Andrographis Reverses Gemcitabine Resistance through Regulation of ERBB3 and Calcium Signaling Pathway in Pancreatic Ductal Adenocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohorts
2.2. Genomewide Expression Profiling and Pathway Enrichment Analysis in Gemcitabine-Resistant PDAC Cells
2.3. Cell Culture and Reagents
2.4. Total RNA Extraction and Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction
2.5. Cell Viability, Colony Formation, and Wound Healing Assays
2.6. Annexin V Binding Assays
2.7. Protein Extraction and Western Immunoblotting
2.8. Fluo-4-Based Calcium Imaging
2.9. Patient-Derived Tumor 3D-Organoids
2.10. Statistical Analysis
3. Results
3.1. The Calcium Signaling Pathway Significantly Correlates with Gemcitabine Resistance in PDAC Cells
3.2. High ERBB3 Expression Associates with Poor Survival Outcomes and Gemcitabine Resistance in PDAC Patients
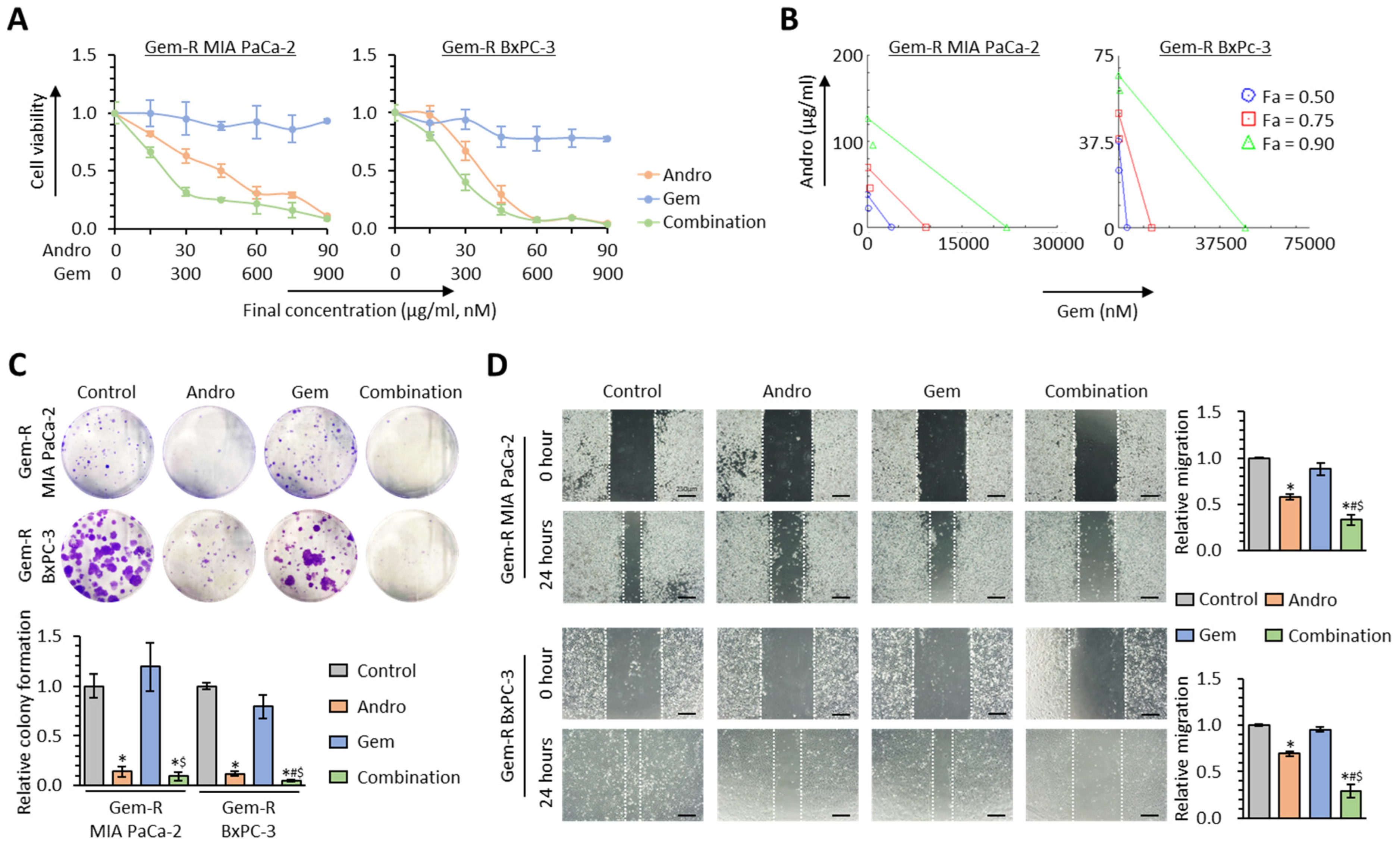
3.3. Andrographis Potentiates the Chemosensitivity to Gemcitabine in Gemcitabine-Resistant PDAC Cells
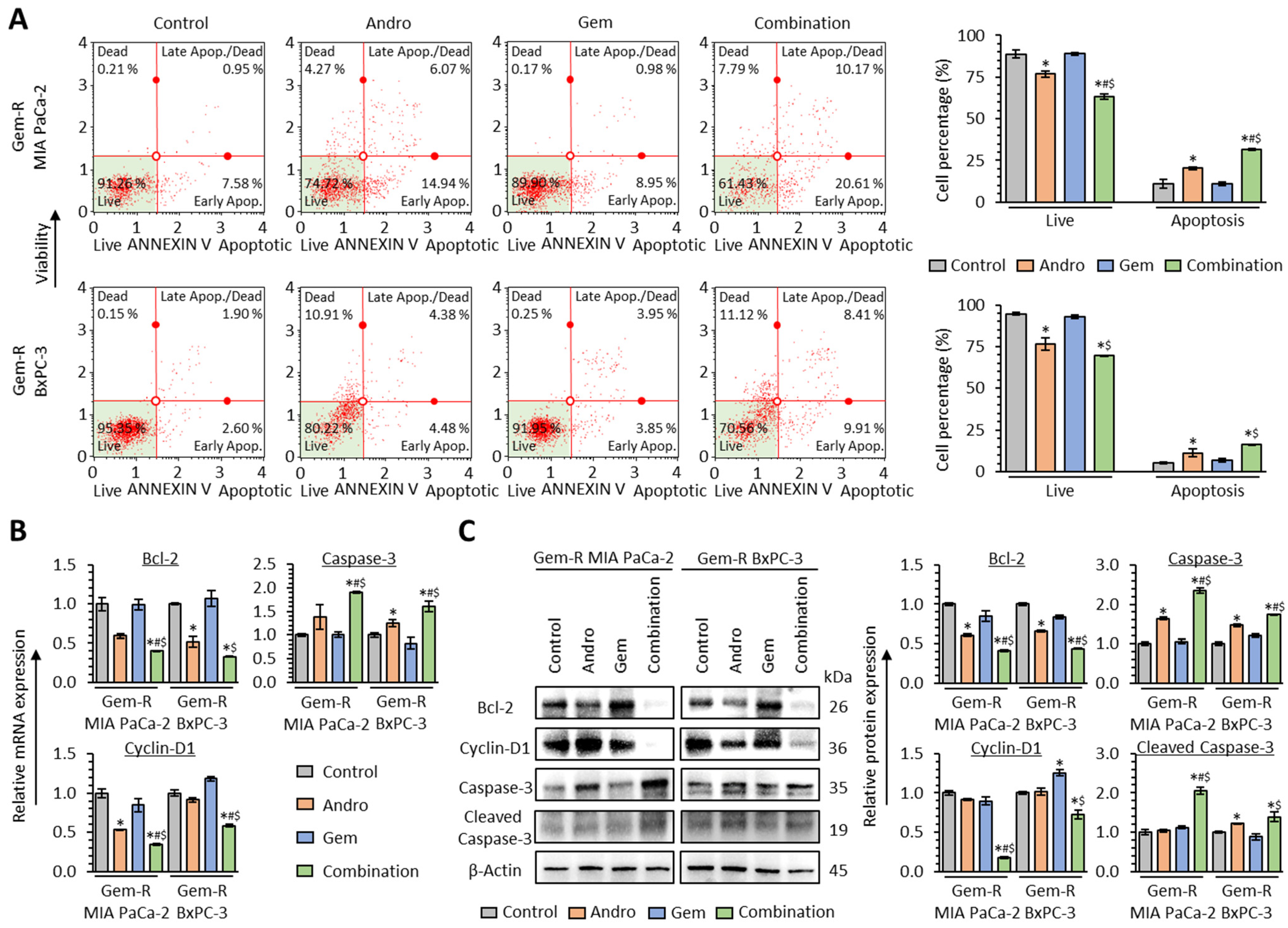
3.4. The Combination of Andrographis and Gemcitabine Promotes Cellular Apoptosis in Gemcitabine-Resistant PDAC Cells
3.5. The Combination of Andrographis and Gemcitabine Decreases Intracellular Calcium Concentration in Gemcitabine-Resistant PDAC Cells
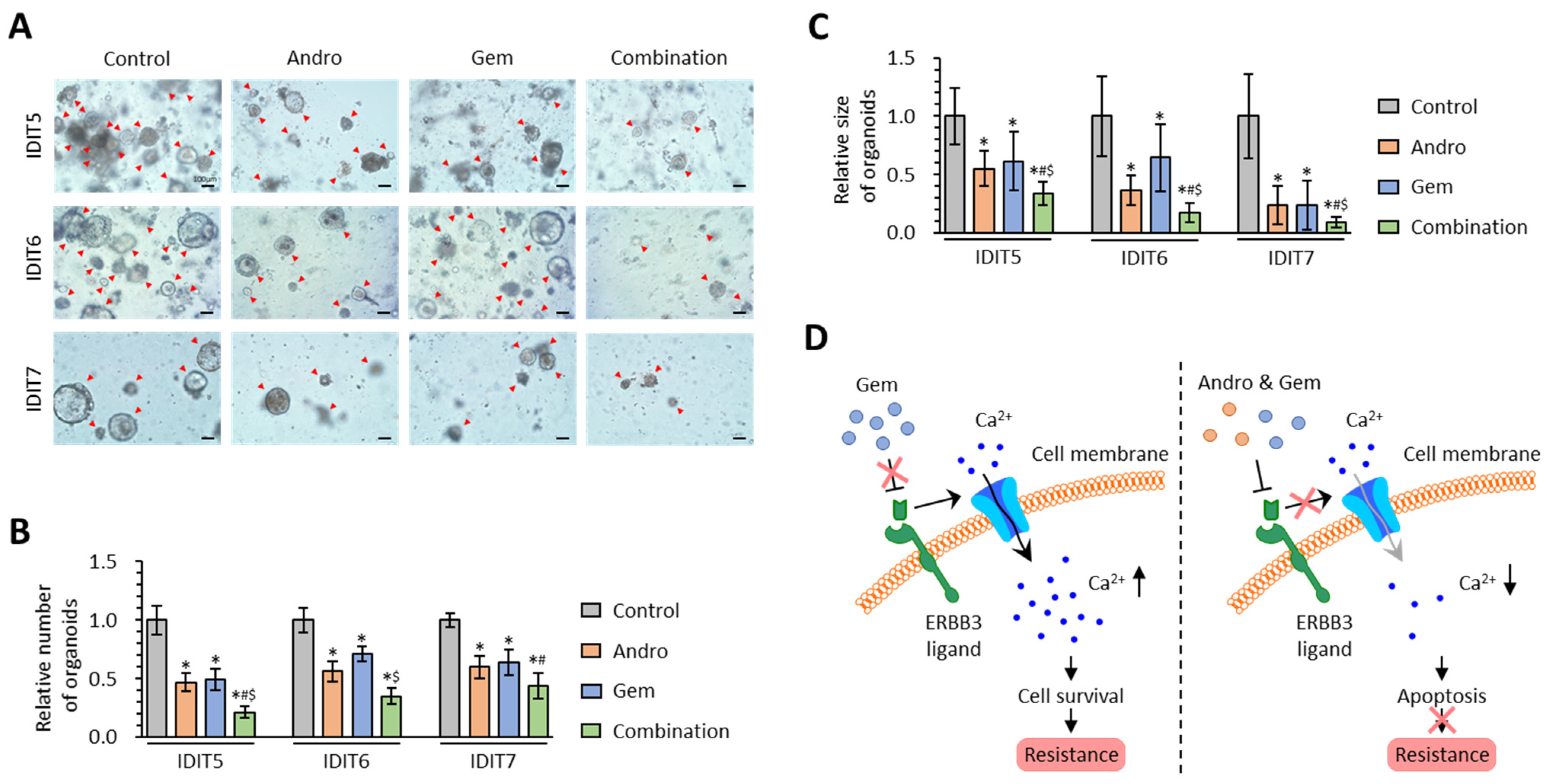
3.6. The Combined Treatment with Andrographis and Gemcitabine Effectively Enhances Anti-Cancer Activity in PDAC Patient-Derived 3D-Organoid Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.Y.K.; Burke, E.; Cunningham, D.; Starling, N. Up-to-Date Tailored Systemic Treatment in Pancreatic Ductal Adenocarcinoma. Gastroenterol. Res. Pract. 2019, 2019, 7135437. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A., 3rd; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef]
- Komarova, N.L.; Boland, C.R. Cancer: Calculated treatment. Nature 2013, 499, 291–292. [Google Scholar] [CrossRef]
- Bozic, I.; Reiter, J.G.; Allen, B.; Antal, T.; Chatterjee, K.; Shah, P.; Moon, Y.S.; Yaqubie, A.; Kelly, N.; Le, D.T.; et al. Evolutionary dynamics of cancer in response to targeted combination therapy. eLife 2013, 2, e00747. [Google Scholar] [CrossRef]
- Louvet, C.; Labianca, R.; Hammel, P.; Lledo, G.; Zampino, M.G.; André, T.; Zaniboni, A.; Ducreux, M.; Aitini, E.; Taïeb, J.; et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 3509–3516. [Google Scholar] [CrossRef]
- Rocha Lima, C.M.; Green, M.R.; Rotche, R.; Miller, W.H., Jr.; Jeffrey, G.M.; Cisar, L.A.; Morganti, A.; Orlando, N.; Gruia, G.; Miller, L.L. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 3776–3783. [Google Scholar] [CrossRef]
- Heinemann, V.; Quietzsch, D.; Gieseler, F.; Gonnermann, M.; Schönekäs, H.; Rost, A.; Neuhaus, H.; Haag, C.; Clemens, M.; Heinrich, B.; et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 3946–3952. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- McAndrew, E.N.; Zhang, H.; Lambert, P.; Rittberg, R.; Dawe, D.E.; Kim, C.A. Treatment Patterns, Toxicity, and Outcomes of Older Adults with Advanced Pancreatic Cancer Receiving First-line Palliative Chemotherapy. Am. J. Clin. Oncol. 2022, 45, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Gharaibeh, M.; McBride, A.; Bootman, J.L.; Patel, H.; Abraham, I. Economic evaluation for the US of nab-paclitaxel plus gemcitabine versus FOLFIRINOX versus gemcitabine in the treatment of metastatic pancreas cancer. J. Med. Econ. 2017, 20, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, M.; Kimura, M.; Usami, E.; Iwai, M.; Hirose, T.; Kawachi, S.; Yoshimura, T. Comparing the cost-effectiveness of FOLFIRINOX, nab-paclitaxel plus gemcitabine, gemcitabine and S-1 for the treatment of metastatic pancreatic cancer. Mol. Clin. Oncol. 2017, 7, 125–130. [Google Scholar] [CrossRef]
- Monteith, G.R.; Prevarskaya, N.; Roberts-Thomson, S.J. The calcium-cancer signalling nexus. Nat. Rev. Cancer 2017, 17, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Merritt, R.; Fu, L.; Pan, Z. Targeting calcium signaling in cancer therapy. Acta Pharm. Sin. B 2017, 7, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Bong, A.H.L.; Monteith, G.R. Calcium signaling and the therapeutic targeting of cancer cells. Biochim. Et Biophys. Acta. Mol. Cell Res. 2018, 1865, 1786–1794. [Google Scholar] [CrossRef]
- Büsselberg, D.; Florea, A.M. Targeting Intracellular Calcium Signaling ([Ca(2+)](i)) to Overcome Acquired Multidrug Resistance of Cancer Cells: A Mini-Overview. Cancers 2017, 9, 48. [Google Scholar] [CrossRef]
- Li, X.; Miao, S.; Li, F.; Ye, F.; Yue, G.; Lu, R.; Shen, H.; Ye, Y. Cellular Calcium Signals in Cancer Chemoprevention and Chemotherapy by Phytochemicals. Nutr. Cancer 2022, 74, 2671–2685. [Google Scholar] [CrossRef]
- Tian, X.; Wang, N. Upregulation of ASPM, BUB1B and SPDL1 in tumor tissues predicts poor survival in patients with pancreatic ductal adenocarcinoma. Oncol. Lett. 2020, 19, 3307–3315. [Google Scholar] [CrossRef]
- Kutschat, A.P.; Hamdan, F.H.; Wang, X.; Wixom, A.Q.; Najafova, Z.; Gibhardt, C.S.; Kopp, W.; Gaedcke, J.; Ströbel, P.; Ellenrieder, V.; et al. STIM1 Mediates Calcium-Dependent Epigenetic Reprogramming in Pancreatic Cancer. Cancer Res. 2021, 81, 2943–2955. [Google Scholar] [CrossRef]
- James, A.D.; Chan, A.; Erice, O.; Siriwardena, A.K.; Bruce, J.I. Glycolytic ATP fuels the plasma membrane calcium pump critical for pancreatic cancer cell survival. J. Biol. Chem. 2013, 288, 36007–36019. [Google Scholar] [CrossRef] [PubMed]
- Kondratska, K.; Kondratskyi, A.; Yassine, M.; Lemonnier, L.; Lepage, G.; Morabito, A.; Skryma, R.; Prevarskaya, N. Orai1 and STIM1 mediate SOCE and contribute to apoptotic resistance of pancreatic adenocarcinoma. Biochim. Et Biophys. Acta 2014, 1843, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Aissa, A.F.; Kumar, S.; Pham, T.N.D.; Underwood, P.W.; Nair, R.; Ke, R.; Rana, B.; Trevino, J.G.; Munshi, H.G.; et al. Calcium channel blockers potentiate gemcitabine chemotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2022, 119, e2200143119. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Y.; Schwarz, B.; Mysliwietz, J.; Hartig, R.; Camaj, P.; Bao, Q.; Jauch, K.W.; Guba, M.; Ellwart, J.W.; et al. Verapamil inhibits tumor progression of chemotherapy-resistant pancreatic cancer side population cells. Int. J. Oncol. 2016, 49, 99–110. [Google Scholar] [CrossRef]
- Khan, H.Y.; Mpilla, G.B.; Sexton, R.; Viswanadha, S.; Penmetsa, K.V.; Aboukameel, A.; Diab, M.; Kamgar, M.; Al-Hallak, M.N.; Szlaczky, M.; et al. Calcium Release-Activated Calcium (CRAC) Channel Inhibition Suppresses Pancreatic Ductal Adenocarcinoma Cell Proliferation and Patient-Derived Tumor Growth. Cancers 2020, 12, 750. [Google Scholar] [CrossRef] [PubMed]
- Poolsup, N.; Suthisisang, C.; Prathanturarug, S.; Asawamekin, A.; Chanchareon, U. Andrographis paniculata in the symptomatic treatment of uncomplicated upper respiratory tract infection: Systematic review of randomized controlled trials. J. Clin. Pharm. Ther. 2004, 29, 37–45. [Google Scholar] [CrossRef]
- Burgos, R.A.; Aguila, M.J.; Santiesteban, E.T.; Sánchez, N.S.; Hancke, J.L. Andrographis paniculata (Ness) induces relaxation of uterus by blocking voltage operated calcium channels and inhibits Ca(+2) influx. Phytother. Res. PTR 2001, 15, 235–239. [Google Scholar] [CrossRef]
- Burgos, R.A.; Imilan, M.; Sánchez, N.S.; Hancke, J.L. Andrographis paniculata (Nees) selectively blocks voltage-operated calcium channels in rat vas deferens. J. Ethnopharmacol. 2000, 71, 115–121. [Google Scholar] [CrossRef]
- Li, C.X.; Li, H.G.; Zhang, H.; Cheng, R.H.; Li, M.; Liang, J.Y.; Gu, Y.; Ling, B.; Yao, Z.R.; Yu, H. Andrographolide suppresses thymic stromal lymphopoietin in phorbol myristate acetate/calcium ionophore A23187-activated mast cells and 2,4-dinitrofluorobenzene-induced atopic dermatitis-like mice model. Drug Des. Dev. Ther. 2016, 10, 781–791. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, Q.; Wang, X.; Sun, X.; Mu, X.; Dong, H. Effect of Andrographolide on Gene Expression Profile and Intracellular Calcium in Primary Rat Myocardium Microvascular Endothelial Cells. J. Cardiovasc. Pharmacol. 2017, 70, 369–381. [Google Scholar] [CrossRef]
- Sriramaneni, R.N.; Omar, A.Z.; Ibrahim, S.M.; Amirin, S.; Mohd Zaini, A. Vasorelaxant effect of diterpenoid lactones from Andrographis paniculata chloroform extract on rat aortic rings. Pharmacogn. Res. 2010, 2, 242–246. [Google Scholar] [CrossRef]
- Ma, R.; Shimura, T.; Yin, C.; Okugawa, Y.; Kitajima, T.; Koike, Y.; Okita, Y.; Ohi, M.; Uchida, K.; Goel, A.; et al. Antitumor effects of Andrographis via ferroptosis-associated genes in gastric cancer. Oncol. Lett. 2021, 22, 523. [Google Scholar] [CrossRef]
- Sharma, P.; Shimura, T.; Banwait, J.K.; Goel, A. Andrographis-mediated chemosensitization through activation of ferroptosis and suppression of β-catenin/Wnt-signaling pathways in colorectal cancer. Carcinogenesis 2020, 41, 1385–1394. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Goel, A. Andrographis overcomes 5-fluorouracil-associated chemoresistance through inhibition of DKK1 in colorectal cancer. Carcinogenesis 2021, 42, 814–825. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Goel, A. A combined treatment with melatonin and andrographis promotes autophagy and anticancer activity in colorectal cancer. Carcinogenesis 2022, 43, 217–230. [Google Scholar] [CrossRef]
- Zhao, Y.; Roy, S.; Wang, C.; Goel, A. A Combined Treatment with Berberine and Andrographis Exhibits Enhanced Anti-Cancer Activity through Suppression of DNA Replication in Colorectal Cancer. Pharmaceuticals 2022, 15, 262. [Google Scholar] [CrossRef]
- Shimura, T.; Sharma, P.; Sharma, G.G.; Banwait, J.K.; Goel, A. Enhanced anti-cancer activity of andrographis with oligomeric proanthocyanidins through activation of metabolic and ferroptosis pathways in colorectal cancer. Sci. Rep. 2021, 11, 7548. [Google Scholar] [CrossRef]
- Yue, Q.; Gao, G.; Zou, G.; Yu, H.; Zheng, X. Natural Products as Adjunctive Treatment for Pancreatic Cancer: Recent Trends and Advancements. BioMed Res. Int. 2017, 2017, 8412508. [Google Scholar] [CrossRef]
- Marasini, B.; Sahu, R.P. Natural Anti-Cancer Agents: Implications in Gemcitabine-Resistant Pancreatic Cancer Treatment. Mini Rev. Med. Chem. 2017, 17, 920–927. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Yoshida, K.; Toden, S.; Ravindranathan, P.; Han, H.; Goel, A. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis 2017, 38, 1036–1046. [Google Scholar] [CrossRef]
- Okuno, K.; Tokunaga, M.; Kinugasa, Y.; Baba, H.; Kodera, Y.; Goel, A. A Transcriptomic Liquid Biopsy Assay for Predicting Resistance to Neoadjuvant Therapy in Esophageal Squamous Cell Carcinoma. Ann. Surg. 2022, 276, 101–110. [Google Scholar] [CrossRef]
- Okuno, K.; Garg, R.; Yuan, Y.C.; Tokunaga, M.; Kinugasa, Y.; Goel, A. Berberine and Oligomeric Proanthocyanidins Exhibit Synergistic Efficacy Through Regulation of PI3K-Akt Signaling Pathway in Colorectal Cancer. Front. Oncol. 2022, 12, 855860. [Google Scholar] [CrossRef]
- Nishiwada, S.; Sho, M.; Banwait, J.K.; Yamamura, K.; Akahori, T.; Nakamura, K.; Baba, H.; Goel, A. A MicroRNA Signature Identifies Pancreatic Ductal Adenocarcinoma Patients at Risk for Lymph Node Metastases. Gastroenterology 2020, 159, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Toden, S.; Okugawa, Y.; Buhrmann, C.; Nattamai, D.; Anguiano, E.; Baldwin, N.; Shakibaei, M.; Boland, C.R.; Goel, A. Novel Evidence for Curcumin and Boswellic Acid-Induced Chemoprevention through Regulation of miR-34a and miR-27a in Colorectal Cancer. Cancer Prev. Res. 2015, 8, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Boland, C.R.; Goel, A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 2015, 36, 355–367. [Google Scholar] [CrossRef]
- Toden, S.; Theiss, A.L.; Wang, X.; Goel, A. Essential turmeric oils enhance anti-inflammatory efficacy of curcumin in dextran sulfate sodium-induced colitis. Sci. Rep. 2017, 7, 814. [Google Scholar] [CrossRef]
- Ravindranathan, P.; Pasham, D.; Balaji, U.; Cardenas, J.; Gu, J.; Toden, S.; Goel, A. A combination of curcumin and oligomeric proanthocyanidins offer superior anti-tumorigenic properties in colorectal cancer. Sci. Rep. 2018, 8, 13869. [Google Scholar] [CrossRef]
- Ravindranathan, P.; Pasham, D.; Balaji, U.; Cardenas, J.; Gu, J.; Toden, S.; Goel, A. Mechanistic insights into anticancer properties of oligomeric proanthocyanidins from grape seeds in colorectal cancer. Carcinogenesis 2018, 39, 767–777. [Google Scholar] [CrossRef]
- Toden, S.; Ravindranathan, P.; Gu, J.; Cardenas, J.; Yuchang, M.; Goel, A. Oligomeric proanthocyanidins (OPCs) target cancer stem-like cells and suppress tumor organoid formation in colorectal cancer. Sci. Rep. 2018, 8, 3335. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Takahashi, N.; Takayama, T.; Goel, A. LAMC2 promotes cancer progression and gemcitabine resistance through modulation of EMT and ATP-binding cassette transporters in pancreatic ductal adenocarcinoma. Carcinogenesis 2021, 42, 546–556. [Google Scholar] [CrossRef]
- Roy, S.; Zhao, Y.; Yuan, Y.C.; Goel, A. Metformin and ICG-001 Act Synergistically to Abrogate Cancer Stem Cells-Mediated Chemoresistance in Colorectal Cancer by Promoting Apoptosis and Autophagy. Cancers 2022, 14, 1281. [Google Scholar] [CrossRef]
- Raimondi, G.; Mato-Berciano, A.; Pascual-Sabater, S.; Rovira-Rigau, M.; Cuatrecasas, M.; Fondevila, C.; Sánchez-Cabús, S.; Begthel, H.; Boj, S.F.; Clevers, H.; et al. Patient-derived pancreatic tumour organoids identify therapeutic responses to oncolytic adenoviruses. EBioMedicine 2020, 56, 102786. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Liles, J.S.; Arnoletti, J.P.; Tzeng, C.W.; Howard, J.H.; Kossenkov, A.V.; Kulesza, P.; Heslin, M.J.; Frolov, A. ErbB3 expression promotes tumorigenesis in pancreatic adenocarcinoma. Cancer Biol. Ther. 2010, 10, 555–563. [Google Scholar] [CrossRef]
- Liles, J.S.; Arnoletti, J.P.; Kossenkov, A.V.; Mikhaylina, A.; Frost, A.R.; Kulesza, P.; Heslin, M.J.; Frolov, A. Targeting ErbB3-mediated stromal-epithelial interactions in pancreatic ductal adenocarcinoma. Br. J. Cancer 2011, 105, 523–533. [Google Scholar] [CrossRef][Green Version]
- Camblin, A.J.; Pace, E.A.; Adams, S.; Curley, M.D.; Rimkunas, V.; Nie, L.; Tan, G.; Bloom, T.; Iadevaia, S.; Baum, J.; et al. Dual Inhibition of IGF-1R and ErbB3 Enhances the Activity of Gemcitabine and Nab-Paclitaxel in Preclinical Models of Pancreatic Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 2873–2885. [Google Scholar] [CrossRef]
- Qian, Y.; Gong, Y.; Fan, Z.; Luo, G.; Huang, Q.; Deng, S.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. Molecular alterations and targeted therapy in pancreatic ductal adenocarcinoma. J. Hematol. Oncol. 2020, 13, 130. [Google Scholar] [CrossRef]
- Kim, A.; Ha, J.; Kim, J.; Cho, Y.; Ahn, J.; Cheon, C.; Kim, S.H.; Ko, S.G.; Kim, B. Natural Products for Pancreatic Cancer Treatment: From Traditional Medicine to Modern Drug Discovery. Nutrients 2021, 13, 3801. [Google Scholar] [CrossRef]
- Sauter, E.R. Cancer prevention and treatment using combination therapy with natural compounds. Expert Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospecting 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Goel, A.; Jhurani, S.; Aggarwal, B.B. Multi-targeted therapy by curcumin: How spicy is it? Mol. Nutr. Food Res. 2008, 52, 1010–1030. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Park, B.; Goel, A.; Aggarwal, B.B. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011, 6, 93–108. [Google Scholar] [CrossRef]
- Weng, W.; Goel, A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin. Cancer Biol. 2022, 80, 73–86. [Google Scholar] [CrossRef]
- Pan, P.; Skaer, C.; Yu, J.; Zhao, H.; Ren, H.; Oshima, K.; Wang, L.S. Berries and other natural products in the pancreatic cancer chemoprevention in human clinical trials. J. Berry Res. 2017, 7, 147–161. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, S.E.; Tan, S.H.; Cao, R.; Chen, Y.; Xia, D.; Zhu, X.; Yang, X.F.; Ong, C.N.; Shen, H.M. Andrographolide sensitizes cisplatin-induced apoptosis via suppression of autophagosome-lysosome fusion in human cancer cells. Autophagy 2012, 8, 338–349. [Google Scholar] [CrossRef]
- Zhou, J.; Ong, C.N.; Hur, G.M.; Shen, H.M. Inhibition of the JAK-STAT3 pathway by andrographolide enhances chemosensitivity of cancer cells to doxorubicin. Biochem. Pharmacol. 2010, 79, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Yunos, N.M.; Mutalip, S.S.; Jauri, M.H.; Yu, J.Q.; Huq, F. Anti-proliferative and pro-apoptotic effects from sequenced combinations of andrographolide and cisplatin on ovarian cancer cell lines. Anticancer Res. 2013, 33, 4365–4371. [Google Scholar]
- Prior, I.A.; Hood, F.E.; Hartley, J.L. The Frequency of Ras Mutations in Cancer. Cancer Res 2020, 80, 2969–2974. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Vander Heiden, M.G.; McCormick, F. The Metabolic Landscape of RAS-Driven Cancers from biology to therapy. Nat. Cancer 2021, 2, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Amrutkar, M.; Gladhaug, I.P. Pancreatic Cancer Chemoresistance to Gemcitabine. Cancers 2017, 9, 157. [Google Scholar] [CrossRef]
- Chio, I.I.C.; Jafarnejad, S.M.; Ponz-Sarvise, M.; Park, Y.; Rivera, K.; Palm, W.; Wilson, J.; Sangar, V.; Hao, Y.; Öhlund, D.; et al. NRF2 Promotes Tumor Maintenance by Modulating mRNA Translation in Pancreatic Cancer. Cell 2016, 166, 963–976. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Goswami, D.; Adiseshaiah, P.P.; Burgan, W.; Yi, M.; Guerin, T.M.; Kozlov, S.V.; Nissley, D.V.; McCormick, F. Undermining Glutaminolysis Bolsters Chemotherapy While NRF2 Promotes Chemoresistance in KRAS-Driven Pancreatic Cancers. Cancer Res. 2020, 80, 1630–1643. [Google Scholar] [CrossRef]
- Chen, W.S.; Lazar, C.S.; Lund, K.A.; Welsh, J.B.; Chang, C.P.; Walton, G.M.; Der, C.J.; Wiley, H.S.; Gill, G.N.; Rosenfeld, M.G. Functional independence of the epidermal growth factor receptor from a domain required for ligand-induced internalization and calcium regulation. Cell 1989, 59, 33–43. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, Z.; Cao, B. The function of human epidermal growth factor receptor-3 and its role in tumors (Review). Oncol. Rep. 2013, 30, 2563–2570. [Google Scholar] [CrossRef][Green Version]
- Lee, D.; Yu, M.; Lee, E.; Kim, H.; Yang, Y.; Kim, K.; Pannicia, C.; Kurie, J.M.; Threadgill, D.W. Tumor-specific apoptosis caused by deletion of the ERBB3 pseudo-kinase in mouse intestinal epithelium. J. Clin. Investig. 2009, 119, 2702–2713. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.; Chin, H.; Kim, K.; Lee, D. ERBB3 knockdown induces cell cycle arrest and activation of Bak and Bax-dependent apoptosis in colon cancer cells. Oncotarget 2014, 5, 5138–5152. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.S.D.; Liao, W.; Zhou, S.; Wong, W.S.F. Is there a future for andrographolide to be an anti-inflammatory drug? Deciphering its major mechanisms of action. Biochem. Pharmacol. 2017, 139, 71–81. [Google Scholar] [PubMed]
- Chern, C.M.; Liou, K.T.; Wang, Y.H.; Liao, J.F.; Yen, J.C.; Shen, Y.C. Andrographolide inhibits PI3K/AKT-dependent NOX2 and iNOS expression protecting mice against hypoxia/ischemia-induced oxidative brain injury. Planta Med. 2011, 77, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Iruretagoyena, M.I.; Tobar, J.A.; González, P.A.; Sepúlveda, S.E.; Figueroa, C.A.; Burgos, R.A.; Hancke, J.L.; Kalergis, A.M. Andrographolide interferes with T cell activation and reduces experimental autoimmune encephalomyelitis in the mouse. J. Pharmacol. Exp. Ther. 2005, 312, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.K.; Hung, L.C.; Kuo, C.D.; Lee, K.Y.; Lee, M.S.; Lin, H.Y.; Chen, Y.J.; Fu, S.L. Andrographolide sensitizes Ras-transformed cells to radiation in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1232–1239. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L. Biological properties of andrographolide, an active ingredient of Andrographis Paniculata: A narrative review. Ann. Transl. Med. 2021, 9, 1186. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuno, K.; Xu, C.; Pascual-Sabater, S.; Tokunaga, M.; Takayama, T.; Han, H.; Fillat, C.; Kinugasa, Y.; Goel, A. Andrographis Reverses Gemcitabine Resistance through Regulation of ERBB3 and Calcium Signaling Pathway in Pancreatic Ductal Adenocarcinoma. Biomedicines 2023, 11, 119. https://doi.org/10.3390/biomedicines11010119
Okuno K, Xu C, Pascual-Sabater S, Tokunaga M, Takayama T, Han H, Fillat C, Kinugasa Y, Goel A. Andrographis Reverses Gemcitabine Resistance through Regulation of ERBB3 and Calcium Signaling Pathway in Pancreatic Ductal Adenocarcinoma. Biomedicines. 2023; 11(1):119. https://doi.org/10.3390/biomedicines11010119
Chicago/Turabian StyleOkuno, Keisuke, Caiming Xu, Silvia Pascual-Sabater, Masanori Tokunaga, Tetsuji Takayama, Haiyong Han, Cristina Fillat, Yusuke Kinugasa, and Ajay Goel. 2023. "Andrographis Reverses Gemcitabine Resistance through Regulation of ERBB3 and Calcium Signaling Pathway in Pancreatic Ductal Adenocarcinoma" Biomedicines 11, no. 1: 119. https://doi.org/10.3390/biomedicines11010119
APA StyleOkuno, K., Xu, C., Pascual-Sabater, S., Tokunaga, M., Takayama, T., Han, H., Fillat, C., Kinugasa, Y., & Goel, A. (2023). Andrographis Reverses Gemcitabine Resistance through Regulation of ERBB3 and Calcium Signaling Pathway in Pancreatic Ductal Adenocarcinoma. Biomedicines, 11(1), 119. https://doi.org/10.3390/biomedicines11010119






