Worsening Thrombotic Complication of Atherosclerotic Plaques Due to Neutrophils Extracellular Traps: A Systematic Review
Abstract
:1. Introduction
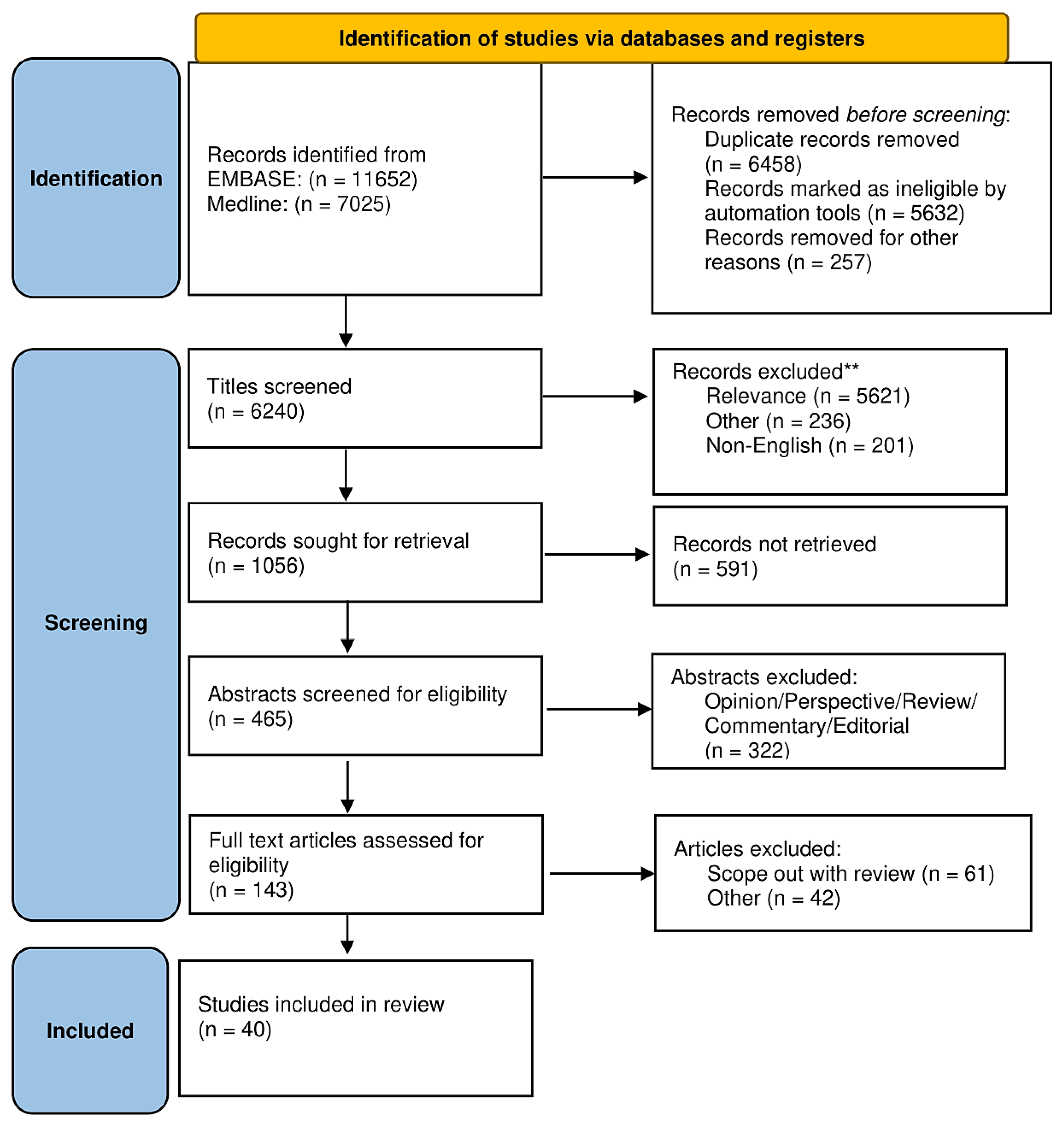
2. Material and Methods
2.1. Data Sources and Systematic Literature Review
2.2. Data Extraction, Quality Assessment, and Aims
3. Results
3.1. Recent Advances in the Biology of Neutrophils
Mechanisms for the Release of NETs and Composition of NETs
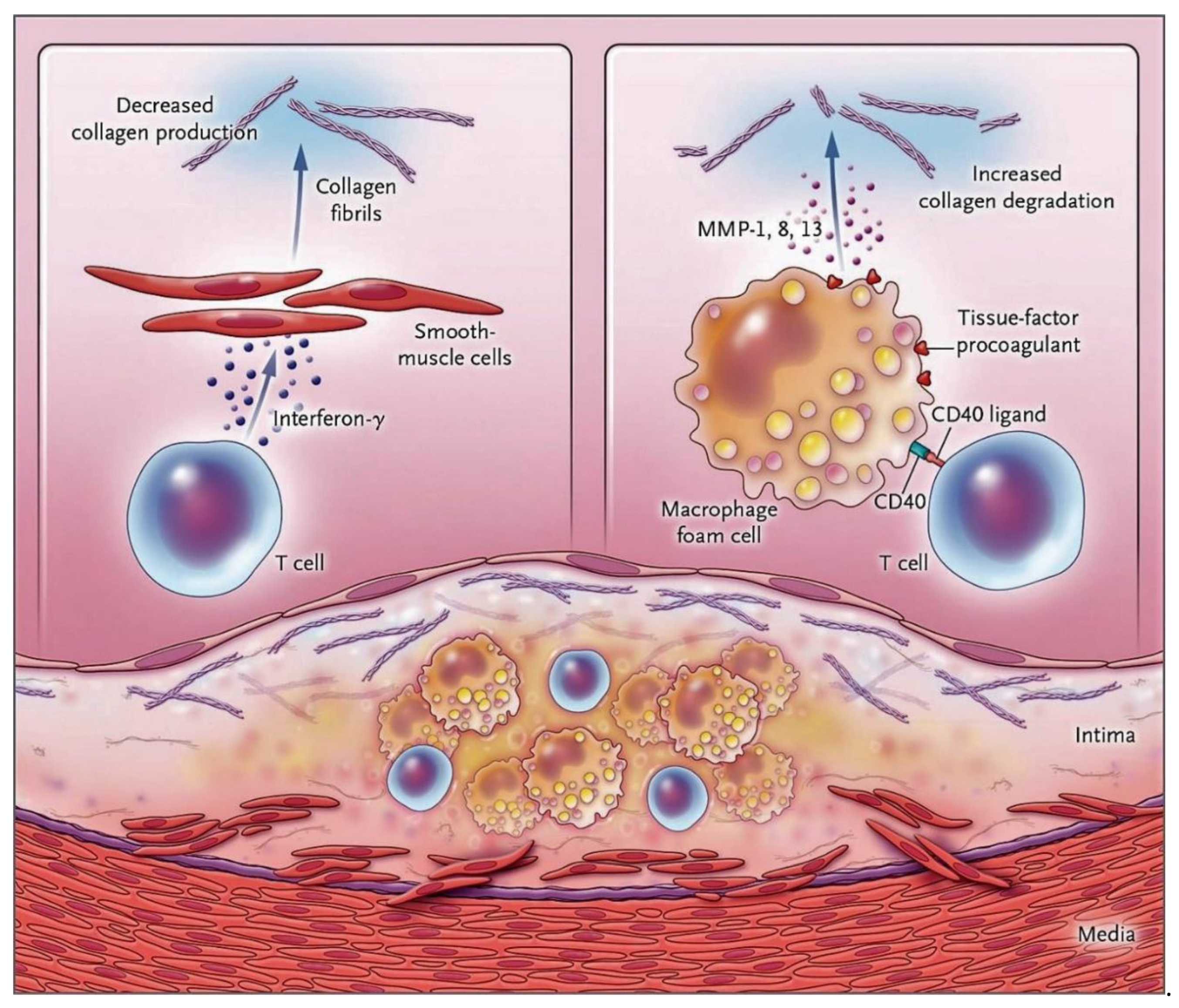
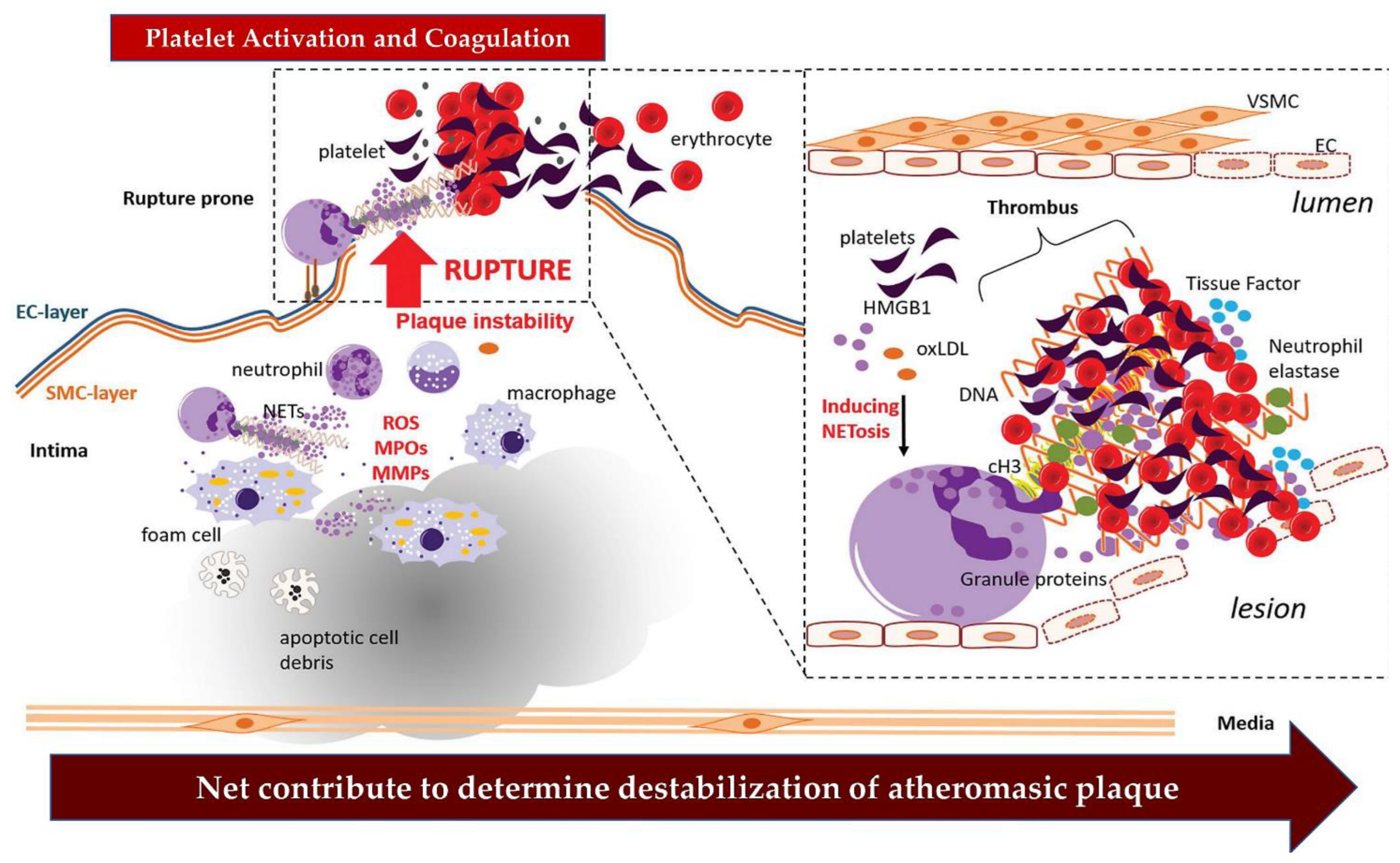
3.2. The NETs Induce a Worsening of the Thrombotic Process Complicating the Atherosclerotic Plaques
Role of Nets in Plaque Destabilization: The New Challenge
4. NETs’ Actors of Arterial Thrombosis
5. The Cardiovascular Clinical Potential Derived from Biomarker Applications of NETs
6. Therapeutic Implications of NETs in Cardiovascular Conditions
7. Limitations
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Gu, Q.; Yang, X.; Lv, J.; Zhang, J.; Xia, B.; Kim, J.D.; Wang, R.; Xiong, F.; Meng, S.; Clements, T.P.; et al. AIBP-mediated cholesterol efflux instructs hematopoietic stem and progenitor cell fate. Science 2019, 363, 1085–1088. [Google Scholar] [CrossRef]
- Nagareddy, P.R.; Murphy, A.J.; Stirzaker, R.A.; Hu, Y.; Yu, S.; Miller, R.G.; Ramkhelawon, B.; Distel, E.; Westerterp, M.; Huang, L.S.; et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013, 17, 695–708. [Google Scholar] [CrossRef]
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 2015, 21, 815–819. [Google Scholar] [CrossRef]
- Lim, C.H.; Adav, S.S.; Sze, S.K.; Choong, Y.K.; Saravanan, R.; Schmidtchen, A. Thrombin and plasmin alter the proteome of neutrophil extracellular traps. Front. Immunol. 2018, 9, 1554. [Google Scholar] [CrossRef]
- Tall, A.R.; Westerterp, M. Inflammasomes, neutrophil extracellular traps, and cholesterol. J. Lipid Res. 2019, 60, 721–727. [Google Scholar] [CrossRef]
- Keitelman, I.A.; Shiromizu, C.M.; Zgajnar, N.R.; Danielián, S.; Jancic, C.C.; Martí, M.A.; Fuentes, F.; Yancoski, J.; Vera Aguilar, D.; Rosso, D.A.; et al. The interplay between serine proteases and caspase-1 regulates the autophagy-mediated secretion of Interleukin-1 beta in human neutrophils. Front. Immunol. 2022, 13, 832306. [Google Scholar] [CrossRef]
- Moschonas, I.C.; Tselepis, A.D. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis 2019, 288, 9–16. [Google Scholar] [CrossRef]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.G.; Shvartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef]
- Wang, W.; Liu, W.; Fidler, T.; Wang, Y.; Tang, Y.; Woods, B.; Welch, C.; Cai, B.; Silvestre-Roig, C.; Ai, D.; et al. Macrophage inflammation, erythrophagocytosis, and accelerated atherosclerosis in Jak2V617F mice. Circ. Res. 2018, 123, e35–e47. [Google Scholar] [CrossRef]
- Wolach, O.; Sellar, R.S.; Martinod, K.; Cherpokova, D.; McConkey, M.; Chappell, R.J.; Silver, A.J.; Adams, D.; Castellano, C.A.; Schneider, R.K.; et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci. Transl. Med. 2018, 10, eaan8292. [Google Scholar] [CrossRef] [PubMed]
- Quillard, T.; Araújo, H.A.; Franck, G.; Shvartz, E.; Sukhova, G.; Libby, P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: Implications for superficial erosion. Eur. Heart J. 2015, 36, 1394–1404. [Google Scholar] [CrossRef]
- Li, G.X.; Jiang, X.H.; Zang, J.N.; Zhu, B.Z.; Jia, C.C.; Niu, K.W.; Liu, X.; Jiang, R.; Wang, B. B-cell receptor associated protein 31 deficiency decreases the expression of adhesion molecule CD11b/CD18 and PSGL-1 in neutrophils to ameliorate acute lung injury. Int. J. Biochem. Cell Biol. 2022, 152, 106299. [Google Scholar] [CrossRef]
- Drechsler, M.; Megens, R.T.; van Zandvoort, M.; Weber, C.; Soehnlein, O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 2010, 122, 1837–1845. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Y.; Xu, S.; Yin, Y.; Ageno, W.; De Stefano, V.; Zhao, Q.; Qi, X. Clinical significance of neutrophil extracellular traps biomarkers in thrombosis. Thromb. J. 2022, 20, 63. [Google Scholar] [CrossRef]
- Pertiwi, K.R.; van der Wal, A.C.; Pabittei, D.R.; Mackaaij, C.; van Leeuwen, M.B.; Li, X.; de Boer, O.J. Neutrophil extracellular traps participate in all different types of thrombotic and haemorrhagic complications of coronary atherosclerosis. Thromb. Haemost. 2018, 118, 1078–1087. [Google Scholar] [CrossRef]
- Megens, R.T.; Vijayan, S.; Lievens, D.; Döring, Y.; van Zandvoort, M.A.; Grommes, J.; Weber, C.; Soehnlein, O. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb. Haemost. 2012, 107, 597–598. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Herre, M.; Cedervall, J.; Mackman, N.; Olsson, A.K. Neutrophil extracellular traps in the pathology of cancer and other inflammatory diseases. Physiol. Rev. 2023, 103, 277–312. [Google Scholar] [CrossRef]
- Rossaint, J.; Herter, J.M.; Van Aken, H.; Napirei, M.; Döring, Y.; Weber, C.; Soehnlein, O.; Zarbock, A. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood J. Am. Soc. Hematol. 2014, 123, 2573–2584. [Google Scholar] [CrossRef]
- Winter, C.; Silvestre-Roig, C.; Ortega-Gomez, A.; Lemnitzer, P.; Poelman, H.; Schumski, A.; Winter, J.; Drechsler, M.; de Jong, R.; Immler, R.; et al. Chrono-pharmacological targeting of the CCL2-CCR2 axis ameliorates atherosclerosis. Cell Metab. 2018, 28, 175–182.e5. [Google Scholar] [CrossRef] [PubMed]
- Adrover, J.M.; Del Fresno, C.; Crainiciuc, G.; Cuartero, M.I.; Casanova-Acebes, M.; Weiss, L.A.; Huerga-Encabo, H.; Silvestre-Roig, C.; Rossaint, J.; Cossío, I.; et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity 2019, 51, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.L.; Hwang, I.Y.; Kamenyeva, O.; Kabat, J.; Kim, J.S.; Park, C.; Kehrl, J.H. Unrestrained Gαi2 Signaling Disrupts Neutrophil Trafficking, Aging, and Clearance. Front. Immunol. 2021, 12, 679856. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Acebes, M.; Nicolás-Ávila, J.A.; Li, J.L.; García-Silva, S.; Balachande, A.; Rubio-Ponce, A.; Weiss, L.A.; Adrover, J.M.; Burrows, K.; A-González, N.; et al. Neutrophils instruct homeostatic and pathological states in naive tissues. J. Exp. Med. 2018, 215, 2778–2795. [Google Scholar] [CrossRef]
- Zheng, Y.; Sefik, E.; Astle, J.; Karatepe, K.; Öz, H.H.; Solis, A.G.; Jackson, R.; Luo, H.R.; Bruscia, E.M.; Halene, S.; et al. Human neutrophil development and functionality are enabled in a humanized mouse model. Proc. Natl. Acad. Sci. USA 2022, 119, e2121077119. [Google Scholar] [CrossRef]
- Wigerblad, G.; Kaplan, M.J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat. Rev. Immunol. 2022; 1–15, online ahead of print. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.Y.; Bae, Y.S. Emerging roles of neutrophils in immune homeostasis. BMB Rep. 2022, 55, 473–480. [Google Scholar] [CrossRef]
- Palmer, L.J.; Cooper, P.R.; Ling, M.R.; Wright, H.J.; Huissoon, A.; Chapple, I.L. Hypochlorous acid regulates neutrophil extracellular trap release in humans. Clin. Exp. Immunol. 2011, 167, 261–268. [Google Scholar] [CrossRef]
- Cassatella, M.A.; Östberg, N.K.; Tamassia, N.; Soehnlein, O. Biological roles of neutrophil-derived granule proteins and cytokines. Trends Immunol. 2019, 40, 648–664. [Google Scholar] [CrossRef]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef]
- Zhong, H.; Lu, R.Y.; Wang, Y. Neutrophil extracellular traps in fungal infections: A seesaw battle in hosts. Front. Immunol. 2022, 13, 977493. [Google Scholar] [CrossRef]
- Subramaniam, S.; Kothari, H.; Bosmann, M. Tissue factor in COVID-19-associated coagulopathy. Thromb. Res. 2022, 220, 35–47. [Google Scholar] [CrossRef]
- Wei, Z.; Cheng, Q.; Xu, N.; Zhao, C.; Xu, J.; Kang, L.; Lou, X.; Yu, L.; Feng, W. Investigation of CRS-associated cytokines in CAR-T therapy with meta-GNN and pathway crosstalk. BMC Bioinform. 2022, 23, 373. [Google Scholar] [CrossRef]
- Wang, H.; Shao, J.; Lu, X.; Jiang, M.; Li, X.; Liu, Z.; Zhao, Y.; Zhou, J.; Lin, L.; Wang, L.; et al. Potential of immune-related genes as promising biomarkers for premature coronary heart disease through high throughput sequencing and integrated bioinformatics analysis. Front. Cardiovasc. Med. 2022, 9, 893502. [Google Scholar] [CrossRef]
- Arazna, M.; Pruchniak, M.P.; Zycinska, K.; Demkow, U. Neutrophil extracellular trap in human diseases. Adv. Exp. Med. Biol. 2013, 756, 1–8. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Brinkmann, V.; Laube, B.; Abu Abed, U.; Goosmann, C.; Zychlinsky, A. Neutrophil extracellular traps: How to generate and visualize them. J. Vis. Exp. 2010, 36, e1724. [Google Scholar] [CrossRef]
- Leung, H.H.L.; Perdomo, J.; Ahmadi, Z.; Zheng, S.S.; Rashid, F.N.; Enjeti, A.; Ting, S.B.; Chong, J.J.H.; Chong, B.H. NETosis and thrombosis in vaccine-induced immune thrombotic thrombocytopenia. Nat. Commun. 2022, 13, 5206. [Google Scholar] [CrossRef]
- Remijsen, Q.; Vanden Berghe, T.; Wirawan, E.; Asselbergh, B.; Parthoens, E.; De Rycke, R.; Noppen, S.; Delforge, M.; Willems, J.; Vandenabeele, P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011, 21, 290–304. [Google Scholar] [CrossRef]
- Amulic, B.; Knackstedt, S.L.; Abu Abed, U.; Deigendesch, N.; Harbort, C.J.; Caffrey, B.E.; Brinkmann, V.; Heppner, F.L.; Hinds, P.W.; Zychlinsky, A. Cell-Cycle proteins control production of neutrophil extracellular traps. Dev. Cell 2017, 43, 449–462.e5. [Google Scholar] [CrossRef]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011, 7, 75–77. [Google Scholar] [CrossRef]
- Branitzki-Heinemann, K.; Möllerherm, H.; Völlger, L.; Husein, D.M.; de Buhr, N.; Blodkamp, S.; Reuner, F.; Brogden, G.; Naim, H.Y.; von Köckritz-Blickwede, M. Formation of neutrophil extracellular traps under low oxygen level. Front. Immunol. 2016, 7, 518. [Google Scholar] [CrossRef]
- Dölling, M.; Eckstein, M.; Singh, J.; Schauer, C.; Schoen, J.; Shan, X.; Bozec, A.; Knopf, J.; Schett, G.; Muñoz, L.E.; et al. Hypoxia Promotes Neutrophil Survival After Acute Myocardial Infarction. Front. Immunol. 2022, 13, 726153. [Google Scholar] [CrossRef]
- Metzler, K.D.; Fuchs, T.A.; Nauseef, W.M.; Reumaux, D.; Roesler, J.; Schulze, I.; Wahn, V.; Papayannopoulos, V.; Zychlinsky, A. Myeloperoxidase is required for neutrophil extracellular trap formation: Implications for innate immunity. Blood 2011, 117, 953–959. [Google Scholar] [CrossRef]
- Björnsdottir, H.; Welin, A.; Michaëlsson, E.; Osla, V.; Berg, S.; Christenson, K.; Sundqvist, M.; Dahlgren, C.; Karlsson, A. Bylund Neutrophil NET formation is regulated from the inside by myeloperoxidase-processed reactive oxygen species. Free Radic. Biol. Med. 2015, 89, 1024–1035. [Google Scholar] [CrossRef]
- Lockhart, J.S.; Sumagin, R. Non-Canonical Functions of Myeloperoxidase in Immune Regulation, Tissue Inflammation and Cancer. Int. J. Mol. Sci. 2022, 23, 12250. [Google Scholar] [CrossRef]
- Kenny, E.F.; Herzig, A.; Kruger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; Bernuth, H.V.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife 2017, 6, e24437. [Google Scholar] [CrossRef]
- Neeli, I.; Radic, M. Opposition between PKC isoforms regulates histone deimination and neutrophil extracellular chromatin release. Front. Immunol. 2013, 4, 38. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Stadler, S.; Correll, S.; Li, P.; Wang, D.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef]
- Leshner, M.; Wang, S.; Lewis, C.; Zheng, H.; Chen, X.A.; Santy, L.; Wang, Y. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 2012, 3, 307. [Google Scholar] [CrossRef]
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010, 207, 1853–1862. [Google Scholar] [CrossRef]
- Martinod, K.; Fuchs, T.A.; Zitomersky, N.L.; Wong, S.L.; Demers, M.; Gallant, M.; Wang, Y.; Wagner, D.D. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood 2015, 125, 1948–1956. [Google Scholar] [CrossRef]
- Boeltz, S.; Amini, P.; Anders, H.J.; Andrade, F.; Bilyy, R.; Chatfield, S.; Cichon, I.; Clancy, D.M.; Desai, J.; Dumych, T.; et al. To NET or not to NET: Current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019, 26, 395–408. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Braster, Q.; Wichapong, K.; Lee, E.Y.; Teulon, J.M.; Berrebeh, N.; Winter, J.; Adrover, J.M.; Santos, G.S.; Froese, A.; et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 2019, 569, 236–240. [Google Scholar] [CrossRef]
- Zhang, Y.; Jian, W.; He, L.; Wu, J. Externalized histone H4: A novel target that orchestrates chronic inflammation by inducing lytic cell death. Acta Biochim. Biophys. Sin. 2020, 52, 336–338. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Penson, P.E.; Banach, M.; Motallebnezhad, M.; Jamialahmadi, T.; Sahebkar, A. Epigenetic control of atherosclerosis via DNA methylation: A new therapeutic target? Life Sci. 2020, 253, 117682. [Google Scholar] [CrossRef]
- Adrover, J.M.; Aroca-Crevillén, A.; Crainiciuc, G.; Ostos, F.; Rojas-Vega, Y.; Rubio-Ponce, A.; Cilloniz, C.; Bonzón-Kulichenko, E.; Calvo, E.; Rico, D.; et al. Programmed ‘disarming’ of the neutrophil proteome reduces the magnitude of inflammation. Nat. Immunol. 2020, 21, 135–144. [Google Scholar] [CrossRef]
- Talal, S.; Mona, K.; Karem, A.; Yaniv, L.; Reut, H.M.; Ariel, S.; Moran, A.K.; Harel, E.; Campisi-Pinto, S.; Mahmoud, A.A.; et al. Neutrophil degranulation and severely impaired extracellular trap formation at the basis of susceptibility to infections of hemodialysis patients. BMC Med. 2022, 20, 364. [Google Scholar] [CrossRef]
- Kolte, D.; Libby, P.; Jang, I.K. New Insights into Plaque Erosion as a Mechanism of Acute Coronary Syndromes. JAMA 2021, 325, 1043–1044. [Google Scholar] [CrossRef]
- Libby, P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef]
- Brezinski, M.; Willard, F.; Rupnick, M. Inadequate Intimal Angiogenesis as a Source of Coronary Plaque Instability: Implications for Healing. Circulation 2019, 140, 1857–1859. [Google Scholar] [CrossRef]
- Fernandez, D.M.; Rahman, A.H.; Fernandez, N.F.; Chudnovskiy, A.; Amir, E.D.; Amadori, L.; Khan, N.S.; Wong, C.K.; Shamailova, R.; Hill, C.A.; et al. Single cell immune landscape of human atherosclerotic plaques. Nat. Med. 2019, 25, 1576–1588. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Prüller, F.; Schnedl, W.; Renner, W.; Almer, G. Beyond Macrophages and T Cells: B Cells and Immunoglobulins Determine the Fate of the Atherosclerotic Plaque. Int. J. Mol. Sci. 2020, 21, 4082. [Google Scholar] [CrossRef] [PubMed]
- Dentali, F.; Nigro, O.; Squizzato, A.; Gianni, M.; Zuretti, F.; Grandi, A.M.; Guasti, L. Impact of neutrophils to lymphocytes ratio on major clinical outcomes in patients with acute coronary syndromes: A systematic review and meta-analysis of the literature. Int. J. Cardiol. 2018, 266, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Guasti, L.; Dentali, F.; Castiglioni, L.; Maroni, L.; Marino, F.; Squizzato, A.; Ageno, W.; Gianni, M.; Gaudio, G.; Grandi, A.M.; et al. Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularization. A systematic review on more than 34,000 subjects. Thromb. Haemost. 2011, 106, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Dahdah, A.; Johnson, J.; Gopalkrishna, S.; Jaggers, R.M.; Webb, D.; Murphy, A.J.; Hanssen, N.M.J.; Hanaoka, B.Y.; Nagareddy, P.R. Neutrophil Migratory Patterns: Implications for Cardiovascular Disease. Front. Cell Dev. Biol. 2022, 10, 795784. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.D.; Klatsky, A.L.; Siegelaub, A.B. The leukocyte count as a predictor of myocardial infarction. N. Engl. J. Med. 1974, 290, 1275–1278. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, S.; Yi, Y.; Qu, T.; Gao, S.; Lin, Y.; Zhu, H. Neutrophil-to-lymphocyte ratio as a predictor for cardiovascular diseases: A cohort study in Tianjin, China. J. Hum. Hypertens. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Zhang, R.; Brennan, M.L.; Fu, X.; Aviles, R.J.; Pearce, G.L.; Penn, M.S.; Topol, E.J.; Sprecher, D.L.; Hazen, S.L. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA 2001, 286, 2136–2142. [Google Scholar] [CrossRef]
- Borissoff, J.I.; Joosen, I.A.; Versteylen, M.O.; Brill, A.; Fuchs, T.A.; Savchenko, A.S.; Gallant, M.; Martinod, K.; Ten Cate, H.; Hofstra, L.; et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arter. Thromb. Vasc. Biol. 2013, 33, 2032–2040. [Google Scholar] [CrossRef]
- Libby, P. The molecular bases of the acute coronary syndromes. Circulation 1995, 91, 2844–2850. [Google Scholar] [CrossRef]
- Narula, J.; Garg, P.; Achenbach, S.; Motoyama, S.; Virmani, R.; Strauss, H.W. Arithmetic of vulnerable plaques for noninvasive imaging. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, S2–S10. [Google Scholar] [CrossRef]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Falk, E.; Nakano, M.; Benton, J.F.; Finn, A.V.; Virmani, R. Update on acute coronary syndromes: The pathologists’ view. Eur. Heart J. 2013, 34, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Liuzzo, G. Pathogenesis of acute coronary syndromes. J. Am. Coll. Cardiol. 2013, 61, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ionita, M.G.; van den Borne, P.; Catanzariti, L.M.; Moll, F.L.; de Vries, J.P.; Pasterkamp, G.; Vink, A.; de Kleijn, D.P. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arter. Thromb. Vasc. Biol. 2010, 30, 1842–1848. [Google Scholar] [CrossRef]
- Lee, M.W.; Luo, E.W.; Silvestre-Roig, C.; Srinivasan, Y.; Akabori, K.; Lemnitzer, P.; Schmidt, N.W.; Lai, G.H.; Santangelo, C.D.; Soehnlein, O.; et al. Apolipoprotein Mimetic Peptide Inhibits Neutrophil-Driven Inflammatory Damage via Membrane Remodeling and Suppression of Cell Lysis. ACS Nano 2021, 26, 15930–15939. [Google Scholar] [CrossRef] [PubMed]
- Locke, M.; Francis, R.J.; Tsaousi, E.; Longstaff, C. Fibrinogen protects neutrophils from the cytotoxic effects of histones and delays neutrophil extracellular trap formation induced by ionomycin. Sci. Rep. 2020, 10, 11694. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones. PLoS ONE 2012, 7, e32366. [Google Scholar] [CrossRef] [PubMed]
- Wildhagen, K.C.; García de Frutos, P.; Reutelingsperger, C.P.; Schrijver, R.; Aresté, C.; Ortega-Gómez, A.; Deckers, N.M.; Hemker, H.C.; Soehnlein, O.; Nicolaes, G.A. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood 2014, 123, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Paulin, N.; Viola, J.R.; Maas, S.L.; de Jong, R.; Fernandes-Alnemri, T.; Weber, C.; Drechsler, M.; Döring, Y.; Soehnlein, O. Double-Strand DNA sensing aim2 inflammasome regulates atherosclerotic plaque vulnerability. Circulation 2018, 138, 321–323. [Google Scholar] [CrossRef]
- Tall, A.R.; Fuster, J.J. Clonal hematopoiesis in cardiovascular disease and therapeutic implications. Nat. Cardiovasc. Res. 2022, 1, 116–124. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, K.; Zhu, L. Emerging Roles of Inflammasomes in Cardiovascular Diseases. Front. Immunol. 2022, 13, 834289. [Google Scholar] [CrossRef] [PubMed]
- Thålin, C.; Hisada, Y.; Lundström, S.; Mackman, N.; Wallén, H. Neutrophil extracellular traps: Villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1724–1738. [Google Scholar] [CrossRef] [PubMed]
- Carestia, A.; Kaufman, T.; Schattner, M. Platelets: New bricks in the building of neutrophil extracellular traps. Front. Immunol. 2016, 7, 271. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.J.; Vu, T.T.; Swystun, L.L.; Dwivedi, D.J.; Mai, S.H.; Weitz, J.I.; Liaw, P.C. Neutrophil extracellular traps promote thrombin generation through platelet dependent and platelet-independent mechanisms. Arter. Thromb. Vasc. Biol. 2014, 34, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers DDJr Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef]
- Von Brühl, M.L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- Noubouossie, D.F.; Whelihan, M.F.; Yu, Y.B.; Sparkenbaugh, E.; Pawlinski, R.; Monroe, D.M.; Key, N.S. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood 2017, 129, 1021–1029. [Google Scholar] [CrossRef]
- Riegger, J.; Byrne, R.A.; Joner, M.; Chandraratne, S.; Gershlick, A.H.; Ten Berg, J.M.; Adriaenssens, T.; Guagliumi, G.; Godschalk, T.C.; Neumann, F.J.; et al. Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort (PRESTIGE) Investigators. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: A report of the prevention of late stent thrombosis by an interdisciplinary global European effort consortium. Eur. Heart J. 2016, 37, 1538–1549. [Google Scholar] [CrossRef]
- Farkas, Á.Z.; Farkas, V.J.; Gubucz, I.; Szabó, L.; Bálint, K.; Tenekedjiev, K.; Nagy, A.I.; Sótonyi, P.; Hidi, L.; Nagy, Z.; et al. Neutrophil extracellular traps in thrombi retrieved during interventional treatment of ischemic arterial diseases. Thromb. Res. 2019, 175, 46–52. [Google Scholar] [CrossRef]
- De Boer, O.J.; Li, X.; Teeling, P.; Mackaay, C.; Ploegmakers, H.J.; van der Loos, C.M.; Daemen, M.J.; de Winter, R.J.; van der Wal, A.C. Neutrophils, neutrophil extracellular traps and interleukin-17 associate with the organization of thrombi in acute myocardial infarction. Thromb. Haemost. 2013, 109, 290–297. [Google Scholar] [CrossRef]
- Mangold, A.; Alias, S.; Scherz, T.; Hofbauer, T.; Jakowitsch, J.; Panzenböck, A.; Simon, D.; Laimer, D.; Bangert, C.; Kammerlander, A.; et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res. 2015, 116, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Stakos, D.A.; Kambas, K.; Konstantinidis, T.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Tsironidou, V.; Giatromanolaki, A.; Skendros, P.; Konstantinides, S.; et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur. Heart J. 2015, 36, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Laridan, E.; Denorme, F.; Desender, L.; François, O.; Andersson, T.; Deckmyn, H.; Vanhoorelbeke, K.; De Meyer, S.F. Neutrophil extracellular traps in ischemic stroke thrombi. Ann. Neurol. 2017, 82, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Pertiwi, K.R.; de Boer, O.J.; Mackaaij, C.; Pabittei, D.R.; de Winter, R.J.; Li, X.; van der Wal, A.C. Extracellular traps derived from macrophages, mast cells, eosinophils and neutrophils are generated in a time-dependent manner during atherothrombosis. J. Pathol. 2019, 247, 505–512. [Google Scholar] [CrossRef]
- Maugeri, N.; Campana, L.; Gavina, M.; Covino, C.; De Metrio, M.; Panciroli, C.; Maiuri, L.; Maseri, A.; D’Angelo, A.; Bianchi, M.E.; et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 2014, 12, 2074–2088. [Google Scholar] [CrossRef]
- Zaid, Y.; Merhi, Y. Implication of Platelets in Immuno-Thrombosis and Thrombo-Inflammation. Front. Cardiovasc. Med. 2022, 9, 863846. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Tsioufis, K.; Tousoulis, D. Factors Associated with Platelet Activation-Recent Pharmaceutical Approaches. Int. J. Mol. Sci. 2022, 23, 3301. [Google Scholar] [CrossRef]
- Novotny, J.; Oberdieck, P.; Titova, A.; Pelisek, J.; Chandraratne, S.; Nicol, P.; Hapfelmeier, A.; Joner, M.; Maegdefessel, L.; Poppert, H.; et al. Thrombus NET content is associated with clinical outcome in stroke and myocardial infarction. Neurology 2020, 94, e2346–e2360. [Google Scholar] [CrossRef]
- Chilingaryan, Z.; Deshmukh, T.; Leung, H.H.L.; Perdomo, J.; Emerson, P.; Kurup, R.; Chong, B.H.; Chong, J.J.H. Erythrocyte interaction with neutrophil extracellular traps in coronary artery thrombosis following myocardial infarction. Pathology 2022, 54, 87–94. [Google Scholar] [CrossRef]
- Knight, J.S.; Luo, W.; O’Dell, A.A.; Yalavarthi, S.; Zhao, W.; Subramanian, V.; Guo, C.; Grenn, R.C.; Thompson, P.R.; Eitzman, D.T.; et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ. Res. 2014, 114, 947–956. [Google Scholar] [CrossRef]
- Bonnard, T.; Hagemeyer, C.E. Ferric Chloride-induced Thrombosis Mouse Model on Carotid Artery Mesentery Vessel. J. Vis. Exp. 2015, e52838. [Google Scholar] [CrossRef] [PubMed]
- Novotny, J.; Chandraratne, S.; Weinberger, T.; Philippi, V.; Stark, K.; Ehrlich, A.; Pircher, J.; Konrad, I.; Oberdieck, P.; Titova, A.; et al. Histological comparison of arterial thrombi in mice and men and the influence of Cl-amidine on thrombus formation. PLoS ONE 2018, 13, e0190728. [Google Scholar] [CrossRef] [PubMed]
- Ducroux, C.; Di Meglio, L.; Loyau, S.; Delbosc, S.; Boisseau, W.; Deschildre, C.; Ben Maacha, M.; Blanc, R.; Redjem, H.; Ciccio, G.; et al. Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke 2018, 49, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Massberg, S.; Grahl, L.; von Bruehl, M.L.; Manukyan, D.; Pfeiler, S.; Goosmann, C.; Brinkmann, V.; Lorenz, M.; Bidzhekov, K.; Khandagale, A.B.; et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010, 16, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Sorvillo, N.; Mizurini, D.M.; Coxon, C.; Martinod, K.; Tilvawala, R.; Cherpokova, D.; Salinger, A.J.; Seward, R.J.; Staudinger, C.; Weerapana, E.; et al. Plasma peptidylarginine deiminase IV promotes VWF-platelet string formation and accelerates thrombosis after vessel injury. Circ. Res. 2019, 125, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Cherpokova, D.; Jouvene, C.C.; Libreros, S.; DeRoo, E.P.; Chu, L.; de la Rosa, X.; Norris, P.C.; Wagner, D.D.; Serhan, C.N. Resolvin D4 attenuates the severity of pathological thrombosis in mice. Blood 2019, 134, 1458–1468. [Google Scholar] [CrossRef]
- Franck, G.; Mawson, T.L.; Folco, E.J.; Molinaro, R.; Ruvkun, V.; Engelbertsen, D.; Liu, X.; Tesmenitsky, Y.; Shvartz, E.; Sukhova, G.K.; et al. Roles of PAD4 and NETosis in experimental atherosclerosis and arterial injury: Implications for superficial erosion. Circ. Res. 2018, 123, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Shimony, A.; Zahger, D.; Gilutz, H.; Goldstein, H.; Orlov, G.; Merkin, M.; Shalev, A.; Ilia, R.; Douvdevani, A. Cell free DNA detected by a novel method in acute ST-elevation myocardial infarction patients. Acute Card. Care 2010, 12, 109–111. [Google Scholar] [CrossRef]
- Cui, M.; Fan, M.; Jing, R.; Wang, H.; Qin, J.; Sheng, H.; Wang, Y.; Wu, X.; Zhang, L.; Zhu, J.; et al. Cell-Free circulating DNA: A new biomarker for the acute coronary syndrome. Cardiology 2013, 124, 76–84. [Google Scholar] [CrossRef]
- Langseth, M.S.; Helseth, R.; Ritschel, V.; Hansen, C.H.; Andersen, G.Ø.; Eritsland, J.; Halvorsen, S.; Fagerland, M.W.; Solheim, S.; Arnesen, H.; et al. Double-Stranded DNA and NETs Components in Relation to Clinical Outcome After ST-Elevation Myocardial Infarction. Sci. Rep. 2020, 10, 5007. [Google Scholar] [CrossRef]
- Helseth, R.; Solheim, S.; Arnesen, H.; Seljeflot, I.; Opstad, T.B. The time course of markers of neutrophil extracellular traps in patients undergoing revascularization for acute myocardial infarction or stable angina pectoris. Mediat. Inflamm. 2016, 2016, 2182358. [Google Scholar] [CrossRef] [PubMed]
- Vallés, J.; Lago, A.; Santos, M.T.; Latorre, A.M.; Tembl, J.I.; Salom, J.B.; Nieves, C.; Moscardó, A. Neutrophil extracellular traps are increased in patients with acute ischemic stroke: Prognostic significance. Thromb. Haemost. 2017, 117, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Hamaguchi, S.; Matsumoto, N.; Irisawa, T.; Seki, M.; Tasaki, O.; Hosotsubo, H.; Yamamoto, N.; Yamamoto, K.; Akeda, Y.; et al. Presence of neutrophil extracellular traps and citrullinated histone H3 in the bloodstream of critically ill patients. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [PubMed]
- Helseth, R.; Shetelig, C.; Andersen, G.Ø.; Langseth, M.S.; Limalanathan, S.; Opstad, T.B.; Arnesen, H.; Hoffmann, P.; Eritsland, J.; Seljeflot, I. Neutrophil extracellular trap components associate with infarct size, ventricular function, and clinical outcome in STEMI. Mediat. Inflamm. 2019, 2019, 7816491. [Google Scholar] [CrossRef] [PubMed]
- Langseth, M.S.; Opstad, T.B.; Bratseth, V.; Solheim, S.; Arnesen, H.; Pettersen, A.Å.; Seljeflot, I.; Helseth, R. Markers of neutrophil extracellular traps are associated with adverse clinical outcome in stable coronary artery disease. Eur. J. Prev. Cardiol. 2018, 25, 762–769. [Google Scholar] [CrossRef]
- Martinod, K.; Witsch, T.; Farley, K.; Gallant, M.; Remold-O’Donnell, E.; Wagner, D.D. Neutrophil elastase-deficient mice form neutrophil extracellular traps in an experimental model of deep vein thrombosis. J. Thromb. Haemost. 2016, 14, 551–558. [Google Scholar] [CrossRef]
- Kim, J.K.; Hong, C.W.; Park, M.J.; Song, Y.R.; Kim, H.J.; Kim, S.G. Increased neutrophil extracellular trap formation in uremia is associated with chronic inflammation and prevalent coronary artery disease. J. Immunol. Res. 2017, 2017, 8415179. [Google Scholar] [CrossRef]
- Bang, O.Y.; Chung, J.W.; Cho, Y.H.; Oh, M.J.; Seo, W.K.; Kim, G.M.; Ahn, M.J. Circulating DNAs, a marker of neutrophil extracellular traposis and cancer-related stroke: The OASIS-Cancer Study. Stroke 2019, 50, 2944–2947. [Google Scholar] [CrossRef]
- Libby, P.; King, K. Biomarkers: A challenging conundrum in cardiovascular disease. Arterioscler Thromb. Vasc. Biol. 2015, 35, 2491–2495. [Google Scholar] [CrossRef]
- Lee, K.H.; Cavanaugh, L.; Leung, H.; Yan, F.; Ahmadi, Z.; Chong, B.H.; Passam, F. Quantification of NETs-associated markers by flow cytometry and serum assays in patients with thrombosis and sepsis. Int. J. Lab. Hematol. 2018, 40, 392–399. [Google Scholar] [CrossRef]
- Langseth, M.S.; Andersen, G.Ø.; Husebye, T.; Arnesen, H.; Zucknick, M.; Solheim, S.; Eritsland, J.; Seljeflot, I.; Opstad, T.B.; Helseth, R. Neutrophil extracellular trap components and myocardial recovery in post-ischemic acute heart failure. PLoS ONE 2020, 15, e0241333. [Google Scholar] [CrossRef]
- Jiménez-Alcázar, M.; Kim, N.; Fuchs, T.A. Circulating Extracellular DNA: Cause or Consequence of Thrombosis? Semin. Thromb. Hemost. 2017, 43, 553–561. [Google Scholar] [CrossRef]
- Kithcart, A.P.; Libby, P. Casting NETs to predict cardiovascular outcomes. Eur. J. Prev. Cardiol. 2018, 25, 759–761. [Google Scholar] [CrossRef] [PubMed]
- De Arriba-Arnau, A.; Dalmau, A.; Soria, V.; Salvat-Pujol, N.; Ribes, C.; Sánchez-Allueva, A.; Menchón, J.M.; Urretavizcaya, M. Protocolized hyperventilation enhances electroconvulsive therapy. J. Affect. Disord. 2017, 217, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Wagner, D.D. NETosis: A new factor in tumor progression and cancer-associated thrombosis. Semin. Thromb. Hemost. 2014, 40, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.B.; de Los Reyes-García, A.M.; Rivera-Caravaca, J.M.; Valledor, P.; García-Barberá, N.; Roldán, V.; Vicente, V.; Martínez, C.; González-Conejero, R. MiR-146a Regulates Neutrophil Extracellular Trap Formation That Predicts Adverse Cardiovascular Events in Patients with Atrial Fibrillation. Arter. Thromb. Vasc. Biol. 2018, 38, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zhou, X.; Ji, W.J.; Lu, R.Y.; Zhang, Y.; Zhang, Y.D.; Ma, Y.Q.; Zhao, J.H.; Li, Y.M. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: Therapeutic potential of DNase-based reperfusion strategy. Am. J. Physiol. Circ. Physiol. 2015, 308, H500–H509. [Google Scholar] [CrossRef]
- Gómez-Moreno, D.; Adrover, J.M.; Hidalgo, A. Neutrophils as effectors of vascular inflammation. Eur. J. Clin. Investig. 2018, 48. [Google Scholar] [CrossRef]
- Gaul, D.S.; Stein, S.; Matter, C.M. Neutrophils in cardiovascular disease. Eur. Heart J. 2017, 38, 1702–1704. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Van Avondt, K.; Maegdefessel, L.; Soehnlein, O. Therapeutic targeting of neutrophil extracellular traps in atherogenic inflammation. Thromb. Haemost. 2019, 119, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Gong, S.; Luan, P.; Shi, Y.; Kou, W.; Zeng, Y.; Shi, J.; Yu, G.; Hou, J.; Yu, Q.; et al. Extracellular traps from activated vascular smooth muscle cells drive the progression of atherosclerosis. Nat. Commun. 2022, 13, 7500. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.; Weimar, C.; Lemmens, R.; Murphy, S.; Purroy, F.; Arsovska, A.; Bornstein, N.M.; Czlonkowska, A.; Fischer, U.; Fonseca, A.C.; et al. Colchicine for prevention of vascular inflammation in non-CardioEmbolic stroke (CONVINCE)—Study protocol for a randomised controlled trial. Eur. Stroke J. 2021, 6, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Katsanos, A.H.; Giannopoulos, G.; Panagopoulou, V.; Jatuzis, D.; Lemmens, R.; Deftereos, S.; Kelly, P.J. The role of colchicine in the prevention of cerebrovascular ischemia. Curr. Pharm. Des. 2018, 24, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Murphy, S.; Coveney, S.; Purroy, F.; Lemmens, R.; Tsivgoulis, G.; Price, C. Anti-inflammatory approaches to ischaemic stroke prevention. J. Neurol. Neurosurg. Psychiatry 2018, 89, 211–218. [Google Scholar] [CrossRef]
- Martínez, G.J.; Celermajer, D.S.; Patel, S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis 2018, 269, 262–271. [Google Scholar] [CrossRef]
- Otani, K.; Watanabe, T.; Shimada, S.; Takeda, S.; Itani, S.; Higashimori, A.; Nadatani, Y.; Nagami, Y.; Tanaka, F.; Kamata, N.; et al. Colchicine prevents NSAID-induced small intestinal injury by inhibiting activation of the NLRP3 inflammasome. Sci. Rep. 2016, 6, 32587. [Google Scholar] [CrossRef]
- Hoss, F.; Latz, E. Inhibitory effects of colchicine on inflammasomes. Atherosclerosis 2018, 273, 153–154. [Google Scholar] [CrossRef]
- Zhang, S.; Diao, J.; Qi, C.; Jin, J.; Li, L.; Gao, X.; Gong, L.; Wu, W. Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: A meta-analysis. BMC Cardiovasc. Disord. 2018, 18, 75. [Google Scholar] [CrossRef]
- Liu, J.; Yang, D.; Wang, X.; Zhu, Z.; Wang, T.; Ma, A.; Liu, P. Neutrophil extracellular traps and dsDNA predict outcomes among patients with STelevation myocardial infarction. Sci. Rep. 2019, 9, 11599. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y. Role of Neutrophils in Cardiac Injury and Repair Following Myocardial Infarction. Cells 2021, 10, 1676. [Google Scholar] [CrossRef] [PubMed]
- Eghbalzadeh, K.; Georgi, L.; Louis, T.; Zhao, H.; Keser, U.; Weber, C.; Mollenhauer, M.; Conforti, A.; Wahlers, T.; Paunel-Görgülü, A. Compromised anti-inflammatory action of neutrophil extracellular traps in PAD4-deficient mice contributes to aggravated acute inflammation after myocardial infarction. Front. Immunol. 2019, 10, 2313. [Google Scholar] [CrossRef]
- Du, M.; Yang, W.; Schmull, S.; Gu, J.; Xue, S. Inhibition of peptidyl arginine deiminase-4 protects against myocardial infarction induced cardiac dysfunction. Int. Immunopharmacol. 2020, 78, 106055. [Google Scholar] [CrossRef] [PubMed]
- NLi, C.; Xing, Y.; Zhang, Y.; Hua, Y.; Hu, J.; Bai, Y. Neutrophil Extracellular Traps Exacerbate Ischemic Brain Damage. Mol. Neurobiol. 2022, 59, 643–656. [Google Scholar] [CrossRef]
| First Author/Year Ref | Type of Study | Cohort | Aims | Finding |
|---|---|---|---|---|
| Gu et al. (2019) Science [2] | Multicenter Center (USA/China) | Animal Model Zebrafish lines and Ldlr−/− (B6) mice | Whether AIBP orchestrates HSPC emergence from the hemogenic endothelium | AIBP-regulated Srebp2-dependent paradigm for HPSC expansion in atherosclerotic cardiovascular disease. |
| Lim et al. (2018) Front Immunol [5] | Prospective Multicenter Center (China/Sweden) | Blood human donors | To evaluate effects of human protease thrombin and plasmin during injury and wounding on NETome | Exogenous proteases present during wounding and inflammation influence the NETome. |
| Keitelman (2022) Front Immunol [7] | Prospective Multicenter Center (Argentina) | Blood human donors. Healthy vs. GOF NLRP3-mutation | To study the role of caspase-1 upon inflammasome activation to release Interleukin-1 beta (IL-1β) | Caspase-1 regulates human neutrophil IL-1β secretion. |
| Jaiswal et al. (2017) NEJM [9] | Prospective Multicenter Center (USA/UK/Spain) | Blood human donors/mice model Prospective CAD (4726 pt.) Retrospective Control (3529 pt.). Hypercholesterolemia-prone mice BM homozygous or heterozygous Tet2 knockout mice BM control | Association between CHIP and atherosclerotic cardiovascular disease using whole-exome sequencing | Double risk of CAD in humans with CHIP. Higher risk of atherosclerosis in mice. With higher macrophages infiltrate in Tet2 knockout mice. Higher expression of chemokine and cytokine genes. |
| Wang et al. (2018) Circ Res. [10] | Multicenter Center (USA/China) | Animal Model Ldlr−/− vs. BM wild-type or Mice Jak2 VF | To evaluate atherosclerosis and mechanisms in hypercholesterolemic mice with hematopoietic Jak2 VF expression | Lesion formation with increased complexity in advanced atherosclerosis hematopoietic Jak2 VF expression led. In early lesion neutrophil and macrophages infiltration. |
| Wolach et al. (2018) Sci Transl Med [11] | Multicenter Center (USA/Israel/UK/ Netherlands) | Human/Animal Model Human 10,000 without MPNs Mice knock-in of Jak2V617F | Whether neutrophils from patients with MPNs are triggered for NET formation | JAK2V617F expression is linked to NET formation and thrombosis. JAK2 inhibition may reduce thrombosis in MPNs through cell-intrinsic effects on neutrophil function. PAD4 is required for NET formation increasing in Jak2V617. |
| Li et al. (2022) Int J Biochem Cell Biol [13] | Single Center (China) | Animal model BAP31 knockdown mice | Whether BAP31 regulates CD11b/CD18 neutrophils | BAP31 depletion exerted a protective effect on ALI. Decreased neutrophil adhesion and infiltration by blocking the expression of adhesion molecules CD11b/CD18 and PSGL-1. |
| Winter (2018) Cell Metab [21] | Multicenter Center (Germany/Netherlands/Spain/Sweden) | Animal model Mice Cx3cr1GFP/WTApoe−/− monocyte BM control | To evaluate that diurnal invasion of the arterial wall could sustain atherogenic growth by CCL2 and CCR2 | In activity phase, chronic inflammation of large vessels nourishes on cadenced myeloid cell recruitment. Inhibition of atherosclerosis by means of pharmacological CCR2 neutralization. |
| Adrover (2019) Immunity [22] | Multicenter Center (Spain/USA/Germany/ France/Singapore) | Animal model. Mice engineered for constitutive neutrophil aging | To identify a neutrophil-intrinsic program that works as efficient anti-microbial defense while preserving vascular health | In mice, engineered diurnal compartmentalization of neutrophils coordinates immune defense and vascular protection becoming resistant to infection. Nevertheless, higher evidence of thrombo-inflammation and death |
| Yan (2021) Front Immunol [23] | Single center (USA) | Animal model C57Bl/6 mouse neutrophils containing a genomic knock-in BM control | Mutation disabling RGS protein-Gαi2 interactions (G184S) lead to worse chemoattractant receptor signaling; compromised response to inflammatory insults | Neutrophil Gαi2/RGS protein interactions limit and facilitate Gαi2 signaling. Promoting normal neutrophil trafficking, aging, and clearance. |
| Casanova-Acebes (2018) J Exp Med [24] | Multicenter (Spain/Singapore/Canada/Germany/ Netherlands/Japan) | Animal model | To investigate neutrophils’ capacity to infiltrate multiple tissues in the steady-state leading to process that follows tissue-specific dynamics | Homeostatic infiltration of tissues unveils a facet of neutrophil biology that sustains organ function inducing pathological states. |
| Zheng (2022) Proc Natl Acad Sci U S A [25] | Multicenter Center (USA) | Animal model * MISTRGGR mice | To evaluate the role of humanized mouse model MISTRGGR The mouse G-CSF was replaced with human G-CSF, and the mouse G-CSF receptor gene was deleted in existing MISTRG mice | MISTRGGR mice represent a unique mouse model that permits the study of human neutrophils in health and disease. |
| Amulic (2017) Dev Cell. [40] | Multicenter Center (Germany, USA) | Animal model | To investigate NETs’ formation induced by mitogens; role of phosphorylation | In neutrophils, CDK6 is required for clearance of the fungal pathogen Candida albicans. CDK4/6 is implicated in immunity. |
| Dölling (2022) Front Immunol. [43] | Multicenter Center (Germany) | Human autopsy samples | To investigate the neutrophil infiltration in cardiac tissue of patients with AMI | Nuclear HIF-1α is associated with prolonged neutrophil survival and enhanced oxidative stress in hypoxic areas of AMI. |
| Silvestre-Roig (2019) Nature [54] | Multicenter (Germany/Netherlands/ USA/France/Spain/Sweden) | Animal model Mouse models of atherosclerosis | To investigate chronic inflammation and its cellular and molecular mediators | Histone H4 binds to and lyses SMCs, leading to the destabilization of plaques. The neutralization of histone H4 prevents cell death of SMCs and stabilizes atherosclerotic lesions. |
| Talal et al. (2022) BMC Med [58] | Multicenter (Israel) | Human HD patients | To investigate mortality from bacterial infections in HD patients and NET role | Targeting NETosis in HD patients may reduce infections, minimize their severity, and decrease the mortality rate from infections in this patient population. |
| First Author/Year Ref | Type of Study | Cohort | Aims | Finding |
|---|---|---|---|---|
| Fernandez et al. (2019) Nat Med [62] | Multicenter Center (USA/Sweden) | Human carotid artery plaques | To investigate the role of T cells and macrophages in carotid artery plaques of patients with recent stroke or transient ischemic attack compared to no recent stroke | In plaques from asymptomatic patients, T cells and macrophages were activated and displayed evidence of IL-1β signaling. |
| Zhao et al. (2022) J Hum Hypertens [68] | Multicenter Center (China/Sweden) | Human 4667 pts > 40 yrs | To study the relationship between NLR and the risk of CVD | When NLR was categorized into tertiles, participants in the top tertile had a significantly higher risk of CVDs (HR 1.61, 95% CI: 1.06, 2.44) and MI (HR 1.88, 95% CI: 1.09, 3.27) relative to those in the bottom tertile. |
| Locke et al. (2020) Sci Rep. [78] | Multicenter (UK) | Human blood donors | Whether formation of fibrinogen/fibrin-histone aggregates prevented cell death | Fibrinogen did not bind to or protect neutrophils stimulated with PMA. The role of fibrinogen in NETosis. |
| Noubouossie et al. (2017) Blood [89] | Multicenter (USA/Taiwan) | Human blood patients’ neutrophils | To investigate the mechanism leading to thrombosis promoted by intact NETs | Recombinant human histones H3 and H4 triggered TG in recalcified human plasma in a platelet-dependent manner. However, human intact NETs do not directly initiate or amplify coagulation in vitro. |
| Riegger et al. (2016) Eur Heart J [90] | Multicenter (Germany /USA/UK/ Netherlands/Belgium/ France/Spain/Polland) | Human 253 thrombus specimens | To evaluate thrombus specimens in patients with ST and presence of NET | Patients with ST revealed thrombus with leukocytes, particularly neutrophils in ST group. The presence of NETs supports their pathophysiological relevance. |
| Farkas et al. (2019) Thromb Res [91] | Multicenter (Hungary/Australia) | Human CAD 66 pts PAD 64 pts | To investigate the NET-related structural features of thrombi retrieved from different arterial localizations | NET content of thrombi was correlated with systemic inflammatory markers and with patients’ age. Evidence of NET-related variations in thrombus structure. |
| Laridan et al. (2017) Ann Neurol [95] | Multicenter (Belgium/Sweden) | Human 68 thrombus specimens | To investigate the presence of neutrophils and NETs in ischemic stroke thrombi | H3Ci higher in cardioembolic thrombi. Older thrombi contained significantly more neutrophils and H3Cit compared to fresh thrombi. |
| Shimony et al. (2010) Acute Card Care [110] | Single Center (Israel) | Human 16 randomly acute STEMI pts vs. 47 healthy individuals | To evaluate detection of CFD in patients with ST STEMI; to study correlation with established markers of necrosis and ventricular function | CFD levels were linked with the levels of established markers of myocardial necrosis but not with EF. |
| Langseth et al. (2020) PLoS One [111] | Multicenter (Norway) | Human 61 randomly STEMI pts | To investigate NETs’ associate to MF and IL 8 in STEMI patients with symptomatic acute HF | NETs’ components higher in acute heart failure and associated to myocardial function and interleukin 8 levels. |
| Langseth et al. (2020) Sci Rep [112] | Multicenter (Norway) | Human 956 cohort STEMI pts | To investigate association between circulating NETs-related components, clinical outcome, and hypercoagulability in STEMI | dsDNA levels were associated with increased all-cause mortality and with hypercoagulability in STEMI patients. |
| Helseth et al. (2016) Mediators Inflamm [113] | Single center (Norway) | Human 20 pts with STEMI vs. 10 pts with AP | To study infarct size and NETs’ markers in STEMI and AP | Higher levels of NETs in STEMI |
| Valles et al. (2017) Thromb Haemost [114] | Single center (Spain) | Human 43 pts with AIS | To evaluate NETs in the plasma of patients with acute ischemic stroke | At one-year follow-up, NETs were associated with severity and mortality. Relevant specific marker of NETs citH3 was higher and independently associated with AF and all-cause mortality. Significant role of NETs in the pathophysiology of stroke. |
| Hirose et al. (2014) PLoS One [115] | Multicenter (Japan) | Human 263 pts in ICU | To evaluate whether NETs and Cit-H3 were correlated with clinical and biological parameters | Crucial role of NETs in the biological defense against the dissemination of pathogens from the respiratory tract to the bloodstream in potentially infected patients. |
| Helseth (2019) Mediators Inflamm [116] | Multicenter Center (Germany) | Human 259 pts with STEMI | To explore circulating NET markers and associations to myocardial injury | dsDNA levels after STEMI were associated with myocardial infarct size, adverse left ventricular remodeling, and clinical outcome. |
| Langseth (2018) Eur J Prev Cardiol [117] | Multicenter (Norway) | Human 1001 pts with AP | To investigate the role of NETs’ markers, dsDNA, and myeloperoxidase-DNA in clinical outcome and hypercoagulability | Double-stranded DNA levels were significantly related to adverse clinical outcome after 2 years, but only weakly associated with hypercoagulability. |
| Martinod et al. (2016) Thromb Haemost [118] | Multicenter (USA) | Animal model WT vs. †NE (−/−) vs. SB1 vs. † NE (−/−) SB1 (−/−) mice. | Whether neutrophils from NE (−/−) mice have a defect in NETosis, similar to PAD4 (−/−) | Neutrophil elastase is not required for NET formation. NE (−/−) mice, which form pathological venous thrombi containing NETs, do not phenocopy PAD4 (−/−) mice in in vitro NETosis assays or experimental venous thrombosis. |
| Kim et al. (2017) J Immunol Res [119] | Multicenter (Korea) | 60 pts in MHD | Whether NET formation was responsible for ESRD leading to higher incidence of CAD | Uremia-associated-increased NET generation may be a sign of increased burden of atherosclerosis. |
| Bang et al. (2019) Stroke [120] | Multicenter (Korea) | Human 138 randomly 38 pts cancer-related stroke 36 pts healthy-controls 27 pts cancer-controls (active cancer but no stroke) 40 pts stroke-controls (acute ischemic stroke but no cancer) | Whether NETs were increased in cancer-related stroke; whether higher NETs levels were associated with coagulopathy | Increased circulating DNA levels were associated with cancer-related stroke. NETosis was one of the molecular mechanisms of cancer-related stroke |
| First Author/Year Ref | Type of Study | Cohort | Aims | Finding |
|---|---|---|---|---|
| Mangold et al. (2015) Circ Res [93] | Single Center (Germany) | Human 111 pts with STEMI | To investigate relationships between CLS -NETs, bacterial components as triggers of NETosis, activity of endogenous deoxyribonuclease, ST-segment resolution, and infarct size | PMNs were highly activated in STEMI with NETosis at the CLS and coronary NET burden. Deoxyribonuclease activity was predictor of ST-segment resolution and myocardial infarct size. |
| Stakos et al. (2015) Eur Heart J [94] | Multicenter Center (Greece/Germany) | Human 18 pts with STEMI | To assess the in vivo importance of NETs during atherothrombosis | NETs by mean PMNs mediated thrombogenic signals during atherothrombosis. |
| Maugeri et al. (2020) Sci Rep [97] | Single center (IT) | Human | To assess the mechanism of platelets induced NETs | Activated platelets were related to HMGB1 in neutrophils. HMGB1 led to autophagy and NET generation. |
| Novotny et al. (2018) PLoS One [104] | Multicenter (Germany) | Human/animal model 81 human arterial thrombi retrieved mice with injury of carotid artery | To evaluate composition of arterial thrombi in mice compared to those of human patients with AMI | Inhibition of PAD was useful for the treatment of arterial thrombosis and to reduce NET generation. |
| Ducrox et al. (2018) Stroke [105] | Multicenter (France) | Human 108 pts with AIS | To determine the occurrence of NETs in thrombi retrieved during endovascular therapy. Impact on tPA-induced thrombolysis | The efficacy of a strategy involving an administration of DNAse 1 in addition to tPA was effective in the setting of AIS. |
| Cui et al. (2013) Cardiology [91] | Single center (China) | Human 137 pts with ACS 60 healthy individuals 13 pts with stable angina (SA) | To investigate cf-DNA concentrations in ACS and their relationship with clinical features | cf-DNA as a new valuable marker for diagnosing and predicting the severity of coronary artery lesions and risk stratification in ACS. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, F.; Bellomo, F.; Avtaar Singh, S.S. Worsening Thrombotic Complication of Atherosclerotic Plaques Due to Neutrophils Extracellular Traps: A Systematic Review. Biomedicines 2023, 11, 113. https://doi.org/10.3390/biomedicines11010113
Nappi F, Bellomo F, Avtaar Singh SS. Worsening Thrombotic Complication of Atherosclerotic Plaques Due to Neutrophils Extracellular Traps: A Systematic Review. Biomedicines. 2023; 11(1):113. https://doi.org/10.3390/biomedicines11010113
Chicago/Turabian StyleNappi, Francesco, Francesca Bellomo, and Sanjeet Singh Avtaar Singh. 2023. "Worsening Thrombotic Complication of Atherosclerotic Plaques Due to Neutrophils Extracellular Traps: A Systematic Review" Biomedicines 11, no. 1: 113. https://doi.org/10.3390/biomedicines11010113
APA StyleNappi, F., Bellomo, F., & Avtaar Singh, S. S. (2023). Worsening Thrombotic Complication of Atherosclerotic Plaques Due to Neutrophils Extracellular Traps: A Systematic Review. Biomedicines, 11(1), 113. https://doi.org/10.3390/biomedicines11010113






