Abstract
Silicosis is a particular form of lung fibrosis attributable to occupational exposure to crystalline silica. The occupational exposure to crystalline silica also increases the risk of chronic obstructive pulmonary disease (COPD), cancer and lung infections, especially pulmonary tuberculosis. Silicosis is currently diagnosed in previously exposed workers by standard chest X-ray, when lesions are visible and irreversible. Therefore, it would be necessary to find specific and non-invasive markers that could detect silicosis in earlier stages, before the occurrence of X-ray opacities. In this narrative review, we present several diagnostic, monitoring and predictive biomarkers with high potential in the management of silicosis, such as: pro- and anti-inflammatory cytokines (TNF (Tumour necrosis factor-α), IL-1 (Interleukin-1), IL-6, IL-10), CC16 (Clara cell 16, an indirect marker of epithelial cell destruction), KL-6 (Krebs von den Lungen 6, an indirect marker of alveolar epithelial damage), neopterin (indicator of cellular immunity) and MUC5B gene (Mucin 5B, a gel-forming mucin in mucus). Studies have shown that all the aforementioned markers have a high potential for early diagnosis or evaluation of progression in silicosis and represent promising alternatives to radiology. We consider that a multicentric study is needed to evaluate these biomarkers in correlation with occupational history, histopathological examination, imaging signs and pulmonary functions tests on large groups of subjects to better evaluate the accuracy of the presented biomarkers.
1. Introduction
Silicosis is a collagenous pneumoconiosis caused by long-term exposure to crystalline silica-rich dust. More precisely, silicosis is a type of pulmonary fibrosis caused by inhaled silica particles [1]. For crystalline silica particles to be biologically active, they must be small enough (“respirable”) to reach the distal airways and alveoli; [2] therefore, their diameter should be less than 5 μm [3]. In addition, the concentration of crystalline silica in inhaled particles must reach a certain threshold (usually >10%), and the exposure time must be at least 5 years [1]. The development of silicosis is a chronic and progressive process; therefore, once it occurs, it is irreversible. There are several individual characteristics and behaviours that increase the risk of occurrence, such as: pre-existing pathology of the respiratory tract (pulmonary tuberculosis, chronic rhinitis and bronchitis, etc.), genetic polymorphisms, alcohol intake, smoking and physical activity [1,4].
2. Occupational Exposure
Crystalline silica is the aetiological agent involved in the development of silicosis. It is a mineral found in the earth’s crust, [5] where it occurs in two distinctive forms: crystalline (quartz) and amorphous (diatom) [1]. Both crystalline and amorphous forms transform into tridymite at high temperatures (800–1000 °C), which, in turn, subjected to even higher temperatures (1100–1400 °C), transforms into cristobalite. These three forms (quartz, tridymite, cristobalite) are the main aetiological agents of silicosis, whose fibrogenic potential increases in the mentioned order. In Romania, the limit values of exposure for quartz are 1 mg/m3, while for tridymite and cristobalite the value is 0.5 mg/m3 [1,2,4]. The National Institute for Occupational Safety and Health (NIOSH) recommends the exposure limit to be less than 0.5 mg/m3 for any allomorphic shape [6].
According to the National Institute of Public Health, in Romania, silicosis registered a significant decrease in the number of new cases in 2019: 87 compared to 149 in 2018 and ranked second in the overall morbidity structure. Later, in 2021, a slight increase in overall number of cases was observed: 55 compared to 30 in 2020. Between 1998 and 2021, the mean value of new silica cases was 294.3 per year [7].
Epidemiological studies have shown that crystalline silica exposure is associated with increased mortality and morbidity rate [8] due to silicosis, chronic obstructive pulmonary disease (COPD) and lung cancer [9]. Worldwide, in 2019, 655.7 thousand disability-adjusted life years were attributed to silicosis [10]. In 2017, 23 695 cases of silicosis were reported globally. Between 1990 and 2017, some geographic regions reported a decrease in the number of silicosis cases (mostly in Europe), but certain regions such as North and South Africa, China and sub-Saharan Africa reported an upward trend [11].
NIOSH identifies the following activities as occupations at risk: (1) manufacturing of glass, pottery, ceramics, bricks, concrete and artificial stone, (2) abrasive blasting, (3) foundry work, (4) hydraulic fracturing, (5) stonecutting and stone countertop, (6) rock drilling, (7) quarry work, (8) tunneling, (9) construction, (10) mining, (11) oil and gas extraction and (12) dentistry [6].
Given the evolution of society and technological processes, physicians must pay particular attention in diagnosing silicosis both in those previously exposed in the former industries, even if some of them have disappeared in certain countries (e.g., mining, foundries) and in those placed in newly emerging professions (e.g., sandblasting jeans, artificial stone benchtop) [9,12].
3. Materials and Methods
This article is a narrative review designed to evaluate and present several diagnostic, monitoring and predictive biomarkers used for patients with occupational exposure to crystalline silica. For this review, existing literature was selected from various databases such as Pubmed, Scopus, ScienceDirect and Google Scholar. After a thorough analysis as shown in Figure 1 (n = 138 274 articles), we selected 33 studies presented in Table 1, aiming to study markers to assess early diagnosis and progression of silicosis. The cytokines (TNF-α (tumour necrosis factor-α), IL-1 (Interleukin-1), IL-10, IL-6), CC16 (Clara cell 16), KL-6 (Krebs von den Lungen 6), MUC5B (Mucin 5B) gene and neopterin were selected as points of interest for further search. We used the names of the aforementioned markers followed by the terms “silicosis”, “inflammation”, “anti-inflammatory”, “immune dysregulation”, “physiopathology”, “evolution”, “early diagnosis”, “genetic polymorphisms”, and “treatment” in different permutations. For each item, we have summarised the biological mechanisms using the search engines described above and the national literature. We then presented a selection of clinical and experimental studies that evaluated several biomarkers for their potential value in the early diagnosis and evolution of silicosis. We did not limit the time range, although more recent studies were preferred.
Figure 1.
Flowchart of the literature search.

Table 1.
Extraction table of the 33 articles included in the narrative review (TNF—Tumour necrosis factor, OR- Odds ratio, CI—Confidence interval, IL—Interleukin, CC16—Clara cell 16, BALF—Broncho-alveolar lavage fluid, FEV1—Forced expiratory volume in 1 s, VC—Vital capacity, ELISA—Enzyme-linked immunosorbent assay, KL-6—Krebs von den Lungen 6, SP-D—Serum surfactant protein D, MMP—Matrix metalloproteinase, MUC5B—Mucin 5B, CWP — Coal workers’ pneumoconiosis, NF-κB—Nuclear factor kappa B, iNOS—Inducible nitric oxide synthase, IHC—Immunohistochemistry, TEM—Transmission electron microscopy).
4. Health Effects
Silicosis is the most common pneumoconiosis and is classified based on the radiological findings into two categories: simple (<10 mm diameter opacities) and complicated (>10 mm diameter opacities). The pathogenesis of silicosis is based on three theories: macrophage destruction, inflammation leading to fibrosis and immunological mechanisms [4].
Macrophage destruction provides the foundation for the onset of inflammation, fibrosis and immunological processes. Crystalline silica particles are phagocytosed by macrophages leading to a series of events that present, in chronological order as follows: (1) rupture of the phagosome wall under the action of lysosomes, (2) release of the contents into the macrophage cytoplasm, (3) disintegration of the macrophage, (4) release of particles and enzymes into the extracellular fluid, (5) resumption of the process. Once activated by respirable-size silica, the macrophages will initiate the inflammatory process, collagen hypersynthesis and immunological processes (Figure 2) [1,4].
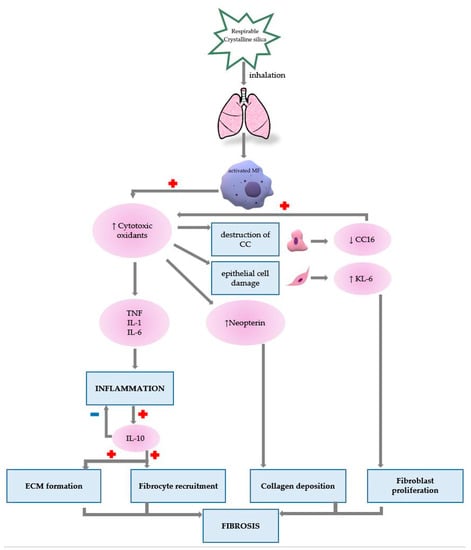
Figure 2.
The role of presented biomarkers in silicosis pathogenesis (MF—macrophage, TNF—Tumour necrosis factor, IL—Interleukin, CC—Clara cell, KL-6—Krebs von den Lungen 6).
Given that the histopathological onset of the disease has no radiological signs (there is a delay between histopathological onset and radiologically visible lesions) [1,3], potential biomarkers useful for early diagnosis have been suggested. We have summarised the most relevant aforementioned biomarkers, as highlighted by specialty literature hitherto.
5. Biomarkers
5.1. TNF
Alveolar macrophages, the first defence line against foreign substances, ingest inhaled silica, leading to cell death and extracellular silica release. The silica is then captured by other macrophages, leading to a repetitive cycle that maintains the inflammatory process. Macrophages release a number of mediators such as cytotoxic oxidants, arachidonic acid metabolites and inflammatory cytokines including TNF-α, IL-1. These mediators initiate the influx of inflammatory cells and induce their infiltration into the alveolar wall, releasing proteolytic enzymes and toxic oxygen derivatives, leading to cell damage and destruction of the extracellular matrix [45].
TNF is a glycoprotein produced primarily by activated macrophages, but also by other cells: mast cells, lymphocytes, fibroblasts, granulocytes, NK (natural killer) cells [46]. The effects of TNF include activation of neutrophils, macrophages, B and T lymphocytes and stimulation of immunoglobulin synthesis. It also induces tumour cell death by necrosis or apoptosis and stimulates synthesis of other cytokines: IL-1, IL-6, and IFN (Interferon). TNF-α stimulates the synthesis and deposition of extracellular matrix and collagen synthesis, hence promoting the development of fibrosis [13]. Therefore, TNF-α plays an important role in maintaining the inflammatory process by activating macrophages, stimulating the production of inflammatory proteins (cytokines) and stimulating T lymphocytes [4]. TNF-α holds several important roles in silicosis, including triggering the influx of inflammatory cells and release of other cytokines [13].
Studies showed that TNF-α levels are elevated before the onset of clinical signs associated with silicosis, making TNF-α a valuable option for early diagnosis [14,47].
Data provided from one study that included 30 controls (healthy individuals), 28 silica-exposed individuals (without clinical disease), and 30 silica subjects showed that TNF-α plasma levels were elevated in exposed workers (p < 0.05) and significantly higher in silicosis patients than in the healthy subjects group (p < 0.01), proving that TNF-α is a contributor in the pathogenesis of silicosis [13].
Evidence from TNF-deficient mice, which are resistant to the development of silica-induced fibrosis, supports the idea that TNF-α plays a significant role in the development of pulmonary fibrosis [16]. Local release of IL-1 and TNF-α on cultures of monocytes and macrophages in humans has been shown to be consistent with disease pathogenesis [39].
Using specific TNF inhibitors that alter the NF-collagen (non-fibrillar), F-collagen (fibrillar), and P4H (Prolyl 4-hydroxylase) gene response (fibrogenic gene expression levels), it was shown that in quartz-treated explant cultures, compared to controls, there was an overexpression of TNF and silica-induced sponge collagen production [36]. Anti-TNF may reduce silica-induced pulmonary inflammation by lowering NF-κB (nuclear factor kappa B), signalling, oxidative stress, and TNF-α, suggesting that anti-TNF may be used to treat silica-induced lung injury [37].
Past studies place promoter polymorphisms of TNF-α in inflammatory and infection-prone conditions [4]. Currently, there is no documented link between TNF polymorphism-related susceptibility to infections and silicosis, [23,48] but it remains open whether a direct association is possible, given the susceptibility of patients with silicosis to pulmonary tuberculosis [4]. A meta-analysis assigns that TNF -308 (11 studies) and -238 (8 studies) polymorphisms are associated with susceptibility of silicosis [17]. Our findings show that also other TNF polymorphisms are linked to silicosis. Corbett et al. identified a strong link between TNF-α polymorphisms -238 and -376 and severe silicosis [18].
A study conducted on subjects working in a cement factory found that individuals who had genetic variation at the TNF -308 gene loci were more susceptible to develop silicosis. These findings were sustained by the higher amount of TNF-α produced by TNF -308 gene variation subjects (p = 0.004) [19].
Overall, the studies showed that increased TNF-α levels in silica-exposed workers without clinical signs of the disease could be a potential biomarker for diagnosing early silicosis. There are still two issues that need further assessment in the interpretation of these results. First, genotyping of the TNF polymorphisms should be taken into account in all studies referring to TNF as a biomarker for silicosis, as TNF was shown to determinate the susceptibility to silicosis, and of developing a complicated form (progressive massive fibrosis).
Second, the updated classification of TNF/TNF-α should be used. TNF was originally attributed to two molecules, TNF-α, a monocyte-derived tumour necrosis factor, and TNF-β, a lymphocyte-derived tumour necrosis factor [46]. Then, at the Seventh International TNF Congress (17–21 May 1998; Hyannis, Massachusetts), the name “TNF-β” was changed to “lymphotoxin-α”. Concomitantly, “TNF-α” became an unnecessary term, with the same meaning as the original term, “TNF”, which was re-established as the official cytokine name [49]. Even though it was renamed more than 20 years ago, TNF continues to be used as TNF-α in a large number of current scientific studies, leading to some sort of misunderstanding of which molecule authors are referring to.
In one experimental study, anti-TNF treatment showed promising potential in reducing silica-related inflammation. However, clinical studies are missing, and at this point we cannot conclude on the efficacy in silicosis.
5.2. IL-1
Macrophages activated by respirable-size crystalline silica produce mediators and initiate the inflammatory process. IL-1 produces synergistic effects with other pro-inflammatory cytokines, such as TNF-α and IL-6. Cytokines secreted by activated macrophages (IL-1, IL-6, IL-12, IL-18) will in turn attract and activate T lymphocytes, leading to stimulation of B lymphocytes by the latter (via IL-11 and IL-14) and initiation of the immunological response. The persistence of silica particles in the lung tissue induces a chronic activation of all these cells, ensuring an ongoing inflammatory process [4].
Experimental animal studies and clinical trials show that TNF-α and IL-1 are important in the regulation of fibrotic mediators in silicosis. Inter-individual differences in IL-1 and TNF-α production sustain the idea that silicosis and the progression to its complicated form are linked to the host’s genetic predisposition to produce these proteins, since in inflammatory diseases, some allelic variants were found to be overexpressed. For example, IL-1 gene polymorphism (IL-1RA +2018) exhibits independent and correlated effects with the susceptibility and severity of silicosis in exposed individuals, thus the occurrence of silicosis would not only rely on the intensity, duration and time of exposure, but also on the cytokine polymorphism [24,39].
These results are in accordance with the findings of Yucesoy B et al., where IL-1RA +2018 was significantly increased in patients with moderate and severe silicosis, suggesting that this variant mainly impacts disease susceptibility [39].
A study conducted on a group of 99 subjects exposed to crystalline silica in a Turkish ceramic plant showed significantly increased levels of the studied interleukins in serum, including IL-1, in comparison to the control group of 81 subjects. Moreover, older subjects had more elevated serum IL-1α values compared to younger subjects [20].
Studies evaluating the relationship between IL-1β levels in blood and exposure to crystalline silica showed significant correlations between the profusion of opacities in the silica group compared to healthy subjects (p < 0.05) [21,38].
Although the homology between IL-1α and IL-1β is 27%, [50] they bind to the same receptor, IL-1R1 (IL-1 type 1 receptor), and induce the same biological functions [50,51]. Hence, in silicosis, IL-1 is involved in collagen deposition and modulation of PDGF (platelet-derived growth factor) activity [40]. Furthermore, a study conducted on cultures of cells sampled from rodent lung tissue showed that after exposure to crystalline silica, IL-1α is rapidly released by alveolar macrophages, stimulating the production of IL-1β, thus promoting lung inflammation [41].
Alongside TNF, results showed that IL-1 polymorphisms genotyping, especially IL-1RA +2018, can be related to the susceptibility and severity of silicosis. The correlation between the levels of IL-1β and the density of radiological opacities in silica patients were reported in only one study and needs to be confirmed in other cohorts. Therefore, further studies on larger groups of subjects are needed in order to eventually implement IL-1β in monitoring the disease and IL-1 in the screening protocol of exposed individuals and determine the specific IL-1 polymorphisms linked to developing silicosis.
5.3. IL-10
Anti-inflammatory cytokines are a group of immunoregulatory molecules adjusting the pro-inflammatory cytokine response. To control the immune response, cytokines act together with certain specific cytokine inhibitors and soluble cytokine receptors. One of the most important anti-inflammatory cytokines is IL-10. IL-10 is an important controller of the differentiation and proliferation of various immune cells and moderating and even suppressing inflammatory reactions [52].
In silicosis, IL-10 is elevated but has a dual effect: on one side, IL-10 limits the amplitude of the inflammatory response by suppression of the production IL-1β, IL-6 and TNF-α in monocytes and macrophages [53]. On the other side, by inducing the fibrotic process, IL-10 contributes to the extension of the pneumoconiotic lesions.
Kurniawidjaja LM started from the hypothesis that the inflammatory process stimulates the production of IL-10, which has an anti-inflammatory role. The TNF-α over IL-10 ratio was evaluated, and the results showed a ratio less than 1 had a protective effect for developing silicosis. The most probable explanation was that the anti-inflammatory effect of IL-10 outweighs the inflammatory effect of TNF-α. If TNF-α/IL-10 ratio is supraunitary, IL-10 is not able to suppress the pro-inflammatory effect of TNF-α, suggesting that the risk factor for silicosis should be derived from this ratio and not from the independent values of TNF-α and IL-10. The significant difference between the TNF-α/IL-10 ratio values was independent of the TNF-α genetic variation [49].
5.4. IL-6
IL-6 is a multifunctional cytokine and plays an essential role in inflammation and immunity. In pulmonary diseases, elevated levels of IL-6 are found in bronchoalveolar lavage fluid, lung tissue and blood. IL-6 facilitates lung infiltration with inflammatory cells by inducing cell expression of adhesion molecules on inflammatory cells and plays a role in regulating fibrosis by modulating the expression of Th2 cytokines [54].
IL-6 controls the production of IL-1 and TNF-α and is known as a primary mediator of the acute phase response and also has anti-inflammatory effects. In the presence of TGF-β (transforming growth factor-β), IL-6 inhibits the development of regulatory T cells and promotes Th17 differentiation, which produces IL-17 [16].
Braz NFT et al. and Blanco-Pérez JJ et al. investigated various cytokines; one of the major findings of both studies was that higher serum levels of IL-6 were found in silicosis patients and in those exposed to crystalline silica than in non-exposed healthy individuals [15,16].
In a recent clinical study, a group of silicosis patients was divided into two categories to evaluate a potential treatment for silicosis (acetylcysteine + tetrandrine) and its effect on serum IL-6 and TNF-α levels. Tetrandrine together with N-acetylcysteine was used on a routine basis to treat patients in the observation group, while the control group received standard, symptomatic treatment. Before therapy, no noticeable difference between the blood levels of IL-6 and TNF-α (p > 0.05) of the two groups was observed. After treatment, the levels of the aforementioned cytokines were reduced in both groups, but in the observation group the reduction was considerably lower (p < 0.05). Tetrandrine together with acetylcysteine may contribute well together to enhance the clinical therapeutic impact in silicosis and reduce the severity of inflammation. The clinical therapeutic effect was assessed by determining FVC (forced vital capacity), FEV1 (forced expiratory volume in 1 s) and RR (respiratory rate). FVC, FEV1 and RR showed an improvement after treatment, but no correlation was performed with the chest X-ray or computed tomography. Based on these results, the authors concluded that peripheral blood IL-6 and TNF-α levels are important for silicosis management, and their detection could reduce the number of X-rays as a follow-up procedure [22].
Along with TNF-α and IL-1, IL-6 has long been considered a pro-inflammatory cytokine produced by lipopolysaccharide. IL-6 is frequently used as a sign of systemic pro-inflammatory cytokine activity. IL-6 possesses pro-inflammatory and anti-inflammatory characteristics, as do many other cytokines, with the acute phase protein response being strongly induced by IL-6. IL-6 has a reduced impact on the production of anti-inflammatory cytokines, such as IL-10 and TGF- β, and reduces the secretion of pro-inflammatory cytokines. In addition to enhancing IL-1Ra (IL-1 receptor antagonist) production and soluble TNF receptor release, IL-6 increases glucocorticoid synthesis. IL-6 also prevents the synthesis of pro-inflammatory cytokines such as GM-CSF (granulocyte macrophage colony-stimulating factor), IFN-γ and MIP-2 (macrophage inflammatory protein-2) [55].
IL-6 showed promising results in diagnosing silicosis, including its incipient stage, when opacities are not radiologically visible. However, IL-6 is a cytokine secreted as a response in many other inflammatory reactions (infections, exposure to other particles, etc.), and these circumstances have to be excluded in the individual judgement of this biomarker significance.
In already diagnosed silicotic patients, the dynamics of the IL-6 could reduce the number of chest X-rays and could be used in monitoring the management of the disease.
5.5. CC16
Clara cell protein (CC16) is a protein secreted by Clara cells, whose name comes from its molecular weight of 16 kD. It is mainly found in the distal respiratory tract, more specifically in the terminal bronchioles [25,42,56]. This protein has an anti-inflammatory, antioxidant, anti-fibrotic and immunosuppressive role [25,57]. Airways inflammation could lead to a reduction in the number of Clara cells, and the degree of reduction may reflect the epithelial cell damage over time [58]. Several studies have even suggested CC16 as a peripheral biomarker of lung epithelial destruction [25,26,27,28]. Different levels of Clara cell damage could lead to a decrease in function, especially in their anti-inflammatory capacity. A potential reason could be the silica dust’s ability to cause inflammatory damage to the lungs; as this inflammation gradually increases with prolonged exposure, it results in decreased secretion of Clara cells via cellular damage. Toxins released by activated phagocytes and free radicals will also contribute to this destruction [26].
One study showed a decrease in CC16 levels in BALF (Broncho-alveolar lavage fluid) in the silicosis group with small opacities (<10 mm) compared to the control group [3,26]. Moreover, the authors reported lower CC16 levels in patients with simple silicosis compared to the group with complicated silicosis (progressive massive fibrosis) (p < 0.05) [3,26]. This result was attributed by the authors to a possible self-repair process of epithelial cells [26] but, to the best of our knowledge, without experimental evidence, such as a lung biopsy, to support the assumption.
Another study comparing three groups (silicosis, exposed and control group) showed that the serum levels of CC16 were lower in the silicosis group, followed by the exposed group, and the highest levels were in the control group (p < 0.001) [25].
A 2020 study suggests that a CC16 serum value below 7.0 ng/mL in workers with an occupational history of crystalline silica exposure could represent a potential marker for detection of silicosis in early stage [27].
Sarkar K et al. investigated the CC16 in the serum of 117 silicosis subjects and 32 non-exposed individuals. The results of the study showed an inversely proportional relationship between the degree of lung damage on chest X-rays and CC16 serum values. The study also suggests that a cut-off value of 9 ng/mL can be correlated with early silicosis [28].
Although the cut-off values of the two studies differ, it is a promising start in recruiting peripheral biomarkers for the diagnosis of early-stage silicosis. Considering the limitations discussed, more studies are needed to accurately determine the cut-off value of CC16, preferably on larger groups of subjects with different radiological stages.
A study conducted on 106 subjects (68 silica-exposed and 38 healthy individuals) measured serum CC16 levels by two methods: ELISA (enzyme-linked immunosorbent assay), the standard reference method, and semi-quantitative lateral flow assay (immunochromatography). By ELISA, all subjects radiologically confirmed with silicosis had CC16 levels below 9 ng/mL, while healthy subjects showed CC16 > 9 ng/mL. In the semi-quantitative lateral flow assay, CC16 values were represented by ranges (<6 ng/mL, 6.1–9 ng/mL, >9 ng/mL), and the results by this method showed a sensitivity of 100% and specificity of 95%, compared to the ELISA results [29]. These findings propose a new approach in CC16 detection, being a much more affordable and reproducible method, which can be easily implemented as a screening method in all silica-related occupational exposure, even in less-advanced geographical areas.
All findings showed that serum and BALF CC16 levels in silica patients were significantly lower than the non-exposed groups. Moreover, serum CC16 levels were reported to decrease in accordance with silicosis stage and correlated to the FEV1/VC ratio. CC16 detection by semi-quantitative lateral flow assay should be applied to larger groups of subjects to better evaluate the sensitivity and specificity compared to ELISA in silica-exposed individuals.
5.6. KL-6
KL-6, also known as MUC-1 (Mucin 1), is a mucin-like glycoprotein with a high molecular weight and is shown to be expressed on type 2 pneumocytes (mostly in the cytoplasm and membrane) [59,60], Clara cells and bronchial glands [61]. Nowadays, it is known that increased levels of KL-6 in serum reflect the presence of an active alveolar epithelial damage [62]. KL-6 can promote the migration and proliferation of fibroblasts and inhibit programmed cell death (apoptosis). Thus, KL-6 could lead to pulmonary fibrosis [63].
KL-6 released by the proliferation of type 2 pneumocytes in pulmonary fibrosis related to occupational exposure to dust or fibres, as pneumoconioses, leads to an increased KL-6 serum concentration. Thus, it may stimulate fibrotic processes in patients with interstitial lung disease and raises the possibility of needing an anti-KL-6 antibody treatment [64].
A study in mice showed that after 45 days of crystalline silica exposure, the pulmonary fibrosis became observable and the level of KL-6 in serum was positively correlated with the severity of fibrotic lesions [43]. Data on human subjects showed that serum KL-6 concentrations are higher in pneumoconioses than healthy controls or exposed individuals [30].
Thus, the results specifically referring to silica are scarce. KL-6 is a potential biomarker for occupational induced fibrosis and for lung fibrosis, in general. The role in diagnosing silicosis is not yet defined.
5.7. MUC5B Gene
MUC5B gene encodes the MUC5B protein, the main gel-forming mucin in humans and mice mucus. Thus, MUC5B contributes to lubrication and viscoelasticity of lungs, saliva and cervical mucus [65].
Studies showed the MUC5B overexpression in the distal airways disturbs the balance required to support efficient mucociliary transport, thereby affecting mucus function. The implication of MUC5B in the development of pulmonary fibrosis proposes two hypotheses. First, the exposure to respirable dust and microparticles, and subsequently their retention in the lungs, could lead to mucociliary dysfunction. Secondly, the inflammation induced by retained substances could represent the beginning of collagen deposition through fibrotic microlesions. Another theory for the occurrence of pulmonary fibrosis linked to MUC5B overexpression is sustained by a decrease in the lung clearance and an increased mucus viscosity [66].
A study in mice showed that silica particles could lead not only to an alteration in the expression of MUC5B, but also to cilia dysfunction and excessive mucus secretion. More studies are needed to better understand if these findings are directly linked to silicosis and should also include data about MUC5B polymorphisms and their implication in the susceptibility of silica-related fibrosis [44].
A study conducted on a Chinese population shows that MUC5B rs2672794 gene polymorphism is in direct association with coal miner’s pneumoconiosis, thus MUC5B rs2672794 CC genotype could increase the risk of developing pneumoconiosis [31].
As MUC5B is a gene extensively studied for its role in lung fibrosis, in future studies, the modification of the gene expression in humans by silica exposure should be considered.
5.8. Neopterin
Neopterin, a pyrazinopyrimidine molecule that belongs to the pteridine class, soluble in plasma or serum, is a crucial and early indicator of cellular immunity. Dendritic cells, macrophages and monocytes that have been stimulated by IFN-γ produce neopterin. Neopterin is a useful prognostic biomarker for immunological stimulation, persistent infection, cell-mediated immunity and oxidative stress [67]. The neopterin secretion induced by IFN-γ is linked to the production of cytotoxic oxidants, making neopterin a candidate for monitoring oxidative stress, not only cellular immunity [32].
Serum neopterin levels could be used as an indicator of the silica-exposure-related impact and other occupational disorders. Elevated levels of neopterin in the serum of silicosis patients raise the possibility of its implication in cellular immunity and ongoing macrophage activation in disease pathogenesis [34]. Neopterin could be considered a potential biomarker for identifying the earliest health effects of crystalline silica [32]. However, in order to be implemented in clinical practice, further studies are needed to investigate oxidative stress parameters besides neopterin [32].
In a study of workers exposed to crystalline silica, significantly higher levels were found in exposed subjects compared to healthy individuals (p < 0.05). The results also demonstrated that the increased neopterin values in exposed subjects are primarily influenced by the presence of crystalline silica in the respirable fraction and are not impacted by individual characteristics or exposure time [35]. Nonetheless, the study’s analysis starts from an incomplete description of the method, without taking into account the subjects’ average exposure to crystalline silica. The study provides limited information on exposure and particularly on long-term exposure. The data rely only on the respirable fraction measured at a given time point, although subjects’ exposure in some cases was over 20 years.
Another study obtained significant statistically differences in serum and urinary neopterin levels between the exposed subjects and healthy individuals. Neopterin levels in urine and serum have been noticeably elevated in silica-exposed workers. Increased neopterin levels in blood, urine and other body fluids could indicate the level of cellular immune activation and estimate the amount of oxidative stress [33].
Overall studies showed that neopterin has a great potential as a biomarker for early detection of silicosis, but, for better accuracy, the oxidative stress parameters should also be measured.
6. Conclusions
Silicosis is still one of the major industrial health issues all over the world. Given the current context where the diagnosis of silicosis is established only on the basis of late and irreversible radiological changes, the lack of specific biomarkers in the screening protocol of silica-exposed patients becomes increasingly necessary.
To integrate the presented results into clinical practice and the diagnostic protocol for early silicosis, further studies are needed to investigate the cytokine profile and functional polymorphisms in silicosis patients. These results should be correlated with occupational history (exposure time, retention time, duration and intensity of exposure), histopathological exam, imaging findings and pulmonary function test results. Moreover, these results should be interpreted in a clinical context and should exclude silica-related respiratory diseases such as industrial bronchitis and possible exacerbations.
Although all the findings show tremendous potential for early diagnosis of silicosis, CC16 detection by immunochromatography seems the most promising and should be applied to larger groups of subjects to demonstrate on a much wider scale the sensitivity and specificity of the method for future introduction into clinical practice and screening protocols.
Author Contributions
Conceptualization, I.-M.C. and R.-A.S.; methodology, I.-M.C., R.-A.S. and A.R.; writing—original draft preparation, I.-M.C.; writing—review and editing, R.-A.S. and A.R.; supervision, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rascu, A.; Naghi, E. Boli Profesionale ale Aparatului Respirator; Silicoza; Ghid Pentru Studenti si Medici; Universitara Carol Davila: Bucharest, Romania, 2019; pp. 76–99. ISBN 978-606-011-103-0. [Google Scholar]
- Hoy, R.F.; Chambers, D.C. Silica-related diseases in the modern world. Allergy 2020, 75, 2785–2797. [Google Scholar] [CrossRef] [PubMed]
- International Labour Organization. Guidelines for the Use of the ILO International Classification of Radiographs of Pneumoconioses, Revised Edition 2011, Geneva. Available online: https://www.ilo.org/wcmsp5/groups/public/---ed_protect/---protrav/---safework/documents/publication/wcms_168260.pdf (accessed on 20 November 2022).
- Cocarla, A. Medicina Ocupațională; Silicoza; Universitara Iuliu Hatieganu: Cluj-Napoca, Romania, 2008; Volume 1, pp. 590–630. ISBN 978-973-693-298-4. [Google Scholar]
- Mohamed, A.M.O.; Paleologos, E.K. Fundamentals of Geoenvironmental Engineering; Fate and effects of pollutants on the land environment; Elsevier Butterworth-Heinemann: Oxford, UK, 2018; pp. 239–281. ISBN 978-012-805-145-0. [Google Scholar]
- Centers for Disease Control and Prevention. The National Institute for Occupational Safety and Health (NIOSH). Workplace Safety and Health Topics. Crystalline Silica. Available online: https://www.cdc.gov/niosh/topics/silica/jobs.html (accessed on 20 November 2022).
- National Institute of Public Health. Occupational Morbidity in Romania. Available online: https://insp.gov.ro/centrul-national-de-monitorizare-a-riscurilor-din-mediul-comunitar-cnmrmc/rapoarte/ (accessed on 22 November 2022).
- Laney, A.S.; Weissman, D.N. Respiratory diseases caused by coal mine dust. J. Occup. Environ. Med. 2014, 56 (Suppl. S10), S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Smărăndescu, R.A.; Căluțu, I.M.; Rașcu, A.; Bușnatu, Ș.S. Diagnostic challenges of radiological opacities in silicosis—Case reports. Occup. Med. 2022, 72, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, M.; Xie, F. Global and national burden and trends of mortality and disability-adjusted life years for silicosis, from 1990 to 2019: Results from the Global Burden of Disease study 2019. BMC Pulm. Med. 2022, 22, 240. [Google Scholar] [CrossRef]
- Shi, P.; Xing, X.; Xi, S.; Jing, H.; Yuan, J.; Fu, Z.; Zhao, H. Trends in global, regional and national incidence of pneumoconiosis caused by different aetiologies: An analysis from the Global Burden of Disease Study 2017. Occup. Environ. Med. 2020, 77, 407–414. [Google Scholar] [CrossRef]
- Glass, D.C.; Dimitriadis, C.; Hansen, J.; Hoy, R.F.; Hore-Lacy, F.; Sim, M.R. Silica exposure estimates in artificial stone benchtop fabrication and adverse respiratory outcomes. Ann. Work. Expo. Health 2022, 66, 5–13. [Google Scholar] [CrossRef]
- Jiang, P.R.; Cao, Z.; Qiu, Z.L.; Pan, J.W.; Zhang, N.; Wu, Y.F. Plasma levels of TNF-α and MMP-9 in patients with silicosis. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1716–1720. [Google Scholar]
- Blanco-Pérez, J.J.; Blanco-Dorado, S.; Rodríguez-García, J.; Gonzalez-Bello, M.E.; Salgado-Barreira, Á.; Caldera-Díaz, A.C.; Pallarés-Sanmartín, A.; Fernandez-Villar, A.; González-Barcala, F.J. Serum levels of inflammatory mediators as prognostic biomarker in silica exposed workers. Sci. Rep. 2021, 11, 13348. [Google Scholar] [CrossRef]
- Braz, N.F.; Carneiro, A.P.; Avelar, N.C.; Miranda, A.S.; Lacerda, A.C.; Teixeira, M.M.; Teixeira, A.L.; Mendonça, V.A. Influence of cytokines and soluble receptors in the quality of life and functional capacity of workers exposed to silica. Occup. Environ. Med. 2016, 58, 272–276. [Google Scholar] [CrossRef]
- Yucesoy, B.; Vallyathan, V.; Landsittel, D.P.; Simeonova, P.; Luster, M.I. Cytokine polymorphisms in silicosis and other pneumoconioses. Mol. Cell Biochem. 2002, 234, 219–224. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, L.L.; Ji, X.L.; Yang, H.B.; Zha, R.S.; Gui, G.P. Tumor necrosis factor gene polymorphisms are associated with silicosis: A systemic review and meta-analysis. Biosci. Rep. 2019, 39, BSR20181896. [Google Scholar] [CrossRef] [PubMed]
- Corbett, E.L.; Mozzato-Chamay, N.; Butterworth, A.E.; De Cock, K.M.; Williams, B.G.; Churchyard, G.J.; Conway, D.J. Polymorphisms in the tumor necrosis factor-alpha gene promoter may predispose to severe silicosis in black South African miners. Am. J. Respir. Crit. Care Med. 2002, 165, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Kurniawidjaja, L.M. Silicosis and its progress influenced by genetic variation on TNF-alpha locus-308, TNF-alpha and IL-10 cytokine on cement factory workers in Indonesia. Pak. J. Biol. Sci. 2014, 17, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Anlar, H.G.; Bacanli, M.; İritaş, S.; Bal, C.; Kurt, T.; Tutkun, E.; Hinc Yilmaz, O.; Basaran, N. Effects of Occupational Silica Exposure on Oxidative Stress and Immune System Parameters in Ceramic Workers in Turkey. J. Toxicol. Environ. Health A 2017, 80, 688–696. [Google Scholar] [CrossRef]
- Lee, J.S.; Shin, J.H.; Lee, J.O.; Lee, W.J.; Hwang, J.H.; Kim, J.H.; Choi, B.S. Blood Levels of IL-Iβ, IL-6, IL-8, TNF-α, and MCP-1 in Pneumoconiosis Patients Exposed to Inorganic Dusts. Toxicol. Res. 2009, 25, 217–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, J.; Song, P.; Wang, Y.; Chen, Y. Clinical efficacy of acetylcysteine combined with tetrandrine tablets in the treatment of silicosis and the effect on serum IL-6 and TNF-α. Exp. Ther. Med. 2019, 18, 3383–3388. [Google Scholar] [CrossRef]
- Salum, K.C.R.; de Castro, M.C.S.; Moreira, V.B.; Nani, A.S.F.; Kohlrausch, F.B. Interleukin 1α and 1β gene variations are associated with tuberculosis in silica exposed subjects. Am. J. Ind. Med. 2020, 63, 74–84. [Google Scholar] [CrossRef]
- Zhou, Y.; Kang, Y.; Zhang, Z.; Liu, J. IL-1RA polymorphism and the susceptivity top pneumoconiosis: A Meta-analysis. Int. J. Clin. Exp. Med. 2014, 7, 2204–2208. [Google Scholar]
- Liu, J.; Song, H.Y.; Zhu, B.L.; Pan, L.P.; Qian, X.L. The Effect of Silica Dust Exposure on the Serum Clara Cell Protein 16 Levels in Chinese Workers. Biomed. Environ. Sci. 2019, 32, 47–50. [Google Scholar] [CrossRef]
- Zhang, S.; Jia, Q.; Song, J.; Tan, Q.; Yu, G.; Guo, X.; Zhang, H. Clinical significance of CC16 and IL-12 in bronchoalveolar lavage fluid of various stages of silicosis. Ann. Palliat. Med. 2020, 9, 3848–3856. [Google Scholar] [CrossRef]
- Naha, N.; Muhamed, J.C.J.; Pagdhune, A.; Sarkar, B.; Sarkar, K. Club cell protein 16 as a biomarker for early detection of silicosis. Indian J. Med. Res. 2020, 151, 319–325. [Google Scholar] [CrossRef]
- Sarkar, K.; Dhatrak, S.; Sarkar, B.; Ojha, U.C.; Raghav, P.; Pagdhune, A. Secondary prevention of silicosis and silico-tuberculosis by periodic screening of silica dust exposed workers using serum club cell protein 16 as a proxy marker. Health Sci. Rep. 2021, 4, e373. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.S.; Lambe, U.P.; Sarkar, K.; Sawant, S.; Deshpande, J. A rapid point of care CC16 kit for screening of occupational silica dust exposed workers for early detection of silicosis/silico-tuberculosis. Sci. Rep. 2021, 11, 23485. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Wu, N.; Li, X.; Qiu, M.; Du, X.; Ye, Q. Serum concentrations of Krebs von den Lungen-6, surfactant protein D, and matrix metalloproteinase-2 as diagnostic biomarkers in patients with asbestosis and silicosis: A case–control study. BMC Pulm. Med. 2017, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Wu, B.; Jin, K.; Luo, C.; Han, R.; Chen, M.; Hou, Z.; Fan, J.; Ni, C. MUC5B promoter polymorphisms and risk of coal workers’ pneumoconiosis in a Chinese population. Mol. Biol. Rep. 2014, 41, 4171–4176. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Dehghan, S.F.; Golbabaei, F.; Ansari, M.; Yaseri, M.; Roshani, S.; Divani, R. Evaluation of Serum and Urinary Neopterin Levels as a Biomarker for Occupational Exposure to Crystalline Silica. Ann. Med. Health Sci. Res. 2016, 6, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Altindag, Z.Z.; Baydar, T.; Isimer, A.; Sahin, G. Neopterin as a new biomarker for the evaluation of occupational exposure to silica. Int. Arch. Occup. Environ. Health 2003, 76, 318–322. [Google Scholar] [CrossRef]
- Prakova, G.; Gidikova, P.; Slavov, E.; Sandeva, G.; Stanilova, S. The potential role of neopterin as a biomarker for silicosis. Trakia J. Sci. 2005, 3, 37–41. [Google Scholar]
- Prakova, G.; Gidikova, P.; Slavov, E.; Sandeva, G.; Stanilova, S. Serum neopterin in workers exposed to inorganic dust containing free crystalline silicon dioxide. Cent. Eur. J. Med. 2009, 4, 104–109. [Google Scholar] [CrossRef]
- Pozzolini, M.; Scarfì, S.; Gallus, L.; Ferrando, S.; Cerrano, C.; Giovine, M. Silica-induced fibrosis: An ancient response from the early metazoans. J. Exp. Biol. 2017, 220, 4007–4015. [Google Scholar] [CrossRef]
- Zhang, H.; Sui, J.N.; Gao, L.; Guo, J. Subcutaneous administration of infliximab-attenuated silica-induced lung fibrosis. Int. J. Occup. Med. Environ. Health 2018, 31, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Rong, Y.; Liu, Y.; Zhou, Y.; Guo, J.; Cheng, W.; Wang, H.; Chen, W. Association between proinflammatory responses of respirable silica dust and adverse health effects among dust-exposed workers. J. Occup. Environ. Med. 2012, 54, 459–465. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Oliver, C.N.; Lepe-Zuniga, J.L.; Green, I.; Gery, I. Silica-stimulated monocytes release fibroblast proliferation factors identical to interleukin 1. A potential role for interleukin 1 in the pathogenesis of silicosis. J. Clin. Invest. 1984, 73, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.D.; Rom, W.N.; Jagirdar, J.; Yie, T.A.; Gordon, T.; Tchou-Wong, K.M. Crucial role of interleukin-1beta and nitric oxide synthase in silica-induced inflammation and apoptosis in mice. Am. J. Respir. Crit. Care Med. 2002, 165, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Rabolli, V.; Badissi, A.A.; Devosse, R.; Uwambayinema, F.; Yakoub, Y.; Palmai-Pallag, M.; Lebrun, A.; De Gussem, V.; Couillin, I.; Ryffel, B.; et al. The alarmin IL-1α is a master cytokine in acute lung inflammation induced by silica micro- and nanoparticles. Part Fibre Toxicol. 2014, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, R.; Wang, H.; Zhang, W. Dynamic changes in expression of clara cell protein and surfactant protein-D expressions in lung tissues and bronchoalveolar lavage fluid of silica-treated rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2014, 32, 168–172. [Google Scholar] [PubMed]
- Xu, B.; Zhang, H.; Xu, J.; Zhang, T.; Hu, C.; Zhou, H.; Shao, S. Correlation between pulmonary fibrosis and KL-6 level in silica-treated mice. Chin. J. Public Health 2014, 30, 1536–1538. [Google Scholar] [CrossRef]
- Yu, Q.; Fu, G.; Lin, H.; Zhao, Q.; Liu, Y.; Zhou, Y.; Shi, Y.; Zhang, L.; Wang, Z.; Zhang, Z.; et al. Influence of silica particles on mucociliary structure and MUC5B expression in airways of C57BL/6 mice. Exp. Lung Res. 2020, 46, 217–225. [Google Scholar] [CrossRef]
- Rimal, B.; Greenberg, A.K.; Rom, W.N. Basic pathogenetic mechanisms in silicosis: Current understanding. Curr. Opin. Pulm. Med. 2005, 11, 169–173. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Slavov, E.; Miteva, L.; Prakova, G.; Gidikova, P.; Stanilova, S. Correlation between TNF-alpha and IL-12p40-containing cytokines in silicosis. Toxicol. Ind. Health 2010, 26, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Tang, Y.; Cao, D.; Wu, F.; Liu, J.; Lu, G.; Zhang, Z.; Xia, Z. Genetic polymorphisms in alveolar macrophage response-related genes, and risk of silicosis and pulmonary tuberculosis in Chinese iron miners. Int. J. Hyg. Environ. Health 2007, 210, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Grimstad, Ø. Tumor Necrosis Factor and the Tenacious α. JAMA Dermatol. 2016, 152, 557. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regener. 2019, 39, 12. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Dotan, S.; Krelin, Y.; Song, X.; Elkabets, M.; Carmi, Y.; Rider, P.; Cohen, I.; Romzova, M.; Kaplanov, I.; et al. Unique Versus Redundant Functions of IL-1α and IL-1β in the Tumor Microenvironment. Front. Immunol. 2013, 4, 177. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Lu, Y.; Zhuang, H.; Gu, W.; Liu, B.; Liu, F.; Sun, J.; Yan, B.; Weng, D.; et al. IL-10-Producing CD1dhiCD5+ Regulatory B Cells May Play a Critical Role in Modulating Immune Homeostasis in Silicosis Patients. Front. Immunol. 2017, 8, 110. [Google Scholar] [CrossRef]
- Tripathi, S.S.; Haushila, P.P.; Bholanath, P. Overview of cytokines and receptors in Silicosis. J. Appl. Pharm. Sci. 2011, 1, 1–5. [Google Scholar]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef]
- Manning, C.M.; Johnston, C.J.; Hernady, E.; Miller, J.N.; Reed, C.K.; Lawrence, B.P.; Williams, J.P.; Finkelstein, J.N. Exacerbation of lung radiation injury by viral infection: The role of Clara cells and Clara cell secretory protein. Radiat. Res. 2013, 179, 617–629. [Google Scholar] [CrossRef]
- Briana, D.D.; Gourgiotis, D.; Boutsikou, M.; Baka, S.; Marmarinos, A.; Liosi, S.; Hassiakos, D.; Malamitsi-Puchner, A. Clara cell protein in full-term pregnancies: The influence of intrauterine growth restriction. Pediatr. Pulmonol. 2010, 45, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xiaoguang, L.; Chen, L.; Fan, Z.; Kang, X.; Bai, L.; Wang, Y.; Liu, J. The expression and significance of protein AQP5 and CC16 in lung injury after hemorrhagic shock resuscitation in rats. Chin. J. Emerg. Med. 2017, 26, 1397–1401. [Google Scholar] [CrossRef]
- Kohno, N.; Inoue, Y.; Hamada, H.; Fujioka, S.; Fujino, S.; Yokoyama, A.; Hiwada, K.; Ueda, N.; Akiyama, M. Difference in serodiagnostic values among KL-6-associated mucins classified as cluster 9. Int. J. Cancer 1994, 8 (Suppl. S8), 81–83. [Google Scholar] [CrossRef]
- Kobayashi, J.; Kitamura, S. KL-6: A serum marker for interstitial pneumonia. Chest 1995, 108, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, H.; Yokoyama, A.; Kondo, K.; Hamada, H.; Abe, M.; Nishimura, K.; Hiwada, K.; Kohno, N. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am. J. Respir. Crit. Care Med. 2002, 165, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Kohno, N.; Awaya, Y.; Oyama, T.; Yamakido, M.; Akiyama, M.; Inoue, Y.; Yokoyama, A.; Hamada, H.; Fujioka, S.; Hiwada, K. KL-6, a mucin-like glycoprotein in bronchoalveolar lavage fluid from patients with interstitial lung disease. Am. Rev. Respir. Dis. 1993, 148, 637–642. [Google Scholar] [CrossRef]
- Ohshimo, S.; Yokoyama, A.; Hattori, N.; Ishikawa, N.; Hirasawa, Y.; Kohno, N. KL-6, a human MUC1 mucin, promotes proliferation and survival of lung fibroblasts. Biochem. Biophys. Res. Commun. 2005, 338, 1845–1852. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Nagata, N.; Kumazoe, H.; Oda, K.; Ishimoto, H.; Yoshimi, M.; Takata, S.; Hamada, M.; Koreeda, Y.; Takakura, K.; et al. Prognostic value of serial serum KL-6 measurements in patients with idiopathic pulmonary fibrosis. Respir. Investig. 2017, 55, 16–23. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. MUC5B Mucin 5B, Oligomeric Mucus/Gel-Forming [Homo Sapiens (Human)]—Gene. Available online: https://www.ncbi.nlm.nih.gov/gene/727897 (accessed on 20 November 2022).
- Hancock, L.A.; Hennessy, C.E.; Solomon, G.M.; Dobrinskikh, E.; Estrella, A.; Hara, N.; Hill, D.B.; Kissner, W.J.; Markovetz, M.R.; Grove Villalon, D.E.; et al. Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat. Commun. 2018, 9, 5363. [Google Scholar] [CrossRef]
- Pingle, S.K.; Thakkar, L.R.; Jawade, A.A.; Tumane, R.G.; Jain, R.K.; Soni, P.N. Neopterin: A candidate biomarker for the early assessment of toxicity of aluminum among bauxite dust exposed mine workers. Indian J. Occup. Environ. Med. 2015, 19, 102–109. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).