, X.; Zeng, Z.; Tunici, P.; Ng, H.; Abdulkadir, I.R.; Lu, L.; Irvin, D.; Black, K.L.; Yu, J.S. Analysis of Gene Expression and Chemoresistance of Cd133+ Cancer Stem Cells in Glioblastoma. Mol. Cancer 2006, 5, 67. [Google Scholar] [CrossRef]
- Sarkar, C.; Suri, V.; Jha, P.; Sharma, M.C. O6 -Methylguanine DNA Methyltransferase Gene Promoter Methylation in High-Grade Gliomas: A Review of Current Status. Neurol. India 2011, 59, 229–235. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Wang, Z.; Shu, F.; Jin, Y.-H.; Liu, H.-Y.; Wang, Q.-J.; Yang, Y. Activation of Amp-Activated Protein Kinase by Temozolomide Contributes to Apoptosis in Glioblastoma Cells Via P53 Activation and Mtorc1 Inhibition. J. Biol. Chem. 2010, 285, 40461–40471. [Google Scholar] [CrossRef]
- Rocha, C.R.R.; Kajitani, G.S.; Quinet, A.; Fortunato, R.S.; Menck, C.F.M. Nrf2 and Glutathione Are Key Resistance Mediators to Temozolomide in Glioma and Melanoma Cells. Oncotarget 2016, 7, 48081–48092. [Google Scholar] [CrossRef]
- Zhu, Z.; Du, S.; Du, Y.; Ren, J.; Ying, G.; Yan, Z. Glutathione Reductase Mediates Drug Resistance in Glioblastoma Cells by Regulating Redox Homeostasis. J. Neurochem. 2017, 144, 93–104. [Google Scholar] [CrossRef]
- Hulst, M.B.; Grocholski, T.; Neefjes, J.J.C.; van Wezel, G.P.; Metsä-Ketelä, M. Anthracyclines: Biosynthesis, engineering and clinical applications. Nat. Prod. Rep. 2021, 39, 814–841. [Google Scholar] [CrossRef]
- Sritharan, S.; Sivalingam, N. A Comprehensive Review on Time-Tested Anticancer Drug Doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef]
- Al-Malky, H.S.; Al Harthi, S.E.; Osman, A.M. Major Obstacles to Doxorubicin Therapy: Cardiotoxicity and Drug Resistance. J. Oncol. Pharm. Pr. 2020, 26, 434–444. [Google Scholar] [CrossRef]
- Ling, G.; Wang, X.; Tan, N.; Cao, J.; Li, W.; Zhang, Y.; Jiang, J.; Sun, Q.; Jiang, Y.; Wang, W.; et al. Mechanisms and Drug Intervention for Doxorubicin-Induced Cardiotoxicity Based on Mitochondrial Bioenergetics. Oxidative Med. Cell. Longev. 2022, 2022, 7176282. [Google Scholar] [CrossRef]
- Watson, C.; Gadikota, H.; Barlev, A.; Beckerman, R. A review of the risks of long-term consequences associated with components of the CHOP chemotherapy regimen. J. Drug Assess. 2022, 11, 1–11. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Hashemi, F.; Zabolian, A.; Farahani, M.V.; Hushmandi, K.; Zarrabi, A.; Goldman, A.; Ashrafizadeh, M.; Orive, G. Advances in Understanding the Role of P-Gp in Doxorubicin Resistance: Molecular Pathways, Therapeutic Strategies, and Prospects. Drug Discov Today 2022, 27, 436–455. [Google Scholar] [CrossRef]
- Genovese, I.; Ilari, A.; Assaraf, Y.G.; Fazi, F.; Colotti, G. Not Only P-Glycoprotein: Amplification of the Abcb1-Containing Chromosome Region 7q21 Confers Multidrug Resistance Upon Cancer Cells by Coordinated Overexpression of an Assortment of Resistance-Related Proteins. Drug Resist Updat. 2017, 32, 23–46. [Google Scholar] [CrossRef]
- Jayaraj, R.; Nayagam, S.g.; Kar, A.; Sathyakumar, S.; Mohammed, H.; Smiti, M.; Sabarimurugan, S.; Kumarasamy, C.; Priyadharshini, T.; Gothandam, K.M.; et al. Clinical Theragnostic Relationship between Drug-Resistance Specific Mirna Expressions, Chemotherapeutic Resistance, and Sensitivity in Breast Cancer: A Systematic Review and Meta-Analysis. Cells 2019, 8, 250. [Google Scholar] [CrossRef]
- Si, Z.; Zhong, Y.; Lao, S.; Wu, Y.; Zhong, G.; Zeng, W. The Role of Mirnas in the Resistance of Anthracyclines in Breast Cancer: A Systematic Review. Front Oncol. 2022, 12, 899145. [Google Scholar] [CrossRef]
- Menéndez, S.; Gallego, B.; Murillo, D.; Rodríguez, A.; Rodríguez, R. Cancer Stem Cells as a Source of Drug Resistance in Bone Sarcomas. J. Clin. Med. 2021, 10, 2621. [Google Scholar] [CrossRef]
- Martins-Neves, S.R.; Sampaio-Ribeiro, G.; Gomes, C.M.F. Chemoresistance-Related Stem Cell Signaling in Osteosarcoma and Its Plausible Contribution to Poor Therapeutic Response: A Discussion That Still Matters. Int. J. Mol. Sci. 2022, 23, 11416. [Google Scholar] [CrossRef]
- Singh, M.S.; Tammam, S.N.; Boushehri, M.A.S.; Lamprecht, A. MDR in cancer: Addressing the underlying cellular alterations with the use of nanocarriers. Pharmacol. Res. 2017, 126, 2–30. [Google Scholar] [CrossRef]
- Tzelepis, K.; Koike-Yusa, H.; De Braekeleer, E.; Li, Y.; Metzakopian, E.; Dovey, O.M.; Mupo, A.; Grinkevich, V.; Li, M.; Mazan, M.; et al. A Crispr Dropout Screen Identifies Genetic Vulnerabilities and Therapeutic Targets in Acute Myeloid Leukemia. Cell Rep. 2016, 17, 1193–1205. [Google Scholar] [CrossRef]
- Yang, F.; Teves, S.S.; Kemp, C.J.; Henikoff, S. Doxorubicin, DNA Torsion, and Chromatin Dynamics. Biochim Biophys Acta 2014, 1845, 84–89. [Google Scholar] [CrossRef]
- Tarpgaard, L.S.; Qvortrup, C.; Nielsen, S.L.; Stenvang, J.; Detlefsen, S.; Brünner, N.; Pfeiffer, P. New use for old drugs: Epirubicin in colorectal cancer. Acta Oncol. 2021, 60, 954–956. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, H.; Bao, G.; Zhang, G.; Wang, H.; Wang, X. Effectiveness and safety of pegylated liposomal doxorubicin versus epirubicin as neoadjuvant or adjuvant chemotherapy for breast cancer: A real-world study. BMC Cancer 2021, 21, 1301. [Google Scholar] [CrossRef]
- Steele, N.G.; Chakrabarti, J.; Wang, J.; Biesiada, J.; Holokai, L.; Chang, J.; Nowacki, L.M.; Hawkins, J.; Mahe, M.; Sundaram, N.; et al. An Organoid-Based Preclinical Model of Human Gastric Cancer. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 161–184. [Google Scholar] [CrossRef]
- Liu, J.-J.; Tang, W.; Fu, M.; Gong, X.-Q.; Kong, L.; Yao, X.-M.; Jing, M.; Cai, F.-Y.; Li, X.-T.; Ju, R.-J. Development of R8 modified epirubicin–dihydroartemisinin liposomes for treatment of non-small-cell lung cancer. Artif. Cells Nanomedicine Biotechnol. 2019, 47, 1947–1960. [Google Scholar] [CrossRef]
- Armenian, S.; Bhatia, S. Predicting and Preventing Anthracycline-Related Cardiotoxicity. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 3–12. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Oeffinger, K.C.; Chen, Y.; Kawashima, T.; Yasui, Y.; Leisenring, W.; Stovall, M.; Chow, E.; Sklar, C.A.; Mulrooney, D.A.; et al. Modifiable Risk Factors and Major Cardiac Events Among Adult Survivors of Childhood Cancer. J. Clin. Oncol. 2013, 31, 3673–3680. [Google Scholar] [CrossRef]
- Effects of Chemotherapy and Hormonal Therapy for Early Breast Cancer on Recurrence and 15-Year Survival: An Overview of the Randomised Trials. Lancet 2005, 365, 1687–1717. [CrossRef]
- Scully, R.E.; Lipshultz, S.E. Anthracycline cardiotoxicity in long-term survivors of childhood cancer. Cardiovasc. Toxicol. 2007, 7, 122–128. [Google Scholar] [CrossRef]
- Calvanese, V.; Lara, E.; Suárez-Álvarez, B.; Abu Dawud, R.; Vázquez-Chantada, M.; Martínez-Chantar, M.L.; Embade, N.; López-Nieva, P.; Horrillo, A.; Hmadcha, A.; et al. Sirtuin 1 regulation of developmental genes during differentiation of stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 13736–13741. [Google Scholar] [CrossRef]
- Robinson, E.L.; Azodi, M.; Heymans, S.; Heggermont, W. Anthracycline-Related Heart Failure: Certain Knowledge and Open Questions: Where Do We Stand with Chemotherapyinduced Cardiotoxicity? Curr. Heart Fail Rep. 2020, 17, 357–364. [Google Scholar] [CrossRef]
- Vitale, D.; Caon, I.; Parnigoni, A.; Sevic, I.; Spinelli, F.; Icardi, A.; Passi, A.; Vigetti, D.; Alaniz, L. Initial Identification of UDP-Glucose Dehydrogenase as a Prognostic Marker in Breast Cancer Patients, Which Facilitates Epirubicin Resistance and Regulates Hyaluronan Synthesis in MDA-MB-231 Cells. Biomolecules 2021, 11, 246. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Wu, Q.; Tu, Y.; Wang, C.; Yu, X.; Li, B.; Wang, Z.; Sun, S.; Sun, S. MEDAG enhances breast cancer progression and reduces epirubicin sensitivity through the AKT/AMPK/mTOR pathway. Cell Death Dis. 2021, 12, 97. [Google Scholar] [CrossRef]
- Qiong, L.; Yin, J. Orosomucoid 1 promotes epirubicin resistance in breast cancer by upregulating the expression of matrix metalloproteinases 2 and 9. Bioengineered 2021, 12, 8822–8832. [Google Scholar] [CrossRef]
- Kopacz-Bednarska, A.; Król, T. Cisplatin—Properties and Clinical Application. Oncol. Clin. Pract. 2022, 18, 166–176. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Crona, D.J.; Faso, A.; Nishijima, T.F.; McGraw, K.A.; Galsky, M.D.; Milowsky, M.I. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist 2017, 22, 609–619. [Google Scholar] [CrossRef]
- Duan, Z.; Cai, G.; Li, J.; Chen, X. Cisplatin-Induced Renal Toxicity in Elderly People. Adv. Med. Oncol. 2020, 12, 1758835920923430. [Google Scholar] [CrossRef]
- Manohar, S.; Leung, N. Cisplatin Nephrotoxicity: A Review of the Literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef]
- Lugones, Y.; Loren, P.; Salazar, L.A. Cisplatin Resistance: Genetic and Epigenetic Factors Involved. Biomolecules 2022, 12, 1365. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Fu, J.; Xu, W.; Yuan, J. The Role of Tumour Metabolism in Cisplatin Resistance. Front. Mol. Biosci. 2021, 8, 691795. [Google Scholar] [CrossRef]
- Han, Y.; Wen, P.; Li, J.; Kataoka, K. Targeted nanomedicine in cisplatin-based cancer therapeutics. J. Control. Release 2022, 345, 709–720. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Zhang, Q.-Y.; Zheng, G.-J.; Feng, B. Phytochemicals: Current strategy to sensitize cancer cells to cisplatin. Biomed. Pharmacother. 2019, 110, 518–527. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Nie, J.; Huang, J.-P.; Zheng, G.-J.; Feng, B. Targeting STAT3 inhibition to reverse cisplatin resistance. Biomed. Pharmacother. 2019, 117, 109135. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Patra, J.; Singh, Y.; Panda, M.; Das, G.; Adetunji, C.; Michael, O.; Sytar, O.; Polito, L.; et al. Paclitaxel: Application in Modern Oncology and Nanomedicine-Based Cancer Therapy. Oxid. Med. Cell Longev. 2021, 2021, 3687700. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, L. Progress in Research on Paclitaxel and Tumor Immunotherapy. Cell Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/Paclitaxel Kills Cancer Cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Woods, C.M.; Zhu, J.; McQueney, P.A.; Bollag, D.; Lazarides, E. Taxol-Induced Mitotic Block Triggers Rapid Onset of a P53-Independent Apoptotic Pathway. Mol. Med. 1995, 1, 506–526. [Google Scholar] [CrossRef]
- Shao, Z.; Zhao, H. Manipulating Natural Product Biosynthetic Pathways Via DNA Assembler. Curr. Protoc. Chem. Biol. 2014, 6, 65–100. [Google Scholar] [CrossRef]
- Murray, S.; Briasoulis, E.; Linardou, H.; Bafaloukos, D.; Papadimitriou, C. Taxane resistance in breast cancer: Mechanisms, predictive biomarkers and circumvention strategies. Cancer Treat. Rev. 2012, 38, 890–903. [Google Scholar] [CrossRef]
- Septin Cooperation with Tubulin Polyglutamylation Contributes to Cancer Cell Adaptation to Taxanes. Oncotarget 2015, 6, 36063. [CrossRef]
- Liu, Z.; Zhu, G.; Getzenberg, R.H.; Veltri, R.W. The Upregulation of PI3K/Akt and MAP Kinase Pathways is Associated with Resistance of Microtubule-Targeting Drugs in Prostate Cancer. J. Cell. Biochem. 2015, 116, 1341–1349. [Google Scholar] [CrossRef]
- Dong, C.; Wu, J.; Chen, Y.; Nie, J.; Chen, C. Activation of PI3K/AKT/mTOR Pathway Causes Drug Resistance in Breast Cancer. Front. Pharmacol. 2021, 12, 628690. [Google Scholar] [CrossRef]
- Quirke, V.M. Tamoxifen from Failed Contraceptive Pill to Best-Selling Breast Cancer Medicine: A Case-Study in Pharmaceutical Innovation. Front Pharm. 2017, 8, 620. [Google Scholar] [CrossRef]
- Chang, M. Tamoxifen Resistance in Breast Cancer. Biomoleculs 2012, 20, 256–267. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Azwar, S.; Seow, H.F.; Abdullah, M.; Jabar, M.F.; Mohtarrudin, N. Recent Updates on Mechanisms of Resistance to 5-Fluorouracil and Reversal Strategies in Colon Cancer Treatment. Biology 2021, 10, 854. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Abak, A.; Tondro Anamag, F.; Shoorei, H.; Fattahi, F.; Javadinia, S.A.; Taheri, M. 5-Fluorouracil: A Narrative Review on the Role of Regulatory Mechanisms in Driving Resistance to This Chemotherapeutic Agent. Front. Oncol. 2021, 11, 658636. [Google Scholar] [CrossRef]
- Blondy, S.; David, V.; Verdier, M.; Mathonnet, M.; Perraud, A.; Christou, N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020, 111, 3142–3154. [Google Scholar] [CrossRef]
- Yan, D.; Cui, H.; Zhu, W.; Nourmohammadi, N.; Milberg, J.; Zhang, L.G.; Sherman, J.H.; Keidar, M. The Specific Vulnerabilities of Cancer Cells to the Cold Atmospheric Plasma-Stimulated Solutions. Sci. Rep. 2017, 7, 4479. [Google Scholar] [CrossRef]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Sherman, J.H.; Cheng, X.; Keidar, M. Toward Understanding the Selective Anticancer Capacity of Cold Atmospheric Plasma—A Model Based on Aquaporins. Biointerphases 2015, 10, 040801. [Google Scholar] [CrossRef]
- Van Der Paal, J.; Verheyen, C.; Neyts, E.C.; Bogaerts, A. Hampering Effect of Cholesterol on the Permeation of Reactive Oxygen Species through Phospholipids Bilayer: Possible Explanation for Plasma Cancer Selectivity. Sci. Rep. 2017, 7, 39526. [Google Scholar] [CrossRef]
- Bauer, G.; Graves, D.B. Mechanisms of Selective Antitumor Action of Cold Atmospheric Plasma-Derived Reactive Oxygen and Nitrogen Species. Plasma Process. Polym. 2016, 13, 1157–1178. [Google Scholar] [CrossRef]
- Bauer, G. Signal Amplification by Tumor Cells: Clue to the Understanding of the Antitumor Effects of Cold Atmospheric Plasma and Plasma-Activated Medium. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 87–98. [Google Scholar] [CrossRef]
- Bauer, G. Targeting Protective Catalase of Tumor Cells with Cold Atmospheric Plasma—Activated Medium (Pam). Anti-Cancer Agents Med. Chem. 2018, 18, 784–804. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting Cancer Cells by Ros-Mediated Mechanisms: A Radical Therapeutic Approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Ghanbari Movahed, Z.; Rastegari-Pouyani, M.; Mohammadi, M.H.; Mansouri, K. Cancer Cells Change Their Glucose Metabolism to Overcome Increased Ros: One Step from Cancer Cell to Cancer Stem Cell? Biomed. Pharmacother. 2019, 112, 108690. [Google Scholar] [CrossRef]
- Jablonowski, L.; Kocher, T.; Schindler, A.; Müller, K.; Dombrowski, F.; Von Woedtke, T.; Arnold, T.; Lehmann, A.; Rupf, S.; Evert, M.; et al. Side Effects by Oral Application of Atmospheric Pressure Plasma on the Mucosa in Mice. PLoS ONE 2019, 14, e0215099. [Google Scholar] [CrossRef]
- Baik, K.Y.; Huh, Y.H.; Kim, Y.H.; Kim, J.; Kim, M.S.; Park, H.-K.; Choi, E.H.; Park, B. The Role of Free Radicals in Hemolytic Toxicity Induced by Atmospheric-Pressure Plasma Jet. Oxidative Med. Cell. Longev. 2017, 2017, 1289041. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer Stem Cells: Current Status and Evolving Complexities. Cell Stem Cell 2012, 10, 717–728. [Google Scholar] [CrossRef]
- Visvader, J.E. Cells of Origin in Cancer. Nature 2011, 469, 314–322. [Google Scholar] [CrossRef]
- Shijie, D.; Li, C.; Cheng, N.; Cui, X.; Xu, X.; Zhou, G. Redox Regulation in Cancer Stem Cells. Oxidative Med. Cell. Longev. 2015, 2015, 750798. [Google Scholar] [CrossRef]
- Dayem, A.A.; Choi, H.-Y.; Kim, J.-H.; Cho, S.-G. Role of Oxidative Stress in Stem, Cancer, and Cancer Stem Cells. Cancers 2010, 2, 859–884. [Google Scholar] [CrossRef]
- Okon, I.S.; Zou, M.-H. Mitochondrial ROS and cancer drug resistance: Implications for therapy. Pharmacol. Res. 2015, 100, 170–174. [Google Scholar] [CrossRef]
- Kahroba, H.; Shirmohamadi, M.; Hejazi, M.S.; Samadi, N. The Role of Nrf2 signaling in cancer stem cells: From stemness and self-renewal to tumorigenesis and chemoresistance. Life Sci. 2019, 239, 116986. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Zheng, J.; Pan, J. Reactive Oxygen Species in Cancer Stem Cells. Antioxid. Redox Signal. 2012, 16, 1215–1228. [Google Scholar] [CrossRef]
- Martinez-Cruzado, L.; Tornin, J.; Santos, L.; Rodriguez, A.; García-Castro, J.; Morís, F.; Rodriguez, R. Aldh1 Expression and Activity Increase During Tumor Evolution in Sarcoma Cancer Stem Cell Populations. Sci. Rep. 2016, 6, 27878. [Google Scholar] [CrossRef]
- Ikeda, J.-I.; Tanaka, H.; Ishikawa, K.; Sakakita, H.; Ikehara, Y.; Hori, M. Plasma-activated medium (PAM) kills human cancer-initiating cells. Pathol. Int. 2018, 68, 23–30. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, S.W.; Jung, M.H.; Kim, Y.S.; Kim, K.S.; Suh, D.S.; Kim, K.H.; Choi, E.H.; Kim, J.; Kwon, B.S. Plasma-activated medium inhibits cancer stem cell-like properties and exhibits a synergistic effect in combination with cisplatin in ovarian cancer. Free. Radic. Biol. Med. 2022, 182, 276–288. [Google Scholar] [CrossRef]
- Han, I.; Choi, E.H. The role of non-thermal atmospheric pressure biocompatible plasma in the differentiation of osteoblastic precursor cells, MC3T3-E1. Oncotarget 2017, 8, 36399–36409. [Google Scholar] [CrossRef]
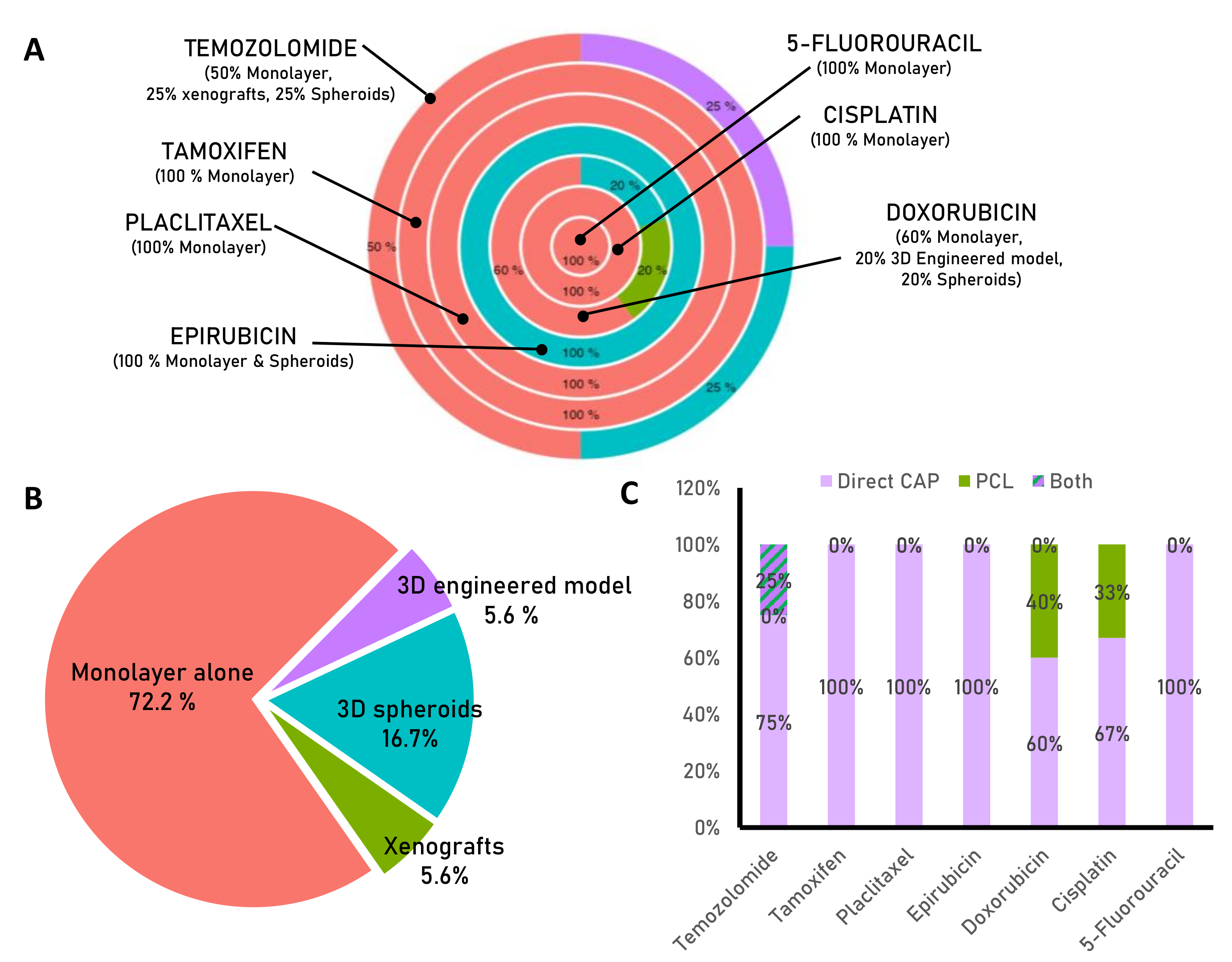
| Drug | Cancer Type | Biological Model | Cold-Plasma Application | Type of Plasma Device | Main Results Obtained and Reference |
|---|---|---|---|---|---|
| Temozolomide | Human Glioblastoma | Monolayer | Direct CAP | Dielectric barrier discharge (DBD) | CAP treatment can reestablish the sensitivity of resistant glioma cells to temozolomide [58] |
| Human Glioblastoma | Monolayer | Direct CAP | Plasma Jet | CAP amplifies cytotoxicity of Temozolomide, causes cell cycle arrest and DNA damage and inhibits cell migration [59]. | |
| Human Glioblastoma | Monolayer/Xenograft | Direct CAP | Plasma Jet | CAP increase the anti-tumoral activity of temozolomide in vitro and in vivo [60]. | |
| Human Glioblastoma | Monolayer/spheroids | Direct CAP + PCLs (PBS as liquid) | Plasma Jet | CAP and PCL are effective treatments against temozolomide resistant cells. Only CAP combined with temozolomide is effective in eliminating 3D spheroids [61]. | |
| Doxorubicin | Murine Melanoma | Monolayer/spheroids | Direct CAP | Plasma Jet | Direct CAP application increase the uptake of DOX and sinergisitically eliminates cell viability. CAP activates inmune system cells [62]. |
| Murine and human melanoma cells | Monolayer | Direct CAP | Plasma Jet | CAP increases the efectiveness of doxorubicin and supports the cancer-selective cytotoxic effect of liposomal nanoparticles loaded with doxorubicin [63]. | |
| Human Melanoma cells | Monolayer | Direct CAP | Plasma Jet | Continuous doxorubicin treatment followed by CAP treatment is more effective than simultaneous drug and plasma treatment [64]. | |
| Human Breast cancer | Monolayer | Indirect PCLs (cell culture media as liquid) | Plasma Jet | Sinergistally elimination of cell viabilityby the combination of PCL and doxorubicin [65]. | |
| Human metastatic bone, prostate cancer | Monolayer/engineered model | Indirect PCLs (cell culture media as liquid) | Plasma Jet | PCL loss cytotoxicty in 3D environments. Combination of low dose of PCL and DOX improves cytotoxic effects of doxorubicin unafeccting healthy counteraparts [39]. | |
| Epirubicin | Murine Melanoma | Monolayer/spheroids | Direct CAP | Plasma Jet | Direct CAP application increase the cytotoxic effects of epirubicin an other chemoterapeutic agents [62]. |
| Cisplatin | Human head and neck squamous carcinoma | Monolayer | Direct CAP | Dielectric barrier discharge (DBD) | Direct CAP increase the efectiveness of low dose of CIS [66] |
| Human oral squamous carcinoma | Monolayer | Direct CAP | Plasma Jet | The combination of CAP and cisplatin has a synergistic anti-cancer effect, although normal fibroblast cells are less sensitive to the combinatory treatment [67]. | |
| Hepatocellular Carcinoma | Monolayer | Direct CAP + PCLs (cell culture media as liquid) | Dielectric barrier discharge (DBD) | PCL in combination with cisplatin has an additive cytotoxic effect that is higher than when PCL is combined with other drugs (sorafenib, doxorubicin, or trametinib). CAP targets cancer stem cell properties and has less impact on healthy cells [68]. | |
| Paclitaxel | Human Breast cancer | Monolayer | Indirect PCLs (cell culture media as liquid) | Dielectric barrier discharge (DBD) | PCLs increase the cytotoxic potential of paclitaxel [69]. |
| Human Breast cancer | Monolayer | Direct CAP | Dielectric barrier discharge (DBD) | CAP restores the paclitaxel sentitive status [70]. | |
| Tamoxifen | Human Breast cancer | Monolayer | Direct CAP | Dielectric barrier discharge (DBD) | CAP restores the paclitaxel sentitive status by modulating gene expression related to drug resistance [71]. |
| 5-Fluorouracil | Human Hepatocarcinoma | Monolayer | Direct CAP | Dielectric barrier discharge (DBD) | Decrease in cell viability of 5-Fluorouracil resistant cells [72]. |
| Human cholangio-carcinoma | Monolayer | Direct CAP | Plasma Jet | Direct CAP application increase the cytotoxic effects of 5-Fluorouracil [73]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murillo, D.; Huergo, C.; Gallego, B.; Rodríguez, R.; Tornín, J. Exploring the Use of Cold Atmospheric Plasma to Overcome Drug Resistance in Cancer. Biomedicines 2023, 11, 208. https://doi.org/10.3390/biomedicines11010208
Murillo D, Huergo C, Gallego B, Rodríguez R, Tornín J. Exploring the Use of Cold Atmospheric Plasma to Overcome Drug Resistance in Cancer. Biomedicines. 2023; 11(1):208. https://doi.org/10.3390/biomedicines11010208
Chicago/Turabian StyleMurillo, Dzohara, Carmen Huergo, Borja Gallego, René Rodríguez, and Juan Tornín. 2023. "Exploring the Use of Cold Atmospheric Plasma to Overcome Drug Resistance in Cancer" Biomedicines 11, no. 1: 208. https://doi.org/10.3390/biomedicines11010208
APA StyleMurillo, D., Huergo, C., Gallego, B., Rodríguez, R., & Tornín, J. (2023). Exploring the Use of Cold Atmospheric Plasma to Overcome Drug Resistance in Cancer. Biomedicines, 11(1), 208. https://doi.org/10.3390/biomedicines11010208






