A Mitochondrion-Targeting Protein (B2) Primes ROS/Nrf2-Mediated Stress Signals, Triggering Apoptosis and Necroptosis in Lung Cancer
Abstract
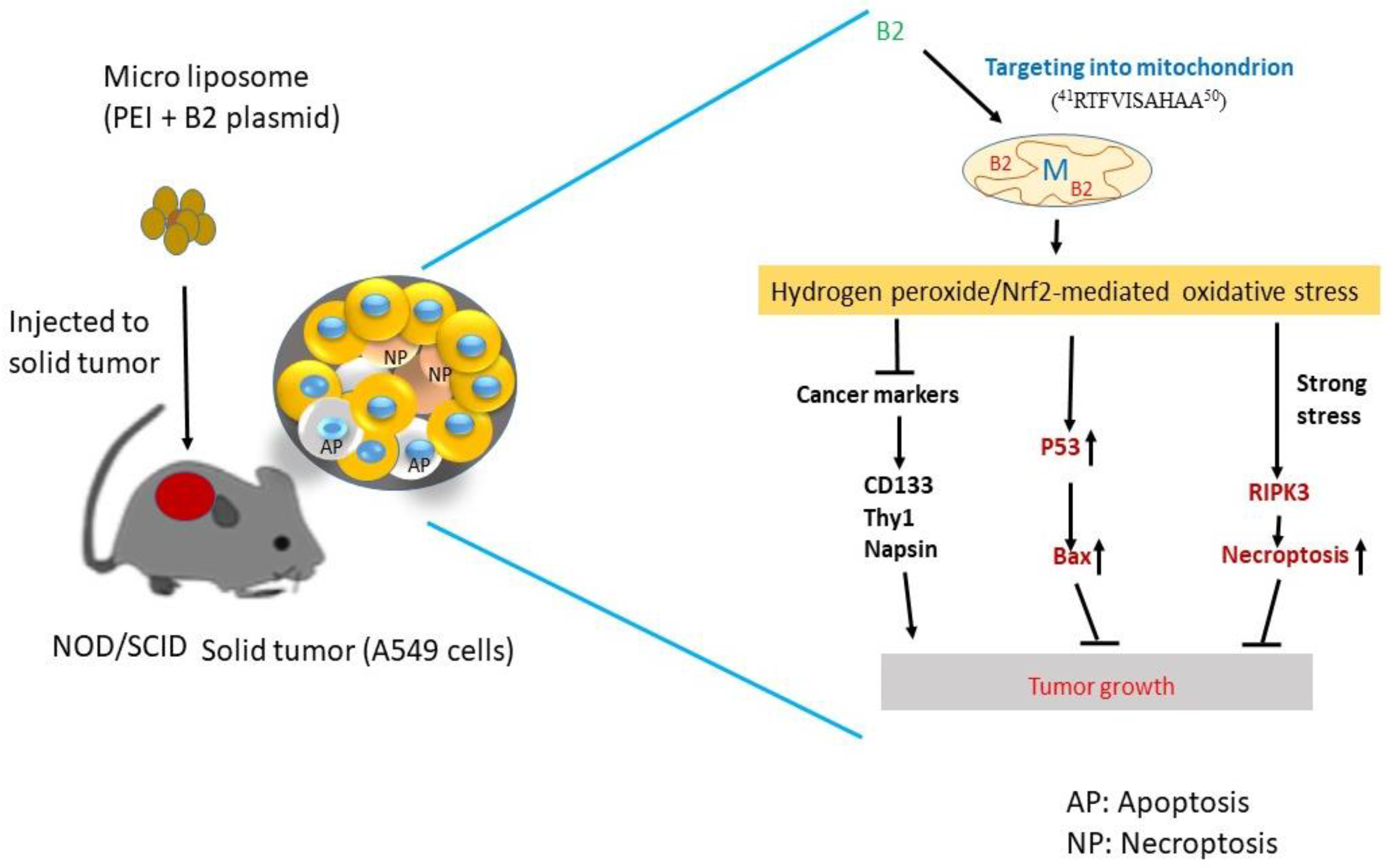
1. Introduction
2. Materials and Methods
2.1. Cancer Cell Culture
2.2. Plasmid Constructions
2.3. Cancer Cells Transfected with Polyethyleneimine and Antioxidant Treatment
2.4. Separation of Mitochondria from B2-Gene-Transfected Cancer Cells
2.5. In Vitro Detection of Relative Hydrogen Peroxide Levels by H2DCFDA
2.6. A549 Human Lung Cancer Cell Xenograft Model in NOD/SCID Mice
2.7. Immunostaining with Antibodies for Tumor Markers
2.8. Analysis of qRT-PCR for Tumor Tissues
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
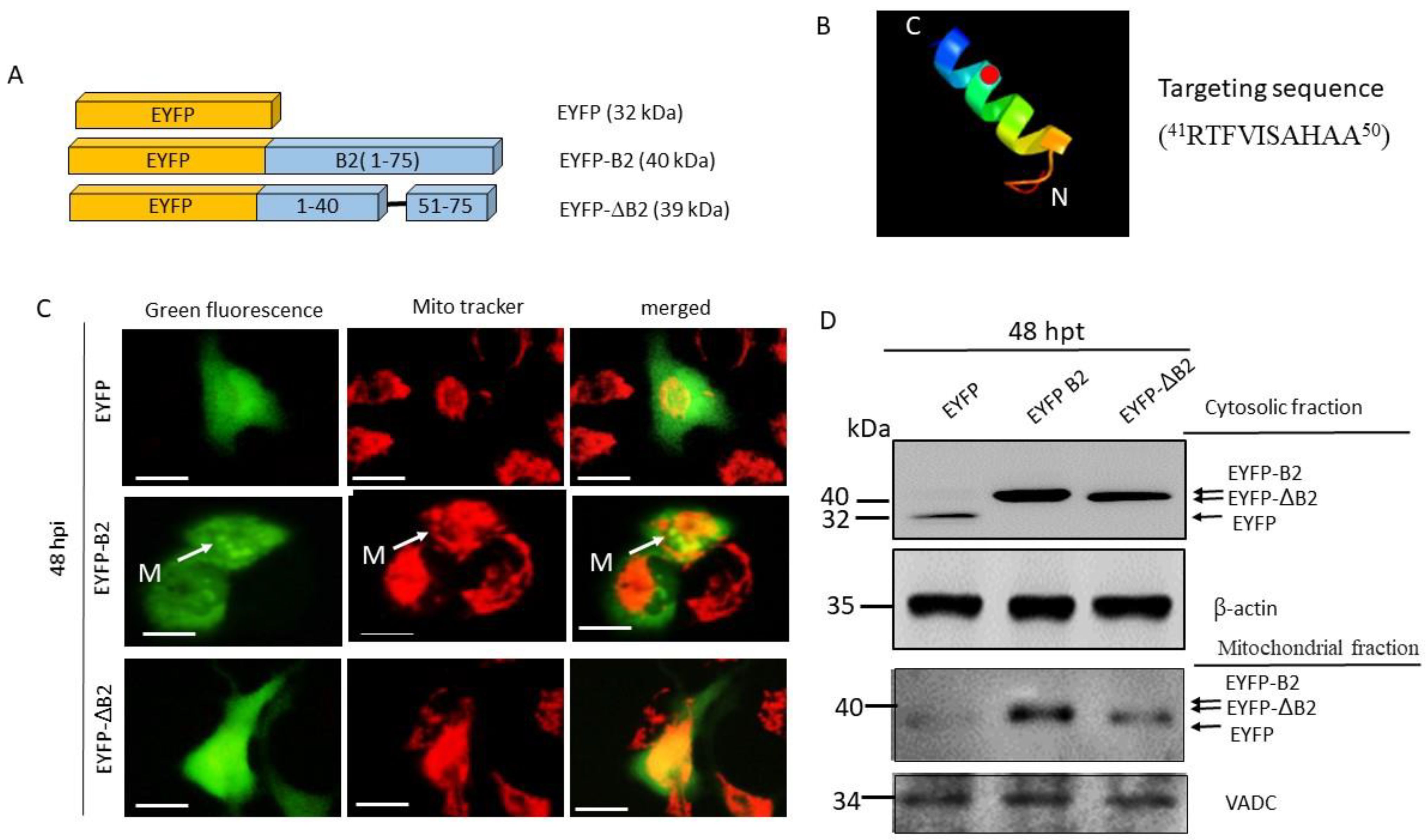
3.1. Use of a Novel Signaling Peptide in Mitochondrial Targeting
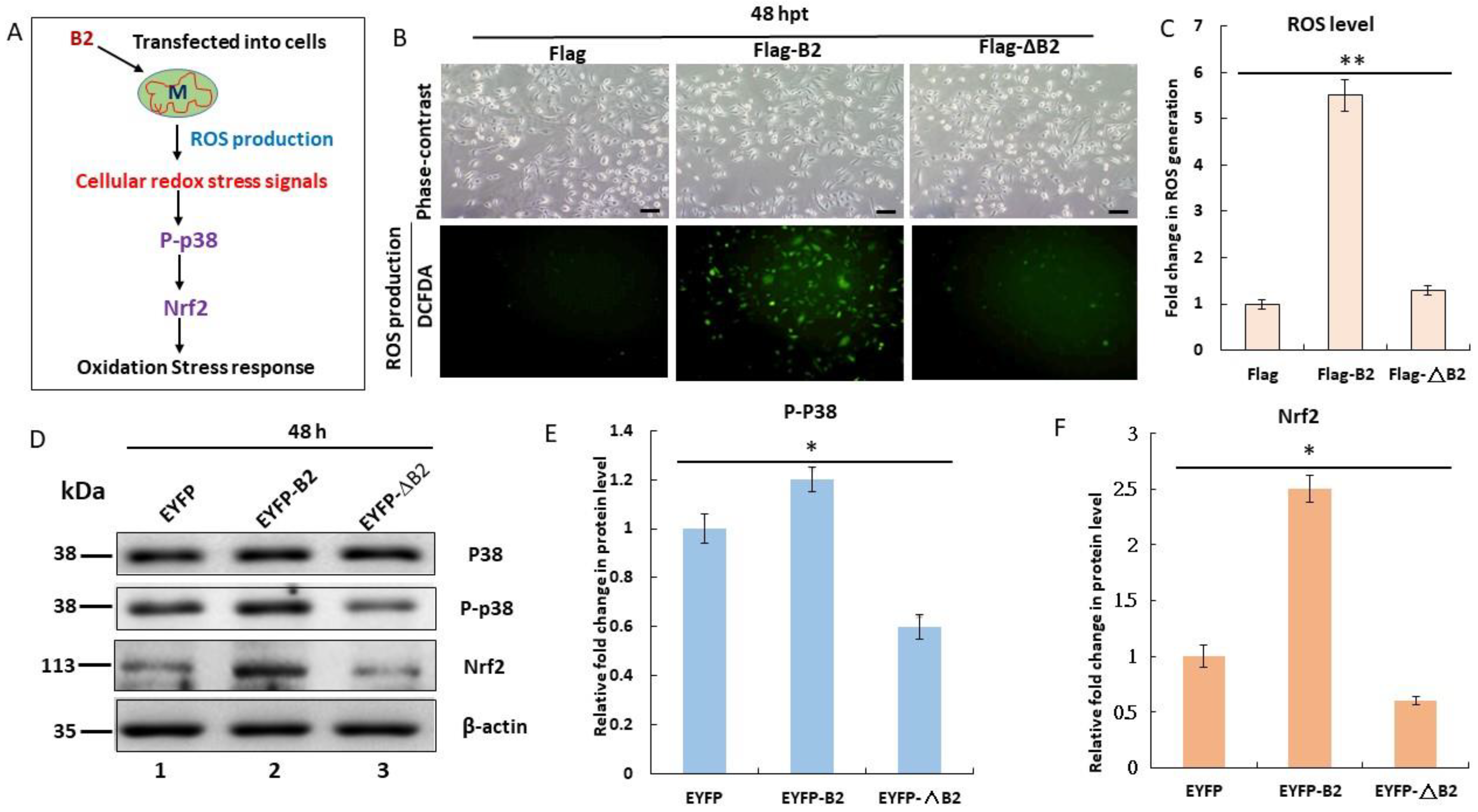
3.2. Triggering of Hydrogen Peroxide/Nrf2-Mediated Stress Signals by B2 Targeting In Vitro
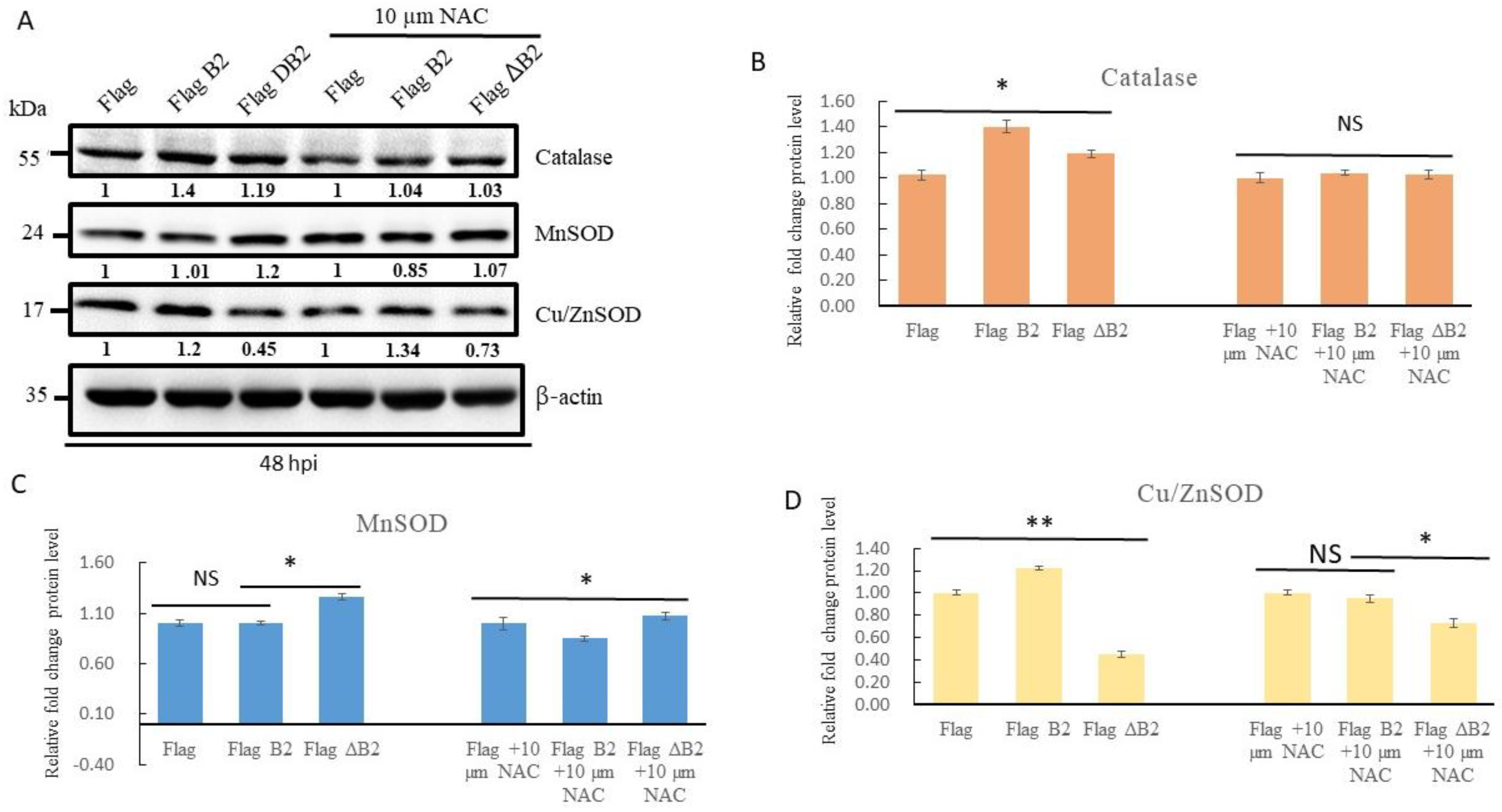
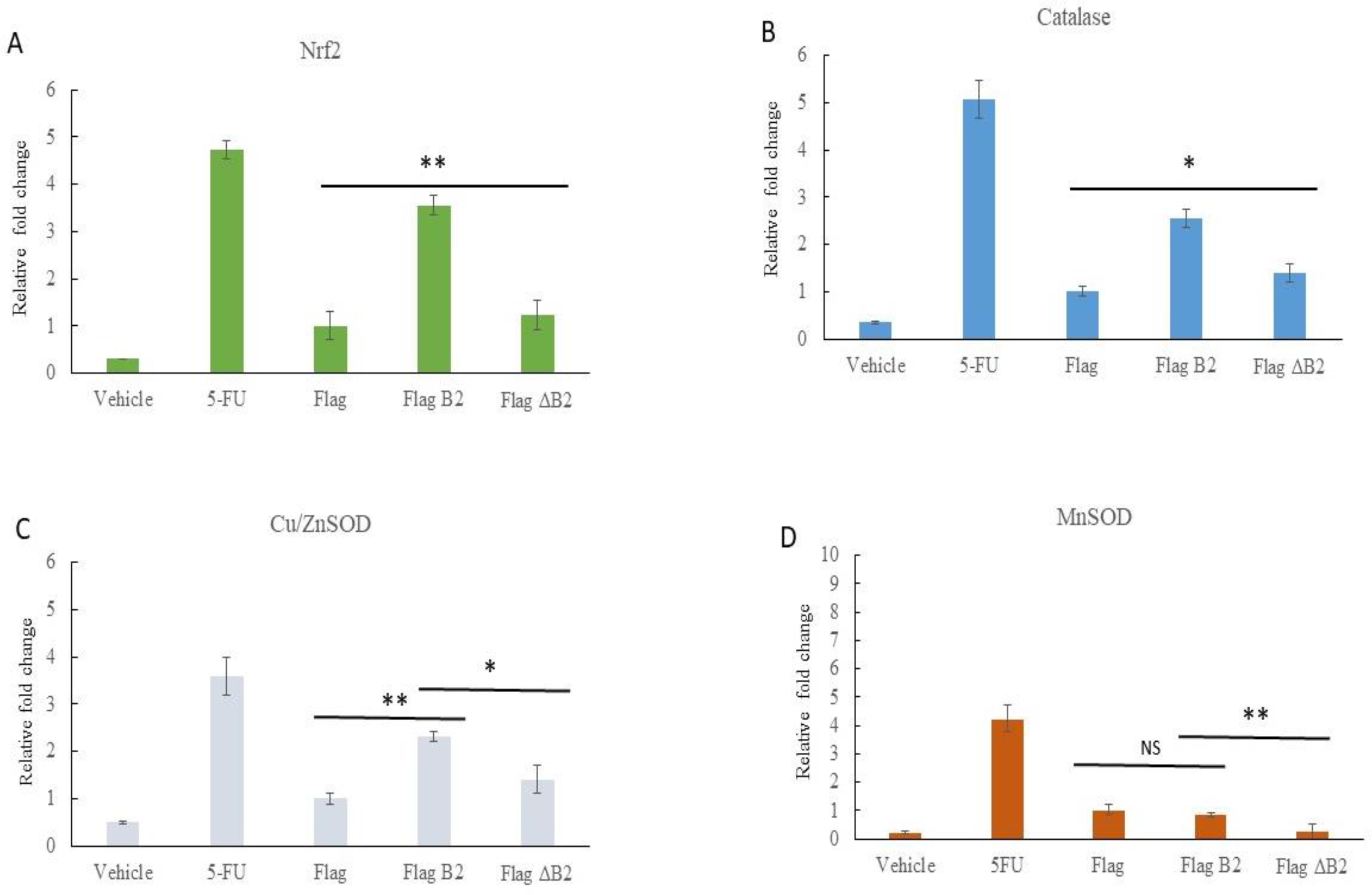
3.3. Blockage of B2-Mediated Nrf2 Stress Signals by Antioxidant NAC, Reducing Antioxidant Enzyme Expression in A549 Cells
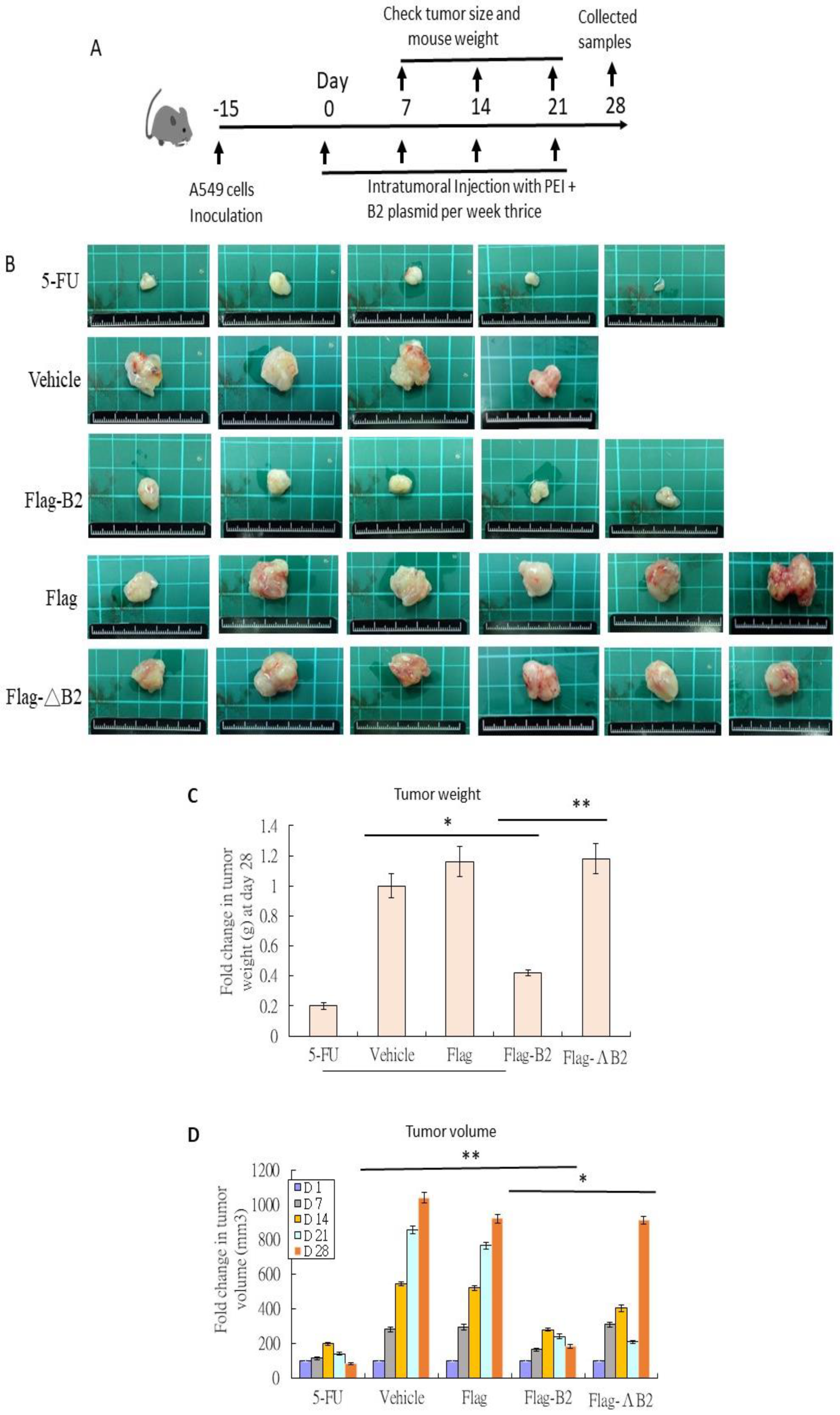
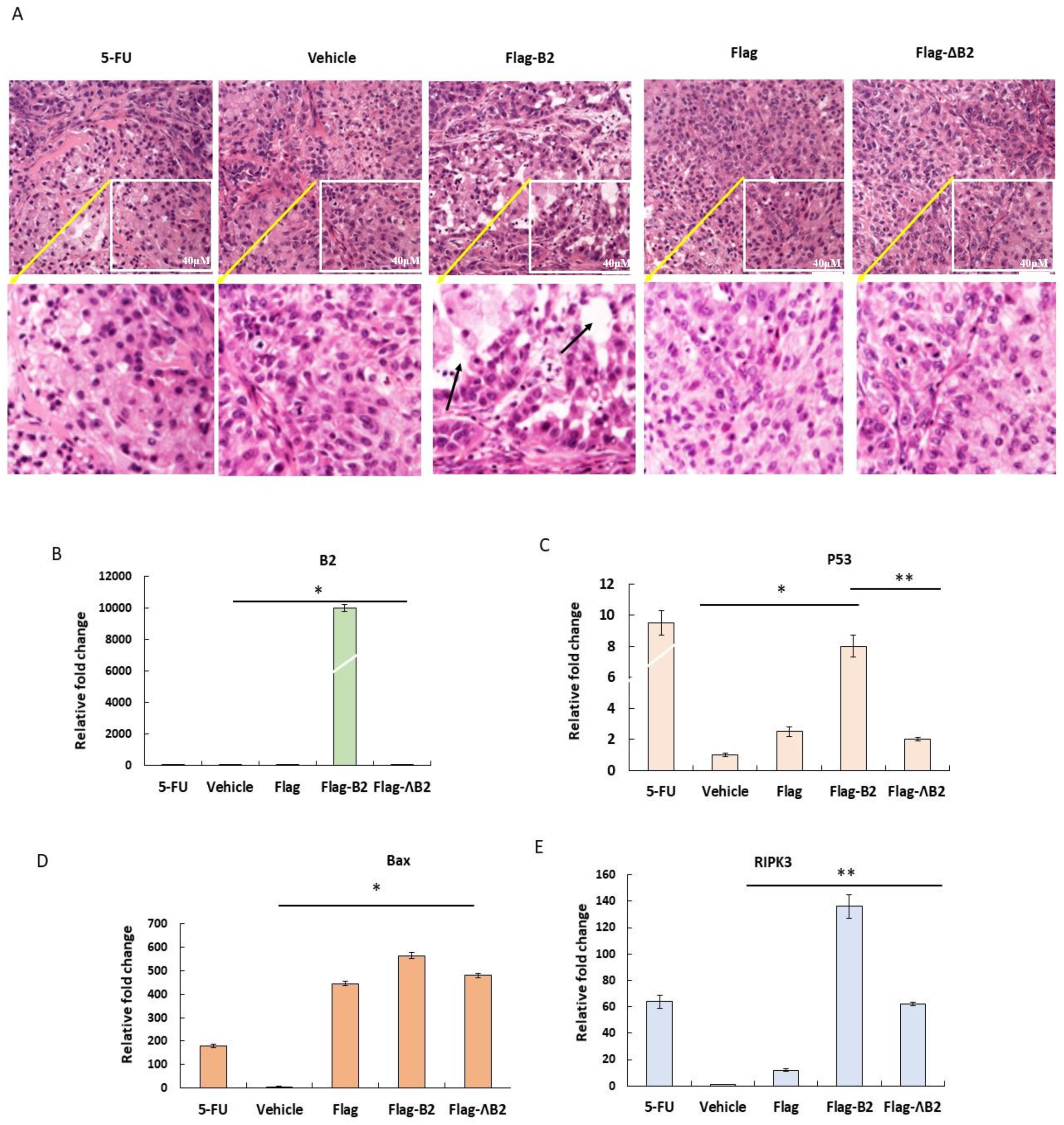
3.4. Reducing Solid Tumors in NOD/SCID Mice by B2 Expression
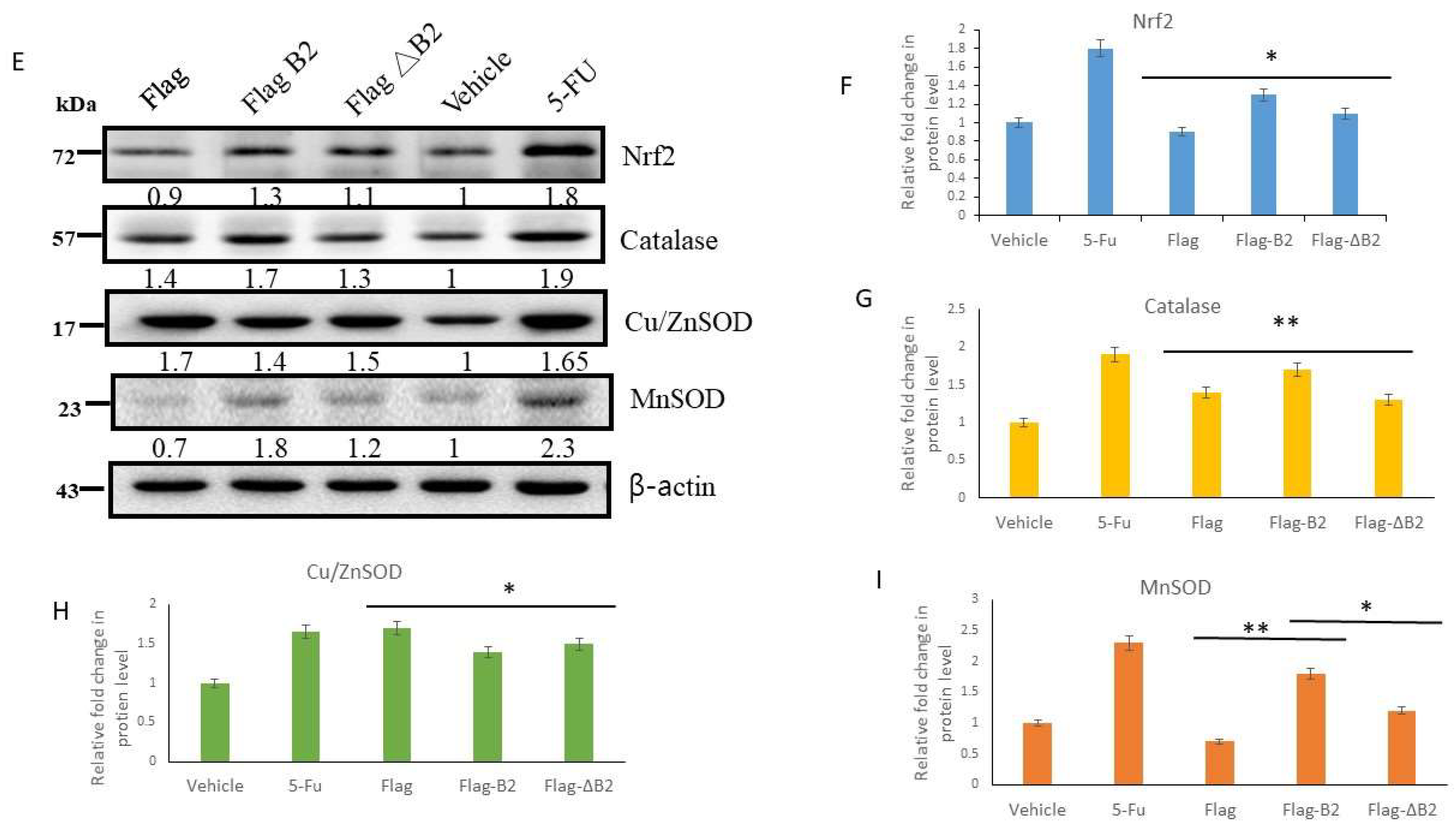
3.5. Reducing B2-Triggering Stress Signals in Solid Tumors in NOD/SCID Mice
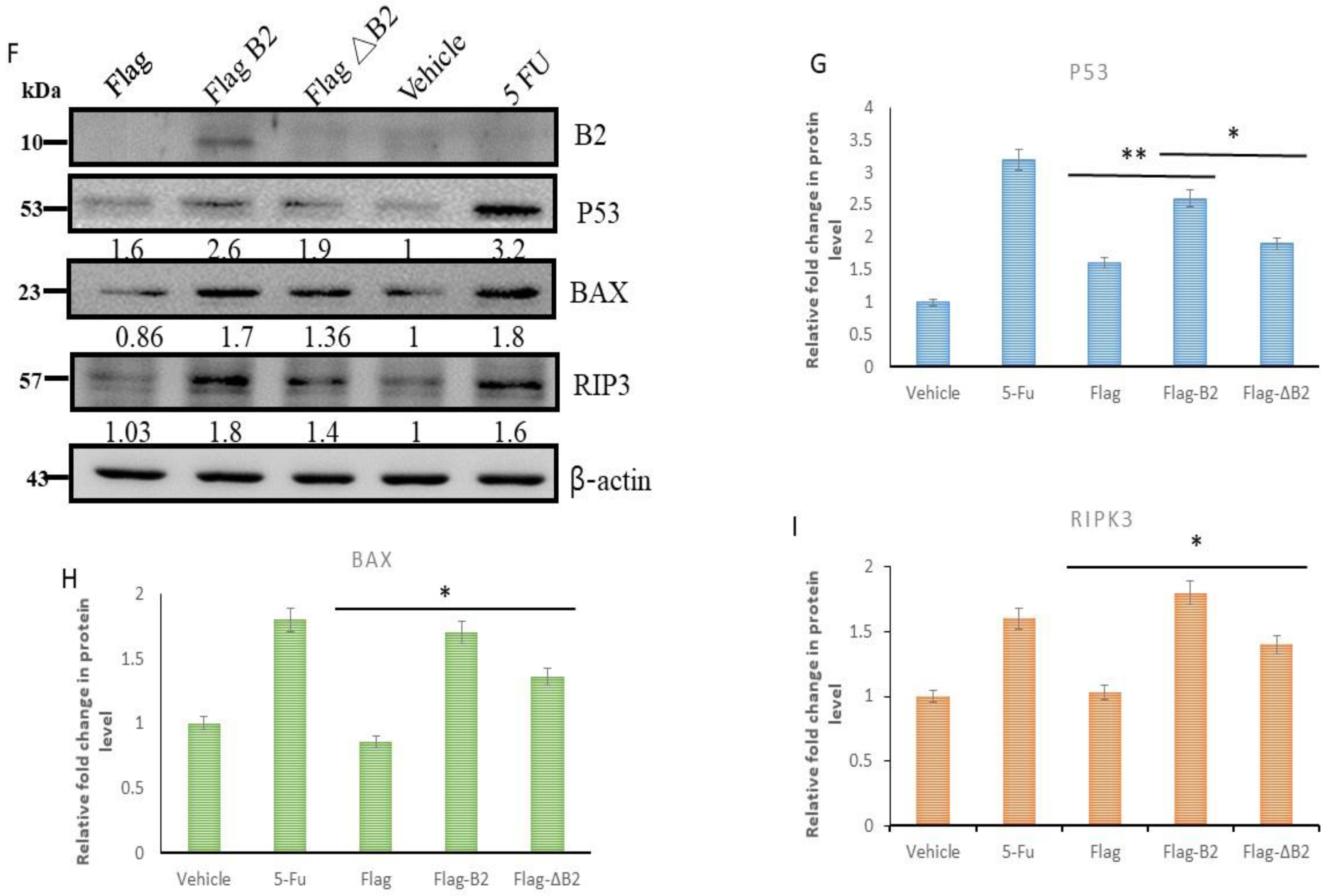
3.6. Triggering Two Death Types in p53/Bax and the RIPK3-Mediated Cell Death Pathway
3.7. In Vivo Inhibition of Cancer Marker Expression In Vivo B2 Protein
4. Discussion
4.1. B2 Induces Mitochondrion-Mediated Hydrogen Peroxide/Nrf2 Signals in Lung Cancer Cells
4.2. Why Can B2 Trigger Multiple Signals for Death Control In Vivo?
4.3. Can a B2-Triggering ROS/Nrf-2 Stress Response Regulate Stem Cell Marker Expression In Vivo?
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ball, L.A.; Johnson, K.L. Reverse genetics of nodaviruses. Adv. Virus Res. 1999, 53, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Bovo, G.; Nishizawa, T.; Maltese, C.; Borghesan, F.; Mutinelli, F.; Montesi, F.; De Mas, S. Viral encephalopathy and retinopathy of farmed marine fish species in Italy. Virus Res. 1999, 63, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Nakai, T.; Muroga, K.; Arimoto, M.; Mushiake, K.; Furusawa, I. Properties of a new virus belonging to nodaviridae found in larval striped jack (Pseudocaranx dentex) with nervous necrosis. Virology 1992, 187, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Munday, B.L.; Kwang, J.; Moody, N. Betanodavirus infections of teleost fish: A review. J. Fish Dis. 2002, 25, 14. [Google Scholar] [CrossRef]
- Delsert, C.; Morin, N.; Comps, M. A fish encephalitis virus that differs from other nodaviruses by its capsid protein processing. Arch. Virol. 1997, 142, 2359–2371. [Google Scholar] [CrossRef]
- Wu, H.C.; Chiu, C.S.; Wu, J.L.; Gong, H.Y.; Chen, M.C.; Lu, M.W.; Hong, J.-R. Zebrafish anti-apoptotic protein zfBcl-xL can block betanodavirus protein alpha-induced mitochondria-mediated secondary necrosis cell death. Fish Shellfish Immunol. 2008, 24, 436–449. [Google Scholar] [CrossRef]
- Iwamoto, T.; Mise, K.; Takeda, A.; Okinaka, Y.; Mori, K.I.; Arimoto, M.; Okuno, T.; Nakai, T. Characterization of Striped jack nervous necrosis virus subgenomic RNA3 and biological activities of its encoded protein B2. J. Gen. Virol. 2005, 86 Pt 10, 2807–2816. [Google Scholar] [CrossRef]
- Su, Y.C.; Wu, J.L.; Hong, J.R. Betanodavirus non-structural protein B2: A novel necrotic death factor that induces mitochondria-mediated cell death in fish cells. Virology 2009, 385, 143–154. [Google Scholar] [CrossRef]
- Chen, L.J.; Su, Y.C.; Hong, J.R. Betanodavirus non-structural protein B1: A novel anti-necrotic death factor that modulates cell death in early replication cycle in fish cells. Virology 2009, 385, 444–454. [Google Scholar] [CrossRef]
- Lu, R.; Maduro, M.; Li, F.; Li, H.W.; Broitman-Maduro, G.; Li, W.X.; Ding, S.W. Animal virus replication and RNAi-mediated antiviral silencing in Caenorhabditis elegans. Nature 2005, 436, 1040–1043. [Google Scholar] [CrossRef]
- Wang, X.H.; Aliyari, R.; Li, W.X.; Li, H.W.; Kim, K.; Carthew, R.; Atkinson, P.; Ding, S.-W. RNA interference directs innate immunity against viruses in adult Drosophila. Science 2006, 312, 452–454. [Google Scholar] [CrossRef]
- Su, Y.C.; Hong, J.R. Betanodavirus B2 causes ATP depletion-induced cell death via mitochondrial targeting and complex II inhibition in vitro and in vivo. J. Biol. Chem. 2010, 285, 39801–39810. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Chiu, H.W.; Hung, J.C.; Hong, J.R. Beta-nodavirus B2 protein induces hydrogen peroxide production, leading to Drp1-recruited mitochondrial fragmentation and cell death via mitochondrial targeting. Apoptosis 2014, 19, 1457–1470. [Google Scholar] [CrossRef]
- Nourazarian, A.R.; Kangari, P.; Salmaninejad, A. Roles of oxidative stress in the development and progression of breast cancer. Asian Pac. J. Cancer Prev. 2014, 15, 4745–4751. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Puar, Y.R.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Sethi, G.; Tergaonkar, V. Evidence for the Involvement of the Master Transcription Factor NF-kappaB in Cancer Initiation and Progression. Biomedicines 2018, 6, 82. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Selvarajoo, N.; Stanslas, J.; Islam, M.K.; Sagineedu, S.R.; Ho, K.L.; Lim, J.C.W. Pharmacological Modulation of Apoptosis and Autophagy in the Treatment of Pancreatic Cancer. Mini Rev. Med. Chem. 2022, 22, 2581–2595. [Google Scholar] [CrossRef]
- Kung, G.; Konstantinidis, K.; Kitsis, R.N. Programmed necrosis, not apoptosis, in the heart. Circ. Res. 2011, 108, 1017–1036. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Kroemer, G. Alternative cell death mechanisms in development and beyond. Genes Dev. 2010, 24, 2592–2602. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Degterev, A.; Jagtap, P.; Xing, X.; Choi, S.; Denu, R.; Yuan, J.; Cuny, G.D. Structure-activity relationship study of novel necroptosis inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 5039–5044. [Google Scholar] [CrossRef]
- Cho, Y.S.; Park, H.L. Exploitation of necroptosis for treatment of caspase-compromised cancers. Oncol. Lett. 2017, 14, 1207–1214. [Google Scholar] [CrossRef]
- Li, C.M.; Haratipour, P.; Lingeman, R.G.; Perry, J.J.P.; Gu, L.; Hickey, R.J.; Malkas, L.H. Novel Peptide Therapeutic Approaches for Cancer Treatment. Cells 2021, 10, 2908. [Google Scholar] [CrossRef]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef]
- Xie, M.; Liu, D.; Yang, Y. Anti-cancer peptides: Classification, mechanism of action, reconstruction and modification. Open Biol. 2020, 10, 200004. [Google Scholar] [CrossRef]
- Chiu, H.W.; Su, Y.C.; Hong, J.R. Betanodavirus B2 protein triggers apoptosis and necroptosis in lung cancer cells that suppresses autophagy. Oncotarget 2017, 8, 94129–94141. [Google Scholar] [CrossRef]
- Longo, P.A.; Kavran, J.M.; Kim, M.S.; Leahy, D.J. Transient mammalian cell transfection with polyethylenimine (PEI). Methods Enzymol. 2013, 529, 227–240. [Google Scholar] [CrossRef]
- Yang, W.; Lam, P.; Kitching, R.; Kahn, H.J.; Yee, A.; Aubin, J.E.; Seth, A. Breast cancer metastasis in a human bone NOD/SCID mouse model. Cancer Biol. Ther. 2007, 6, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Huang, P.-S.; Lin, H.-R.; Lu, C.-H. Transactivation of Protein Expression by Rice HSP101 in Planta and Using Hsp101 as a Selection Marker for Transformation. Plant Cell Physiol. 2007, 48, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Herreros-Pomares, A.; de-Maya-Girones, J.D.; Calabuig-Farinas, S.; Lucas, R.; Martinez, A.; Pardo-Sanchez, J.M.; Alonso, S.; Blasco, A.; Guijarro, R.; Martorell, M.; et al. Lung tumorspheres reveal cancer stem cell-like properties and a score with prognostic impact in resected non-small-cell lung cancer. Cell Death Dis. 2019, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Barzegar Behrooz, A.; Syahir, A.; Ahmad, S. CD133: Beyond a cancer stem cell biomarker. J. Drug Target. 2019, 27, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Rege, T.A.; Hagood, J.S. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006, 20, 1045–1054. [Google Scholar] [CrossRef]
- Sauzay, C.; Voutetakis, K.; Chatziioannou, A.; Chevet, E.; Avril, T. CD90/Thy-1, a Cancer-Associated Cell Surface Signaling Molecule. Front. Cell Dev. Biol. 2019, 7, 66. [Google Scholar] [CrossRef]
- Turner, B.M.; Cagle, P.T.; Sainz, I.M.; Fukuoka, J.; Shen, S.S.; Jagirdar, J. Napsin A, a new marker for lung adenocarcinoma, is complementary and more sensitive and specific than thyroid transcription factor 1 in the differential diagnosis of primary pulmonary carcinoma: Evaluation of 1674 cases by tissue microarray. Arch. Pathol. Lab. Med. 2012, 136, 163–171. [Google Scholar] [CrossRef]
- Yatabe, Y.; Dacic, S.; Borczuk, A.C.; Warth, A.; Russell, P.A.; Lantuejoul, S.; Beasley, M.B.; Thunnissen, E.; Pelosi, G.; Rekhtman, N.; et al. Best Practices Recommendations for Diagnostic Immunohistochemistry in Lung Cancer. J. Thorac. Oncol. 2019, 14, 377–407. [Google Scholar] [CrossRef]
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef]
- Chio, I.I.C.; Tuveson, D.A. ROS in Cancer: The Burning Question. Trends Mol. Med. 2017, 23, 411–429. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Conklin, K.A. Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004, 3, 294–300. [Google Scholar] [CrossRef] [PubMed]
- de Sa Junior, P.L.; Camara, D.A.D.; Porcacchia, A.S.; Fonseca, P.M.M.; Jorge, S.D.; Araldi, R.P.; Ferreira, A.K. The Roles of ROS in Cancer Heterogeneity and Therapy. Oxid. Med. Cell. Longev. 2017, 2017, 2467940. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.P. Cancer. p53, guardian of the genome. Nature 1992, 358, 15–16. [Google Scholar] [CrossRef]
- Prives, C. Signaling to p53: Breaking the MDM2-p53 circuit. Cell 1998, 95, 5–8. [Google Scholar] [CrossRef]
- Kubbutat, M.H.; Jones, S.N.; Vousden, K.H. Regulation of p53 stability by Mdm2. Nature 1997, 387, 299–303. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E.; Werbin, H. Defining the molecular mechanisms of human cell immortalization. Biochim. Biophys. Acta 1991, 1072, 1–7. [Google Scholar] [CrossRef]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 2013, 1833, 3448–3459. [Google Scholar] [CrossRef]
- Prives, C.; Hall, P.A. The p53 pathway. J. Pathol. 1999, 187, 112–126. [Google Scholar] [CrossRef]
- Werness, B.A.; Levine, A.J.; Howley, P.M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990, 248, 76–79. [Google Scholar] [CrossRef]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. p53 signaling in cancer progression and therapy. Cancer Cell Int. 2021, 21, 703. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Kinzler, K.W. p53 function and dysfunction. Cell 1992, 70, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.; Haughton, M.; Blaydes, J.; Gire, V.; Wynford-Thomas, D.; Wyllie, F. Evidence that transcriptional activation by p53 plays a direct role in the induction of cellular senescence. Oncogene 1996, 13, 2097–2104. [Google Scholar] [PubMed]
- Levine, A.J. p53, the cellular gatekeeper for growth and division. Cell 1997, 88, 323–331. [Google Scholar] [CrossRef]
- Vousden, K.H. p53: Death star. Cell 2000, 103, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Hu, W.; Feng, Z. The P53 pathway: What questions remain to be explored? Cell Death Differ. 2006, 13, 1027–1036. [Google Scholar] [CrossRef]
- Gilbertson, R.J.; Rich, J.N. Making a tumour’s bed: Glioblastoma stem cells and the vascular niche. Nat. Rev. Cancer 2007, 7, 733–736. [Google Scholar] [CrossRef]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef]
- Zhu, T.S.; Costello, M.A.; Talsma, C.E.; Flack, C.G.; Crowley, J.G.; Hamm, L.L.; He, X.; Hervey-Jumper, S.L.; Heth, J.A.; Muraszko, K.M.; et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 2011, 71, 6061–6072. [Google Scholar] [CrossRef]
- Prager, B.C.; Xie, Q.; Bao, S.; Rich, J.N. Cancer Stem Cells: The Architects of the Tumor Ecosystem. Cell Stem Cell 2019, 24, 41–53. [Google Scholar] [CrossRef]
- Lim, S.D.; Sun, C.; Lambeth, J.D.; Marshall, F.; Amin, M.; Chung, L.; Petros, J.A.; Arnold, R.S. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate 2005, 62, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.; Asoodeh, A.; Klonisch, T.; Halayko, A.J.; Kadkhoda, K.; Kroczak, T.J.; Gibson, S.B.; Booy, E.P.; Naderi-Manesh, H.; Los, M. Brevinin-2R(1) semi-selectively kills cancer cells by a distinct mechanism, which involves the lysosomal-mitochondrial death pathway. J. Cell. Mol. Med. 2008, 12, 1005–1022. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Niu, X.; Chen, Y.; Hu, Q.; Shi, G.; Wu, H.; Wang, J.; Yi, J. Emodin-induced generation of reactive oxygen species inhibits RhoA activation to sensitize gastric carcinoma cells to anoikis. Neoplasia 2008, 10, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Koren, E.; Fuchs, Y. Modes of Regulated Cell Death in Cancer. Cancer Discov. 2021, 11, 245–265. [Google Scholar] [CrossRef]
- Parga, J.A.; Rodriguez-Perez, A.I.; Garcia-Garrote, M.; Rodriguez-Pallares, J.; Labandeira-Garcia, J.L. NRF2 Activation and Downstream Effects: Focus on Parkinson’s Disease and Brain Angiotensin. Antioxidants 2021, 10, 1649. [Google Scholar] [CrossRef]
- Hu, Q.; Ren, J.; Li, G.; Wu, J.; Wu, X.; Wang, G.; Gu, G.; Ren, H.; Hong, Z.; Li, J. The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway. Cell Death Dis. 2018, 9, 403. [Google Scholar] [CrossRef]
- Amorim, R.; Cagide, F.; Tavares, L.C.; Simões, R.F.; Soares, P.; Benfeito, S.; Baldeiras, I.; Jones, J.G.; Borges, F.; Oliveira, P.J.; et al. Mitochondriotropic antioxidant based on caffeic acid AntiOxCIN(4) activates Nrf2-dependent antioxidant defenses and quality control mechanisms to antagonize oxidative stress-induced cell damage. Free Radic. Biol. Med. 2022, 179, 119–132. [Google Scholar] [CrossRef]
- Panieri, E.; Pinho, S.A.; Afonso, G.J.M.; Oliveira, P.J.; Cunha-Oliveira, T.; Saso, L. NRF2 and Mitochondrial Function in Cancer and Cancer Stem Cells. Cells 2022, 11, 2401. [Google Scholar] [CrossRef]

| Group | No | Agents | Dose | Characteristics of Test Drugs | |
|---|---|---|---|---|---|
| A | A549 | 5 | 5-FU | 20 mg/kg body weight | |
| B | A549 | 4 | 0.9% saline | 50 µL | |
| C | A549 | 6 | PEI/Flag | 25 µg/25 µg in 50 µL | Agents (PEI, Flag, Flag b2, or Flag ΔB2) are mixed for 30 min at 40 °C and will be stable for 1–2 h at 4 °C |
| D | A549 | 5 | PEI/Flag b2 | 25 µg/25 µg in 50 µL | |
| E | A549 | 6 | PEI/Flag ΔB2 | 25 µg/25 µg in 50 µL | |
| Name | Sequence (5′-3′) |
|---|---|
| p53 Forward primer | AGGGTTAGTTTACAATCAGC |
| p53 Reverse primer | GGTAGGTGCAAATGCC |
| Bax Forward primer | GGTGCCTCAGGATGCG |
| Bax Reverse primer | GGAGTCTGTGTCCACG |
| Actin Forward primer | ATCCGCAAAGACCTGT |
| Actin Reverse primer | GGGTGTAACGCAACTAAG |
| RGNNV B2 Forward primer | ATGGCAAATCCAACAAGC |
| RGNNV B2 Reverse primer | CTAGTCCGTCTCCATCGGCT |
| Ripk3 Forward primer | GACTCCCGGCTTAGAAGGACT |
| Ripk3 Reverse primer | CTGCTCTTGAGCTGAGACAGG |
| Catalase Forward primer | AACTGGGATCTTGTGGGAA |
| Catalase Reverse primer | GACAGTTCACAGGTATCTG |
| Cu/Zn Forward primer | GCGACGAAGGCCGTGTGCGTTG |
| Cu/Zn Reverse primer | TGTGCGGCCAATGATGCAATG |
| Mn Forward primer | CGACCTGCCCTACGACTACGG |
| Mn Reverse primer | CAAGCCAACCCCAACCTGAGC |
| Nrf2 Forward primer | ACACGGTCCACAGCTCATC |
| Nrf2 Reverse primer | TGTCAATCAAATCCATGTCCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, H.-W.; Hung, S.-W.; Chiu, C.-F.; Hong, J.-R. A Mitochondrion-Targeting Protein (B2) Primes ROS/Nrf2-Mediated Stress Signals, Triggering Apoptosis and Necroptosis in Lung Cancer. Biomedicines 2023, 11, 186. https://doi.org/10.3390/biomedicines11010186
Chiu H-W, Hung S-W, Chiu C-F, Hong J-R. A Mitochondrion-Targeting Protein (B2) Primes ROS/Nrf2-Mediated Stress Signals, Triggering Apoptosis and Necroptosis in Lung Cancer. Biomedicines. 2023; 11(1):186. https://doi.org/10.3390/biomedicines11010186
Chicago/Turabian StyleChiu, Hsuan-Wen, Shao-Wen Hung, Ching-Feng Chiu, and Jiann-Ruey Hong. 2023. "A Mitochondrion-Targeting Protein (B2) Primes ROS/Nrf2-Mediated Stress Signals, Triggering Apoptosis and Necroptosis in Lung Cancer" Biomedicines 11, no. 1: 186. https://doi.org/10.3390/biomedicines11010186
APA StyleChiu, H.-W., Hung, S.-W., Chiu, C.-F., & Hong, J.-R. (2023). A Mitochondrion-Targeting Protein (B2) Primes ROS/Nrf2-Mediated Stress Signals, Triggering Apoptosis and Necroptosis in Lung Cancer. Biomedicines, 11(1), 186. https://doi.org/10.3390/biomedicines11010186








