Quantitative Measurement of Swallowing Performance Using Iowa Oral Performance Instrument: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Focused PICO Question
- Participants: Patients with swallowing disorders or pathologies and conditions that imply dysphagia;
- Intervention: IOPI;
- Comparison: HC;
- IOPI is a reliable tool to distinguish HC from patients with swallowing disorders or pathologies and conditions that imply dysphagia.
- IOPI reliability is similar for children and adults.
- IOPI is able to measure an improvement in swallowing performance following traditional treatments and tongue training exercises in HC and in patients.
2.4. Selection of Studies
2.5. Data Extraction and Analysis
2.6. Assessment of Methodological Quality
2.7. Statistical Analysis
3. Results
3.1. Review Analysis
3.2. Characteristics of the Studies
3.2.1. Case-Control Studies
3.2.2. Intervention Studies
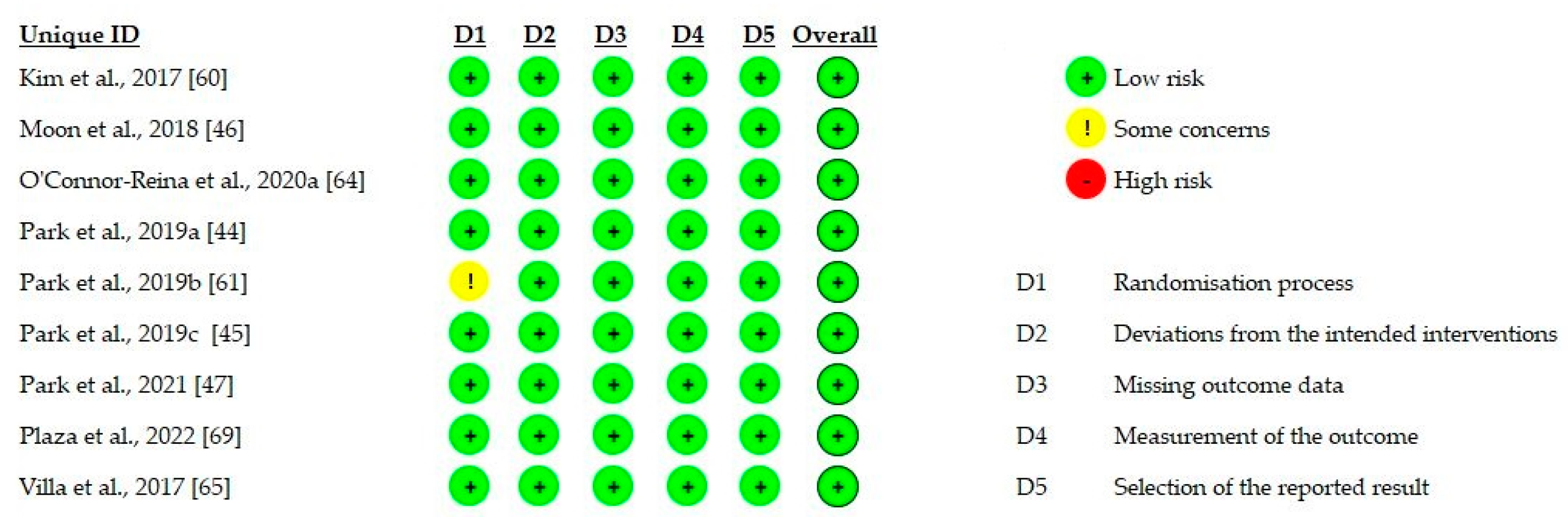
3.3. Evaluation of Methodological Quality
3.3.1. Case-Control Studies
3.3.2. Intervention Studies
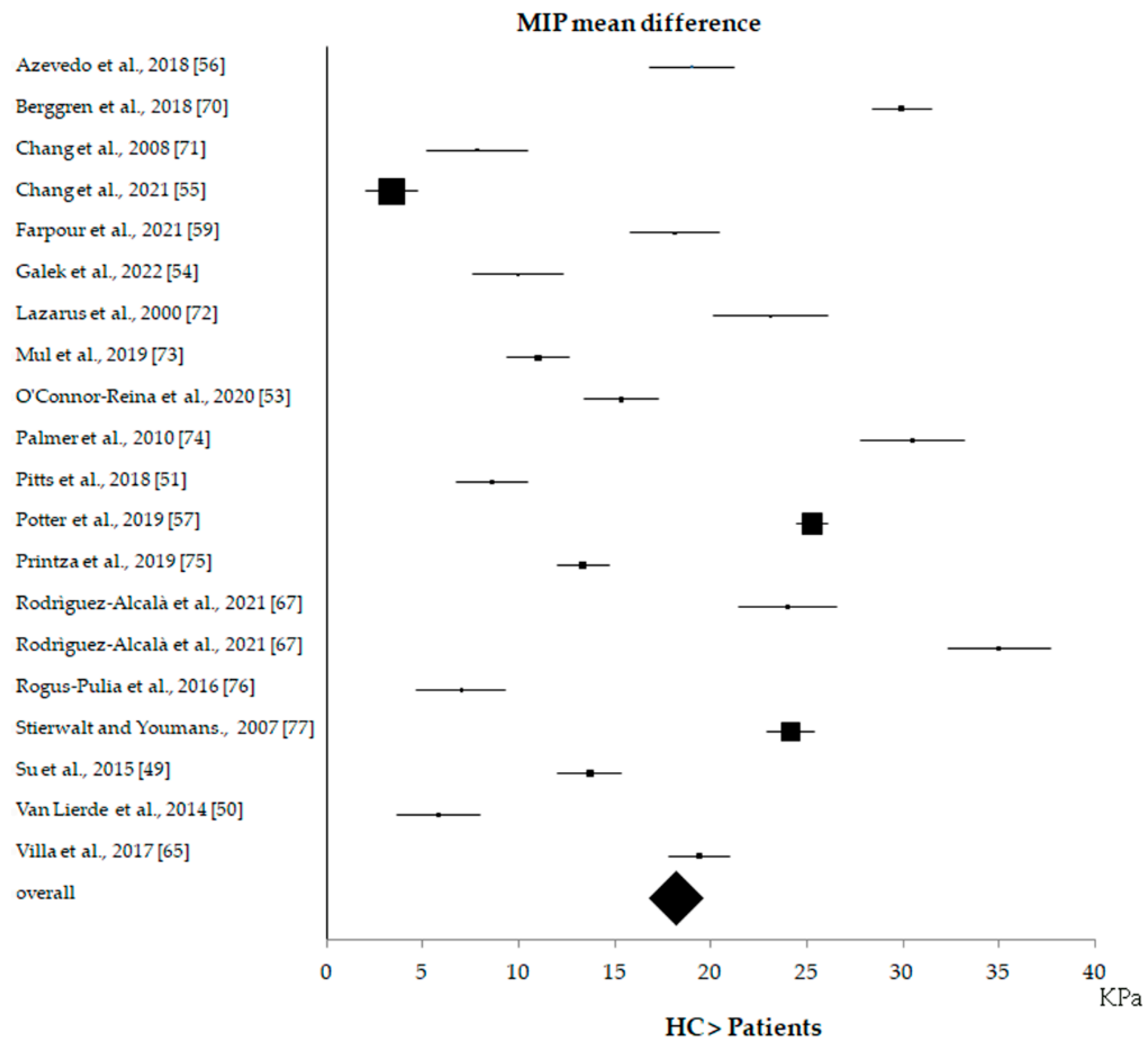
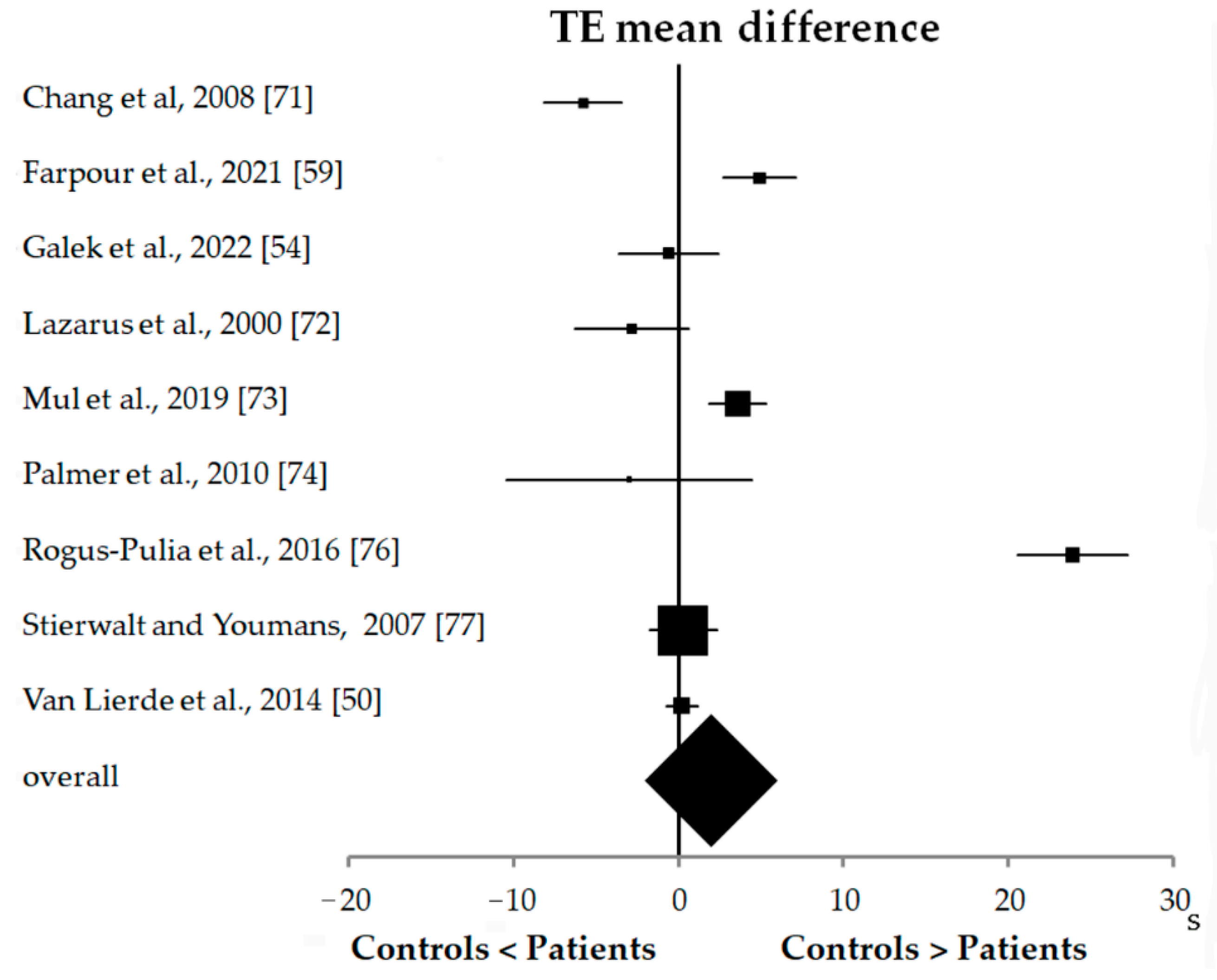
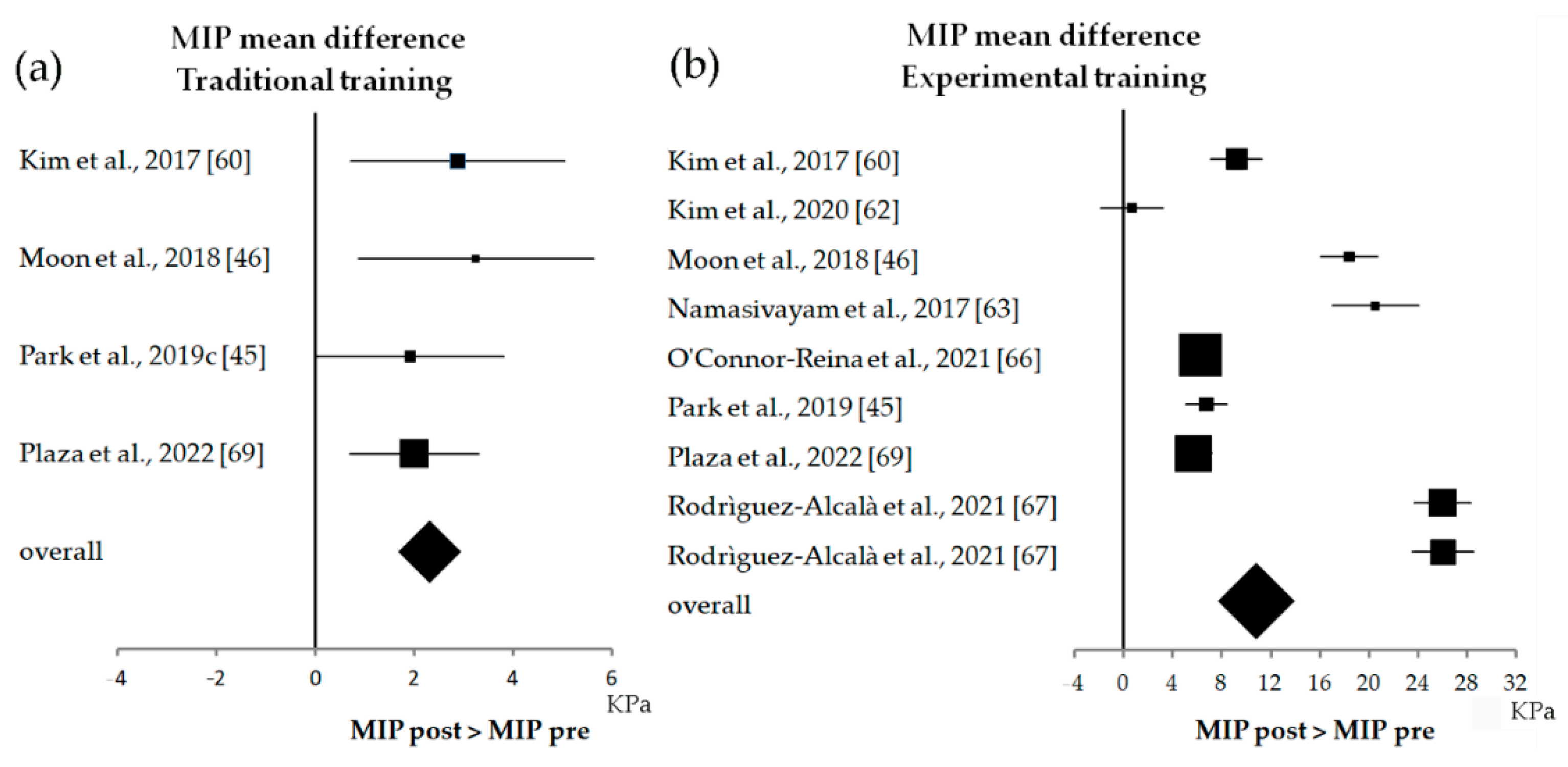
3.4. Statistical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jean, A. Brain Stem Control of Swallowing: Neuronal Network and Cellular Mechanisms. Physiol. Rev. 2001, 81, 929–969. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Palmer, J.B. Anatomy and Physiology of Feeding and Swallowing—Normal and Abnormal. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, C.; Aydogdu, I. Neurophysiology of Swallowing. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2003, 114, 2226–2244. [Google Scholar] [CrossRef]
- Carucci, L.R.; Turner, M.A. Dysphagia Revisited: Common and Unusual Causes. Radiographics 2015, 35, 105–122. [Google Scholar] [CrossRef]
- Domenech, E.; Kelly, J. Swallowing disorders. Med. Clin. N. Am. 1999, 83, 97–113. [Google Scholar] [CrossRef]
- Bhattacharyya, N. The Prevalence of Pediatric Voice and Swallowing Problems in the United States. The Laryngoscope 2015, 125, 746–750. [Google Scholar] [CrossRef]
- Lawlor, C.M.; Choi, S. Diagnosis and Management of Pediatric Dysphagia: A Review. JAMA Otolaryngol. Neck Surg. 2020, 146, 183. [Google Scholar] [CrossRef]
- Kalf, J.G.; de Swart, B.J.M.; Bloem, B.R.; Munneke, M. Prevalence of Oropharyngeal Dysphagia in Parkinson’s Disease: A Meta-Analysis. Parkinsonism Relat. Disord. 2012, 18, 311–315. [Google Scholar] [CrossRef]
- Crary, M.; Sura, L.; Madhavan, A.; Carnaby-Mann, G. Dysphagia in the Elderly: Management and Nutritional Considerations. Clin. Interv. Aging 2012, 7, 287–298. [Google Scholar] [CrossRef]
- Takizawa, C.; Gemmell, E.; Kenworthy, J.; Speyer, R. A Systematic Review of the Prevalence of Oropharyngeal Dysphagia in Stroke, Parkinson’s Disease, Alzheimer’s Disease, Head Injury, and Pneumonia. Dysphagia 2016, 31, 434–441. [Google Scholar] [CrossRef]
- Barczi, S.R.; Sullivan, P.A.; Robbins, J. How Should Dysphagia Care of Older Adults Differ? Establishing Optimal Practice Patterns. Semin. Speech Lang. 2000, 21, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Serra-Prat, M.; Palomera, M.; Gomez, C.; Sar-Shalom, D.; Saiz, A.; Montoya, J.G.; Navajas, M.; Palomera, E.; Clavé, P. Oropharyngeal Dysphagia as a Risk Factor for Malnutrition and Lower Respiratory Tract Infection in Independently Living Older Persons: A Population-Based Prospective Study. Age Ageing 2012, 41, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, O.; Hamdy, S.; Woisard, V.; Wuttge-Hannig, A.; Ortega, P. Social and Psychological Burden of Dysphagia: Its Impact on Diagnosis and Treatment. Dysphagia 2002, 17, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Azpeitia Armán, J.; Lorente-Ramos, R.M.; Gete García, P.; Collazo Lorduy, T. Videofluoroscopic Evaluation of Normal and Impaired Oropharyngeal Swallowing. RadioGraphics 2019, 39, 78–79. [Google Scholar] [CrossRef]
- Langmore, S.E.; Kenneth, S.M.A.; Olsen, N. Fiberoptic Endoscopic Examination of Swallowing Safety: A New Procedure. Dysphagia 1988, 2, 216–219. [Google Scholar] [CrossRef]
- Nacci, A.; Ursino, F.; La Vela, R.; Matteucci, F.; Mallardi, V.; Fattori, B. Fiberoptic Endoscopic Evaluation of Swallowing (FEES): Proposal for Informed Consent. Acta Otorhinolaryngol. Ital. 2008, 28, 206–211. [Google Scholar]
- Schatz, K.; Langmore, S.E.; Olson, N. Endoscopic and Videofluoroscopic Evaluations of Swallowing and Aspiration. Ann. Otol. Rhinol. Laryngol. 1991, 100, 678–681. [Google Scholar] [CrossRef]
- Langmore, S.E. Evaluation of Oropharyngeal Dysphagia: Which Diagnostic Tool Is Superior? : Curr. Opin. Otolaryngol. Head Neck Surg. 2003, 11, 485–489. [Google Scholar] [CrossRef]
- Vaiman, M.; Segal, S.; Eviatar, E. Surface Electromyographic Studies of Swallowing in Normal Children, Age 4–12 Years. Int. J. Pediatr. Otorhinolaryngol. 2004, 68, 65–73. [Google Scholar] [CrossRef]
- Adams, V.; Mathisen, B.; Baines, S.; Lazarus, C.; Callister, R. A Systematic Review and Meta-Analysis of Measurements of Tongue and Hand Strength and Endurance Using the Iowa Oral Performance Instrument (IOPI). Dysphagia 2013, 28, 350–369. [Google Scholar] [CrossRef]
- Robbins, J.; Kays, S.A.; Gangnon, R.E.; Hind, J.A.; Hewitt, A.L.; Gentry, L.R.; Taylor, A.J. The Effects of Lingual Exercise in Stroke Patients with Dysphagia. Arch. Phys. Med. Rehabil. 2007, 88, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, S.-Y. Criteria to Assess Tongue Strength for Predicting Penetration and Aspiration in Patients with Stroke Having Dysphagia. Eur. J. Phys. Rehabil. Med. 2020, 56, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Solomon, N.P.; Robin, D.A.; Luschei, E.S. Strength, Endurance, and Stability of the Tongue and Hand in Parkinson Disease. J. Speech Lang. Hear. Res. 2000, 43, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Clark, H.M.; Solomon, N.P. Age and Sex Differences in Orofacial Strength. Dysphagia 2012, 27, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J. Normal Swallowing and Aging. Semin. Neurol. 1996, 16, 309–317. [Google Scholar] [CrossRef]
- Maeda, K.; Akagi, J. Decreased Tongue Pressure Is Associated with Sarcopenia and Sarcopenic Dysphagia in the Elderly. Dysphagia 2015, 30, 80–87. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Hara, M.; Ishida, R.; Ohkubo, M.; Sugiyama, T.; Abe, S. Effects of Varying Fixed Lingual Apex Positions on Tongue Pressure during Straw Drinking. J. Oral Rehabil. 2014, 41, 374–380. [Google Scholar] [CrossRef]
- Kondoh, J.; Ono, T.; Tamine, K.; Fujiwara, S.; Minagi, Y.; Hori, K.; Maeda, Y.; Kreissl, M.; Nitschke, I. Effect of Complete Denture Wearing on Tongue Motor Biomechanics during Swallowing in Edentulous Older Adults. Geriatr. Gerontol. Int. 2015, 15, 565–571. [Google Scholar] [CrossRef]
- Mori, T.; Fujishima, I.; Wakabayashi, H.; Oshima, F.; Itoda, M.; Kunieda, K.; Kayashita, J.; Nishioka, S.; Sonoda, A.; Kuroda, Y.; et al. Development, Reliability, and Validity of a Diagnostic Algorithm for Sarcopenic Dysphagia. JCSM Clin. Rep. 2017, 2, 1–10. [Google Scholar] [CrossRef]
- Borrmann, P.F.; O’Connor-Reina, C.; Ignacio, J.M.; Rodriguez Ruiz, E.; Rodriguez Alcala, L.; Dzembrovsky, F.; Baptista, P.; Garcia Iriarte, M.T.; Casado Alba, C.; Plaza, G. Muscular Assessment in Patients With Severe Obstructive Sleep Apnea Syndrome: Protocol for a Case-Control Study. JMIR Res. Protoc. 2021, 10, e30500. [Google Scholar] [CrossRef] [PubMed]
- Guzel, H.C.; Tuncer, A. Evaluation of Tongue Strength and Symptoms of Oral Dysphagia in Patients Accompanying Temporomandibular Disorder. Adv. Rehabil. 2021, 35, 9–16. [Google Scholar] [CrossRef]
- Keskool, P.; Warnpeurch, L.; Ongard, S.; Pitaksurachai, P.; Nujchanart, N.; Kerdnoppakhun, J. The Relationships among Objective Measures of Tongue Strength and Risk of Aspiration. Siriraj Med. J. 2018, 70, 302–309. [Google Scholar] [CrossRef]
- University of South Carolina; O’Day, C.; Frank, E.; Montgomery, A.; Nichols, M.; McDade, H. Repeated Tongue and Hand Strength Measurements in Normal Adults and Individuals with Parkinson’s Disease. Int. J. Orofacial Myology 2005, 31, 15–25. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, H.-J.; Oh, D.-H. Effect of Tongue Strength Training Using the Iowa Oral Performance Instrument in Stroke Patients with Dysphagia. J. Phys. Ther. Sci. 2015, 27, 3631–3634. [Google Scholar] [CrossRef]
- Park, H.-S.; Park, J.-Y.; Kwon, Y.-H.; Choi, H.S.; Kim, H.J. Effect of Orbicularis Oris Muscle Training on Muscle Strength and Lip Closure Function in Patients with Stroke and Swallowing Disorder. J. Phys. Ther. Sci. 2018, 30, 1355–1356. [Google Scholar] [CrossRef][Green Version]
- Potter, N.L.; Nievergelt, Y.; Shriberg, L.D. Motor and Speech Disorders in Classic Galactosemia. JIMD Rep. 2013, 11, 31–41. [Google Scholar] [CrossRef]
- Hewitt, A.; Hind, J.; Kays, S.; Nicosia, M.; Doyle, J.; Tompkins, W.; Gangnon, R.; Robbins, J. Standardized Instrument for Lingual Pressure Measurement. Dysphagia 2008, 23, 16–25. [Google Scholar] [CrossRef]
- Park, J.-S.; Oh, D.-H.; Chang, M.-Y. Effect of Expiratory Muscle Strength Training on Swallowing-Related Muscle Strength in Community-Dwelling Elderly Individuals: A Randomized Controlled Trial. Gerodontology 2017, 34, 121–128. [Google Scholar] [CrossRef]
- Steele, C.M.; Bailey, G.L.; Polacco, R.E.C.; Hori, S.F.; Molfenter, S.M.; Oshalla, M.; Yeates, E.M. Outcomes of Tongue-Pressure Strength and Accuracy Training for Dysphagia Following Acquired Brain Injury. Int. J. Speech Lang. Pathol. 2013, 15, 492–502. [Google Scholar] [CrossRef]
- Ottawa Hospital Research Institute. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 7 June 2022).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Park, J.-S.; Hwang, N.-K.; Kim, H.-H.; Choi, J.-B.; Chang, M.-Y.; Jung, Y.-J. Effects of Lingual Strength Training on Oropharyngeal Muscles in South Korean Adults. J. Oral Rehabil. 2019, 46, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Oh, D.-H.; Yoon, T.; Park, J.-S. Effect of Effortful Swallowing Training on Tongue Strength and Oropharyngeal Swallowing Function in Stroke Patients with Dysphagia: A Double-Blind, Randomized Controlled Trial. Int. J. Lang. Commun. Disord. 2019, 54, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-H.; Hahm, S.-C.; Won, Y.S.; Cho, H.-Y. The Effects of Tongue Pressure Strength and Accuracy Training on Tongue Pressure Strength, Swallowing Function, and Quality of Life in Subacute Stroke Patients with Dysphagia: A Preliminary Randomized Clinical Trial. Int. J. Rehabil. Res. 2018, 41, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Oh, C.-H.; Choi, B.-U.; Hong, H.-J.; Park, J.-H.; Kim, T.-Y.; Cho, Y.-J. Effect of Progressive Head Extension Swallowing Exercise on Lingual Strength in the Elderly: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 3419. [Google Scholar] [CrossRef]
- Franciotti, R.; Moharrami, M.; Quaranta, A.; Bizzoca, M.E.; Piattelli, A.; Aprile, G.; Perrotti, V. Use of Fractal Analysis in Dental Images for Osteoporosis Detection: A Systematic Review and Meta-Analysis. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2021, 32, 1041–1052. [Google Scholar] [CrossRef]
- Su, H.; Hsiao, T.-Y.; Ku, S.-C.; Wang, T.-G.; Lee, J.-J.; Tzeng, W.-C.; Huang, G.-H.; Chen, C.C.-H. Tongue Weakness and Somatosensory Disturbance Following Oral Endotracheal Extubation. Dysphagia 2015, 30, 188–195. [Google Scholar] [CrossRef]
- Van Lierde, K.M.; Bettens, K.; Luyten, A.; Plettinck, J.; Bonte, K.; Vermeersch, H.; Roche, N. Oral Strength in Subjects with a Unilateral Cleft Lip and Palate. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1306–1310. [Google Scholar] [CrossRef]
- Pitts, L.L.; Morales, S.; Stierwalt, J.A.G. Lingual Pressure as a Clinical Indicator of Swallowing Function in Parkinson’s Disease. J. Speech Lang. Hear. Res. 2018, 61, 257–265. [Google Scholar] [CrossRef]
- Marim, G.C.; Machado, B.C.Z.; Trawitzki, L.V.V.; de Felício, C.M. Tongue Strength, Masticatory and Swallowing Dysfunction in Patients with Chronic Temporomandibular Disorder. Physiol. Behav. 2019, 210, 112616. [Google Scholar] [CrossRef]
- O’Connor-Reina, C.; Plaza, G.; Garcia-Iriarte, M.T.; Ignacio-Garcia, J.M.; Baptista, P.; Casado-Morente, J.C.; De Vicente, E. Tongue Peak Pressure: A Tool to Aid in the Identification of Obstruction Sites in Patients with Obstructive Sleep Apnea/Hypopnea Syndrome. Sleep Breath. Schlaf Atm. 2020, 24, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Galek, K.; Bice, E.M.; Marquez, G. Tongue and Lip Comparisons between Healthy and Nondysphagic Poststroke Individuals. Folia Phoniatr. Logop. 2022, 74, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-V.; Wu, W.-T.; Chen, L.-R.; Wang, H.-; Wang, T.-G.; Han, D.-S. Suboptimal Tongue Pressure Is Associated with Risk of Malnutrition in Community-Dwelling Older Individuals. Nutrients 2021, 13, 1821. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, N.D.; Lima, J.C.; Furlan, R.M.M.M.; Motta, A.R. Tongue Pressure Measurement in Children with Mouth-Breathing Behaviour. J. Oral Rehabil. 2018, 45, 612–617. [Google Scholar] [CrossRef]
- Potter, N.L.; Nievergelt, Y.; Vandam, M. Tongue Strength in Children with and without Speech Sound Disorders. Am. J. Speech Lang. Pathol. 2019, 28, 612–622. [Google Scholar] [CrossRef]
- Zanin, M.C.; Garcia, D.M.; Rocha, E.M.; de Felício, C.M. Orofacial Motor Functions and Temporomandibular Disorders in Patients With Sjögren’s Syndrome. Arthritis Care Res. 2020, 72, 1057–1065. [Google Scholar] [CrossRef]
- Farpour, H.R.; Moosavi, S.A.; Mohammadian, Z.; Farpour, S. Comparing the Tongue and Lip Strength and Endurance of Children with Down Syndrome with Their Typical Peers Using IOPI. Dysphagia. 2022, 37, 966–972. [Google Scholar] [CrossRef]
- Kim, H.D.; Choi, J.B.; Yoo, S.J.; Chang, M.Y.; Lee, S.W.; Park, J.S. Tongue-to-Palate Resistance Training Improves Tongue Strength and Oropharyngeal Swallowing Function in Subacute Stroke Survivors with Dysphagia. J. Oral Rehabil. 2017, 44, 59–64. [Google Scholar] [CrossRef]
- Park, J.-S.; Lee, S.-H.; Jung, S.-H.; Choi, J.-B.; Jung, Y.-J. Tongue Strengthening Exercise Is Effective in Improving the Oropharyngeal Muscles Associated with Swallowing in Community-Dwelling Older Adults in South Korea: A Randomized Trial. Medicine 2019, 98, e17304. [Google Scholar] [CrossRef]
- Kim, H.; Cho, N.-B.; Kim, J.; Kim, K.M.; Kang, M.; Choi, Y.; Kim, M.; You, H.; Nam, S.I.; Shin, S. Implementation of a Home-Based Mhealth App Intervention Program with Human Mediation for Swallowing Tongue Pressure Strengthening Exercises in Older Adults: Longitudinal Observational Study. JMIR MHealth UHealth 2020, 8, e22080. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam-MacDonald, A.M.; Burnett, L.; Nagy, A.; Waito, A.A.; Steele, C.M. Effects of Tongue Strength Training on Mealtime Function in Long-Term Care. Am. J. Speech Lang. Pathol. 2017, 26, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- O’Connor-Reina, C.; Ignacio Garcia, J.M.; Rodriguez Ruiz, E.; Morillo Dominguez, M.D.C.; Ignacio Barrios, V.; Baptista Jardin, P.; Casado Morente, J.C.; Garcia Iriarte, M.T.; Plaza, G. Myofunctional Therapy App for Severe Apnea-Hypopnea Sleep Obstructive Syndrome: Pilot Randomized Controlled Trial. Jmir Mhealth Uhealth 2020, 8, e23123. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.P.; Evangelisti, M.; Martella, S.; Barreto, M.; Del Pozzo, M. Can Myofunctional Therapy Increase Tongue Tone and Reduce Symptoms in Children with Sleep-Disordered Breathing? Sleep Breath. 2017, 21, 1025–1032. [Google Scholar] [CrossRef]
- O’Connor-Reina, C.; Ignacio Garcia, J.M.; Rodriguez Alcala, L.; Rodriguez Ruiz, E.; Garcia Iriarte, M.T.; Casado Morente, J.C.; Baptista, P.; Plaza, G. Improving Adherence to Myofunctional Therapy in the Treatment of Sleep-Disordered Breathing. J. Clin. Med. 2021, 10, 5772. [Google Scholar] [CrossRef]
- Rodríguez-Alcalá, L.; Martínez, J.M.-L.; Baptista, P.; Ríos Fernández, R.; Javier Gómez, F.; Parejo Santaella, J.; Plaza, G. Sensorimotor Tongue Evaluation and Rehabilitation in Patients with Sleep-Disordered Breathing: A Novel Approach. J. Oral Rehabil. 2021, 48, 1363–1372. [Google Scholar] [CrossRef]
- Mozzanica, F.; Pizzorni, N.; Scarponi, L.; Crimi, G.; Schindler, A. Impact of Oral Myofunctional Therapy on Orofacial Myofunctional Status and Tongue Strength in Patients with Tongue Thrust. Folia Phoniatr. Logop. 2021, 73, 413–421. [Google Scholar] [CrossRef]
- Plaza, E.; Ruviaro Busanello-Stella, A. Effects of a Tongue Training Program in Parkinson’s Disease: Analysis of Electrical Activity and Strength of Suprahyoid Muscles. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2022, 63, 102642. [Google Scholar] [CrossRef]
- Berggren, K.N.; Hung, M.; Dixon, M.M.; Bounsanga, J.; Crockett, B.; Foye, M.D.; Gu, Y.; Campbell, C.; Butterfield, R.J.; Johnson, N.E. Orofacial Strength, Dysarthria, and Dysphagia in Congenital Myotonic Dystrophy. Muscle Nerve 2018, 58, 413–417. [Google Scholar] [CrossRef]
- Chang, C.-W.; Chen, S.H.; Ko, J.-Y.; Lin, Y.-H. Early Radiation Effects on Tongue Function for Patients with Nasopharyngeal Carcinoma: A Preliminary Study. Dysphagia 2008, 23, 193–198. [Google Scholar] [CrossRef]
- Lazarus, C.L.; Logemann, J.A.; Pauloski, B.R.; Rademaker, A.W.; Larson, C.R.; Mittal, B.B.; Pierce, M. Swallowing and Tongue Function Following Treatment for Oral and Oropharyngeal Cancer. J. Speech Lang. Hear. Res. 2000, 43, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Mul, K.; Berggren, K.N.; Sills, M.Y.; McCalley, A.; Van Engelen, B.G.M.; Johnson, N.E.; Statland, J.M. Effects of Weakness of Orofacial Muscles on Swallowing and Communication in FSHD. Neurology 2019, 92, E957–E963. [Google Scholar] [CrossRef] [PubMed]
- Palmer, P.M.; Neel, A.T.; Sprouls, G.; Morrison, L. Swallow Characteristics in Patients with Oculopharyngeal Muscular Dystrophy. J. Speech Lang. Hear. Res. 2010, 53, 1567–1578. [Google Scholar] [CrossRef]
- Printza, A.; Goutsikas, C.; Triaridis, S.; Kyrgidis, A.; Haidopoulou, K.; Constantinidis, J.; Pavlou, E. Dysphagia Diagnosis with Questionnaire, Tongue Strength Measurement, and FEES in Patients with Childhood-Onset Muscular Dystrophy. Int. J. Pediatr. Otorhinolaryngol. 2019, 117, 198–203. [Google Scholar] [CrossRef]
- Rogus-Pulia, N.M.; Larson, C.; Mittal, B.B.; Pierce, M.; Zecker, S.; Kennelty, K.; Kind, A.; Connor, N.P. Effects of Change in Tongue Pressure and Salivary Flow Rate on Swallow Efficiency Following Chemoradiation Treatment for Head and Neck Cancer. Dysphagia 2016, 31, 687–696. [Google Scholar] [CrossRef]
- Stierwalt, J.A.G.; Youmans, S.R. Tongue Measures in Individuals with Normal and Impaired Swallowing. Am. J. Speech Lang. Pathol. 2007, 16, 148–156. [Google Scholar] [CrossRef]
- Baudelet, M.; Van Den Steen, L.; Duprez, F.; De Bodt, M.; Deschuymer, S.; Goeleven, A.; Hutsebaut, I.; Mariën, S.; Meersschout, S.; Nevens, D.; et al. Study Protocol for a Randomized Controlled Trial: Prophylactic Swallowing Exercises in Head-and-Neck Cancer Patients Treated with (Chemo)Radiotherapy (PRESTO Trial). Trials 2020, 21, 237. [Google Scholar] [CrossRef]
- McCarty, E.B.; Chao, T.N. Dysphagia and Swallowing Disorders. Med. Clin. N. Am. 2021, 105, 939–954. [Google Scholar] [CrossRef]
- Adams, V.; Mathisen, B.; Baines, S.; Lazarus, C.; Callister, R. Reliability of Measurements of Tongue and Hand Strength and Endurance Using the Iowa Oral Performance Instrument with Healthy Adults. Dysphagia 2014, 29, 83–95. [Google Scholar] [CrossRef]
- Dotan, R.; Mitchell, C.; Cohen, R.; Klentrou, P.; Gabriel, D.; Falk, B. Child—Adult Differences in Muscle Activation—A Review. Pediatr. Exerc. Sci. 2012, 24, 2–21. [Google Scholar] [CrossRef]
- Potter, N.L.; Short, R. Maximal Tongue Strength in Typically Developing Children and Adolescents. Dysphagia 2009, 24, 391. [Google Scholar] [CrossRef]
- Suzuki, M.; Okamoto, T.; Akagi, Y.; Matsui, K.; Sekiguchi, H.; Satoya, N.; Inoue, Y.; Tatsuta, A.; Hagiwara, N. Efficacy of Oral Myofunctional Therapy in Middle-Aged to Elderly Patients with Obstructive Sleep Apnoea Treated with Continuous Positive Airway Pressure. J. Oral Rehabil. 2021, 48, 176–182. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chung, S.-Y.; Lin, C.-T.; Hwu, Y.-J. Effect of Tongue-to-Palate Resistance Training on Tongue Strength in Healthy Adults. Auris. Nasus. Larynx 2021, 48, 116–123. [Google Scholar] [CrossRef]
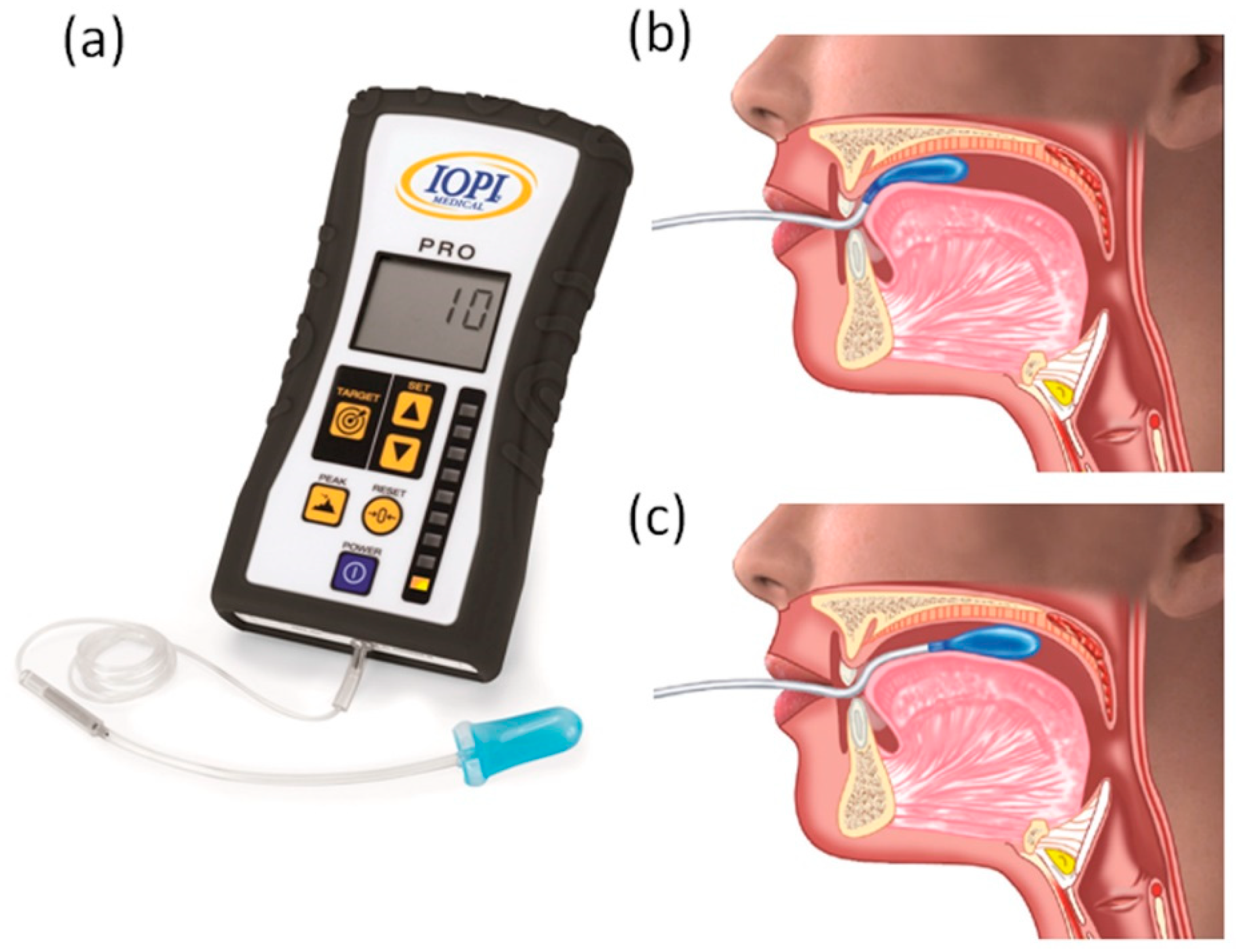


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Studies in English | Studies not available in the English Language |
| Human studies Case-control studies, intervention studies | Preclinical/Animal studies Case reports, case series, preclinical studies, systematic reviews, meta-analyses |
| Studies including healthy as well as pathological populations of any age or gender Employment of IOPI | Studies referring to any diagnostic tool other than IOPI Studies published on non-impacted journals |
| Author, Year | Reason for Exclusion |
|---|---|
| Baudelet et al., 2020 [78] | Ongoing trial |
| Borrman et al., 2021 [31] | No impact factor |
| Guzel and Tuncer, 2021 [32] | No Impact factor |
| Hara et al., 2014 [28] | No employment of IOPI |
| Hewitt et al., 2008 [38] | Raw data not available |
| Keskool et al., 2018 [33] | No impact factor |
| Kondoh et al., 2015 [29] | No employment of IOPI |
| Mori et al., 2017 [30] | No employment of IOPI |
| O’Day et al., 2005 [34] | No impact factor |
| Park et al., 2015 [35] | No Impact factor |
| Park et al., 2017 [39] | No tongue pressure or endurance measurement |
| Park et al., 2018 [36] | No impact factor |
| Potter et al., 2013 [37] | No impact factor |
| Steele et al., 2013 [40] | No control group and no post-intervention data |
| Author, Year | Healthy Controls | Patients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Age Mean ± sd | Age Range | Sex (Females) | n | Age Mean ± sd | Age Range | Sex (Females) | Pathologies | |
| Azevedo et al., 2018 [56] | 20 | 8.3 ± 2.0 | 5.1–12 | 7 | 20 | 7.6 ± 2.2 | 5.1–12 | 7 | Mouth breathing behaviour |
| Berggren et al., 2018 [70] | 29 | 9.1 ± 3.1 | 1.3–13.9 | 17 | 41 | 6.8 ± 3.3 | 0.5–13.2 | 20 | Congenital myotonic dystrophy |
| Chang et al., 2008 [71] | 12 | 45.7 | 30–65 | 1 | 12 | 45.4 | 33–63 | 1 | Nasopharyngeal carcinoma |
| Chang et al., 2021 [55] | 336 | 71.6 ± 5.2 | >65 | 196 | 26 | 75.5 ± 6.3 | >65 | 17 | Malnutrition |
| Farpour et al., 2021 [59] | 33 | 10.7 ± 1.5 | 8.1–13 | 23 | 8 | 10.4 ± 1.7 | 8.1–13 | 6 | Down syndrome |
| Galek et al., 2022 [54] | 18 | 46–95 | 11 | 18 | 46–95 | 11 | Stroke | ||
| Kim et al., 2017 [60] | ET: 18 TT: 17 | ET: 62.2 ± 11.0 TT: 59.3 ± 10.2 | ET: 7 TT: 9 | Dysphagia following subacute stroke | |||||
| Kim et al., 2020 [62] | 11 | 75.7 ± 5.0 | 10 | Older than 65 years, with complaints of swallowing difficulties | |||||
| Lazarus et al., 2000 [72] | 13 | 56 | 36–77 | 3 | 13 | 57 | 38–72 | 3 | H and N cancer |
| Marim et al., 2019 [52] | 23 | 25.5 ± 4.8 | 18 | 23 | 28.7 ± 6.2 | 19 | Temporomandibular disorder | ||
| Moon et al., 2018 [46] | ET: 8 TT: 8 | ET: 62 ± 4.2 TT: 63.5 ± 6.0 | ET: 5 TT: 4 | Stroke | |||||
| Mozzanica et al., 2020 [68] | ET1: 10 ET2: 12 | ET1: 8.8 ± 1.1 ET2: 19.8 ± 4.7 | ET1: 6 ET2: 7 | Tongue thrust | |||||
| Mul et al., 2019 [73] | 35 | 40.2 ± 13.2 | 18 | 43 | 52.5 ± 13.1 | 19 | Facioscapulohumeral muscular dystrophy | ||
| Namasivayam-MacDonald et al., 2017 [63] | 8 | 90.4 | 84–99 | 6 | Seniors, with mild to moderately severe cognitive impairment in the long-term care setting | ||||
| O’Connor-Reina et al., 2020a [64] | ET: 18 AI: 10 | ET: 59.17 α; 53.7–64.6 β AI: 63.90 α; 56.4–71.38 β | ET: 4 AI: 2 | Sleep breathing disorders | |||||
| O’Connor-Reina et al., 2020b [53] | 20 | 35 | 40.6 ± 14.25 | 6 | Sleep breathing disorders | ||||
| O’Connor-Reina et al., 2021 [66] | AP: 35 NA: 19 | AP: 45.9 ± 17.8 NA: 50.3 | NA: 36.2–64.22 | AP: 6 NA: 7 | Sleep breathing disorders | ||||
| Palmer et al., 2010 [74] | 9 | 61.0 | 52–76 | 5 | 11 | 61.1 | 50–73 | 8 | Oculopharyngeal muscular dystrophy |
| Park et al., 2019a [44] | ET:15 AI:15 | ET: 24.5 ± 5.3 AI: 25.1 ± 4.2 | ET: 7 AI: 8 | ||||||
| Park et al., 2019b [61] | ET:20 AI:20 | ET: 69.5 ± 4.3 AI: 68.4 ± 3.9 | ET: 10 AI: 9 | ||||||
| Park et al., 2019c [45] | ET: 15 TT: 15 | ET: 66.5 ± 9.5 TT: 64.8 ± 11.2 | ET: 51–81 TT: 53–78 | ET: 6 TT: 7 | Stroke | ||||
| Park et al., 2021 [47] | ET1:13 ET2:13 | ET1: 72.7 ± 7.3 ET2: 73.2 ± 5.7 | ET1: 9 ET2: 12 | ||||||
| Pitts et al., 2018 [51] | 28 | 70.7 ± 8.1 | 11 | 28 | 71.1 ± 8.0 | 11 | Parkinson’s disease | ||
| Plaza et al., 2022 [69] | ET: 30 TT: 30 | ET: 71.23 ± 7.64 TT: 67.46 ± 6.90 | ET: 1 TT: 14 | Parkinson’s disease | |||||
| Potter et al., 2019 [57] | 228 | 3.1–17 | 118 | 58 | 3.1–17 | 20 | Sound and motor speech disorders | ||
| Printza et al., 2019 [75] | 56 | 7–27 | 58 | 7–27 | Muscular dystrophies | ||||
| Rodrìguez-Alcalà et al., 2021 [67] | 20 | 44 ± 6.4 | 18–75 | ET1: 22 ET2: 21 | ET1: 49.70 ± 7.90 ET2: 51.40 ± 9.30 | 18–75 | Sleep breathing disorders | ||
| Rogus-Pulia et al., 2016 [76] | 21 | 56.0 | 31–77 | 4 | 21 | 56.0 | 36–80 | 4 | H and N cancer |
| Stierwalt and Youmans, 2007 [77] | 200 | 43.8 ± 20.4 | 19–91 | 120 | 50 | 70.0 ± 13.2 | 44–91 | Dysphagia | |
| Su et al., 2015 [49] | 36 | 61.5 ± 14.8 | 15 | 30 | 61.8 ± 15.6 | 8 | Post-extubated patients | ||
| Van Lierde et al., 2014 [50] | 25 | 10.6 | 6.7–18.2 | 8 | 25 | 10.7 | 6–17.9 | 8 | Unilateral cleft lip and palate |
| Villa et al., 2017 [65] | 38 | 7.8 ± 2.2 | 13 | ET: 36 TT: 18 | ET: 6.7 ± 2.3 TT: 6.7 ± 2.8 | ET: 22 TT: 10 | Sleep breathing disorders | ||
| Zanin et al., 2020 [58] | 20 | 31.9 ± 9.3 | 20–51 | 20 | 19 | 33.2 ± 8.7 | 18–53 | 19 | Sjogren’s syndrome |
| Author, Year | Case Definition | Rapresentativeness | Controls Selection | Controls Definition | Comparability | Ascertainment of Exposure | Same Method | Non-Response Rate | Total Score | |
|---|---|---|---|---|---|---|---|---|---|---|
| Azevedo et al., 2018 [56] | * | / | * | * | ** | / | * | * | 7 | Low |
| Berggren et al., 2018 [70] | * | / | * | * | * | / | * | * | 5 | Intermediate |
| Chang et al., 2008 [71] | * | / | * | * | ** | / | * | * | 7 | Low |
| Chang et al., 2021 [55] | * | / | * | * | / | / | * | * | 5 | Intermediate |
| Farpour et al., 2021 [59] | * | / | * | * | ** | / | * | * | 7 | Low |
| Galek et al., 2022 [54] | * | / | * | * | ** | / | * | * | 7 | Low |
| Lazarus et al., 2000 [72] | * | * | * | * | ** | / | * | * | 7 | Low |
| Marim et al., 2019 [52] | * | / | * | * | ** | / | * | * | 5 | Intermediate |
| Mul et al. 2019 [73] | * | / | * | / | / | / | * | * | 4 | Intermediate |
| O’Connor-Reina et al., 2020b [53] | * | * | / | / | ** | / | * | * | 6 | Intermediate |
| Palmer et al., 2010 [74] | * | / | / | * | * | / | * | * | 5 | Intermediate |
| Pitts et al., 2018 [51] | * | / | * | * | ** | / | * | / | 6 | Intermediate |
| Potter et al., 2019 [57] | * | / | * | * | ** | / | * | / | 6 | Intermediate |
| Printza et al., 2019 [75] | * | * | * | * | ** | / | * | * | 8 | Low |
| Rogus-Pulia et al., 2016 [76] | * | / | * | * | ** | / | * | * | 7 | Low |
| Stierwalt and Youmans, 2007 [77] | * | / | * | * | ** | / | * | * | 7 | Low |
| Su et al., 2015 [49] | * | * | * | * | ** | / | * | / | 7 | Low |
| Van Lierde et al., 2014 [50] | * | / | * | * | ** | / | * | * | 7 | Low |
| Zanin et al., 2020 [58] | * | / | * | * | ** | * | * | * | 8 | Low |
| Author, Year | Confounding | Selection of Participants | Classification of Interventions | Deviations from Intended Interventions | Missing Data | Measurement of Outcomes | Selection of the Reported Result | Overall Bias |
|---|---|---|---|---|---|---|---|---|
| Kim et al., 2020 [62] | Low | Low | Low | Low | Low | Low | Low | Low |
| Mozzanica et al., 2020 [68] | Low | Low | Low | Low | Low | Low | Low | Low |
| Namasivayam-MacDonald et al., 2017 [63] | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| O’Connor-Reina et al., 2021 [66] | Low | Low | Low | Low | Low | Low | Low | Low |
| Rodriguez-Alcalà et al., 2021 [67] | Low | Low | Low | Low | Low | Low | Low | Low |
| Author, Year | Primary Outcomes | Secondary Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|
| MIP (kPa) Mean ± sd | LSP (kPa) Mean ± sd | TE (s) Mean ± sd | TPS (kPa) Mean ± sd | |||||
| HC | Patients | HC | Patients | HC | Patients | HC | Patients | |
| Azevedo et al., 2018 [56] | 51.4 | 32.4 | ||||||
| Berggren et al., 2018 [70] | 41.81 | 11.88 | ||||||
| Chang et al., 2008 [71] | 64.5 ± 12.57 | 56.67 ± 9.35 | 18.75 ± 6.22 | 24.58 ± 10.72 | ||||
| Chang et al., 2021 [55] | 38.2 ± 14.01 | 34.84 ± 11.57 | ||||||
| Farpour et al., 2021 [59] | 31.43 ± 15.39 | 13.29 ± 7.46 | 11.5 ± 7.66 | 6.6 ± 5.12 | ||||
| Galek et al., 2022 [54] | 42 ± 11.16 | 32.05 ± 14.66 | 30.33 ± 11.89 | 28.22 ± 13.36 | 43.77 ± 20.24 | 44.38 ± 22.25 | ||
| Lazarus et al., 2000 [72] | 60.15 ± 3.68 | 37.05 ± 4.56 | 37.77 | 40.62 ± 7.8 | ||||
| Marim et al., 2019 [52] | 30.3 ± 12 | 23.1 ± 9 | 47.2 ± 17.2 | 35.3 ± 17 | ||||
| Mul et al. 2019 [73] | 61.45 ± 10.7 | 50.45 ± 15.65 | 29 ± 14.7 | 25.4 ± 14.8 | ||||
| O’Connor-Reina et al., 2020b [53] | 59.34 ± 12.3 | 44.01 ± 12.2 | ||||||
| Palmer et al., 2010 [74] | 57.4 ± 10.4 | 26.9 ± 7.8 | 22.3 ± 8.9 | 10.4 ± 2.4 | 119 ± 63 | 122 ± 43 | ||
| Pitts et al., 2018 [51] | 54.5 ± 10.6 | 45.9 ± 15 | 18.7 ± 10 | 17.55 ± 9.8 | ||||
| Potter et al., 2019 [57] | 51.53 ± 7.69 | 26.24 ± 7.75 | ||||||
| Printza et al., 2019 [75] | 49.86 ± 13.7 | 36.51 ± 14.68 | ||||||
| Rogus-Pulia et al., 2016 [76] | 58 ± 15.8 | 51 ± 13.7 | 68.9 ± 43.8 | 45 ± 16.4 | ||||
| Stierwalt and Youmans, 2007 [77] | 59.78 ± 13.73 | 35.64 | 39.62 | 39.34 ± 43.28 | ||||
| Su et al., 2015 [49] | 47.4 ± 9.6 | 33.7 ± 13.67 | ||||||
| Van Lierde et al., 2014 [50] | 43 ± 14.8 | 37.2 ± 15.3 | 3.9 ± 3.7 | 3.7 ± 2.3 | ||||
| Zanin et al., 2020 [58] | 41.3 ± 14.4 | 24.2 ± 12.8 | ||||||
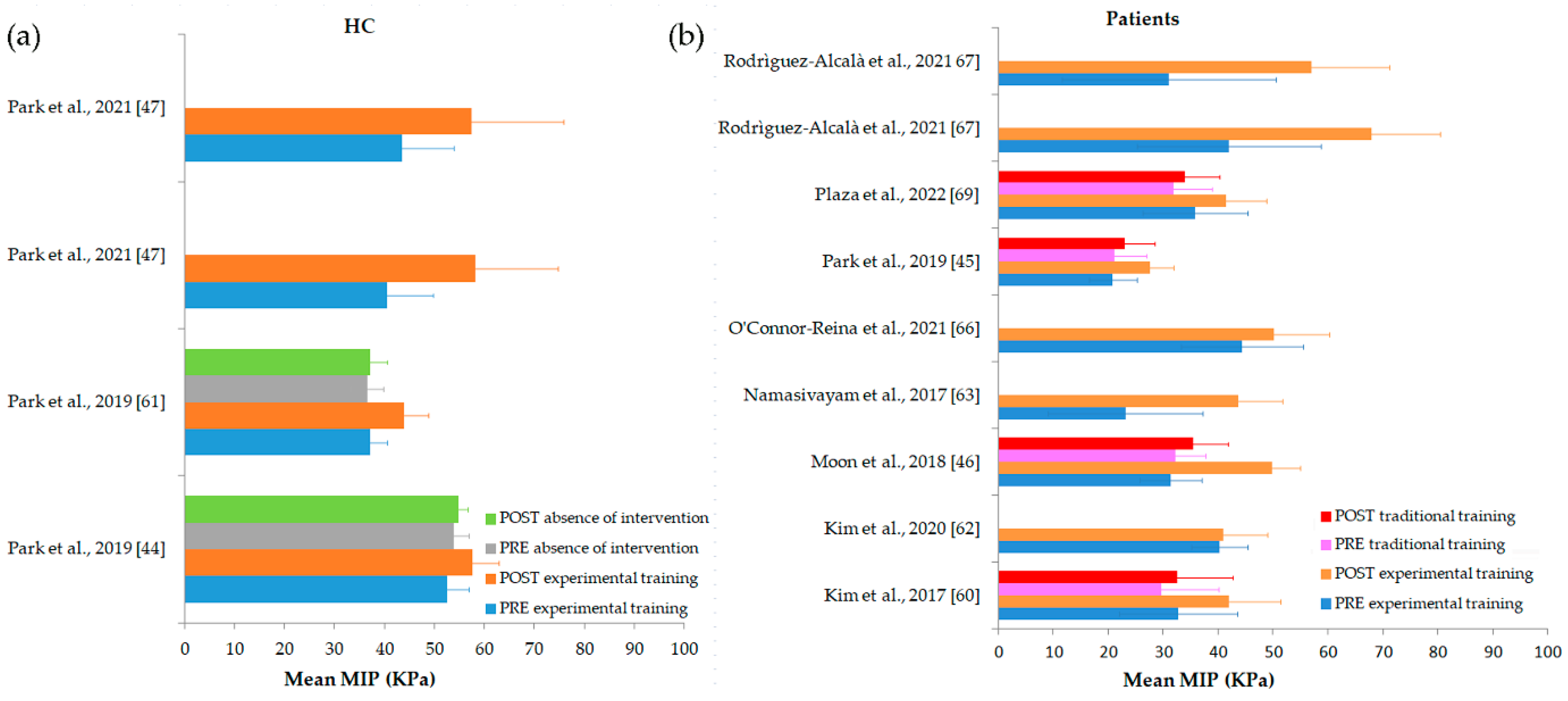
| Author, Year | Intervention | Follow Up (Weeks) | Primary Outcomes | Secondary Outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIP (kPa) | LSP (kPa) | TE (s) | |||||||||||||
| HC | Patients | HC | Patients | HC | Patients | HC | Patients | ||||||||
| Pre- Treatment | Post- Treatment | Pre- Treatment | Post- Treatment | Pre- Treatment | Post- Treatment | Pre- Treatment | Post-Treatment | Pre-Treatment | Post-Treatment | Pre-Treatment | Post-Treatment | ||||
| Kim et al., 2017 [60] | ET: Tongue-to-palate resistance training + traditional dysphagia therapy TT: Traditional dysphagia therapy | 4 | ET: 32.67 ± 10.78 TT: 29.65 ± 10.41 | ET: 41.89 ± 9.54 TT: 32.53 ± 10.17 | |||||||||||
| Kim et al., 2020 [62] | mHealth app therapy program | 12 | ET: 40.30 ± 5.10 | TT: 41 ± 8 | ET: 18.30 ± 6.50 | ET: 27.40 ± 9.40 | |||||||||
| Moon et al., 2018 [46] | ET: Tongue pressure strength and accuracy training TT: Traditional dysphagia therapy | 8 | ET: 31.38 ± 5.68 TT: 32.25 ± 5.37 | ET: 49.75 ± 5.26 TT: 35.50 ± 6.35 | |||||||||||
| Mozzanica et al., 2020 [68] | Oral myofunctional therapy | 10 | ET1: 31 α; 27–42.5 β ET2: 23.50 α; 19.5–33.2 β | ET1: 47 α; 39.5–49.5 β ET2: 41.50 α; 36.75–45.75 β | |||||||||||
| Namasivayam-MacDonald et al., 2017 [63] | Tongue strengthening training | ET: 23.10 ± 14.08 | ET: 43.62 ± 8.10 | ||||||||||||
| O’Connor-Reina et al., 2020a [64] | mHealth app myofunctional therapy | 12 | ET: 40.26 α; 35.32–45.2 β AI: 42 α; 32.67–51.33 β | ET: 59.06 α; 54.74–64 β AI: 44.2 α; 34.1–54.2 β | |||||||||||
| O’Connor-Reina et al., 2021 [66] | AirwayGym app myofunctional therapy | 12 | AP: 44.40 ± 11.08 NA: 51.30 ± 11.40 | AP: 50.66 ± 10.20 NA: 51.10 ± 11.17 | |||||||||||
| Park et al., 2019a [44] | Lingual strength training | 6 | ET: 52.50 ± 4.44 AI: 53.81 ± 3.01 | ET: 57.66 ± 5.21 AI: 54.72 ± 1.95 | |||||||||||
| Park et al., 2019b [61] | Tongue strengthening exercise | ET: 37.08 ± 3.50 AI: 36.55 ± 3.29 | ET: 43.92 ± 4.88 AI: 37.09 ± 3.36 | ||||||||||||
| Park et al., 2019c [45] | ET: Effortful swallowing training + conventional dysphagia treatment TT: Saliva swallowing + conventional dysphagia treatment | 4 | ET: 20.83 ± 4.32 TT: 21.16 ± 5.78 | ET: 27.58 ± 4.27 TT: 23.08 ± 5.42 | |||||||||||
| Park et al., 2021 [47] | ET1: Tongue resistance exercise ET2: Tongue isometric exercise | 4 | ET1: 40.50 ± 9.20 ET2: 43.50 ± 10.40 | ET1: 58.10 ± 16.70 ET2: 57.50 ± 18.30 | ET1: 26.10 ± 12.40 ET2: 31.30 ± 12.60 | ET1: 38 ± 22.70 ET2: 32.50 ± 22.70 | |||||||||
| Plaza et al., 2022 [69] | ET: Tongue isometric pressure exercises + traditional tongue therapy TT: Traditional tongue therapy | 12 | ET: 35.80 ± 9.58 TT: 31.90 ± 6.96 | ET: 41.50 ± 7.39 TT: 33.90 ± 6.38 | |||||||||||
| Rodriguez-Alcalà et al., 2021 [67] | AirwayGym app myofunctional therapy | 12 | 66 ± 18.20 | ET1: 42.00 ± 16.70 ET2: 31.00 ± 19.50 | ET1: 68.00 ± 12.40 ET2: 57.00 ± 14.20 | ||||||||||
| Villa et al., 2017 [65] | ET: Myofunctional therapy + nasal washing TT: nasal washing | 8 | 51.30 ± 13.60 | ET: 31.90 ± 10.70 TT: 32.40 ± 9.40 | 15.80 ± 7.20 | ET: 28.10 ± 8.90 TT: 23.30 ± 5.90 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franciotti, R.; Di Maria, E.; D’Attilio, M.; Aprile, G.; Cosentino, F.G.; Perrotti, V. Quantitative Measurement of Swallowing Performance Using Iowa Oral Performance Instrument: A Systematic Review and Meta-Analysis. Biomedicines 2022, 10, 2319. https://doi.org/10.3390/biomedicines10092319
Franciotti R, Di Maria E, D’Attilio M, Aprile G, Cosentino FG, Perrotti V. Quantitative Measurement of Swallowing Performance Using Iowa Oral Performance Instrument: A Systematic Review and Meta-Analysis. Biomedicines. 2022; 10(9):2319. https://doi.org/10.3390/biomedicines10092319
Chicago/Turabian StyleFranciotti, Raffaella, Erica Di Maria, Michele D’Attilio, Giuseppe Aprile, Federica Giulia Cosentino, and Vittoria Perrotti. 2022. "Quantitative Measurement of Swallowing Performance Using Iowa Oral Performance Instrument: A Systematic Review and Meta-Analysis" Biomedicines 10, no. 9: 2319. https://doi.org/10.3390/biomedicines10092319
APA StyleFranciotti, R., Di Maria, E., D’Attilio, M., Aprile, G., Cosentino, F. G., & Perrotti, V. (2022). Quantitative Measurement of Swallowing Performance Using Iowa Oral Performance Instrument: A Systematic Review and Meta-Analysis. Biomedicines, 10(9), 2319. https://doi.org/10.3390/biomedicines10092319






