Comprehensive Profiling of Early Neoplastic Gastric Microenvironment Modifications and Biodynamics in Impaired BMP-Signaling FoxL1+-Telocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
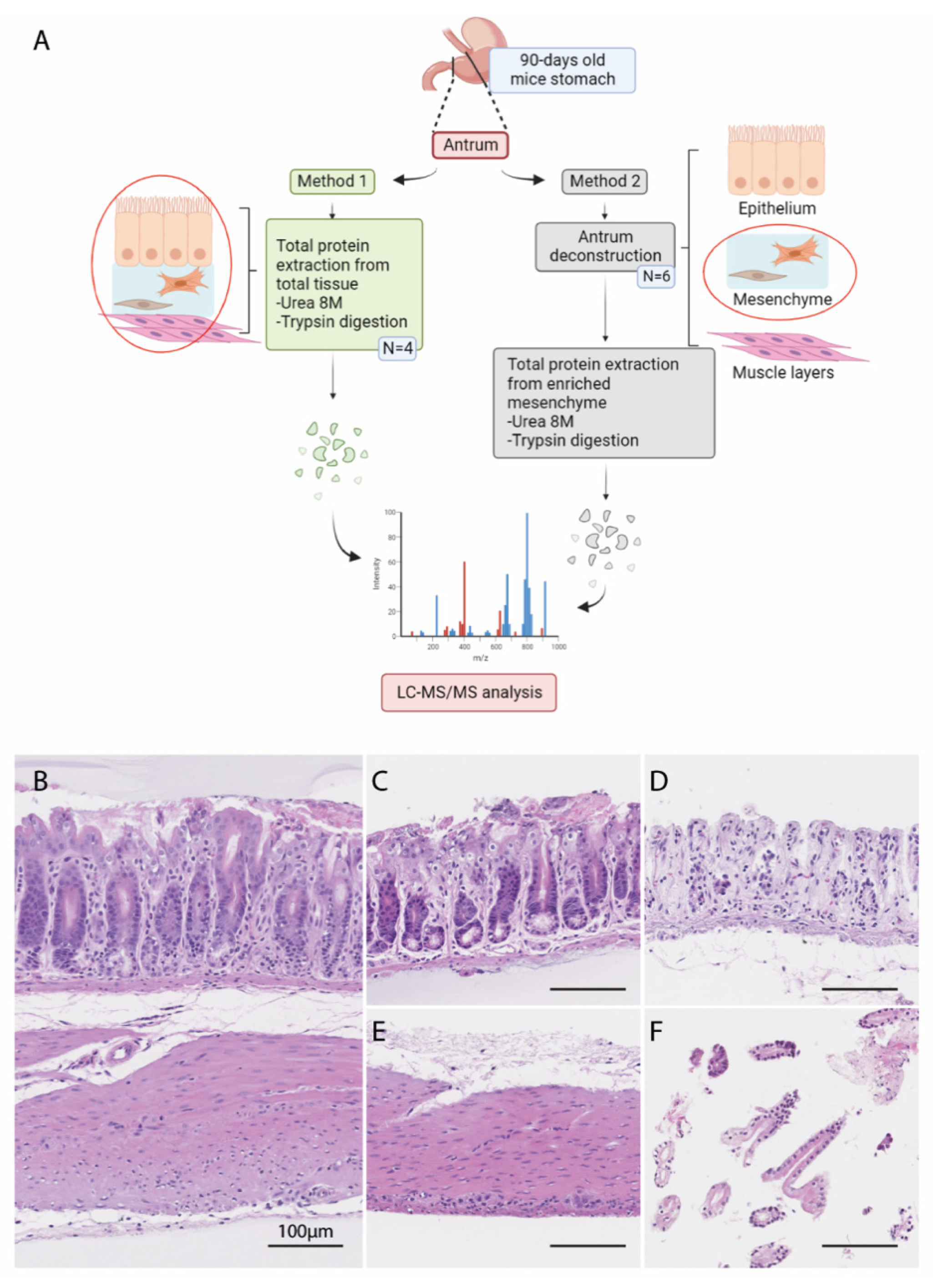
2.2. Deconstruction of Mouse Ex Vivo Stomach Tissues
2.3. Histological Analysis
2.4. In-Solution Digestion of Proteins to Peptides for Mass Spectrometry Analysis
2.5. Purification and Desalting of the Peptides on C18 Columns
2.6. LC-MS/MS Analysis
2.7. Protein Identification Using MaxQuant Analysis
2.8. Differential and Statistical Analyses of Mass Spectrometry Data
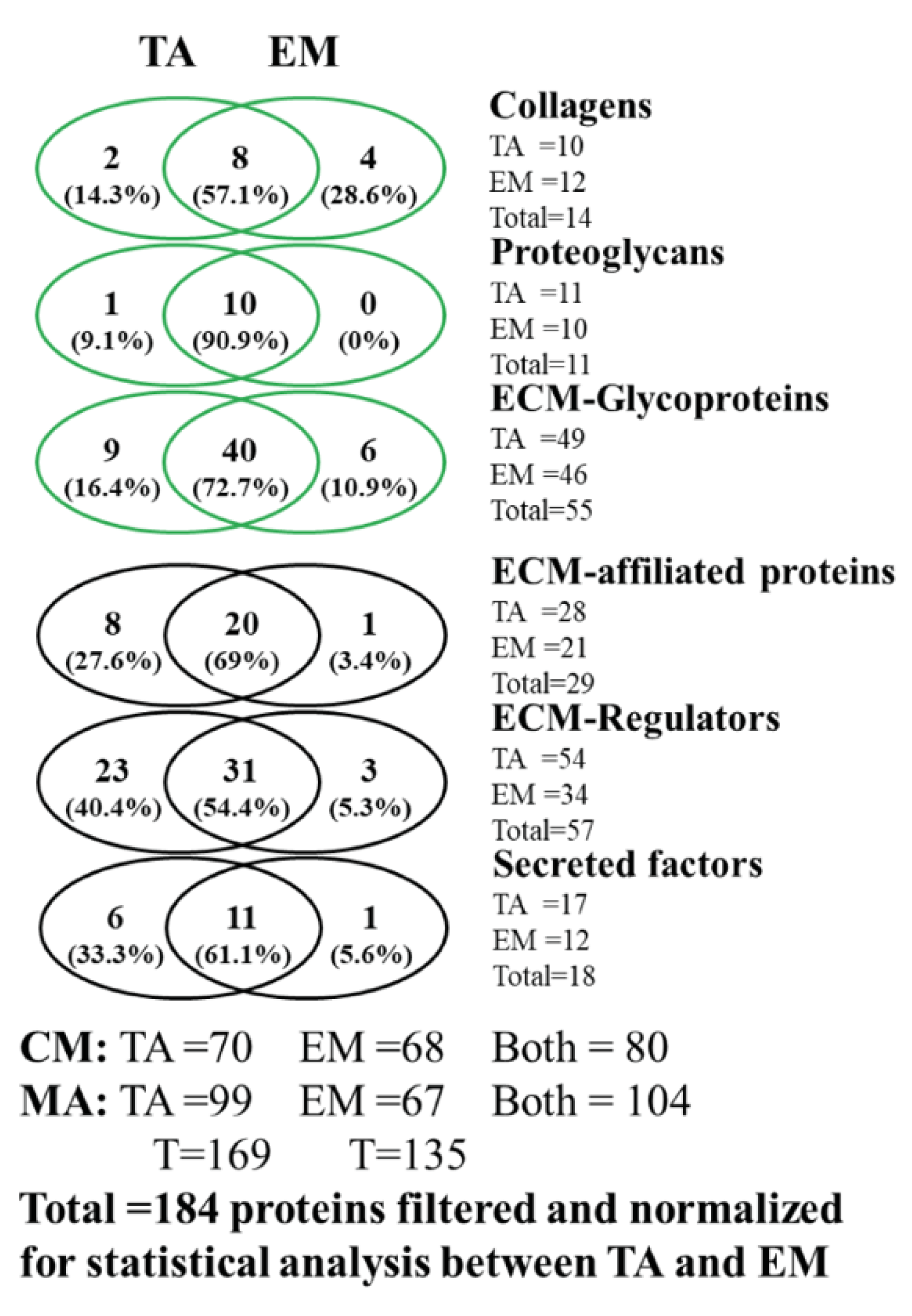
2.9. Matrisome Identification
2.10. Indirect Immunofluorescence
2.11. Picro-Sirius Red Staining
2.12. Immunoblot Analysis
3. Results
3.1. Analysis of the Matrisome from Total Antrum of BmpR1a△FoxL1+ Mouse
3.2. Analysis of the Matrisome from Enriched Mesenchymal Antrum of BmpR1a△FoxL1+ Mouse
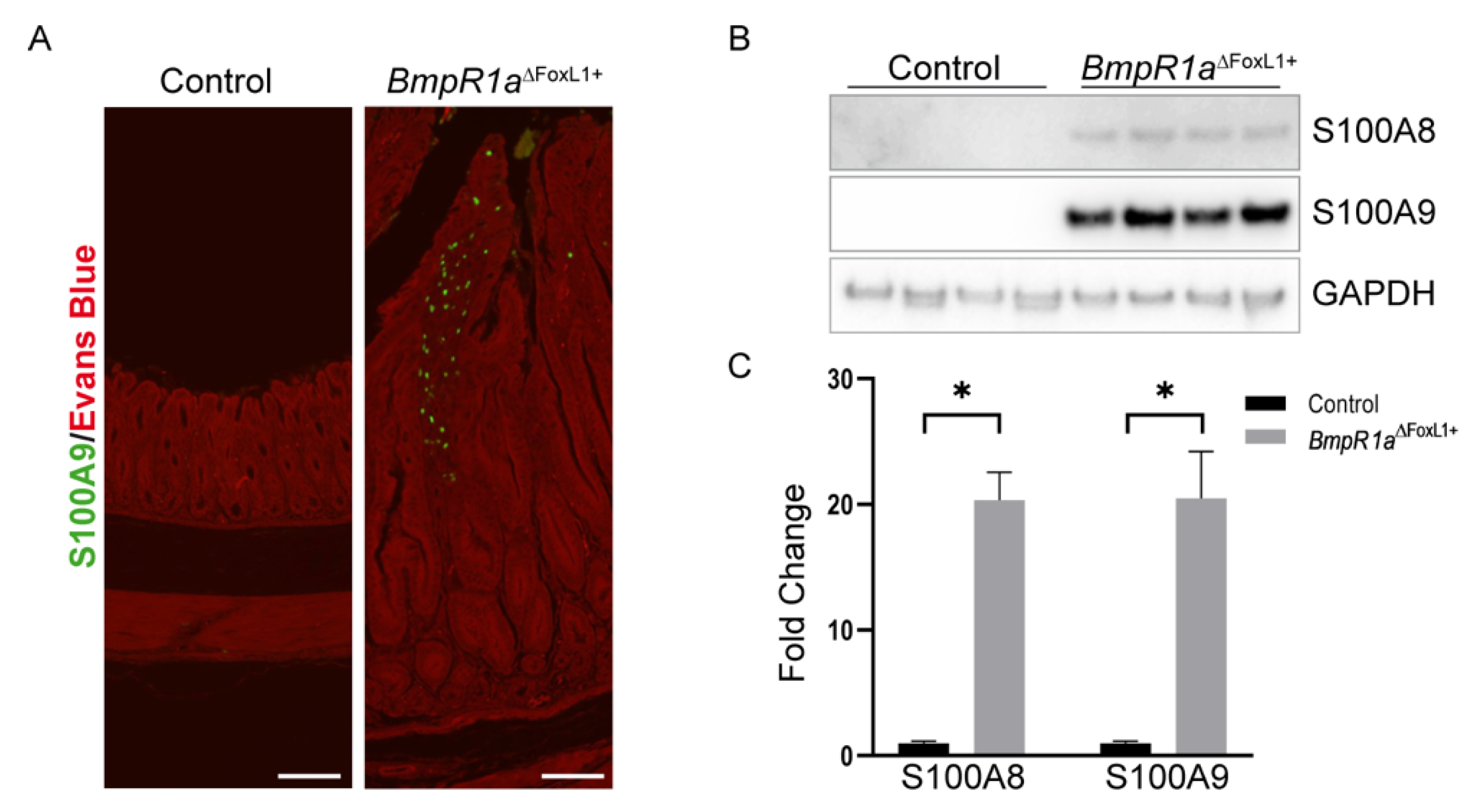
3.3. Loss of BMP Signaling in Gastric TCFoxL1+ Induces Dysregulations in ECM Biodynamics Associated with Inflammation
3.4. Disruption of the CL Network in Mice with Impaired Gastric BMP Signaling in TCFoxL1+
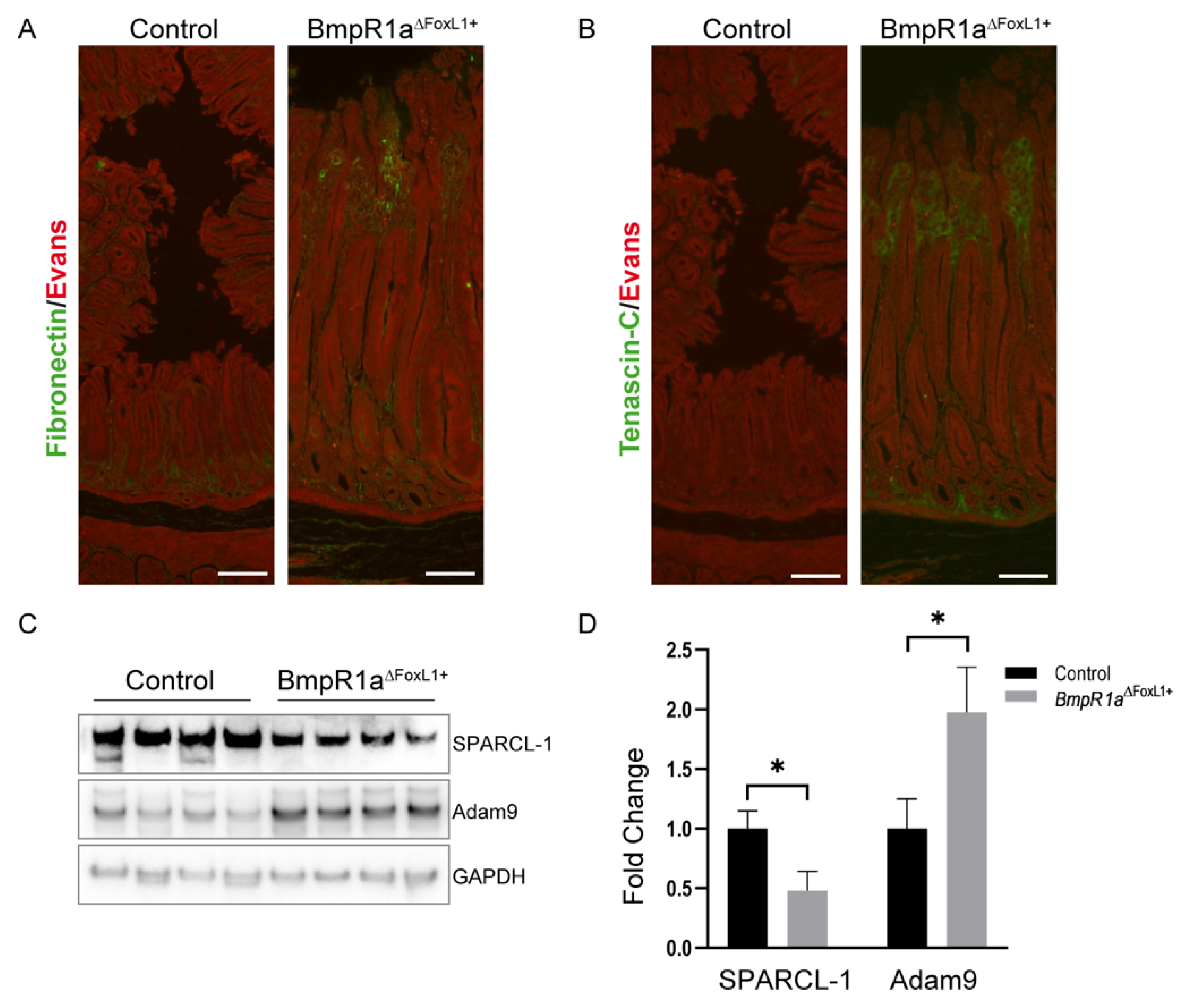
3.5. Loss of BMP Signaling in Gastric TCFoxL1+ Causes Remodeling of ECM Glycoproteins Associated with Early Gastric Neoplasia
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Mojares, E.; Del Rio Hernandez, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef]
- Filipe, E.C.; Chitty, J.L.; Cox, T.R. Charting the unexplored extracellular matrix in cancer. Int. J. Exp. Pathol. 2018, 99, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014, 15, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.M.; Pereira, J.; Melo, S.; Fernandes, M.S.; Carneiro, P.; Seruca, R.; Figueiredo, J. The Extracellular Matrix: An Accomplice in Gastric Cancer Development and Progression. Cells 2020, 9, 394. [Google Scholar] [CrossRef]
- Gerarduzzi, C.; Hartmann, U.; Leask, A.; Drobetsky, E. The Matrix Revolution: Matricellular Proteins and Restructuring of the Cancer Microenvironment. Cancer Res. 2020, 80, 2705–2717. [Google Scholar] [CrossRef]
- Brauchle, E.; Kasper, J.; Daum, R.; Schierbaum, N.; Falch, C.; Kirschniak, A.; Schaffer, T.E.; Schenke-Layland, K. Biomechanical and biomolecular characterization of extracellular matrix structures in human colon carcinomas. Matrix Biol. 2018, 68–69, 180–193. [Google Scholar] [CrossRef]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef]
- Yang, Z.; Xue, F.; Li, M.; Zhu, X.; Lu, X.; Wang, C.; Xu, E.; Wang, X.; Zhang, L.; Yu, H.; et al. Extracellular Matrix Characterization in Gastric Cancer Helps to Predict Prognosis and Chemotherapy Response. Front. Oncol. 2021, 11, 753330. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Hynes, R.O.; Naba, A. Overview of the matrisome—An inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Hoersch, S.; Liu, H.; Carr, S.A.; Hynes, R.O. The matrisome: In silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell. Proteom. 2012, 11, M111.014647. [Google Scholar] [CrossRef]
- McCarthy, N.; Manieri, E.; Storm, E.E.; Saadatpour, A.; Luoma, A.M.; Kapoor, V.N.; Madha, S.; Gaynor, L.T.; Cox, C.; Keerthivasan, S.; et al. Distinct Mesenchymal Cell Populations Generate the Essential Intestinal BMP Signaling Gradient. Cell Stem Cell 2020, 26, 391–402.e5. [Google Scholar] [CrossRef]
- Powell, D.W.; Pinchuk, I.V.; Saada, J.I.; Chen, X.; Mifflin, R.C. Mesenchymal cells of the intestinal lamina propria. Annu. Rev. Physiol. 2011, 73, 213–237. [Google Scholar] [CrossRef]
- Shoshkes-Carmel, M.; Wang, Y.J.; Wangensteen, K.J.; Toth, B.; Kondo, A.; Massasa, E.E.; Itzkovitz, S.; Kaestner, K.H. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature 2018, 557, 242–246. [Google Scholar] [CrossRef]
- Allaire, J.M.; Roy, S.A.; Ouellet, C.; Lemieux, E.; Jones, C.; Paquet, M.; Boudreau, F.; Perreault, N. Bmp signaling in colonic mesenchyme regulates stromal microenvironment and protects from polyposis initiation. Int. J. Cancer 2016, 138, 2700–2712. [Google Scholar] [CrossRef]
- Langlois, M.J.; Servant, R.; Reyes Nicolas, V.; Jones, C.; Roy, S.A.B.; Paquet, M.; Carrier, J.C.; Rivard, N.; Boudreau, F.; Perreault, N. Loss of PTEN Signaling in Foxl1(+) Mesenchymal Telocytes Initiates Spontaneous Colonic Neoplasia in Mice. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 530–533.e5. [Google Scholar] [CrossRef]
- McCarthy, N.; Kraiczy, J.; Shivdasani, R.A. Cellular and molecular architecture of the intestinal stem cell niche. Nature 2020, 22, 1033–1041. [Google Scholar] [CrossRef]
- Reyes Nicolas, V.; Allaire, J.M.; Alfonso, A.B.; Pupo Gomez, D.; Pomerleau, V.; Giroux, V.; Boudreau, F.; Perreault, N. Altered Mucus Barrier Integrity and Increased Susceptibility to Colitis in Mice upon Loss of Telocyte Bone Morphogenetic Protein Signalling. Cells 2021, 10, 2954. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.A.; Allaire, J.M.; Ouellet, C.; Maloum-Rami, F.; Pomerleau, V.; Lemieux, E.; Babeu, J.P.; Rousseau, J.; Paquet, M.; Garde-Granger, P.; et al. Loss of mesenchymal bone morphogenetic protein signaling leads to development of reactive stroma and initiation of the gastric neoplastic cascade. Sci. Rep. 2016, 6, 32759. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Shoshkes-Carmel, M.; Gao, N.; Shin, S.; May, C.L.; Golson, M.L.; Zahm, A.M.; Ray, M.; Wiser, C.L.; Wright, C.V.; et al. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Greicius, G.; Kabiri, Z.; Sigmundsson, K.; Liang, C.; Bunte, R.; Singh, M.K.; Virshup, D.M. PDGFRalpha(+) pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc. Natl. Acad. Sci. USA 2018, 115, E3173–E3181. [Google Scholar] [CrossRef]
- Kaestner, K.H. The Intestinal Stem Cell Niche: A Central Role for Foxl1-Expressing Subepithelial Telocytes. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 111–117. [Google Scholar] [CrossRef]
- Kondo, A.; Kaestner, K.H. Emerging diverse roles of telocytes. Development 2019, 146, dev175018. [Google Scholar] [CrossRef]
- Sackett, S.D.; Fulmer, J.T.; Friedman, J.R.; Kaestner, K.H. Foxl1-Cre BAC transgenic mice: A new tool for gene ablation in the gastrointestinal mesenchyme. Genesis 2007, 45, 518–522. [Google Scholar] [CrossRef]
- Mishina, Y.; Hanks, M.C.; Miura, S.; Tallquist, M.D.; Behringer, R.R. Generation of Bmpr/Alk3 conditional knockout mice. Genesis 2002, 32, 69–72. [Google Scholar] [CrossRef]
- Pomerleau, V.; Nicolas, V.R.; Jurkovic, C.M.; Faucheux, N.; Lauzon, M.A.; Boisvert, F.M.; Perreault, N. FOXL1+ Telocytes in mouse colon orchestrate extracellular matrix biodynamics and wound repair resolution. J. Proteom. 2023, 21, 104755. [Google Scholar] [CrossRef]
- Wieczorek, S.; Combes, F.; Lazar, C.; Giai Gianetto, Q.; Gatto, L.; Dorffer, A.; Hesse, A.M.; Coute, Y.; Ferro, M.; Bruley, C.; et al. DAPAR & ProStaR: Software to perform statistical analyses in quantitative discovery proteomics. Bioinformatics 2017, 33, 135–136. [Google Scholar] [CrossRef]
- Allaire, J.M.; Darsigny, M.; Marcoux, S.S.; Roy, S.A.; Schmouth, J.F.; Umans, L.; Zwijsen, A.; Boudreau, F.; Perreault, N. Loss of Smad5 leads to the disassembly of the apical junctional complex and increased susceptibility to experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G586–G597. [Google Scholar] [CrossRef] [PubMed]
- Gagne-Sansfacon, J.; Allaire, J.M.; Jones, C.; Boudreau, F.; Perreault, N. Loss of Sonic hedgehog leads to alterations in intestinal secretory cell maturation and autophagy. PLoS ONE 2014, 9, e98751. [Google Scholar] [CrossRef] [PubMed]
- Maloum, F.; Allaire, J.M.; Gagne-Sansfacon, J.; Roy, E.; Belleville, K.; Sarret, P.; Morisset, J.; Carrier, J.C.; Mishina, Y.; Kaestner, K.H.; et al. Epithelial BMP signaling is required for proper specification of epithelial cell lineages and gastric endocrine cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G1065–G1079. [Google Scholar] [CrossRef] [PubMed]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Perreault, N.; Beaulieu, J.F. Primary cultures of fully differentiated and pure human intestinal epithelial cells. Exp. Cell Res. 1998, 245, 34–42. [Google Scholar] [CrossRef]
- Naba, A.; Pearce, O.M.T.; Del Rosario, A.; Ma, D.; Ding, H.; Rajeeve, V.; Cutillas, P.R.; Balkwill, F.R.; Hynes, R.O. Characterization of the Extracellular Matrix of Normal and Diseased Tissues Using Proteomics. J. Proteome Res. 2017, 16, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Stretching the boundaries of extracellular matrix research. Nat. Rev. Mol. Cell Biol. 2014, 15, 761–763. [Google Scholar] [CrossRef]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordonez-Moran, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Grevers, L.C.; de Vries, T.J.; Vogl, T.; Abdollahi-Roodsaz, S.; Sloetjes, A.W.; Leenen, P.J.; Roth, J.; Everts, V.; van den Berg, W.B.; van Lent, P.L. S100A8 enhances osteoclastic bone resorption in vitro through activation of Toll-like receptor 4: Implications for bone destruction in murine antigen-induced arthritis. Arthritis Rheum. 2011, 63, 1365–1375. [Google Scholar] [CrossRef]
- Ehrchen, J.M.; Sunderkotter, C.; Foell, D.; Vogl, T.; Roth, J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 2009, 86, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Foell, D.; Wittkowski, H.; Roth, J. Mechanisms of disease: A ‘DAMP’ view of inflammatory arthritis. Nat. Clin. Pr. Rheumatol. 2007, 3, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Ji, C.D.; Xiao, H.L.; Zhao, H.B.; Cui, Y.H.; Bian, X.W. Reorganized Collagen in the Tumor Microenvironment of Gastric Cancer and Its Association with Prognosis. J. Cancer 2017, 8, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Min, S.; Choi, Y.S.; Jo, S.H.; Jung, J.H.; Han, K.; Kim, J.; An, S.; Ji, Y.W.; Kim, Y.G.; et al. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat. Commun. 2022, 13, 1692. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadegan, M.R.; Mojarrad, M.; Moghbeli, M. Role of extra cellular proteins in gastric cancer progression and metastasis: An update. Genes Environ. 2020, 42, 18. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, Z.; Zhao, Y.; Zhang, Q.; Wu, X.; Miao, B.; Cao, J.; Fei, S. FN1, SPARC, and SERPINE1 are highly expressed and significantly related to a poor prognosis of gastric adenocarcinoma revealed by microarray and bioinformatics. Sci. Rep. 2019, 9, 7827. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.Y.; Cheong, J.H. The Matrisome Is Associated with Metabolic Reprograming in Stem-like Phenotypes of Gastric Cancer. Cancers 2022, 14, 1438. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Hynes, R.O. Enrichment of Extracellular Matrix Proteins from Tissues and Digestion into Peptides for Mass Spectrometry Analysis. J. Vis. Exp. 2015, e53057. [Google Scholar] [CrossRef]
- Socovich, A.M.; Naba, A. The cancer matrisome: From comprehensive characterization to biomarker discovery. Semin. Cell Dev. Biol. 2019, 89, 157–166. [Google Scholar] [CrossRef]
- Taha, I.N.; Naba, A. Exploring the extracellular matrix in health and disease using proteomics. Essays Biochem. 2019, 63, 417–432. [Google Scholar] [CrossRef]
- Shao, X.; Taha, I.N.; Clauser, K.R.; Gao, Y.T.; Naba, A. MatrisomeDB: The ECM-protein knowledge database. Nucleic Acids Res. 2020, 48, D1136–D1144. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. A human model of gastric carcinogenesis. Cancer Res. 1988, 48, 3554–3560. [Google Scholar] [PubMed]
- Piazuelo, M.B.; Correa, P. Gastric cancer: Overview. Colomb. Med. 2013, 44, 192–201. [Google Scholar]
- De Re, V. Molecular Features Distinguish Gastric Cancer Subtypes. Int. J. Mol. Sci. 2018, 19, 3121. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, S.; Watanabe, A.; Aburatani, H.; Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 2006, 8, 1369–1375. [Google Scholar] [CrossRef]
- Salama, I.; Malone, P.S.; Mihaimeed, F.; Jones, J.L. A review of the S100 proteins in cancer. Eur. J. Surg. Oncol. 2008, 34, 357–364. [Google Scholar] [CrossRef]
- Foell, D.; Wittkowski, H.; Vogl, T.; Roth, J. S100 proteins expressed in phagocytes: A novel group of damage-associated molecular pattern molecules. J. Leukoc. Biol. 2007, 81, 28–37. [Google Scholar] [CrossRef]
- Emberley, E.D.; Murphy, L.C.; Watson, P.H. S100 proteins and their influence on pro-survival pathways in cancer. Biochem. Cell Biol. 2004, 82, 508–515. [Google Scholar] [CrossRef]
- Gebhardt, C.; Nemeth, J.; Angel, P.; Hess, J. S100A8 and S100A9 in inflammation and cancer. Biochem. Pharmacol. 2006, 72, 1622–1631. [Google Scholar] [CrossRef]
- Hermani, A.; De Servi, B.; Medunjanin, S.; Tessier, P.A.; Mayer, D. S100A8 and S100A9 activate MAP kinase and NF-kappaB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Exp. Cell Res. 2006, 312, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.W.; Lindner, H.; Sarg, B.; Mueller-Holzner, E.; Abendstein, B.; Bergant, A.; Fessler, S.; Schwaerzler, P.; Zeimet, A.; Marth, C.; et al. Calgranulins in cystic fluid and serum from patients with ovarian carcinomas. Cancer Res. 2003, 63, 7507–7514. [Google Scholar] [PubMed]
- Bresnick, A.R.; Weber, D.J.; Zimmer, D.B. S100 proteins in cancer. Nat. Rev. Cancer 2015, 15, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Efthymiou, G.; Saint, A.; Ruff, M.; Rekad, Z.; Ciais, D.; Van Obberghen-Schilling, E. Shaping Up the Tumor Microenvironment With Cellular Fibronectin. Front. Oncol. 2020, 10, 641. [Google Scholar] [CrossRef]
- Liu, A.Y.; Zheng, H.; Ouyang, G. Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol. 2014, 37, 150–156. [Google Scholar] [CrossRef]
- Sirica, A.E. Matricellular proteins in intrahepatic cholangiocarcinoma. Adv. Cancer Res. 2022, 156, 249–281. [Google Scholar] [CrossRef]
- Shen, C.; Wang, C.; Yin, Y.; Chen, H.; Yin, X.; Cai, Z.; Chen, Z.; Zhang, B.; Zhou, Z. Tenascin-C expression is significantly associated with the progression and prognosis in gastric GISTs. Medicine 2019, 98, e14045. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, C.; Ye, Y.; Wang, Z.; He, Y.; Li, Y.; Mao, H. High expression of fibronectin 1 indicates poor prognosis in gastric cancer. Oncol. Lett. 2020, 19, 93–102. [Google Scholar] [CrossRef]
- Wang, J.; Deng, L.; Huang, J.; Cai, R.; Zhu, X.; Liu, F.; Wang, Q.; Zhang, J.; Zheng, Y. High expression of Fibronectin 1 suppresses apoptosis through the NF-kappaB pathway and is associated with migration in nasopharyngeal carcinoma. Am. J. Transl. Res. 2017, 9, 4502–4511. [Google Scholar]
- Jakharia, A.; Borkakoty, B.; Singh, S. Expression of SPARC like protein 1 (SPARCL1), extracellular matrix-associated protein is down regulated in gastric adenocarcinoma. J. Gastrointest. Oncol. 2016, 7, 278–283. [Google Scholar] [CrossRef]
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. J. Cell Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Fei, X.; Chen, X.; Yan, J.; Liu, B.; Zhu, Z. ADAM9 functions as a promoter of gastric cancer growth which is negatively and post-transcriptionally regulated by miR-126. Oncol. Rep. 2017, 37, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; Li, H.; Yu, H.; Chen, S.; Liu, S.; Zhang, C.; He, Y. FN1 is a prognostic biomarker and correlated with immune infiltrates in gastric cancers. Front. Oncol. 2022, 12, 918719. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Orend, G. The role of tenascin-C in tissue injury and tumorigenesis. J. Cell Commun. Signal. 2009, 3, 287–310. [Google Scholar] [CrossRef] [PubMed]
- Bella, J.; Hulmes, D.J. Fibrillar Collagens. Subcell. Biochem. 2017, 82, 457–490. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a double-edged sword in tumor progression. Tumor Biol. 2014, 35, 2871–2882. [Google Scholar] [CrossRef]
- Taufalele, P.V.; VanderBurgh, J.A.; Munoz, A.; Zanotelli, M.R.; Reinhart-King, C.A. Fiber alignment drives changes in architectural and mechanical features in collagen matrices. PLoS ONE 2019, 14, e0216537. [Google Scholar] [CrossRef]
- Kirkness, M.W.; Lehmann, K.; Forde, N.R. Mechanics and structural stability of the collagen triple helix. Curr. Opin. Chem. Biol. 2019, 53, 98–105. [Google Scholar] [CrossRef]
- Krasny, L.; Paul, A.; Wai, P.; Howard, B.A.; Natrajan, R.C.; Huang, P.H. Comparative proteomic assessment of matrisome enrichment methodologies. Biochem. J. 2016, 473, 3979–3995. [Google Scholar] [CrossRef]
- McCabe, M.C.; Schmitt, L.R.; Hill, R.C.; Dzieciatkowska, M.; Maslanka, M.; Daamen, W.F.; van Kuppevelt, T.H.; Hof, D.J.; Hansen, K.C. Evaluation and Refinement of Sample Preparation Methods for Extracellular Matrix Proteome Coverage. Mol. Cell. Proteom. 2021, 20, 100079. [Google Scholar] [CrossRef] [PubMed]



| Core Matrisome | Matrisome-Associated | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ECM-Glycoproteins | Collagen Chains | ECM-Regulators | ECM-Affiliated Proteins | ||||||||
| Name | FC | p-Value | Name | FC | p-Value | Name | FC | p-Value | Name | FC | p-Value |
| Agrn | 2372 | 0.0076 | Col18a1 | 1.94 | 0.0099 | Adam9 | 510 | 0.0063 | Muc4 | 1594 | 0.0084 |
| Dmbt1 | 3.60 | 0.0188 | Col15a1 | 1.19 | NC/0.1689 | Prss3 | 2.51 | NC/0.0528 | Mbl2 | 1382 | 0.0066 |
| Fgb | 2.96 | 0.0200 | Col6a5 | −1.15 | 0.4277 | Leprel1 | 2.38 | NC/0.0002 | Lgals7 | 4.40 | NC/0.0210 |
| Fgg | 2.67 | 0.0206 | Col4a1 | −1.36 | 0.1037 | Serpinb1a | 2.29 | 0.0044 | Lgals4 | 2.09 | 0.0276 |
| Fga | 2.54 | 0.0175 | Col12a1 | −1.73 | 0.0030 | Serpinb5 | 2.07 | 0.0183 | Lgals9 | 1.63 | 0.0293 |
| Vtn | 2.49 | 0.0191 | Col14a1 | −2.08 | 0.0558 | Ctss | 2.05 | 0.0043 | Anxa3 | 1.54 | 0.0224 |
| Thbs1 | 2.10 | 0.0049 | Col6a1 | −3.10 | 0.0178 | Ctsc | 1.85 | 0.0134 | Anxa10 | 1.51 | 0.0454 |
| Mfge8 | 1.51 | 0.071 | Col6a2 | −4.52 | 0.0124 | Serpinb12 | 1.84 | NC/0.0554 | Anxa1 | 1.48 | 0.0121 |
| Tnc | 1.40 | 0.0253 | Col4a2 | −553 | 0.0071 | Ctsh | 1.73 | 0.0113 | Lgalsl | 1.43 | 0.0206 |
| Igfbp7 | 1.39 | 0.1144 | Col1a2 | −1496 | 0.0049 | F2 | 1.56 | 0.0280 | Reg1 | 1.35 | 0.6373 |
| Creld2 | 1.35 | 0.1078 | Try10 | 1.53 | NC/0.0394 | Hpx | 1.31 | 0.0656 | |||
| Fn1 | 1.33 | 0.0094 | Proteoglycans | Hrg | 1.51 | 0.0357 | Reg2 | 1.29 | NC/0.1715 | ||
| Vwa1 | 1.28 | NC/0.0437 | Name | FC | p-Value | Plg | 1.39 | 0.0466 | Anxa2 | 1.26 | 0.0574 |
| Fbln2 | 1.24 | 0.174 | Bgn | 1.31 | 0.0160 | Ctse | 1.38 | 0.0548 | Anxa7 | 1.25 | 0.0696 |
| Sparc | 1.23 | 0.1481 | Prg2 | 1.09 | 0.7556 | Serpinf2 | 1.37 | 0.0572 | Lman1 | 1.12 | 0.4453 |
| Postn | 1.22 | 0.0344 | Vcan | −1.27 | 0.0841 | Itih3 | 1.36 | 0.0472 | Anxa5 | 1.10 | 0.1127 |
| Lrg1 | 1.14 | 0.2343 | Hspg2 | −1.39 | 0.0302 | Ctsa | 1.35 | 0.0365 | Anxa4 | 1.08 | 0.2235 |
| Vwa5a | 1.13 | 0.1869 | Prelp | −1.41 | 0.0513 | Serpini2 | 1.34 | NC/0.3921 | Muc6 | 1.05 | 0.7641 |
| Tgfbi | 1.12 | 0.1154 | Lum | −1.47 | 0.0188 | Serping1 | 1.33 | 0.0997 | Sema4b | 1.01 | 0.9016 |
| Aebp1 | 1.03 | 0.8022 | Aspn | −1.56 | 0.0104 | Serpinc1 | 1.30 | 0.1275 | Lgals3 | −1.03 | 0.7888 |
| Efemp1 | 1.02 | 0.7931 | Ogn | −1.85 | 0.0065 | Itih2 | 1.26 | 0.1419 | Anxa11 | −1.05 | 0.3729 |
| Ltbp4 | 1.01 | 0.906 | Dcn | −2.05 | 0.0094 | Kng1 | 1.24 | 0.0814 | Plxnb2 | −1.06 | 0.3421 |
| Fbln1 | −1.01 | 0.8928 | Podn | −2835 | 3 × 10−5 | F13a1 | 1.23 | 0.0278 | Lgals1 | −1.10 | 0.2588 |
| Thbs4 | −1.12 | NC/0.5325 | Fmod | −12,933 | 7 × 10−15 | Cst3 | 1.23 | 0.0257 | Anxa6 | −1.47 | 0.0357 |
| Nid2 | −1.20 | 0.1529 | Adam10 | 1.21 | 0.1819 | Muc5ac | −1.50 | 0.0477 | |||
| Fbln5 | −1.23 | 0.1405 | Ctsb | 1.17 | 0.1297 | Sdc1 | −1.55 | NC/0.0175 | |||
| Lamb1 | −1.27 | 0.0300 | Ctsl | 1.15 | 0.1851 | Lgals2 | −2.76 | 0.0067 | |||
| Pcolce | −1.31 | NC/0.0097 | Ctsz | 1.14 | 0.1260 | Cspg4 | −9977 | 1 × 10−14 | |||
| Lamb3 | −1.32 | NC/0.0020 | A2m | 1.13 | 0.3687 | ||||||
| Sbspon | −1.34 | NC/0.0423 | Itih1 | 1.13 | 0.2482 | Secreted factors | |||||
| Tsku | −1.44 | NC/0.0188 | Serpinb9 | 1.10 | 0.4599 | Name | FC | p-Value | |||
| Dpt | −1.58 | 0.0069 | Serpina1e | 1.08 | 0.8936 | S100a9 | 13058 | 0.0059 | |||
| Mfap4 | −1.60 | 0.0210 | Serpinh1 | 1.06 | 0.5231 | S100a8 | 11412 | 0.0043 | |||
| Adipoq | −1.61 | 0.0623 | Ctsd | 1.05 | 0.4297 | Sfrp1 | 3720 | NC/1 × 10−13 | |||
| Lama4 | −1.67 | 0.0145 | Cstb | 1.04 | 0.5912 | Il1rn | 948 | 0.0100 | |||
| Lamc1 | −1.69 | 0.0269 | Ngly1 | 1.03 | 0.8976 | S100a6 | 1.99 | 0.0389 | |||
| Mfap5 | −1.69 | 0.0429 | Serpinf1 | −1.00 | 0.9864 | Rptn | 1.86 | NC/1 × 10−13 | |||
| Nid1 | −1.70 | 0.0116 | Serpind1 | −1.02 | 0.8891 | S100g | 1.58 | 0.0410 | |||
| Lama2 | −1.70 | 0.0377 | Cela2a | −1.05 | 0.9419 | S100a4 | 1.52 | 0.0059 | |||
| Tinagl1 | −1.74 | 0.0283 | Fam20b | −1.06 | 0.5655 | S100a1 | 1.46 | 0.0119 | |||
| Lama5 | −1.77 | 0.0121 | St14 | −1.09 | 0.4949 | S100a13 | 1.38 | 0.0825 | |||
| Emilin1 | −1.79 | NC/4 × 10−6 | Cela3b | −1.11 | 0.8716 | S100a11 | 1.36 | 0.0774 | |||
| Lamb2 | −2.33 | 0.0140 | Serpina3k | −1.18 | 0.5193 | S100a14 | 1.32 | 0.0266 | |||
| Tnxb | −2.38 | 0.0193 | Prss2 | −1.20 | 0.7794 | Il18 | 1.20 | 0.2779 | |||
| Fbn1 | −243 | 1 × 10−5 | Serpina1d | −1.25 | 0.1443 | S100a16 | 1.18 | 0.1155 | |||
| Abi3bp | −1117 | 0.0038 | Tgm2 | −1.29 | 0.0344 | Hcfc1 | −1.12 | 0.0848 | |||
| Mfap2 | −2262 | NC/1 × 10−12 | Serpina1c | −1.30 | 0.3113 | S100a10 | −1.23 | 0.0344 | |||
| Sparcl1 | −15,377 | 1 × 10−13 | Cela1 | −1.30 | 0.6149 | S100b | −2.20 | 1 × 10−5 | |||
| Spp1 | −24,277 | NC/1 × 10−12 | F12 | −1.38 | 0.0016 | ||||||
| P4ha1 | −1.41 | NC/0.0071 | |||||||||
| Serpina1b | −1.47 | 0.1053 | |||||||||
| P4ha2 | −1.63 | NC/0.0159 | |||||||||
| Serpina6 | −1.76 | 0.1680 | |||||||||
| Ambp | −671 | 0.0064 | |||||||||
| Core Matrisome | Matrisome-Associated | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ECM-Glyucoproteins | Collagen Chains | ECM-Regulators | ECM-Affiliated Proteins | ||||||||
| Name | FC | p-Value | Name | FC | p-Value | Name | FC | p-Value | Name | FC | p-Value |
| Fbln1 | 5535 | 3 × 10−18 | Col18a1 | 2.28 | 2 × 10−5 | Serpinb5 | 15,966 | 7 × 10−21 | Muc4 | 7357 | 4 × 10−21 |
| Dmbt1 | 74,2 | 5 × 10−12 | Col4a1 | −1.17 | 0.4016 | Plg | 8304 | 4 × 10−20 | Muc5ac | 409 | NC/3 × 10−16 |
| Fgb | 18,4 | 2 × 10−6 | Col6a4 | −1.49 | 0.0575 | Mmp9 | 8201 | 2 × 10−19 | Anxa10 | 90.47 | 8 × 10−9 |
| Fgg | 10,4 | 6 × 10−6 | Col4a2 | −1.72 | 0.0288 | Loxl2 | 7570 | NC/6 × 10−17 | Lgals4 | 25.55 | 1 × 10−10 |
| Fga | 8.91 | 3 × 10−5 | Col15a1 | −2.72 | 2 × 10−5 | Fam20b | 5265 | 5 × 10−19 | Lgals9 | 4.89 | 9 × 10−8 |
| Tnc | 1.96 | 9 × 10−5 | Col6a2 | −2.84 | 6 × 10−7 | Adam10 | 4586 | 2 × 10−5 | Lgals2 | 3.87 | 2 × 10−5 |
| Vtn | 1.93 | NC/4 × 10−5 | Col6a1 | −2.96 | 3 × 10−7 | P4ha2 | 4433 | NC/2 × 10−17 | Anxa3 | 1.74 | 0.0003 |
| Mfge8 | 1.78 | 0.0003 | Col6a3 | −3.08 | 1 × 10−7 | Ctse | 3.81 | 3 × 10−7 | Anxa11 | 1.35 | 0.0461 |
| Fn1 | 1.46 | 0.0760 | Col1a2 | −6.44 | 7 × 10−5 | Cst3 | 2.08 | NC/0.0088 | Anxa7 | 1.16 | 0.1634 |
| Fbln5 | 1.41 | NC/0.0454 | Col1a1 | −9.15 | 4 × 10−5 | Serpinc1 | 1.88 | 0.1149 | Lman1 | 1.15 | 0.2975 |
| Igfbp7 | 1.30 | 0.1291 | Col6a5 | −10.97 | 3 × 10−7 | F13a1 | 1.66 | 0.1621 | Plxnb2 | 1.12 | 0.4665 |
| Ecm1 | 1.10 | NC/0.3423 | Col4a6 | −12,771 | NC/2 × 10−21 | Serpinb9 | 1.64 | NC/0.0025 | Anxa4 | −1.07 | 0.4975 |
| Creld2 | 1.09 | 0.6556 | Serpinb1a | 1.27 | 0.0296 | Anxa1 | −1.17 | 0.2537 | |||
| Ltbp4 | −1.04 | 0.8548 | Proteoglycans | A2m | 1.20 | 0.1905 | Muc6 | −1.22 | 0.2380 | ||
| Tgfbi | −1.06 | 0.5927 | Name | FC | p-Value | Ctsc | −1.05 | 0.6744 | Cspg4 | −1.25 | NC/0.0437 |
| Agrn | −1.08 | 0.4679 | Prg2 | 2.27 | 0.0003 | Itih1 | −1.08 | 0.7179 | Lgals3 | −1.28 | 0.0832 |
| Vwf | −1.32 | 0.0853 | Hspg2 | −1.4 | 0.0134 | Ctsh | −1.12 | 0.2113 | Anxa2 | −1.31 | 0.0187 |
| Vwa1 | −1.32 | NC/0.0617 | Bgn | −1.50 | 0.1212 | Ctsb | −1.13 | 0.2275 | Sema3d | −1.44 | NC/0.0015 |
| Postn | −1.58 | 0.0054 | Podn | −2.23 | 0.0010 | Ctsa | −1.20 | 0.1233 | Anxa5 | −2.05 | 5 × 10−5 |
| Vwa5a | −1.61 | 0.0076 | Prelp | −3.01 | 5 × 10−7 | Serpina1c | −1.22 | 0.1968 | Lgals1 | −2.78 | 8 × 10−5 |
| Mfap4 | −1.61 | 0.0311 | Aspn | −3.42 | 1 × 10−7 | Ctsz | −1.26 | 0.0404 | Anxa6 | −3.53 | 2 × 10−6 |
| Lamb1 | −1.73 | 0.0002 | Dcn | −3.62 | 3 × 10−9 | Itih3 | −1.30 | 0.2241 | |||
| Emilin1 | −1.79 | 0.0432 | Lum | −3.78 | 9 × 10−10 | Ctsd | −1.40 | 0.0005 | Secreted factors | ||
| Adipoq | −1.95 | NC/0.0357 | Ogn | −4.29 | 7 × 10−10 | Itih2 | −1.49 | 0.2789 | Name | FC | p-Value |
| Papln | −1.96 | NC/9 × 10−7 | Vcan | −12.30 | 2 × 10−12 | Cstb | −1.52 | 0.0248 | S100a16 | 16,177 | 1 × 10−20 |
| Aebp1 | −2.00 | 0.0004 | Serpinh1 | −1.66 | 0.0003 | S100a14 | 12,545 | 2 × 10−20 | |||
| Nid2 | −2.09 | 9 × 10−7 | Serpina3k | −1.68 | NC/7 × 10−5 | S100a9 | 85.2 | NC/3 × 10−12 | |||
| Lamc1 | −2.33 | 9 × 10−6 | Serping1 | −1.93 | NC/3 × 10−5 | S100a8 | 37.9 | 6 × 10−7 | |||
| Lama4 | −2.34 | 1 × 10−7 | P4ha1 | −2.01 | 8 × 10−5 | S100a1 | 3.1 | NC/0.0003 | |||
| Nid1 | −2.50 | 5 × 10−7 | Ctss | −2.13 | 5 × 10−5 | S100a4 | 1.3 | 0.2283 | |||
| Sbspon | −2.54 | NC/3 × 10−5 | Tgm2 | −2.88 | 6 × 10−8 | Angptl2 | 1.22 | NC/0.0289 | |||
| Lama5 | −2.57 | 2 × 10−5 | Cela1 | −3.38 | 4 × 10−6 | Hcfc1 | 1.20 | 0.2782 | |||
| Tinagl1 | −3.45 | 1 × 10−6 | Ambp | −13,221 | 3 × 10−16 | S100a6 | 1.20 | 0.1979 | |||
| Lamb2 | −3.98 | 1 × 10−6 | Adamts20 | −68,487 | NC/6 × 10−22 | S100a11 | 1.12 | 0.3482 | |||
| Mfap5 | −4.09 | 3 × 10−6 | S100a13 | −1.12 | 0.4822 | ||||||
| Tnxb | −4.37 | 1 × 10−5 | S100a10 | −2.16 | 0.0004 | ||||||
| Lama2 | −4.66 | 6 × 10−7 | |||||||||
| Dpt | −5.49 | 3 × 10−11 | |||||||||
| Fbn1 | −5.99 | 2 × 10−5 | |||||||||
| Sparc | −3620 | 0.0001 | |||||||||
| Spp1 | −4710 | NC/8 × 10−19 | |||||||||
| Mmrn2 | −7450 | 3 × 10−18 | |||||||||
| Mfap2 | −7588 | 3 × 10−13 | |||||||||
| Abi3bp | −11,200 | 1 × 10−22 | |||||||||
| Fbn2 | −46,287 | 1 × 10−20 | |||||||||
| Spon1 | −51,711 | NC/9 × 10−23 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfonso, A.B.; Pomerleau, V.; Nicolás, V.R.; Raisch, J.; Jurkovic, C.-M.; Boisvert, F.-M.; Perreault, N. Comprehensive Profiling of Early Neoplastic Gastric Microenvironment Modifications and Biodynamics in Impaired BMP-Signaling FoxL1+-Telocytes. Biomedicines 2023, 11, 19. https://doi.org/10.3390/biomedicines11010019
Alfonso AB, Pomerleau V, Nicolás VR, Raisch J, Jurkovic C-M, Boisvert F-M, Perreault N. Comprehensive Profiling of Early Neoplastic Gastric Microenvironment Modifications and Biodynamics in Impaired BMP-Signaling FoxL1+-Telocytes. Biomedicines. 2023; 11(1):19. https://doi.org/10.3390/biomedicines11010019
Chicago/Turabian StyleAlfonso, Alain B., Véronique Pomerleau, Vilcy Reyes Nicolás, Jennifer Raisch, Carla-Marie Jurkovic, François-Michel Boisvert, and Nathalie Perreault. 2023. "Comprehensive Profiling of Early Neoplastic Gastric Microenvironment Modifications and Biodynamics in Impaired BMP-Signaling FoxL1+-Telocytes" Biomedicines 11, no. 1: 19. https://doi.org/10.3390/biomedicines11010019
APA StyleAlfonso, A. B., Pomerleau, V., Nicolás, V. R., Raisch, J., Jurkovic, C.-M., Boisvert, F.-M., & Perreault, N. (2023). Comprehensive Profiling of Early Neoplastic Gastric Microenvironment Modifications and Biodynamics in Impaired BMP-Signaling FoxL1+-Telocytes. Biomedicines, 11(1), 19. https://doi.org/10.3390/biomedicines11010019






