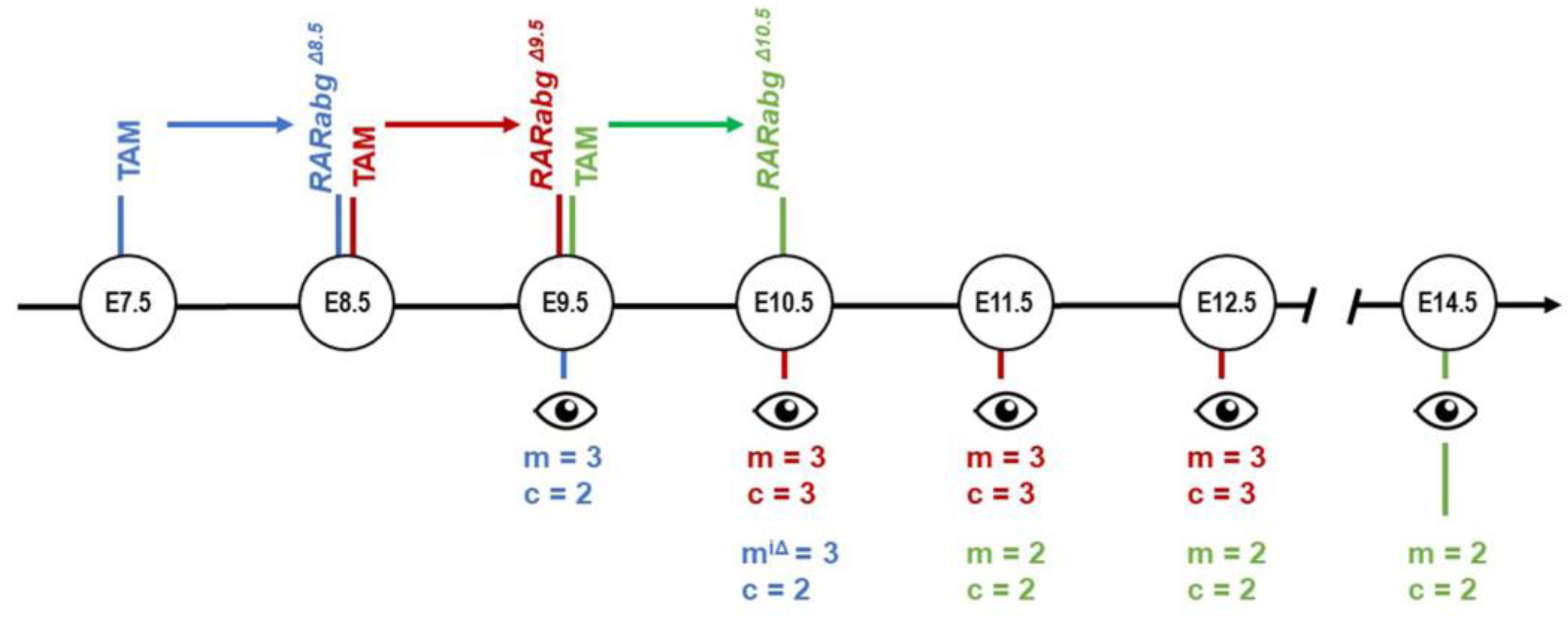
Timeline of Developmental Defects Generated upon Genetic Inhibition of the Retinoic Acid Receptor Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Collection, Staging, Tissue Processing and Reconstruction of Embryos
3. Results
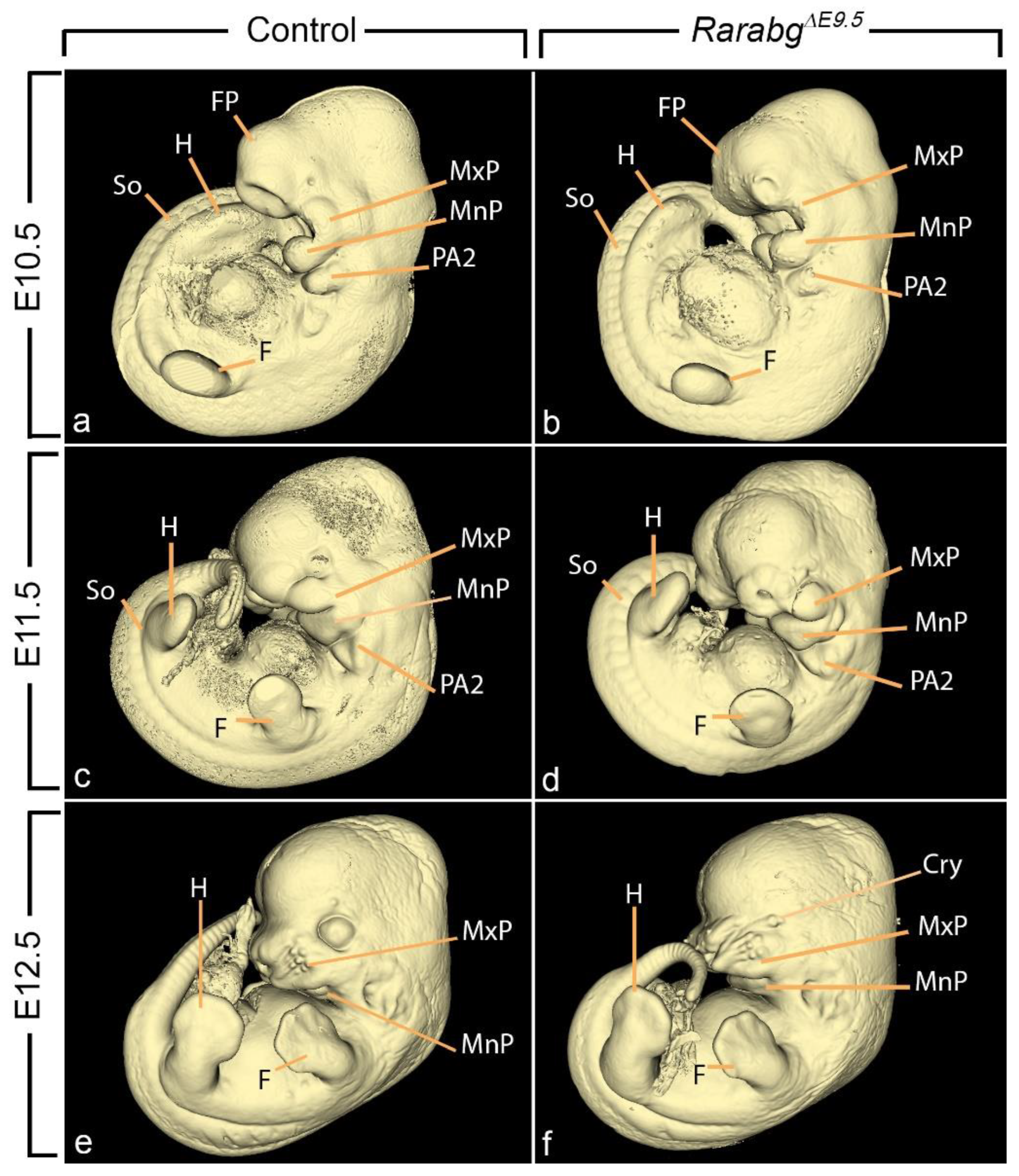
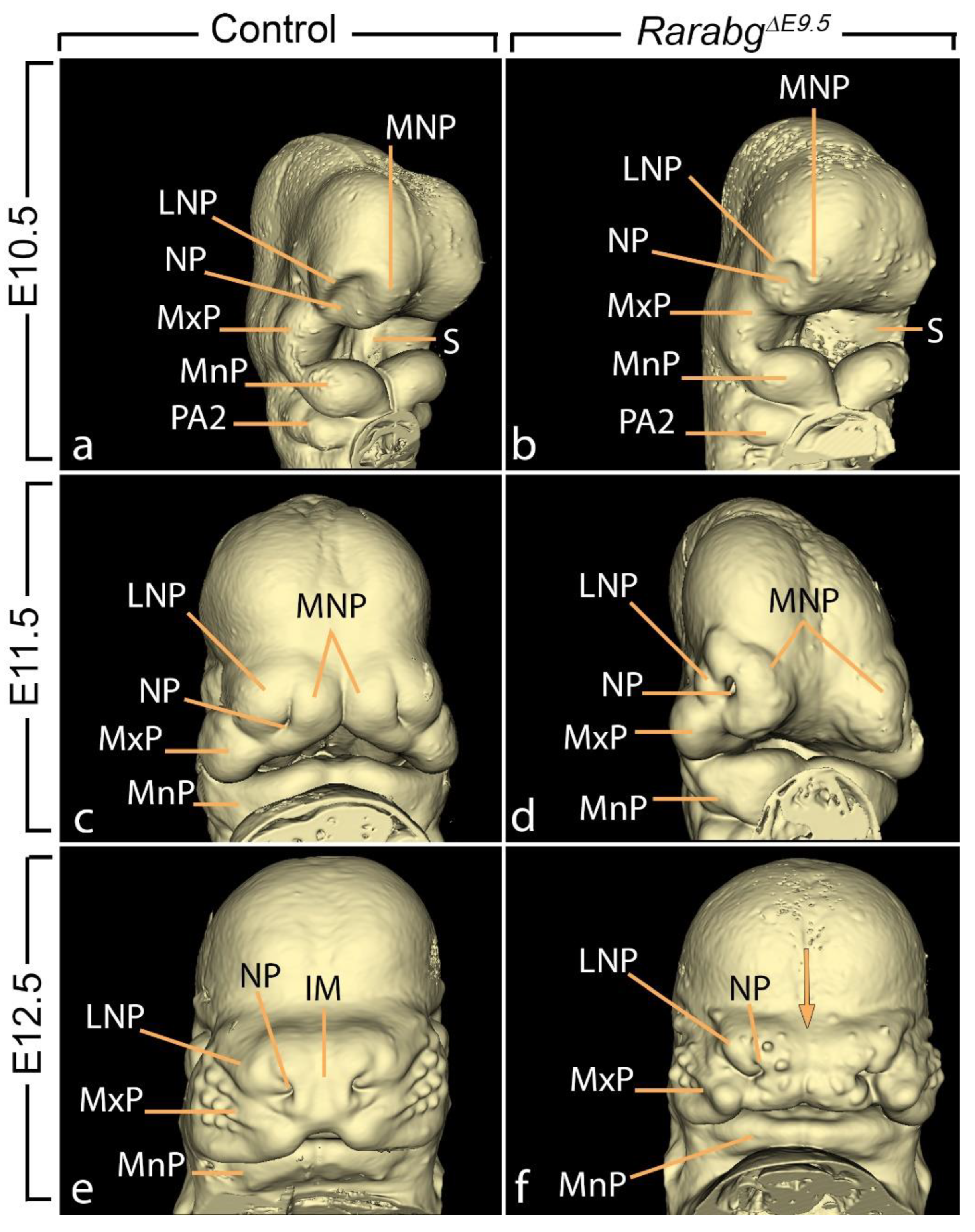
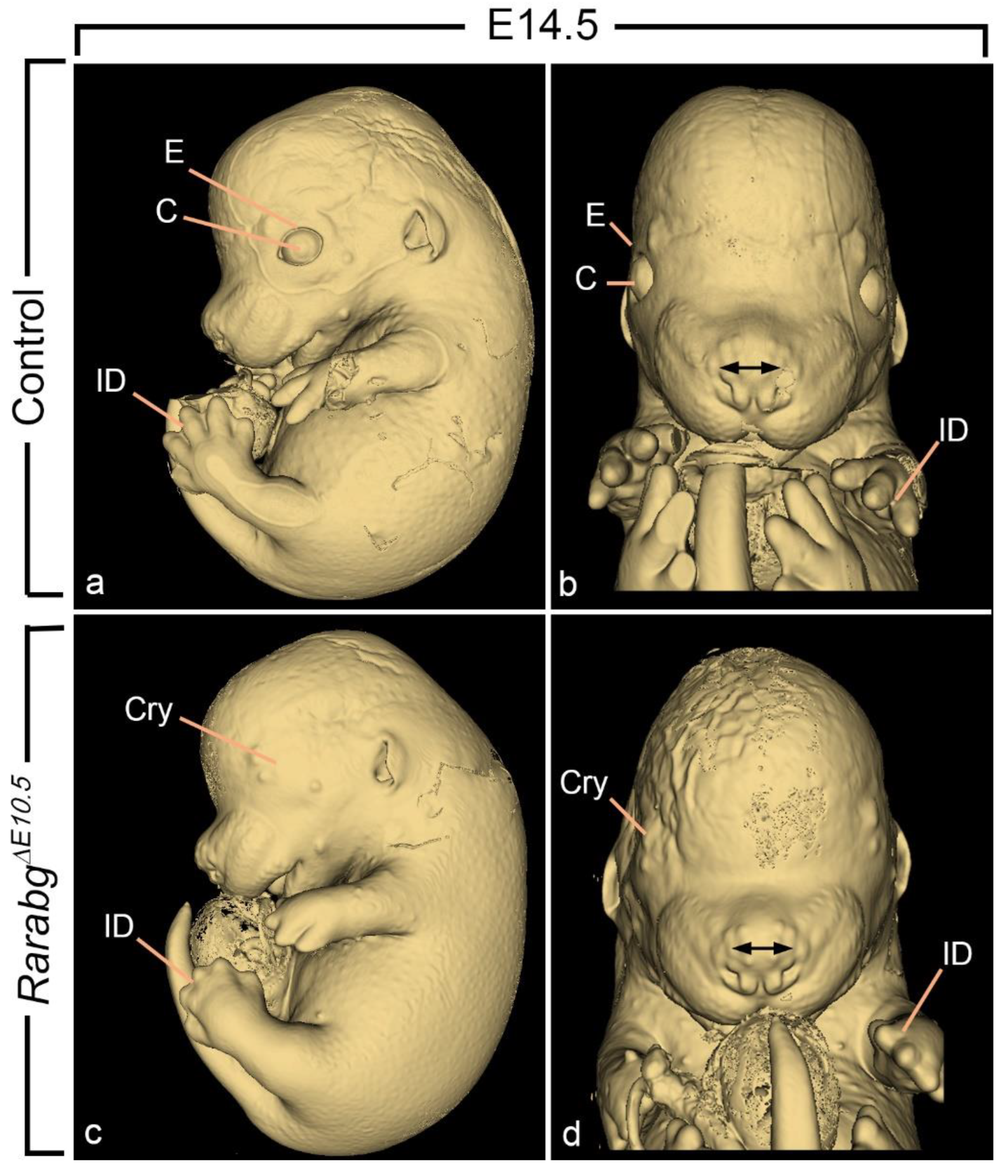
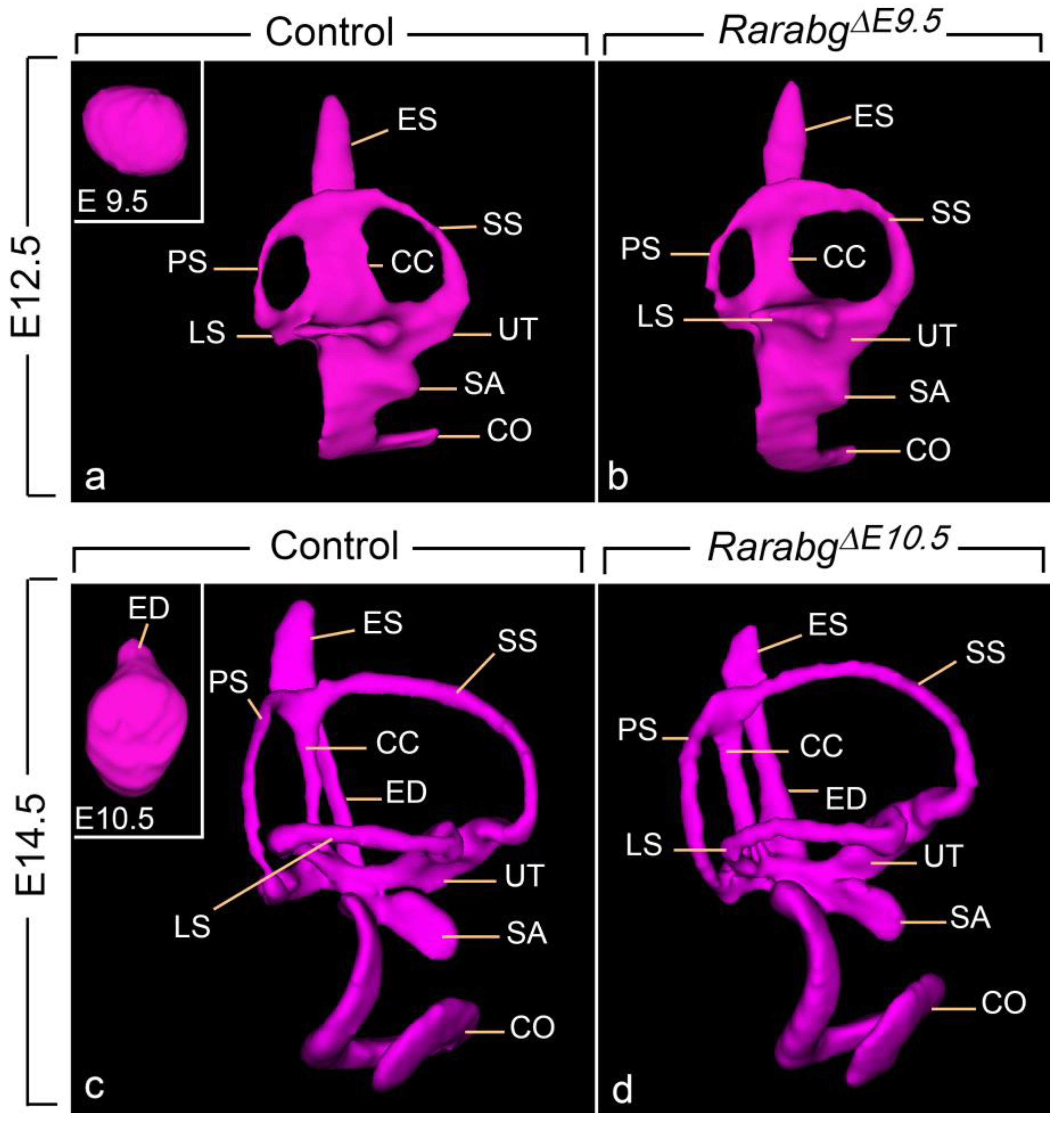
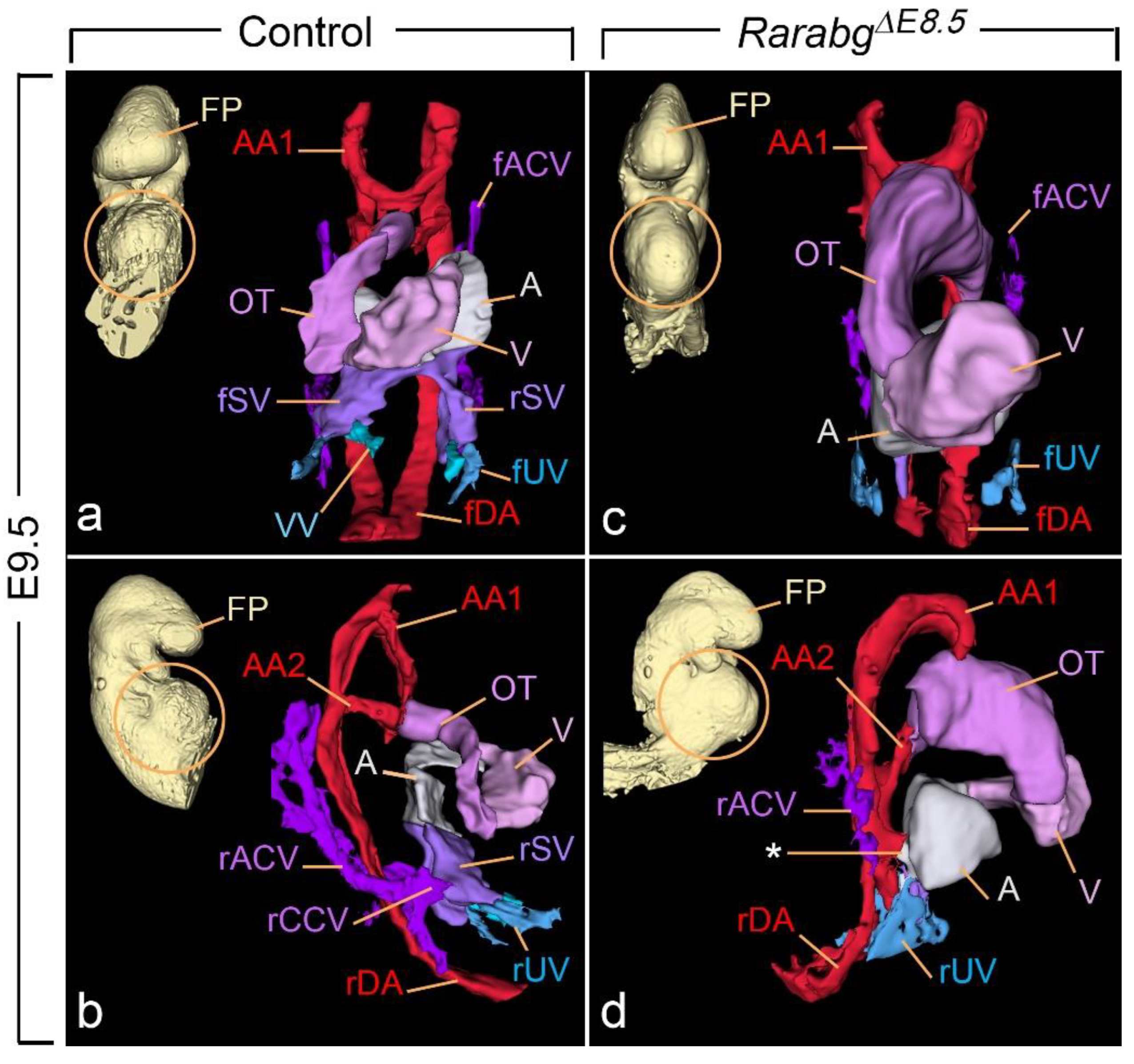
3.1. Abnormal Phenotype of RarabgΔE9.5 Embryos and Comparison with RarabgΔE10.5 Embryos
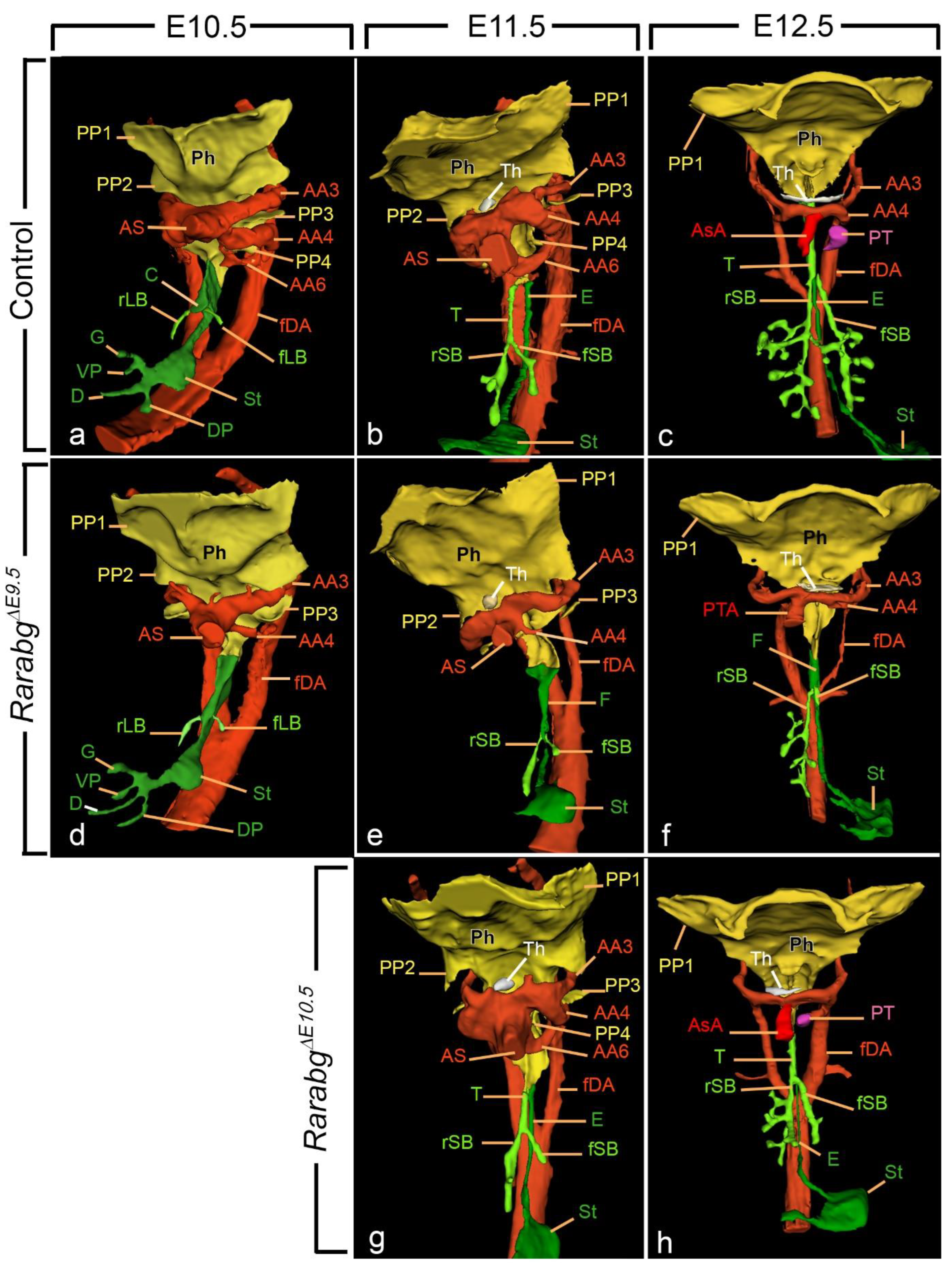
3.1.1. Tracheal Agenesis
3.1.2. Delayed Lung Branching Morphogenesis
3.1.3. Persistent Truncus Arteriosus
3.1.4. Ageneses of the 3rd, 4th, and 6th Aortic Arches
3.1.5. Reduced Development of the 3rd and 4th Pharyngeal Pouches
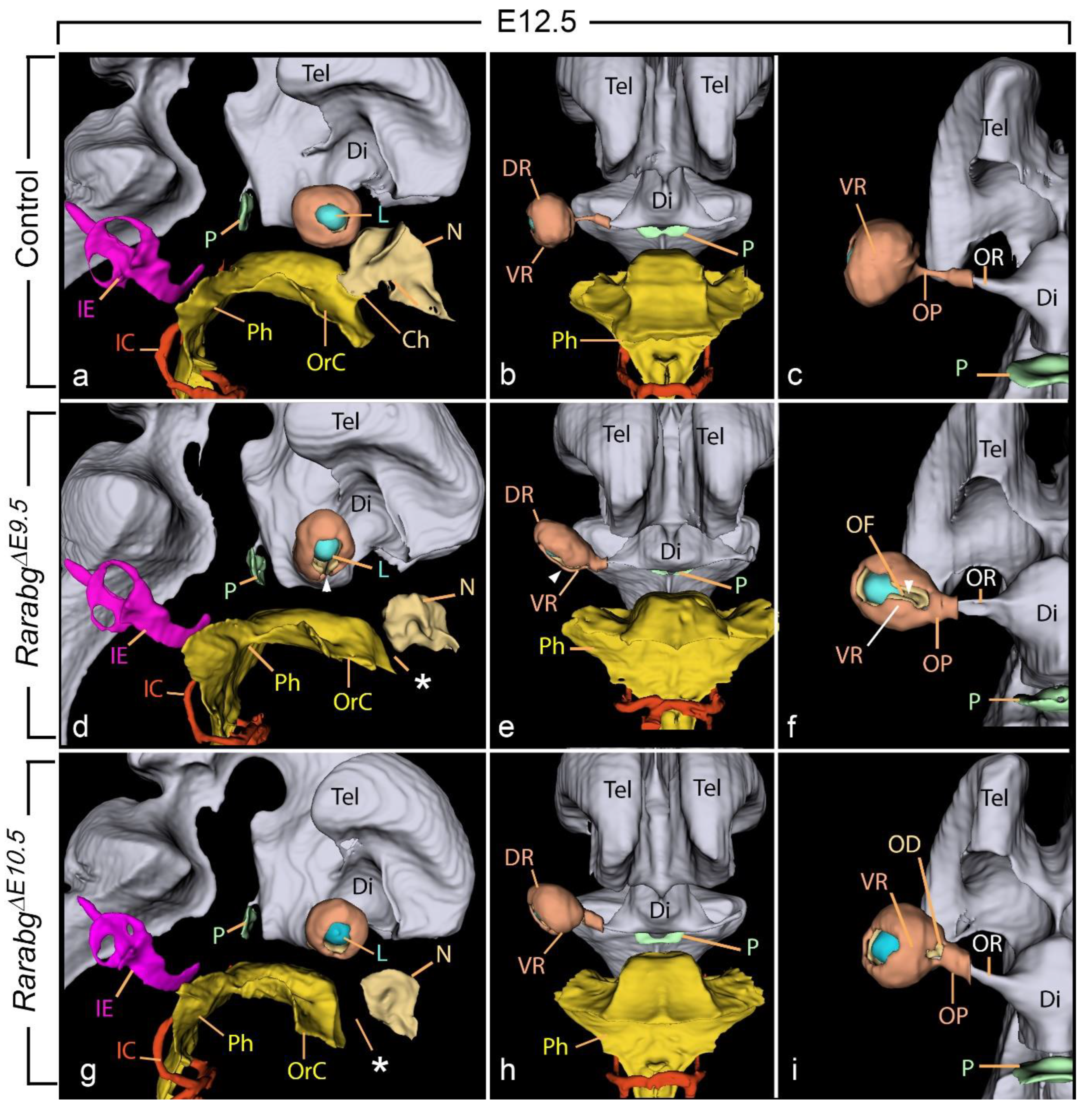
3.1.6. Absence of Closure of the Optic Fissure and Associated Ocular Defects
3.1.7. Hypoplasia of the Nasal Processes and Cavities
3.1.8. Near Normal Morphogenesis of the Inner Ear
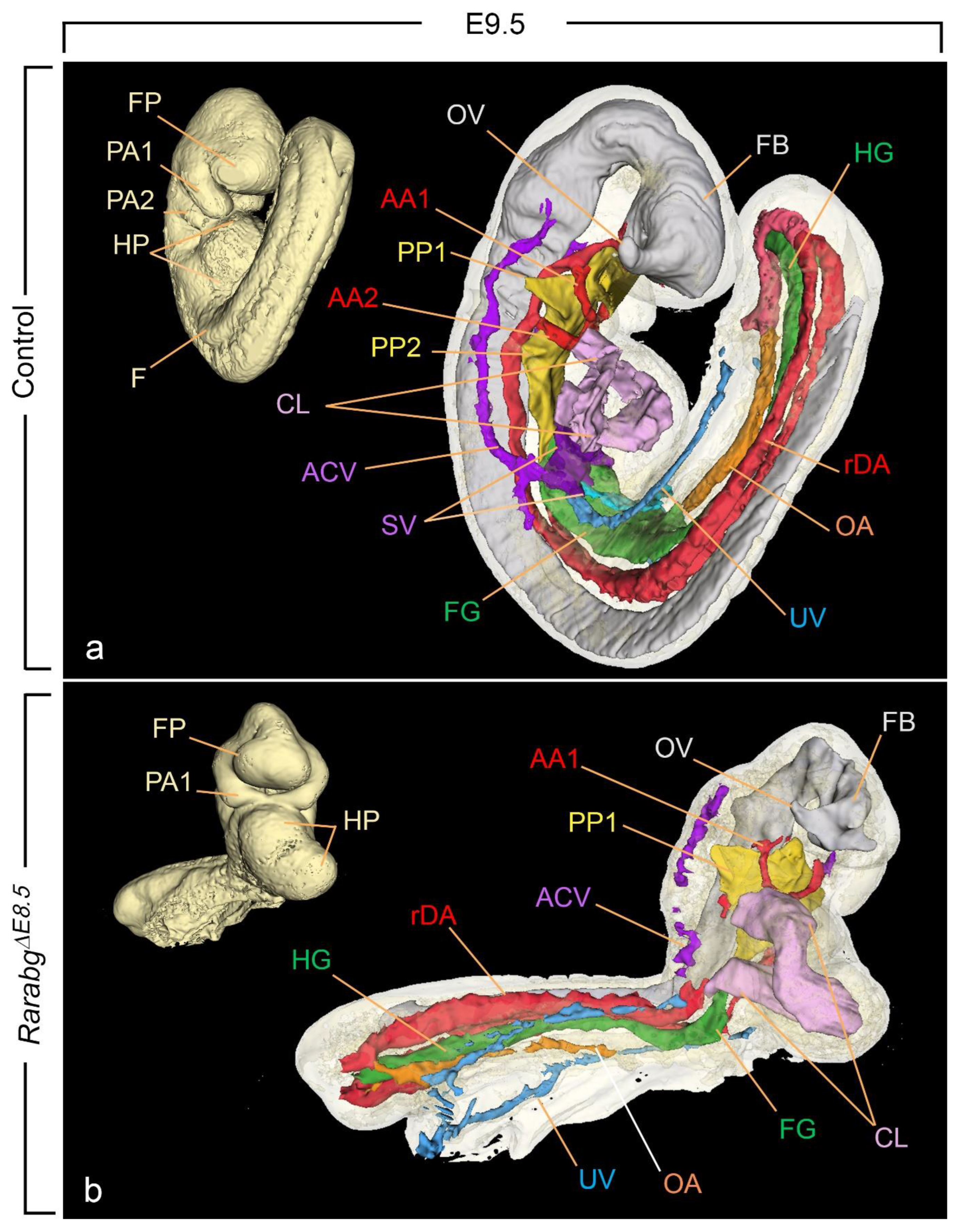
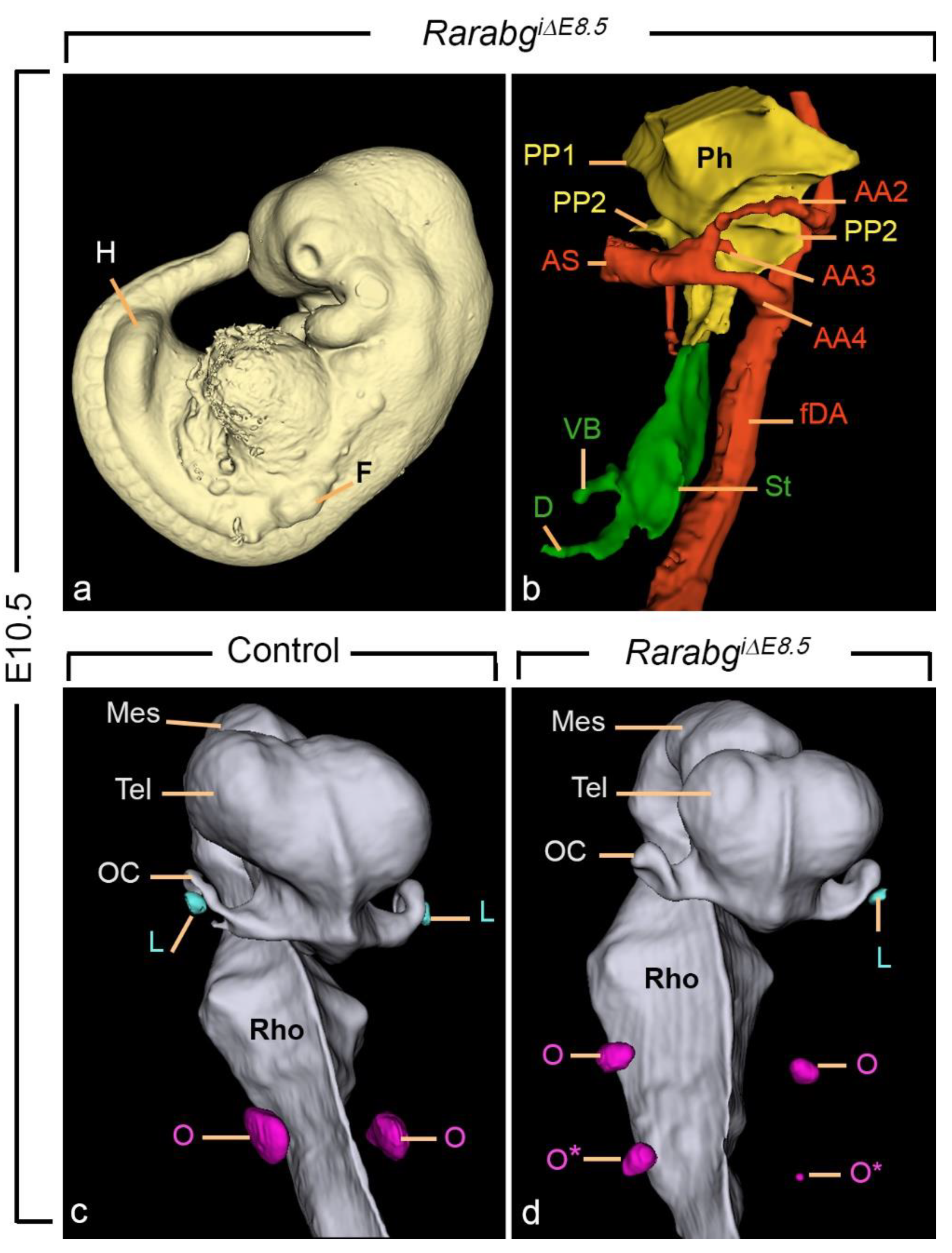
3.2. The Overall Development of RarabgΔE8.5 Mutants Is Arrested Shortly after Rar Excision
3.3. Incomplete Excision of Rara and Rarg at E8.5 Yields Severe Developmental Defects
4. Discussion
4.1. RARs Have Essential Roles of in the Establishment of the Respiratory System
4.2. RARs Exert Functions at Different Stages of Cardiovascular Development
4.3. Distinct Timing of RAR Signalling Pathway in Pharyngeal Pouches
4.4. Different Events in Eye Morphogenesis Require a Functional RAR Signalling Pathway
4.5. A Crucial Role of the RAR Signalling Pathway before the Otocyst Stage
4.6. Early Functions of the RAR Signalling Pathway in the Midface
4.7. The RAR Signalling Pathway Is Instrumental to Axial Rotation
4.8. RAR Signalling in the Development of the Urogenital System and Rectum
4.9. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Function of retinoic acid receptors during embryonic development. Nucl. Recept. Signal. 2009, 7, e002. [Google Scholar] [CrossRef] [PubMed]
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Function of retinoid nuclear receptors: Lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 451–480. [Google Scholar] [CrossRef] [PubMed]
- Vernet, N.; Condrea, D.; Mayere, C.; Féret, B.; Klopfenstein, M.; Magnant, W.; Alunni, V.; Teletin, M.; Souali-Crespo, S.; Nef, S.; et al. Meiosis occurs normally in the fetal ovary of mice lacking all retinoic acid receptors. Sci. Adv. 2020, 6, eaaz1139. [Google Scholar] [CrossRef] [PubMed]
- Mark, M.; Teletin, M.; Wendling, O.; Vonesch, J.L.; Féret, B.; Hérault, Y.; Ghyselinck, N.B. Pathogenesis of anorectal malformations in retinoic acid receptor knockout mice studied by HREM. Biomedicines 2021, 9, 742. [Google Scholar] [CrossRef]
- Ruzankina, Y.; Pinzon-Guzman, C.; Asare, A.; Ong, T.; Pontano, L.; Cotsarelis, G.; Zediak, V.P.; Velez, M.; Bhandoola, A.; Brown, E.J. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 2007, 1, 113–126. [Google Scholar] [CrossRef]
- Theiler, K. The House Mouse. Development and Normal Stages from Fertilization to 4 Weeks of Age; Springer: Berlin/Heidelberg, Germany, 1972. [Google Scholar]
- Mohun, T.J.; Weninger, W.J. Embedding embryos for high-resolution episcopic microscopy (HREM). Cold Spring Harb. Protoc. 2012, 2012, 678–680. [Google Scholar] [CrossRef]
- Wendling, O.; Hentsch, D.; Jacobs, H.; Lemercier, N.; Taubert, S.; Pertuy, F.; Vonesch, J.L.; Sorg, T.; Di Michele, M.; Le Cam, L.; et al. High Resolution Episcopic Microscopy for qualitative and quantitative data in phenotyping altered embryos and adult mice using the new “Histo3D” system. Biomedicines 2021, 9, 767. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Mendelsohn, C.; Lohnes, D.; Décimo, D.; Lufkin, T.; LeMeur, M.; Chambon, P.; Mark, M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development 1994, 120, 2749–2771. [Google Scholar] [CrossRef]
- Ghyselinck, N.B.; Dupé, V.; Dierich, A.; Messaddeq, N.; Garnier, J.M.; Rochette-Egly, C.; Chambon, P.; Mark, M. Role of the retinoic acid receptor beta (RARbeta) during mouse development. Int. J. Dev. Biol. 1997, 41, 425–447. [Google Scholar] [PubMed]
- Lohnes, D.; Mark, M.; Mendelsohn, C.; Dollé, P.; Dierich, A.; Gorry, P.; Gansmuller, A.; Chambon, P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development 1994, 120, 2723–2748. [Google Scholar] [CrossRef] [PubMed]
- Romand, R.; Krezel, W.; Beraneck, M.; Cammas, L.; Fraulob, V.; Messaddeq, N.; Kessler, P.; Hashino, E.; Dollé, P. Retinoic acid deficiency impairs the vestibular function. J. Neurosci. 2013, 33, 5856–5866. [Google Scholar] [CrossRef] [PubMed]
- Dupé, V.; Matt, N.; Garnier, J.M.; Chambon, P.; Mark, M.; Ghyselinck, N.B. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc. Natl. Acad. Sci. USA 2003, 100, 14036–14041. [Google Scholar] [CrossRef] [PubMed]
- Spooner, B.S.; Wessells, N.K. Mammalian lung development: Interactions in primordium formation and bronchial morphogenesis. J. Exp. Zool. 1970, 175, 445–454. [Google Scholar] [CrossRef]
- Henick, D.H. Three-dimensional analysis of murine laryngeal development. Ann. Otol. Rhinol. Laryngol. Suppl. 1993, 159, 3–24. [Google Scholar] [CrossRef]
- Billmyre, K.K.; Hutson, M.; Klingensmith, J. One shall become two: Separation of the esophagus and trachea from the common foregut tube. Dev. Dyn. 2015, 244, 277–288. [Google Scholar] [CrossRef]
- Cardoso, W.V.; Lü, J. Regulation of early lung morphogenesis: Questions, facts and controversies. Development 2006, 133, 1611–1624. [Google Scholar] [CrossRef]
- Metzger, R.J.; Klein, O.D.; Martin, G.R.; Krasnow, M.A. The branching programme of mouse lung development. Nature 2008, 453, 745–750. [Google Scholar] [CrossRef]
- Jiang, X.; Choudhary, B.; Merki, E.; Chien, K.R.; Maxson, R.E.; Sucov, H.M. Normal fate and altered function of the cardiac neural crest cell lineage in retinoic acid receptor mutant embryos. Mech. Dev. 2002, 117, 115–122. [Google Scholar] [CrossRef]
- Anderson, R.H.; Chaudhry, B.; Mohun, T.J.; Bamforth, S.D.; Hoyland, D.; Phillips, H.M.; Webb, S.; Moorman, A.F.; Brown, N.A.; Henderson, D.J. Normal and abnormal development of the intrapericardial arterial trunks in humans and mice. Cardiovasc. Res. 2012, 95, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Bamforth, S.D. Morphogenesis of the Mammalian Aortic Arch Arteries. Front. Cell Dev. Biol. 2022, 10, 892900. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, T.; Nakajima, Y.; Nakamura, H. Development of pharyngeal arch arteries in early mouse embryo. J. Anat. 2002, 201, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Cordier, A.C.; Haumont, S.M. Development of thymus, parathyroids, and ultimo-branchial bodies in NMRI and nude mice. Am. J. Anat. 1980, 157, 227–263. [Google Scholar] [CrossRef]
- Kameda, Y.; Nishimaki, T.; Chisaka, O.; Iseki, S.; Sucov, H.M. Expression of the epithelial marker E-cadherin by thyroid C cells and their precursors during murine development. J. Histochem. Cytochem. 2007, 55, 1075–1088. [Google Scholar] [CrossRef]
- Patel, A.; Sowden, J.C. Genes and pathways in optic fissure closure. Semin. Cell. Dev. Biol. 2019, 91, 55–65. [Google Scholar] [CrossRef]
- Jiang, R.; Bush, J.O.; Lidral, A.C. Development of the upper lip: Morphogenetic and molecular mechanisms. Dev. Dyn. 2006, 235, 1152–1166. [Google Scholar] [CrossRef]
- Diewert, V.M.; Wang, K.Y. Recent advances in primary palate and midface morphogenesis research. Crit. Rev. Oral. Biol. Med. 1992, 4, 111–130. [Google Scholar] [CrossRef]
- Morsli, H.; Choo, D.; Ryan, A.; Johnson, R.; Wu, D.K. Development of the mouse inner ear and origin of its sensory organs. J. Neurosci. 1998, 18, 3327–3335. [Google Scholar] [CrossRef]
- Wu, D.K.; Kelley, M.W. Molecular mechanisms of inner ear development. Cold Spring Harb. Perspect. Biol. 2012, 4, a008409. [Google Scholar] [CrossRef]
- Faisst, A.M.; Alvarez-Bolado, G.; Treichel, D.; Gruss, P. Rotatin is a novel gene required for axial rotation and left-right specification in mouse embryos. Mech. Dev. 2002, 113, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Niederreither, K.; Subbarayan, V.; Dollé, P.; Chambon, P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 1999, 21, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Bohnsack, B.L.; Niederreither, K.; Hirschi, K.K. Retinoic acid regulates endothelial cell proliferation during vasculogenesis. Development 2003, 130, 6465–6474. [Google Scholar] [CrossRef] [PubMed]
- Jainandunsing, J.S.; Linnemann, R.; Maessen, J.; Natour, N.E.; Lorusso, R.; Gelsomino, S.; Johnson, D.M.; Natour, E. Aorto-atrial fistula formation and therapy. J. Thorac. Dis. 2019, 11, 1016–1021. [Google Scholar] [CrossRef]
- Webb, S.; Brown, N.A.; Wessels, A.; Anderson, R.H. Development of the murine pulmonary vein and its relationship to the embryonic venous sinus. Anat. Rec. 1998, 250, 325–334. [Google Scholar] [CrossRef]
- Niederreither, K.; Vermot, J.; Messaddeq, N.; Schuhbaur, B.; Chambon, P.; Dollé, P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 2001, 128, 1019–1031. [Google Scholar] [CrossRef]
- Mollard, R.; Ghyselinck, N.B.; Wendling, O.; Chambon, P.; Mark, M. Stage-dependent responses of the developing lung to retinoic acid signaling. Int. J. Dev. Biol. 2000, 44, 457–462. [Google Scholar]
- Pawlikowski, B.; Wragge, J.; Siegenthaler, J.A. Retinoic acid signaling in vascular development. Genesis 2019, 57, e23287. [Google Scholar] [CrossRef]
- Mic, F.A.; Haselbeck, R.J.; Cuenca, A.E.; Duester, G. Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development 2002, 129, 2271–2282. [Google Scholar] [CrossRef]
- Chazaud, C.; Chambon, P.; Dollé, P. Retinoic acid is required in the mouse embryo for left-right asymmetry determination and heart morphogenesis. Development 1999, 126, 2589–2596. [Google Scholar] [CrossRef]
- Wendling, O.; Dennefeld, C.; Chambon, P.; Mark, M. Retinoid signaling is essential for patterning the endoderm of the third and fourth pharyngeal arches. Development 2000, 127, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Dupé, V.; Ghyselinck, N.B.; Wendling, O.; Chambon, P.; Mark, M. Key roles of retinoic acid receptors alpha and beta in the patterning of the caudal hindbrain, pharyngeal arches and otocyst in the mouse. Development 1999, 126, 5051–5059. [Google Scholar] [CrossRef] [PubMed]
- Cvekl, A.; Ashery-Padan, R. The cellular and molecular mechanisms of vertebrate lens development. Development 2014, 141, 4432–4447. [Google Scholar] [CrossRef] [PubMed]
- Gage, P.J.; Rhoades, W.; Prucka, S.K.; Hjalt, T. Fate maps of neural crest and mesoderm in the mammalian eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4200–4208. [Google Scholar] [CrossRef]
- Matt, N.; Dupé, V.; Garnier, J.M.; Dennefeld, C.; Chambon, P.; Mark, M.; Ghyselinck, N.B. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development 2005, 132, 4789–4800. [Google Scholar] [CrossRef]
- Frenz, D.A.; Liu, W.; Galinovic-Schwartz, V.; Van De Water, T.R. Retinoic acid-induced embryopathy of the mouse inner ear. Teratology 1996, 53, 292–303. [Google Scholar] [CrossRef]
- Choo, D.; Sanne, J.L.; Wu, D.K. The differential sensitivities of inner ear structures to retinoic acid during development. Dev. Biol. 1998, 204, 136–150. [Google Scholar] [CrossRef]
- Bok, J.; Raft, S.; Kong, K.A.; Koo, S.K.; Dräger, U.C.; Wu, D.K. Transient retinoic acid signaling confers anterior-posterior polarity to the inner ear. Proc. Natl. Acad. Sci. USA 2011, 108, 161–166. [Google Scholar] [CrossRef]
- Romand, R.; Hashino, E.; Dollé, P.; Vonesch, J.L.; Chambon, P.; Ghyselinck, N.B. The retinoic acid receptors RARalpha and RARgamma are required for inner ear development. Mech. Dev. 2002, 119, 213–223. [Google Scholar] [CrossRef]
- Romand, R.; Kondo, T.; Fraulob, V.; Petkovich, M.; Dollé, P.; Hashino, E. Dynamic expression of retinoic acid-synthesizing and -metabolizing enzymes in the developing mouse inner ear. J. Comp. Neurol. 2006, 496, 643–654. [Google Scholar] [CrossRef]
- Niederreither, K.; Vermot, J.; Schuhbaur, B.; Chambon, P.; Dollé, P. Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development. 2000, 127, 75–85. [Google Scholar] [CrossRef]
- Wendling, O.; Ghyselinck, N.B.; Chambon, P.; Mark, M. Roles of retinoic acid receptors in early embryonic morphogenesis and hindbrain patterning. Development 2001, 128, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- Dupé, V.; Pellerin, I. Retinoic acid receptors exhibit cell-autonomous functions in cranial neural crest cells. Dev. Dyn. 2009, 238, 2701–2711. [Google Scholar] [CrossRef] [PubMed]
- Serbedzija, G.N.; Bronner-Fraser, M.; Fraser, S.E. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development 1992, 116, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dollé, P.; Cardoso, W.V.; Niederreither, K. Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev. Biol. 2006, 297, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Molotkov, A.; Molotkova, N.; Duester, G. Retinoic acid generated by Raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev. Dyn. 2005, 232, 950–957. [Google Scholar] [CrossRef]
- Martín, M.; Gallego-Llamas, J.; Ribes, V.; Kedinger, M.; Niederreither, K.; Chambon, P.; Dollé, P.; Gradwohl, G. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev. Biol. 2005, 284, 399–411. [Google Scholar] [CrossRef]
- Sajithlal, G.; Zou, D.; Silvius, D.; Xu, P.X. Eya 1 acts as a critical regulator for specifying the metanephric mesenchyme. Dev. Biol. 2005, 284, 323–336. [Google Scholar] [CrossRef]


| Predicted Outcome | |
|---|---|
| Respiratory defects | |
| No left lung bud | Left lung agenesis * |
| Delayed formation of secondary bronchi # | Lung hypoplasia * |
| Agenesis of the trachea # | |
| Cardiovascular defects | |
| Persistent truncus arteriosus # | |
| Abnormal patterning of the aortic arches # | Abnormal cephalic arteries * |
| No remodelling of umbilical roots # § | Absent iliac arteries ** |
| Ocular defects | |
| Cryptophthalmos # § | No cornea, iris and eyelid folds * |
| Short ventral retina # § | |
| Ventral rotation of the optic cup and lens # § | |
| Absence of closure of the optic fissure # | Colobomatous microphtalmia* |
| Inner ear defects | |
| Reduced development of the saccule and endolymphatic sac # | Hypoplasia of the saccule and endolymphatic sac *** |
| Facial defects | |
| Hypoplastic nasal processes # | Midfacial cleft * |
| Persistent oronasal membrane # § | Choanal atresia *** |
| Small nasal cavities # § | |
| Urogenital defects | |
| • Hypoplastic urogenital sinus # § | Agenesis of the rectum ** |
| • Kidney hypoplasia # § | |
| • Kidney ectopia # § | |
| • Abnormal endings of Wolffian ducts # § |
| Individual Mutant Embryos | Control Embryos | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E10.5 | E11.5 | E10.5 | E11.5 | |||||||||||||
| a | b | c | d | e | f | (n = 3) | (n = 3) | |||||||||
| L | R | L | R | L | R | L | R | L | R | L | R | L | R | L | R | |
| AA1 | +/− | +/− | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| AA2 | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − |
| AA3 | +/− | − | +/− | − | + | + | + | + | + | + | +/− | − | + | + | + | + |
| AA4 | + | +/− | + | + | + | + | + | + | +/− | +/− | − | + | + | + | + | + |
| AA6 | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | + |
| PP3 | hy | abs | hy | abs | pre | pre | pre | pre | pre | pre | hy | hy | pre | pre | pre | pre |
| PP4 | abs | abs | abs | abs | abs | abs | abs | abs | abs | abs | abs | abs | pre | pre | pre | pre |
| Type of Defect | RarabgΔE8.5 | Aldh1a2−/− KO * |
|---|---|---|
| Failure of axial rotation | + # | + |
| Absence of the limb buds | + # | + |
| Open ventral body wall | + # | + |
| Small and densely packed somites | + # | + |
| Narrow and folded neural tube | + # | + |
| Open neural tube | + | + |
| Absence of the 2nd aortic arch | + | + |
| Dilated heart | + # | + |
| Absence of the sinus venosus | + # | + |
| Absence of large vitelline veins | + | + |
| Hypoplastic otocyst | NA | + |
| No looping of the heart tube | − | + |
| Aorto-atrial fistula | + # | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teletin, M.; Mark, M.; Wendling, O.; Vernet, N.; Féret, B.; Klopfenstein, M.; Herault, Y.; Ghyselinck, N.B. Timeline of Developmental Defects Generated upon Genetic Inhibition of the Retinoic Acid Receptor Signaling Pathway. Biomedicines 2023, 11, 198. https://doi.org/10.3390/biomedicines11010198
Teletin M, Mark M, Wendling O, Vernet N, Féret B, Klopfenstein M, Herault Y, Ghyselinck NB. Timeline of Developmental Defects Generated upon Genetic Inhibition of the Retinoic Acid Receptor Signaling Pathway. Biomedicines. 2023; 11(1):198. https://doi.org/10.3390/biomedicines11010198
Chicago/Turabian StyleTeletin, Marius, Manuel Mark, Olivia Wendling, Nadège Vernet, Betty Féret, Muriel Klopfenstein, Yann Herault, and Norbert B. Ghyselinck. 2023. "Timeline of Developmental Defects Generated upon Genetic Inhibition of the Retinoic Acid Receptor Signaling Pathway" Biomedicines 11, no. 1: 198. https://doi.org/10.3390/biomedicines11010198
APA StyleTeletin, M., Mark, M., Wendling, O., Vernet, N., Féret, B., Klopfenstein, M., Herault, Y., & Ghyselinck, N. B. (2023). Timeline of Developmental Defects Generated upon Genetic Inhibition of the Retinoic Acid Receptor Signaling Pathway. Biomedicines, 11(1), 198. https://doi.org/10.3390/biomedicines11010198






