Adipose Tissue Development Relies on Coordinated Extracellular Matrix Remodeling, Angiogenesis, and Adipogenesis
Abstract
1. Introduction
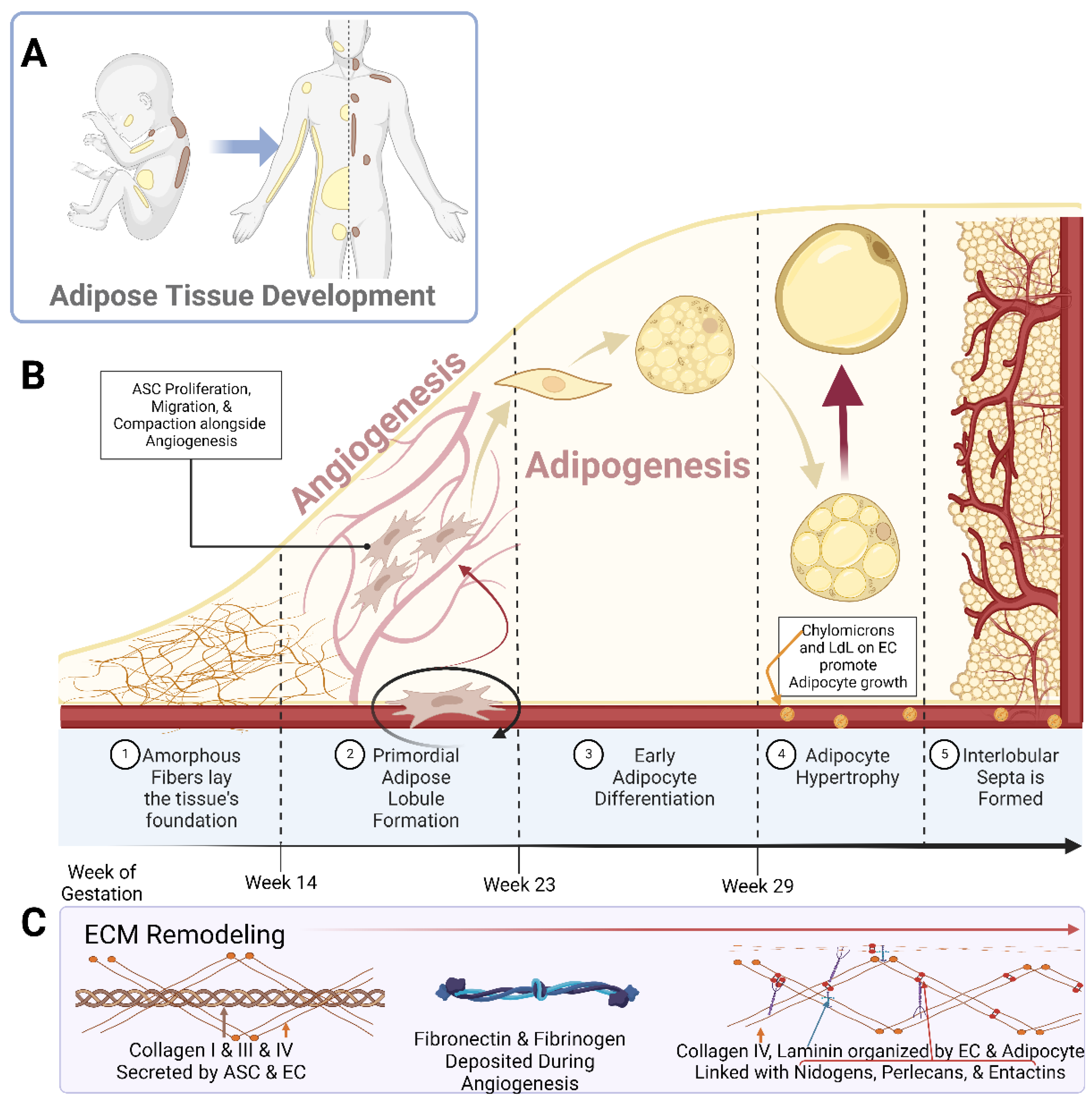
2. Adipose Tissue Remodeling during Fetal Development
2.1. Ultrastructural Changes during Adipose Tissue Development
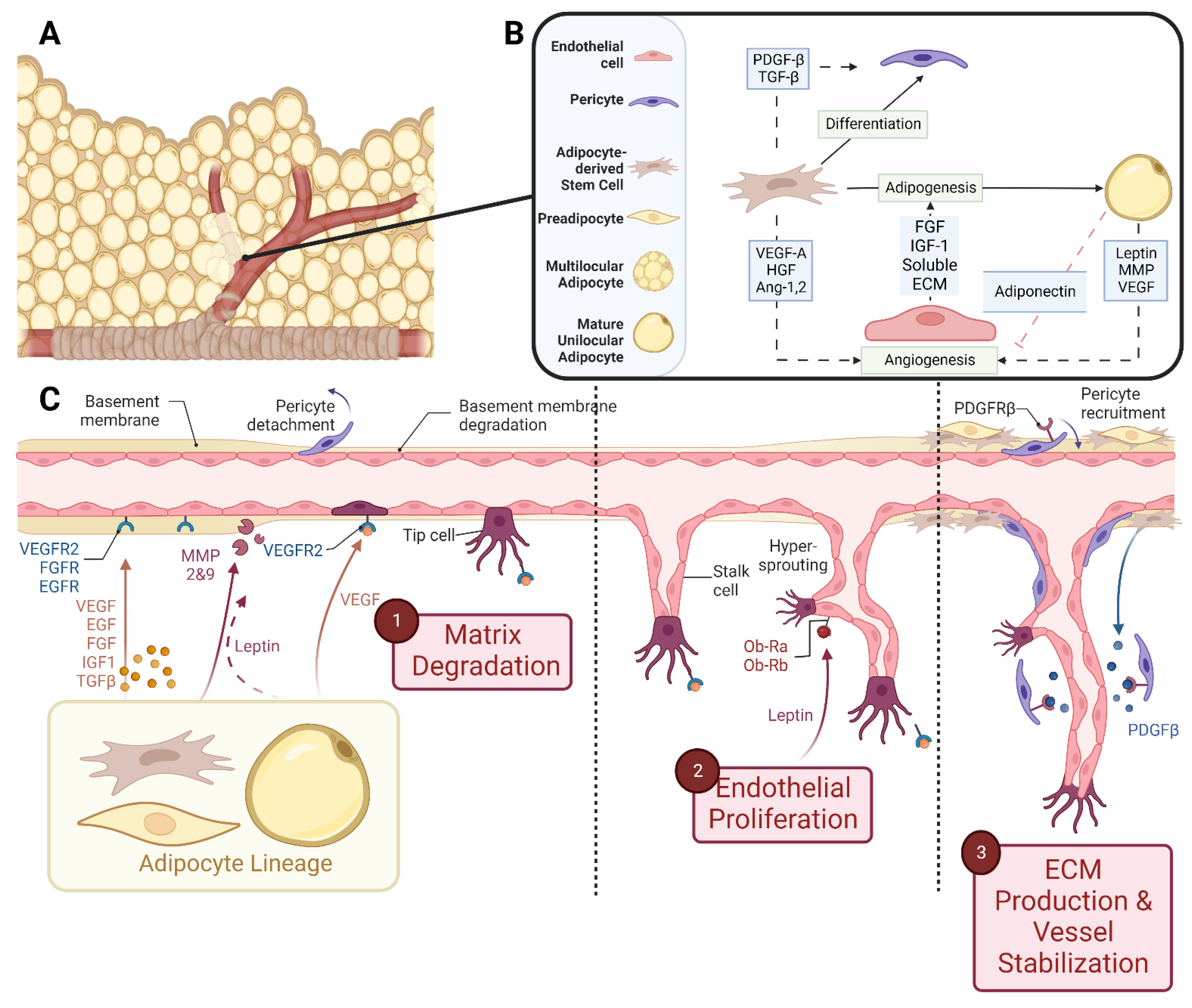
2.2. Remodeling during Angiogenesis and Adipogenesis
2.2.1. Angiogenesis
2.2.2. Adipogenesis
3. Cellular Crosstalk and Paracrine Signaling during Simultaneous Adipogenesis and Angiogenesis
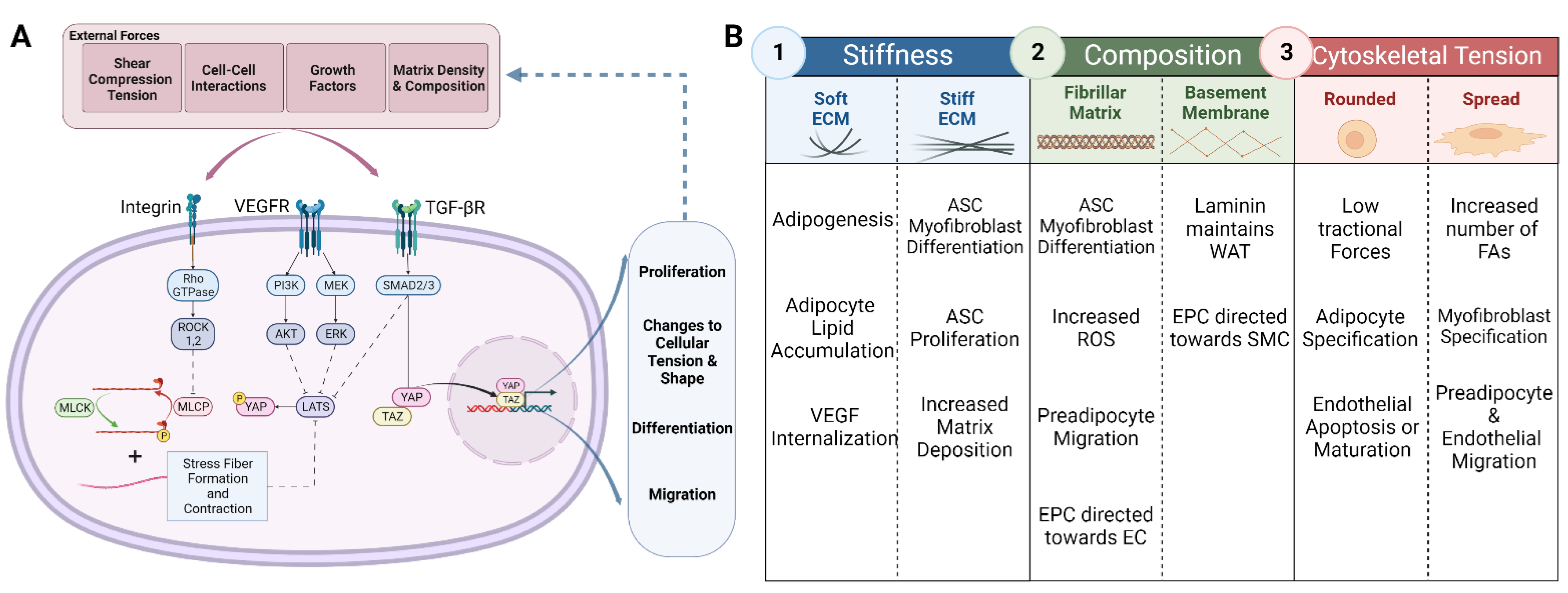
4. Mechanical Regulation of Adipogenesis and Angiogenesis
4.1. ECM Regulation
4.1.1. Stiffness
4.1.2. Matrix Composition
4.2. Tractional Regulation of Cell Fate
4.2.1. Cell Shape
4.2.2. Tractional Forces
5. Regulation of ECM Remodeling
5.1. Proteolytic Involvement during Adipose Tissue Development
| Proteolytic System | Enzymes | Activators | Inhibitors | Activity | Citation |
|---|---|---|---|---|---|
| Fibrinolytic | Plasminogen- inactive |
|
|
| [147,152,156] |
| Plasmin-Active | |||||
| Collagenases | MMP1 (Collagenase 1) |
|
|
| [160,161,162] |
| MMP13 (Collagenase 3) |
|
| |||
| MT1-MMP |
|
|
| [158,159,163] | |
| Gelatinases | MMP2 (Gelatinase A) |
|
|
| [42,43,158,164,165,166,167] |
| MMP9 (Gelatinase B) |
|
|
| [40,41,42,43,44,89] |
5.2. Adipokine Regulation of ECM Remodeling
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berg, A.H.; Scherer, P.E. Adipose Tissue, Inflammation, and Cardiovascular Disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Papadopoulos, V.; Vihma, V. Steroid biosynthesis in adipose tissue. Steroids 2015, 103, 89–104. [Google Scholar] [CrossRef]
- Gregory, E.L. Thermoregulatory aspects of adipose tissue. Clin. Dermatol. 1989, 7, 78–92. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef]
- Widdowson, E.M. Chemical composition of newly born mammals. Nature 1950, 166, 626–628. [Google Scholar] [CrossRef]
- Gilsanz, V.; Smith, M.L.; Goodarzian, F.; Kim, M.; Wren, T.A.L.; Hu, H.H. Changes in Brown Adipose Tissue in Boys and Girls during Childhood and Puberty. J. Pediatr. 2012, 160, 604–609.e1. [Google Scholar] [CrossRef]
- Leitner, B.P.; Huang, S.; Brychta, R.J.; Duckworth, C.J.; Baskin, A.S.; McGehee, S.; Tal, I.; Dieckmann, W.; Gupta, G.; Kolodny, G.M.; et al. Mapping of human brown adipose tissue in lean and obese young men. Proc. Natl. Acad. Sci. USA 2017, 114, 8649–8654. [Google Scholar] [CrossRef]
- Symonds, M.E.; Lomax, M.A. Maternal and environmental influences on thermoregulation in the neonate. Proc. Nutr. Soc. 1992, 51, 165–172. [Google Scholar] [CrossRef]
- Harrington, T.A.M.; Thomas, E.L.; Modi, N.; Frost, G.; Coutts, G.A.; Bell, J.D. Fast and reproducible method for the direct quantitation of adipose tissue in newborn infants. Lipids 2002, 37, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Harrington, T.A.M.; Thomas, E.L.; Frost, G.; Modi, N.; Bell, J.D. Distribution of Adipose Tissue in the Newborn. Pediatr. Res. 2004, 55, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Mittal, B. Subcutaneous adipose tissue & visceral adipose tissue. Indian J. Med. Res. 2019, 149, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; He, Y.; Zhou, T.; Zhang, P.; Gao, J.; Lu, F. Adipose Extracellular Matrix/Stromal Vascular Fraction Gel Secretes Angiogenic Factors and Enhances Skin Wound Healing in a Murine Model. BioMed Res. Int. 2017, 2017, 3105780. [Google Scholar] [CrossRef]
- Yoshimura, K.; Suga, H.; Eto, H. Adipose-derived stem/progenitor cells: Roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen. Med. 2009, 4, 265–273. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, S.; Zhang, X.; Pei, M. Significance of Cellular Cross-Talk in Stromal Vascular Fraction of Adipose Tissue in Neovascularization. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1034–1044. [Google Scholar] [CrossRef]
- Herold, J.; Kalucka, J. Angiogenesis in Adipose Tissue: The Interplay Between Adipose and Endothelial Cells. Front. Physiol. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Cao, Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 2013, 18, 478–489. [Google Scholar] [CrossRef]
- Mammoto, T.; Mammoto, A.; Ingber, D.E. Mechanobiology and Developmental Control. Annu. Rev. Cell Dev. Biol. 2013, 29, 27–61. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Chen, C.S. Mechanotransduction in development: A growing role for contractility. Nat. Rev. Mol. Cell Biol. 2009, 10, 34–43. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Nava, M.M.; Raimondi, M.T.; Pietrabissa, R. Controlling Self-Renewal and Differentiation of Stem Cells via Mechanical Cues. J. Biomed. Biotechnol. 2012, 2012, 797410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cai, J.; Li, Y.; He, Y.; Dong, Z.; Dai, J.; Lu, F. External Volume Expansion Adjusted Adipose Stem Cell by Shifting the Ratio of Fibronectin to Laminin. Tissue Eng. Part A 2020, 26, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.-J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Linder-Ganz, E.; Shabshin, N.; Itzchak, Y.; Gefen, A. Assessment of mechanical conditions in sub-dermal tissues during sitting: A combined experimental-MRI and finite element approach. J. Biomech. 2007, 40, 1443–1454. [Google Scholar] [CrossRef]
- Slomka, N.; Or-Tzadikario, S.; Sassun, D.; Gefen, A. Membrane-stretch-induced cell death in deep tissue injury: Computer model studies. Cell. Mol. Bioeng. 2009, 2, 118–132. [Google Scholar] [CrossRef]
- Poissonnet, C.M.; Burdi, A.R.; Bookstein, F.L. Growth and development of human adipose tissue during early gestation. Early Hum. Dev. 1983, 8, 1–11. [Google Scholar] [CrossRef]
- Poissonnet, C.M.; Burdi, A.R.; Garn, S.M. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum. Dev. 1984, 10, 1–11. [Google Scholar] [CrossRef]
- Hausman, G.J.; Martin, R.J. Subcutaneous Adipose Tissue Development in Yorkshire (Lean) and Ossabaw (Obese) Pigs. J. Anim. Sci. 1981, 52, 1442–1449. [Google Scholar] [CrossRef]
- Hausman, G.J.; Richardson, L.R. Histochemical and Ultrastructural Analysis of Developing Adipocytes in the Fetal Pig. Cells Tissues Organs 1982, 114, 228–247. [Google Scholar] [CrossRef]
- Hausman, G.J.; Richardson, R.L. Cellular and vascular development in immature rat adipose tissue. J. Lipid Res. 1983, 24, 522–532. [Google Scholar] [CrossRef]
- Hausman, G.J.; Thomas, G.B. Enzyme histochemical differentiation of white adipose tissue in the rat. Am. J. Anat. 1984, 169, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Hausman, G.J.; Thomas, G.B. Differentiation of blood vessels in the adipose tissue of lean and obese fetal pigs, studied by differential enzyme histochemistry. Acta Anat. 1985, 123, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.T.; Hausman, G.J. Adipose tissue development in the fetal pig examined using monoclonal antibodies. J. Anim. Sci. 1990, 68, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Hausman, G.J.; Wright, J.T.; Thomas, G.B. Vascular and cellular development in fetal adipose tissue: Lectin binding studies and immunocytochemistry for laminin and type IV collagen. Microvasc. Res. 1991, 41, 111–125. [Google Scholar] [CrossRef]
- Hausman, G.J.; Wright, J.T. Cytochemical studies of adipose tissue-associated blood vessels in untreated and thyroxine-treated hypophysectomized pig fetuses. J. Anim. Sci. 1996, 74, 354–362. [Google Scholar] [CrossRef]
- Estève, D.; Boulet, N.; Belles, C.; Zakaroff-Girard, A.; Decaunes, P.; Briot, A.; Veeranagouda, Y.; Didier, M.; Remaury, A.; Guillemot, J.C.; et al. Lobular architecture of human adipose tissue defines the niche and fate of progenitor cells. Nat. Commun. 2019, 10, 2549. [Google Scholar] [CrossRef]
- Asahara, T.; Kawamoto, A. Endothelial progenitor cells for postnatal vasculogenesis. Am. J. Physiol. Cell Physiol. 2004, 287, C572–C579. [Google Scholar] [CrossRef]
- Hallmann, R.; Horn, N.; Selg, M.; Wendler, O.; Pausch, F.; Sorokin, L.M. Expression and Function of Laminins in the Embryonic and Mature Vasculature. Physiol. Rev. 2005, 85, 979–1000. [Google Scholar] [CrossRef]
- Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 2003, 9, 685–693. [Google Scholar] [CrossRef]
- Cleaver, O.; Melton, D.A. Endothelial signaling during development. Nat. Med. 2003, 9, 661–668. [Google Scholar] [CrossRef]
- Bouloumié, A.; Sengenès, C.; Portolan, G.; Galitzky, J.; Lafontan, M. Adipocyte produces matrix metalloproteinases 2 and 9: Involvement in adipose differentiation. Diabetes 2001, 50, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Bourlier, V.; Zakaroff-Girard, A.; De Barros, S.; Pizzacalla, C.; de Saint Front, V.D.; Lafontan, M.; Bouloumié, A.; Galitzky, J. Protease inhibitor treatments reveal specific involvement of matrix metalloproteinase-9 in human adipocyte differentiation. J. Pharm. Exp. 2005, 312, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, H.R.; Maquoi, E.; Demeulemeester, D.; Van Hoef, B.; Collen, D. Modulation of fibrinolytic and gelatinolytic activity during adipose tissue development in a mouse model of nutritionally induced obesity. Thromb. Haemost. 2002, 88, 345–353. [Google Scholar] [PubMed]
- Evensen, L.; Micklem, D.R.; Blois, A.; Berge, S.V.; Aarsæther, N.; Littlewood-Evans, A.; Wood, J.; Lorens, J.B. Mural Cell Associated VEGF Is Required for Organotypic Vessel Formation. PLoS ONE 2009, 4, e5798. [Google Scholar] [CrossRef]
- Leung, D.W.; Cachianes, G.; Kuang, W.-J.; Goeddel, D.V.; Ferrara, N. Vascular Endothelial Growth Factor Is a Secreted Angiogenic Mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef]
- Igarashi, J.; Erwin, P.A.; Dantas, A.P.V.; Chen, H.; Michel, T. VEGF induces S1P1 receptors in endothelial cells: Implications for cross-talk between sphingolipid and growth factor receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 10664–10669. [Google Scholar] [CrossRef]
- Pepper, M.S. Transforming growth factor-beta: Vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997, 8, 21–43. [Google Scholar] [CrossRef]
- Goumans, M.J.; Valdimarsdottir, G.; Itoh, S.; Rosendahl, A.; Sideras, P.; ten Dijke, P. Balancing the activation state of the endothelium via two distinct TGF-β type I receptors. EMBO J. 2002, 21, 1743–1753. [Google Scholar] [CrossRef]
- Laurie, G.W.; Leblond, C.P.; Martin, G.R. Localization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. J. Cell Biol. 1982, 95, 340–344. [Google Scholar] [CrossRef]
- Varzaneh, F.E.; Shillabeer, G.; Wong, K.L.; Lau, D.C.W. Extracellular matrix components secreted by microvascular endothelial cells stimulate preadipocyte differentiation in vitro. Metabolism 1994, 43, 906–912. [Google Scholar] [CrossRef]
- Abuhattum, S.; Gefen, A.; Weihs, D. Ratio of total traction force to projected cell area is preserved in differentiating adipocytes. Integr. Biol. 2015, 7, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Shitaye, H.; Friedman, M.; Bennett, C.N.; Miller, J.; Macdougald, O.A.; Hankenson, K.D. Disruption of cell-matrix interactions by heparin enhances mesenchymal progenitor adipocyte differentiation. Exp. Cell Res. 2008, 314, 3382–3391. [Google Scholar] [CrossRef] [PubMed]
- Shoham, N.; Girshovitz, P.; Katzengold, R.; Shaked, N.T.; Benayahu, D.; Gefen, A. Adipocyte stiffness increases with accumulation of lipid droplets. Biophys. J. 2014, 106, 1421–1431. [Google Scholar] [CrossRef]
- Antras, J.; Hilliou, F.; Redziniak, G.; Pairault, J. Decreased biosynthesis of actin and cellular fibronectin during adipose conversion of 3T3-F442A cells. Reorganization of the cytoarchitecture and extracellular matrix fibronectin. Biol. Cell 1989, 66, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Fernández, J.L.; Ben-Ze’ev, A. Regulation of fibronectin, integrin and cytoskeleton expression in differentiating adipocytes: Inhibition by extracellular matrix and polylysine. Differentiation 1989, 42, 65–74. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, K.; Chen, Y.; Yang, Y.; Kawazoe, N.; Chen, G. Preparation of Stepwise Adipogenesis-Mimicking ECM-Deposited PLGA–Collagen Hybrid Meshes and Their Influence on Adipogenic Differentiation of hMSCs. ACS Biomater. Sci. Eng. 2019, 5, 6099–6108. [Google Scholar] [CrossRef]
- Zhang, Z.; Qu, R.; Fan, T.; Ouyang, J.; Lu, F.; Dai, J. Stepwise Adipogenesis of Decellularized Cellular Extracellular Matrix Regulates Adipose Tissue-Derived Stem Cell Migration and Differentiation. Stem. Cells Int. 2019, 2019, 1845926. [Google Scholar] [CrossRef]
- Spiegelman, B.M.; Ginty, C.A. Fibronectin modulation of cell shape and lipogenic gene expression in 3t3-adipocytes. Cell 1983, 35, 657–666. [Google Scholar] [CrossRef]
- Paganelli, A.; Benassi, L.; Rossi, E.; Magnoni, C. Extracellular matrix deposition by adipose-derived stem cells and fibroblasts: A comparative study. Arch. Dermatol. Res. 2020, 312, 295–299. [Google Scholar] [CrossRef]
- Zhou, Z.Q.; Chen, Y.; Chai, M.; Tao, R.; Lei, Y.H.; Jia, Y.Q.; Shu, J.; Ren, J.; Li, G.; Wei, W.X.; et al. Adipose extracellular matrix promotes skin wound healing by inducing the differentiation of adipose-derived stem cells into fibroblasts. Int. J. Mol. Med. 2019, 43, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, K.; Kuismanen, E.; Myllylä, R.; Kiistala, U.; Asko-Seljavaara, S.; Vaheri, A. Extracellular matrix proteins of human epidermal keratinocytes and feeder 3T3 cells. J. Cell Biol. 1982, 94, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.; Hatzmann, F.M.; Hammerle, S.; Ritthammer, H.; Mattesich, M.; Zwierzina, M.; Waldegger, P.; Zwerschke, W. Fibroblast feeder layer supports adipogenic differentiation of human adipose stromal/progenitor cells. Adipocyte 2019, 8, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, I.; Yamaguchi, T.; Ozutsumi, K.; Aso, H. Adipose tissue extracellular matrix: Newly organized by adipocytes during differentiation. Differentiation 1998, 63, 193–200. [Google Scholar] [CrossRef]
- Nakajima, I.; Muroya, S.; Tanabe, R.; Chikuni, K. Extracellular matrix development during differentiation into adipocytes with a unique increase in type V and VI collagen. Biol. Cell 2002, 94, 197–203. [Google Scholar] [CrossRef]
- Nakajima, I.; Muroya, S.; Tanabe, R.-i.; Chikuni, K. Positive effect of collagen V and VI on triglyceride accumulation during differentiation in cultures of bovine intramuscular adipocytes. Differentiation 2002, 70, 84–91. [Google Scholar] [CrossRef]
- Aratani, Y.; Kitagawa, Y. Enhanced synthesis and secretion of type IV collagen and entactin during adipose conversion of 3T3-L1 cells and production of unorthodox laminin complex. J. Biol. Chem. 1988, 263, 16163–16169. [Google Scholar] [CrossRef]
- Weiner, F.R.; Shah, A.; Smith, P.J.; Rubin, C.S.; Zern, M.A. Regulation of collagen gene expression in 3T3-L1 cells. Effects of adipocyte differentiation and tumor necrosis factor alpha. Biochemistry 1989, 28, 4094–4099. [Google Scholar] [CrossRef]
- Wang, P.; Mariman, E.; Keijer, J.; Bouwman, F.; Noben, J.P.; Robben, J.; Renes, J. Profiling of the secreted proteins during 3T3-L1 adipocyte differentiation leads to the identification of novel adipokines. Cell. Mol. Life Sci. CMLS 2004, 61, 2405–2417. [Google Scholar] [CrossRef]
- Wang, T.; Hill, R.C.; Dzieciatkowska, M.; Zhu, L.; Infante, A.M.; Hu, G.; Hansen, K.C.; Pei, M. Site-Dependent Lineage Preference of Adipose Stem Cells. Front. Cell Dev. Biol. 2020, 8, 1–16. [Google Scholar] [CrossRef]
- Li, H.; Zimmerlin, L.; Marra, K.G.; Donnenberg, V.S.; Donnenberg, A.D.; Rubin, J.P. Adipogenic potential of adipose stem cell subpopulations. Plast. Reconstr. Surg. 2011, 128, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Planat-Benard, V.; Silvestre, J.S.; Cousin, B.; André, M.; Nibbelink, M.; Tamarat, R.; Clergue, M.; Manneville, C.; Saillan-Barreau, C.; Duriez, M.; et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation 2004, 109, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Zannettino, A.C.; Paton, S.; Arthur, A.; Khor, F.; Itescu, S.; Gimble, J.M.; Gronthos, S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J. Cell Physiol. 2008, 214, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Cigolini, M.; Bosello, O.; Björntorp, P. A morphological study of the adipocyte precursor. J. Submicrosc. Cytol. 1984, 16, 243–251. [Google Scholar] [PubMed]
- Iyama, K.; Ohzono, K.; Usuku, G. Electron microscopical studies on the genesis of white adipocytes: Differentiation of immature pericytes into adipocytes in transplanted preadipose tissue. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1979, 31, 143–155. [Google Scholar] [CrossRef]
- Tang, W.; Zeve, D.; Suh, J.M.; Bosnakovski, D.; Kyba, M.; Hammer, R.E.; Tallquist, M.D.; Graff, J.M. White Fat Progenitor Cells Reside in the Adipose Vasculature. Science 2008, 322, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef]
- Castillo, G.; Hauser, S.; Rosenfield, J.K.; Spiegelman, B.M. Role and Regulation of PPARγ During Adipogenesis. J. Anim. Sci. 1999, 77, 9–15. [Google Scholar] [CrossRef]
- Otto, T.C.; Lane, M.D. Adipose development: From stem cell to adipocyte. Crit. Rev. Biochem. Mol. Biol. 2005, 40, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Gao, Y.; Zhao, X.; Gao, M.; Wu, Y.; Han, Y.; Qiao, Y.; Luo, Z.; Yang, L.; Chen, J.; et al. FSP1-positive fibroblasts are adipogenic niche and regulate adipose homeostasis. PLoS Biol. 2018, 16, e2001493. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Li, H.; Zhang, C.; Mao, Y.; Nie, F.; Xing, Y.; Sha, W.; Wang, X.; Irwin, D.M.; Tan, H. Stromal vascular fraction promotes migration of fibroblasts and angiogenesis through regulation of extracellular matrix in the skin wound healing process. Stem. Cell Res. Ther. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, G.; Janowicz, K.; Moncrieff, L.; Dompe, C.; Strauss, E.; Kocherova, I.; Nawrocki, M.J.; Kruszyna, Ł.; Wąsiatycz, G.; Antosik, P.; et al. The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis. Int. J. Mol. Sci. 2020, 21, 3790. [Google Scholar] [CrossRef] [PubMed]
- Gan, F.; Liu, L.; Zhou, Q.; Huang, W.; Huang, X.; Zhao, X. Effects of adipose-derived stromal cells and endothelial progenitor cells on adipose transplant survival and angiogenesis. PLoS ONE 2022, 17, e0261498. [Google Scholar] [CrossRef] [PubMed]
- Hutley, L.J.; Herington, A.C.; Shurety, W.; Cheung, C.; Vesey, D.A.; Cameron, D.P.; Prins, J.B. Human adipose tissue endothelial cells promote preadipocyte proliferation. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1037–E1044. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, T.G.; Rao, S.V.; Wolverton, C.K. In Vitro Systems for the Analysis of the Development of Adipose Tissue in Domestic Animals. J. Nutr. 1992, 122, 806–817. [Google Scholar] [CrossRef]
- Crandall, D.L.; Hausman, G.J.; Kral, J.G. A Review of the Microcirculation of Adipose Tissue: Anatomic, Metabolic, and Angiogenic Perspectives. Microcirculation 1997, 4, 211–232. [Google Scholar] [CrossRef]
- Fukumura, D.; Ushiyama, A.; Duda, D.G.; Xu, L.; Tam, J.; Krishna, V.; Chatterjee, K.; Garkavtsev, I.; Jain, R.K. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ. Res. 2003, 93, e88–e97. [Google Scholar] [CrossRef]
- Shao, M.; Hepler, C.; Vishvanath, L.; MacPherson, K.A.; Busbuso, N.C.; Gupta, R.K. Fetal development of subcutaneous white adipose tissue is dependent on Zfp423. Mol. Metab. 2017, 6, 111–124. [Google Scholar] [CrossRef]
- Nigro, E.; Mallardo, M.; Polito, R.; Scialò, F.; Bianco, A.; Daniele, A. Adiponectin and Leptin Exert Antagonizing Effects on HUVEC Tube Formation and Migration Modulating the Expression of CXCL1, VEGF, MMP-2 and MMP-9. Int. J. Mol. Sci. 2021, 22, 7516. [Google Scholar] [CrossRef]
- Briffa, J.F.; McAinch, A.J.; Romano, T.; Wlodek, M.E.; Hryciw, D.H. Leptin in pregnancy and development: A contributor to adulthood disease? Am. J. Physiol. Endocrinol. Metab. 2015, 308, E335–E350. [Google Scholar] [CrossRef]
- Bouloumie, A.; Drexler, H.C.; Lafontan, M.; Busse, R. Leptin, the product of Ob gene, promotes angiogenesis. Circ. Res. 1998, 83, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Honigmann, M.R.o.; Nath Anjali, K.; Murakami, C.; García-Cardeña, G.; Papapetropoulos, A.; Sessa William, C.; Madge Lisa, A.; Schechner Jeffrey, S.; Schwabb Michael, B.; Polverini Peter, J.; et al. Biological Action of Leptin as an Angiogenic Factor. Science 1998, 281, 1683–1686. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Brakenhielm, E.; Wahlestedt, C.; Thyberg, J.; Cao, Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc. Natl. Acad. Sci. USA 2001, 98, 6390–6395. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Kwon, H.M.; Lim, H.J.; Hong, B.K.; Lee, J.Y.; Park, B.E.; Jang, Y.S.; Cho, S.Y.; Kim, H.-S. Potential role of leptin in angiogenesis: Leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp. Mol. Med. 2001, 33, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Aronis, K.; Diakopoulos, K.; Fiorenza, C.; Chamberland, J.; Mantzoros, C. Leptin administered in physiological or pharmacological doses does not regulate circulating angiogenesis factors in humans. Diabetologia 2011, 54, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.; Barkan, D.; Levy, Y.; Goldberg, I.; Fridman, E.; Kopolovic, J.; Rubinstein, M. Leptin induces angiopoietin-2 expression in adipose tissues. J. Biol. Chem. 2001, 276, 7697–7700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.X.; Magovern, C.J.; Mack, C.A.; Budenbender, K.T.; Ko, W.; Rosengart, T.K. Vascular endothelial growth factor is the major angiogenic factor in omentum: Mechanism of the omentum-mediated angiogenesis. J. Surg. Res. 1997, 67, 147–154. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Young, D.A.; Choi, Y.S.; Engler, A.J.; Christman, K.L. Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials 2013, 34, 8581–8588. [Google Scholar] [CrossRef]
- Lee, J.; Abdeen, A.A.; Kilian, K.A. Rewiring mesenchymal stem cell lineage specification by switching the biophysical microenvironment. Sci. Rep. 2014, 4, 5188. [Google Scholar] [CrossRef]
- Berger, A.J.; Anvari, G.; Bellas, E. Mechanical Memory Impairs Adipose-Derived Stem Cell (ASC) Adipogenic Capacity After Long-Term In Vitro Expansion. Cell. Mol. Bioeng. 2021, 14, 397–408. [Google Scholar] [CrossRef]
- Yang, C.; Tibbitt, M.W.; Basta, L.; Anseth, K.S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 2014, 13, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Deng, Z.; He, Y.; Lu, F.; Yuan, Y. Mechanical Strain Promotes Proliferation of Adipose-Derived Stem Cells Through the Integrin β1-Mediated RhoA/Myosin Light Chain Pathway. Tissue Eng. Part A 2020, 26, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, M.; Zhang, Z.; Xia, J.; Wang, Z.; Chen, X.; Xiao, X.; Lu, F.; Dong, Z. Application of External Force Regulates the Migration and Differentiation of Adipose-Derived Stem/Progenitor Cells by Altering Tissue Stiffness. Tissue Eng. Part A 2019, 25, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Ida, Y.; Hikage, F.; Ohguro, H. ROCK inhibitors enhance the production of large lipid-enriched 3D organoids of 3T3-L1 cells. Sci. Rep. 2021, 11, 5479. [Google Scholar] [CrossRef]
- Sordella, R.; Jiang, W.; Chen, G.-C.; Curto, M.; Settleman, J. Modulation of Rho GTPase Signaling Regulates a Switch between Adipogenesis and Myogenesis. Cell 2003, 113, 147–158. [Google Scholar] [CrossRef]
- Martínez-Estrada, O.M.; Muñoz-Santos, Y.; Julve, J.; Reina, M.; Vilaró, S. Human adipose tissue as a source of Flk-1+ cells: New method of differentiation and expansion. Cardiovasc. Res. 2005, 65, 328–333. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, X.; Sun, Z.; Chen, B.; Han, Q.; Li, J.; Zhao, R.C. Flk-1+ adipose-derived mesenchymal stem cells differentiate into skeletal muscle satellite cells and ameliorate muscular dystrophy in mdx mice. Stem. Cells Dev. 2007, 16, 695–706. [Google Scholar] [CrossRef]
- Saito, N.; Shirado, T.; Funabashi-Eto, H.; Wu, Y.; Mori, M.; Asahi, R.; Yoshimura, K. Purification and characterization of human adipose-resident microvascular endothelial progenitor cells. Sci. Rep. 2022, 12, 1775. [Google Scholar] [CrossRef]
- Froehlich, H.; Simari, R.D.; Boilson, B.A. Differential phenotype and behavior in culture of CD34 positive cells from peripheral blood and adipose tissue. Heliyon 2021, 7, e07779. [Google Scholar] [CrossRef]
- Yamashita, J.; Itoh, H.; Hirashima, M.; Ogawa, M.; Nishikawa, S.; Yurugi, T.; Naito, M.; Nakao, K.; Nishikawa, S.-I. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 2000, 408, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, P.; Cefaloni, G.; Vajhadin, F.; Lee, K.; Kim, H.-J.; Cho, H.-J.; Hartel, M.C.; Zhang, S.; Sun, W.; Goudie, M.J.; et al. Mechanical Cues Regulating Proangiogenic Potential of Human Mesenchymal Stem Cells through YAP-Mediated Mechanosensing. Small 2020, 16, e2001837. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, A.; Connor, K.M.; Mammoto, T.; Yung, C.W.; Huh, D.; Aderman, C.M.; Mostoslavsky, G.; Smith, L.E.H.; Ingber, D.E. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 2009, 457, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Sack, K.D.; Teran, M.; Nugent, M.A. Extracellular Matrix Stiffness Controls VEGF Signaling and Processing in Endothelial Cells. J. Cell. Physiol. 2016, 231, 2026–2039. [Google Scholar] [CrossRef]
- Numaguchi, Y.; Huang, S.; Polte, T.R.; Eichler, G.S.; Wang, N.; Ingber, D.E. Caldesmon-dependent switching between capillary endothelial cell growth and apoptosis through modulation of cell shape and contractility. Angiogenesis 2003, 6, 55–64. [Google Scholar] [CrossRef]
- Bordeleau, F.; Mason, B.N.; Lollis, E.M.; Mazzola, M.; Zanotelli, M.R.; Somasegar, S.; Califano, J.P.; Montague, C.; LaValley, D.J.; Huynh, J.; et al. Matrix stiffening promotes a tumor vasculature phenotype. Proc. Natl. Acad. Sci. USA 2017, 114, 492–497. [Google Scholar] [CrossRef]
- Liu, X.; Long, X.; Liu, W.; Zhao, Y.; Hayashi, T.; Yamato, M.; Mizuno, K.; Fujisaki, H.; Hattori, S.; Tashiro, S.-i.; et al. Type I collagen induces mesenchymal cell differentiation into myofibroblasts through YAP-induced TGF-β1 activation. Biochimie 2018, 150, 110–130. [Google Scholar] [CrossRef]
- Liu, X.; Long, X.; Gao, Y.; Liu, W.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ogura, T.; Onodera, S.; et al. Type I collagen inhibits adipogenic differentiation via YAP activation in vitro. J. Cell. Physiol. 2020, 235, 1821–1837. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, X.; Liu, W.; Hayashi, T.; Yamato, M.; Fujisaki, H.; Hattori, S.; Tashiro, S.-i.; Onodera, S.; Ikejima, T. Type I collagen-induced YAP nuclear expression promotes primary cilia growth and contributes to cell migration in confluent mouse embryo fibroblast 3T3-L1 cells. Mol. Cell. Biochem. 2019, 450, 87–96. [Google Scholar] [CrossRef]
- Liu, X.; Long, X.; Liu, W.; Yao, G.; Zhao, Y.; Hayashi, T.; Hattori, S.; Fujisaki, H.; Ogura, T.; Tashiro, S.-i.; et al. Differential levels of reactive oxygen species in murine preadipocyte 3T3-L1 cells cultured on type I collagen molecule-coated and gel-covered dishes exert opposite effects on NF-κB-mediated proliferation and migration. Free Radic. Res. 2018, 52, 913–928. [Google Scholar] [CrossRef]
- Gonzalez Porras, M.A.; Stojkova, K.; Vaicik, M.K.; Pelowe, A.; Goddi, A.; Carmona, A.; Long, B.; Qutub, A.A.; Gonzalez, A.; Cohen, R.N.; et al. Integrins and extracellular matrix proteins modulate adipocyte thermogenic capacity. Sci. Rep. 2021, 11, 5442. [Google Scholar] [CrossRef]
- Vaicik, M.K.; Blagajcevic, A.; Ye, H.; Morse, M.C.; Yang, F.; Goddi, A.; Brey, E.M.; Cohen, R.N. The Absence of Laminin α4 in Male Mice Results in Enhanced Energy Expenditure and Increased Beige Subcutaneous Adipose Tissue. Endocrinology 2018, 159, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Vaicik, M.K.; Thyboll Kortesmaa, J.; Movérare-Skrtic, S.; Kortesmaa, J.; Soininen, R.; Bergström, G.; Ohlsson, C.; Chong, L.Y.; Rozell, B.; Emont, M.; et al. Laminin α4 Deficient Mice Exhibit Decreased Capacity for Adipose Tissue Expansion and Weight Gain. PLoS ONE 2014, 9, e109854. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimi, A.; Bonino, F.; Bardon, S.; Ailhaud, G.; Dani, C. Essential role of collagens for terminal differentiation of preadipocytes. Biochem. Biophys. Res. Commun. 1992, 187, 1314–1322. [Google Scholar] [CrossRef]
- Zuidema, A.; Wang, W.; Sonnenberg, A. Crosstalk between Cell Adhesion Complexes in Regulation of Mechanotransduction. BioEssays 2020, 42, 2000119. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Moscona, A. Role of cell shape in growth control. Nature 1978, 273, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis: Initiation and control. Ann. N. Y. Acad. Sci. 1982, 401, 212–227. [Google Scholar] [CrossRef]
- Ingber, D.E.; Folkman, J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: Role of extracellular matrix. J. Cell Biol. 1989, 109, 317–330. [Google Scholar] [CrossRef]
- Reinhart-King, C.A.; Dembo, M.; Hammer, D.A. The Dynamics and Mechanics of Endothelial Cell Spreading. Biophys. J. 2005, 89, 676–689. [Google Scholar] [CrossRef]
- Mor-Yossef Moldovan, L.; Lustig, M.; Naftaly, A.; Mardamshina, M.; Geiger, T.; Gefen, A.; Benayahu, D. Cell shape alteration during adipogenesis is associated with coordinated matrix cues. J. Cell. Physiol. 2019, 234, 3850–3863. [Google Scholar] [CrossRef]
- Lustig, M.; Zadka, Y.; Levitsky, I.; Gefen, A.; Benayahu, D. Adipocytes Migration is Altered Through Differentiation. Microsc. Microanal. 2019, 25, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Abuhattum, S.; Weihs, D. Asymmetry in traction forces produced by migrating preadipocytes is bounded to 33%. Med. Eng. Phys. 2016, 38, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Darling, E.M.; Topel, M.; Zauscher, S.; Vail, T.P.; Guilak, F. Viscoelastic properties of human mesenchymally-derived stem cells and primary osteoblasts, chondrocytes, and adipocytes. J. Biomech. 2008, 41, 454–464. [Google Scholar] [CrossRef]
- Shoham, N.; Gefen, A. Mechanotransduction in adipocytes. J. Biomech. 2012, 45, 1–8. [Google Scholar] [CrossRef]
- Levy, A.; Enzer, S.; Shoham, N.; Zaretsky, U.; Gefen, A. Large, but not Small Sustained Tensile Strains Stimulate Adipogenesis in Culture. Ann. Biomed. Eng. 2012, 40, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Abuhattum, S.; Kotzbeck, P.; Schlüßler, R.; Harger, A.; Schellenberger, A.A.; Kim, K.; Escolano, J.-C.; Müller, T.; Braun, J.; Wabitsch, M.; et al. Adipose cells and tissues soften with lipid accumulation while in diabetes adipose tissue stiffens. bioRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Nobusue, H.; Onishi, N.; Shimizu, T.; Sugihara, E.; Oki, Y.; Sumikawa, Y.; Chiyoda, T.; Akashi, K.; Saya, H.; Kano, K. Regulation of MKL1 via actin cytoskeleton dynamics drives adipocyte differentiation. Nat. Commun. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Yuan, Y.; Gao, J.; Ogawa, R. Mechanobiology and Mechanotherapy of Adipose Tissue-Effect of Mechanical Force on Fat Tissue Engineering. Plast. Reconstr. Surg. Glob. Open 2016, 3, e578. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nishita, M.; Mishima, T.; Ohashi, K.; Mizuno, K. MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J. 2006, 25, 713–726. [Google Scholar] [CrossRef]
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial Cell Migration During Angiogenesis. Circ. Res. 2007, 100, 782–794. [Google Scholar] [CrossRef]
- Li, S.; Huang, N.F.; Hsu, S. Mechanotransduction in endothelial cell migration. J. Cell. Biochem. 2005, 96, 1110–1126. [Google Scholar] [CrossRef] [PubMed]
- Bastounis, E.E.; Yeh, Y.-T.; Theriot, J.A. Subendothelial stiffness alters endothelial cell traction force generation while exerting a minimal effect on the transcriptome. Sci. Rep. 2019, 9, 18209. [Google Scholar] [CrossRef] [PubMed]
- Ghajar, C.M.; Blevins, K.S.; Hughes, C.C.; George, S.C.; Putnam, A.J. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng. 2006, 12, 2875–2888. [Google Scholar] [CrossRef]
- Ghajar, C.M.; Chen, X.; Harris, J.W.; Suresh, V.; Hughes, C.C.; Jeon, N.L.; Putnam, A.J.; George, S.C. The effect of matrix density on the regulation of 3-D capillary morphogenesis. Biophys. J. 2008, 94, 1930–1941. [Google Scholar] [CrossRef]
- Kniazeva, E.; Putnam, A.J. Endothelial cell traction and ECM density influence both capillary morphogenesis and maintenance in 3-D. Am. J. Physiol. Cell Physiol. 2009, 297, C179–C187. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, R.; Sabatier, F.; Mialhe, A.; Basire, A.; Pannell, R.; Borghi, H.; Robert, S.; Lamy, E.; Plawinski, L.; Camoin-Jau, L.; et al. Activation of plasminogen into plasmin at the surface of endothelial microparticles: A mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood 2007, 110, 2432–2439. [Google Scholar] [CrossRef]
- Morange, P.E.; Lijnen, H.R.; Alessi, M.C.; Kopp, F.; Collen, D.; Juhan-Vague, I. Influence of PAI-1 on adipose tissue growth and metabolic parameters in a murine model of diet-induced obesity. Arter. Thromb. Vasc. Biol. 2000, 20, 1150–1154. [Google Scholar] [CrossRef]
- Morange, P.E.; Alessi, M.C.; Verdier, M.; Casanova, D.; Magalon, G.; Juhan-Vague, I. PAI-1 Produced Ex Vivo by Human Adipose Tissue Is Relevant to PAI-1 Blood Level. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1361–1365. [Google Scholar] [CrossRef]
- Alessi, M.C.; Peiretti, F.; Morange, P.; Henry, M.; Nalbone, G.; Juhan-Vague, I. Production of Plasminogen Activator Inhibitor 1 by Human Adipose Tissue: Possible Link Between Visceral Fat Accumulation and Vascular Disease. Diabetes 1997, 46, 860–867. [Google Scholar] [CrossRef]
- Wu, J.; Strawn, T.L.; Luo, M.; Wang, L.; Li, R.; Ren, M.; Xia, J.; Zhang, Z.; Ma, W.; Luo, T.; et al. Plasminogen activator inhibitor-1 inhibits angiogenic signaling by uncoupling vascular endothelial growth factor receptor-2-αVβ3 integrin cross talk. Arter. Thromb. Vasc. Biol. 2015, 35, 111–120. [Google Scholar] [CrossRef]
- Isogai, C.; Laug, W.E.; Shimada, H.; Declerck, P.J.; Stins, M.F.; Durden, D.L.; Erdreich-Epstein, A.; DeClerck, Y.A. Plasminogen Activator Inhibitor-1 Promotes Angiogenesis by Stimulating Endothelial Cell Migration toward Fibronectin1. Cancer Res. 2001, 61, 5587–5594. [Google Scholar] [PubMed]
- Crandall, D.L.; Busler, D.E.; McHendry-Rinde, B.; Groeling, T.M.; Kral, J.G. Autocrine Regulation of Human Preadipocyte Migration by Plasminogen Activator Inhibitor-1. J. Clin. Endocrinol. Metab. 2000, 85, 2609–2614. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crandall, D.L.; Quinet, E.M.; Morgan, G.A.; Busler, D.E.; McHendry-Rinde, B.; Kral, J.G. Synthesis and Secretion of Plasminogen Activator Inhibitor-1 by Human Preadipocytes. J. Clin. Endocrinol. Metab. 1999, 84, 3222–3227. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Kanjanabuch, T.; Mao, S.-L.; Hao, C.-M.; Tang, Y.-W.; Declerck, P.J.; Hasty, A.H.; Wasserman, D.H.; Fogo, A.B.; Ma, L.-J. Plasminogen activator inhibitor-1 modulates adipocyte differentiation. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E103–E113. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, V.; Lijnen, H.R. Role of the fibrinolytic and matrix metalloproteinase systems in development of adipose tissue. Arch. Physiol. Biochem. 2006, 112, 254–259. [Google Scholar] [CrossRef]
- Christiaens, V.; Scroyen, I.; Lijnen, H.R. Role of proteolysis in development of murine adipose tissue. Thromb Haemost 2008, 99, 290–294. [Google Scholar] [CrossRef]
- Brown, L.M.; Fox, H.L.; Hazen, S.A.; LaNoue, K.F.; Rannels, S.R.; Lynch, C.J. Role of the matrixin MMP-2 in multicellular organization of adipocytes cultured in basement membrane components. Am. J. Physiol. Cell Physiol. 1997, 272, C937–C949. [Google Scholar] [CrossRef]
- Chun, T.H.; Sabeh, F.; Ota, I.; Murphy, H.; McDonagh, K.T.; Holmbeck, K.; Birkedal-Hansen, H.; Allen, E.D.; Weiss, S.J. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J. Cell Biol. 2004, 167, 757–767. [Google Scholar] [CrossRef]
- Chun, T.-H.; Hotary, K.B.; Sabeh, F.; Saltiel, A.R.; Allen, E.D.; Weiss, S.J. A Pericellular Collagenase Directs the 3-Dimensional Development of White Adipose Tissue. Cell 2006, 125, 577–591. [Google Scholar] [CrossRef]
- Unemori, E.N.; Ferrara, N.; Bauer, E.A.; Amento, E.P. Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J. Cell. Physiol. 1992, 153, 557–562. [Google Scholar] [CrossRef]
- Song, Y.H.; Shon, S.H.; Shan, M.; Stroock, A.D.; Fischbach, C. Adipose-derived stem cells increase angiogenesis through matrix metalloproteinase-dependent collagen remodeling. Integr. Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Maquoi, E.; Munaut, C.; Colige, A.; Collen, D.s.; Lijnen, H.R. Modulation of Adipose Tissue Expression of Murine Matrix Metalloproteinases and Their Tissue Inhibitors With Obesity. Diabetes 2002, 51, 1093–1101. [Google Scholar] [CrossRef]
- Yana, I.; Weiss, S.J. Regulation of Membrane Type-1 Matrix Metalloproteinase Activation by Proprotein Convertases. Mol. Biol. Cell 2000, 11, 2387–2401. [Google Scholar] [CrossRef] [PubMed]
- Demeulemeester, D.; Collen, D.; Lijnen, H.R. Effect of matrix metalloproteinase inhibition on adipose tissue development. Biochem. Biophys. Res. Commun. 2005, 329, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Rupnick, M.A.; Panigrahy, D.; Zhang, C.Y.; Dallabrida, S.M.; Lowell, B.B.; Langer, R.; Folkman, M.J. Adipose tissue mass can be regulated through the vasculature. Proc. Natl. Acad. Sci. USA 2002, 99, 10730–10735. [Google Scholar] [CrossRef] [PubMed]
- Demeulemeester, D.; Scroyen, I.; Voros, G.; Snoeys, J.; De Geest, B.; Collen, D.; Lijnen, H.R. Overexpression of tissue inhibitor of matrix metalloproteinases-1 (TIMP-1) in mice does not affect adipogenesis or adipose tissue development. Thromb. Haemost. 2006, 95, 1019–1024. [Google Scholar] [CrossRef]
- Johnson, M.D.; Kim, H.-R.C.; Chesler, L.; Tsao-Wu, G.; Polverini, P.J.; Bouck, N. Inhibition of angiogenesis by tissue inhibitor of metalloproteinase. J. Cell. Physiol. 1994, 160, 194–202. [Google Scholar] [CrossRef]
- Arroyo-Jousse, V.; Jaramillo, A.; Castaño-Moreno, E.; Lépez, M.; Carrasco-Negüe, K.; Casanello, P. Adipokines underlie the early origins of obesity and associated metabolic comorbidities in the offspring of women with pregestational obesity. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165558. [Google Scholar] [CrossRef]
- Moon, H.-S.; Lee, H.-G.; Seo, J.-H.; Chung, C.-S.; Guo, D.-D.; Kim, T.-G.; Choi, Y.-J.; Cho, C.-S. Leptin-induced matrix metalloproteinase-2 secretion is suppressed by trans-10,cis-12 conjugated linoleic acid. Biochem. Biophys. Res. Commun. 2007, 356, 955–960. [Google Scholar] [CrossRef]
- Dadson, K.; Chasiotis, H.; Wannaiampikul, S.; Tungtrongchitr, R.; Xu, A.; Sweeney, G. Adiponectin mediated APPL1-AMPK signaling induces cell migration, MMP activation, and collagen remodeling in cardiac fibroblasts. J. Cell Biochem. 2014, 115, 785–793. [Google Scholar] [CrossRef]
- Chavey, C.; Mari, B.; Monthouel, M.-N.; Bonnafous, S.; Anglard, P.; Van Obberghen, E.; Tartare-Deckert, S. Matrix Metalloproteinases Are Differentially Expressed in Adipose Tissue during Obesity and Modulate Adipocyte Differentiation. J. Biol. Chem. 2003, 278, 11888–11896. [Google Scholar] [CrossRef]
- Schram, K.; Wong, M.M.C.; Palanivel, R.; No, E.K.; Dixon, I.M.C.; Sweeney, G. Increased expression and cell surface localization of MT1-MMP plays a role in stimulation of MMP-2 activity by leptin in neonatal rat cardiac myofibroblasts. J. Mol. Cell Cardiol. 2008, 44, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Adya, R.; Tan, B.K.; Chen, J.; Randeva, H.S. Protective actions of globular and full-length adiponectin on human endothelial cells: Novel insights into adiponectin-induced angiogenesis. J. Vasc. Res. 2012, 49, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lee, J.-E.; Jin, J.; Lim, J.S.; Oh, N.; Kim, K.; Chang, S.-I.; Shibuya, M.; Kim, H.; Koh, G.Y. The spatiotemporal development of adipose tissue. Development 2011, 138, 5027–5037. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnston, E.K.; Abbott, R.D. Adipose Tissue Development Relies on Coordinated Extracellular Matrix Remodeling, Angiogenesis, and Adipogenesis. Biomedicines 2022, 10, 2227. https://doi.org/10.3390/biomedicines10092227
Johnston EK, Abbott RD. Adipose Tissue Development Relies on Coordinated Extracellular Matrix Remodeling, Angiogenesis, and Adipogenesis. Biomedicines. 2022; 10(9):2227. https://doi.org/10.3390/biomedicines10092227
Chicago/Turabian StyleJohnston, Elizabeth K., and Rosalyn D. Abbott. 2022. "Adipose Tissue Development Relies on Coordinated Extracellular Matrix Remodeling, Angiogenesis, and Adipogenesis" Biomedicines 10, no. 9: 2227. https://doi.org/10.3390/biomedicines10092227
APA StyleJohnston, E. K., & Abbott, R. D. (2022). Adipose Tissue Development Relies on Coordinated Extracellular Matrix Remodeling, Angiogenesis, and Adipogenesis. Biomedicines, 10(9), 2227. https://doi.org/10.3390/biomedicines10092227






