Clinical Prospective Assessment of Genotoxic and Cytotoxic Effects of Fluoride Toothpaste and Mouthwash in Buccal Mucosal Cells
Abstract
1. Introduction
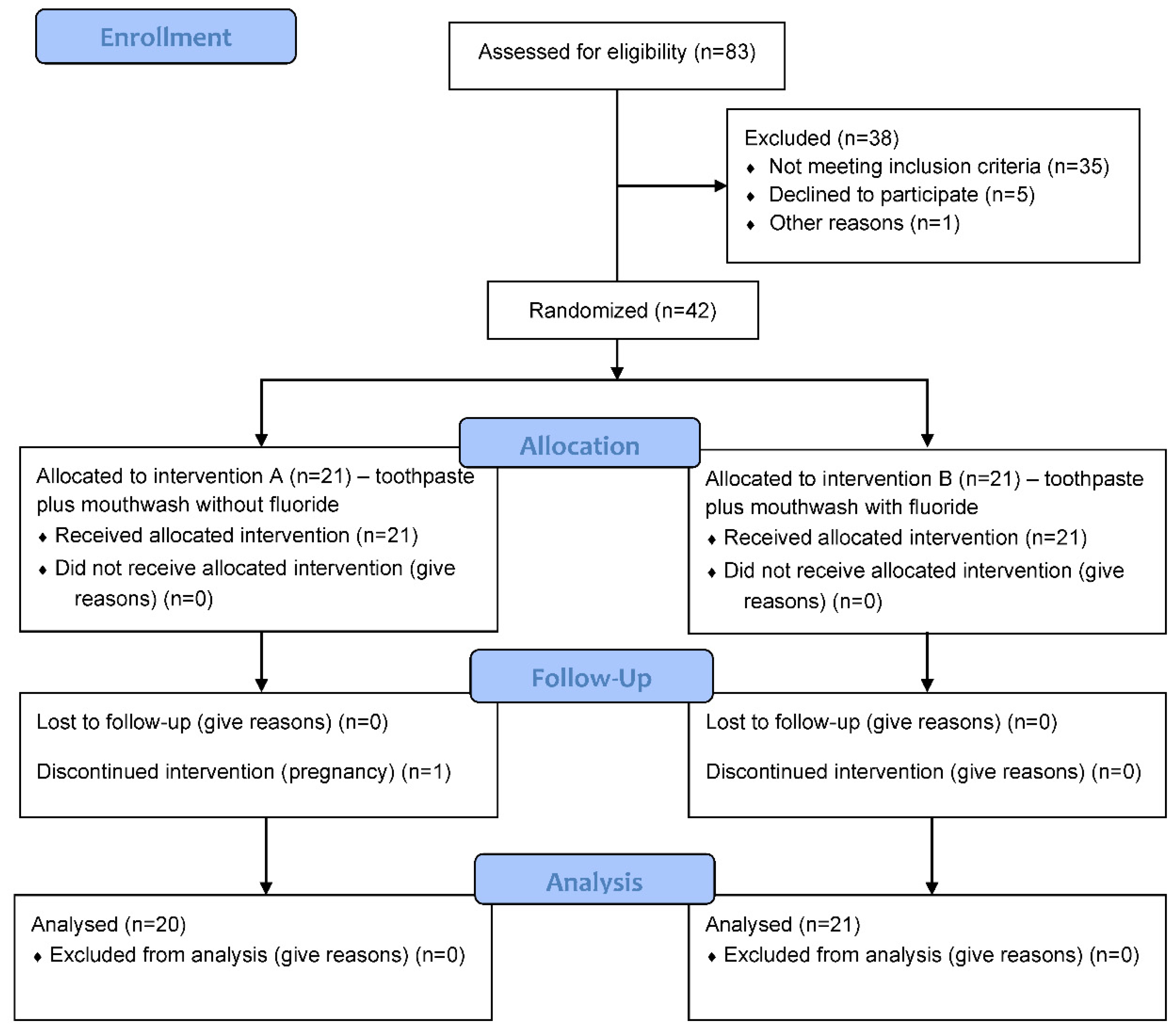
2. Material and Methods
2.1. Study Design
2.2. Participants and Sample Size
2.3. Materials and Clinical Procedure
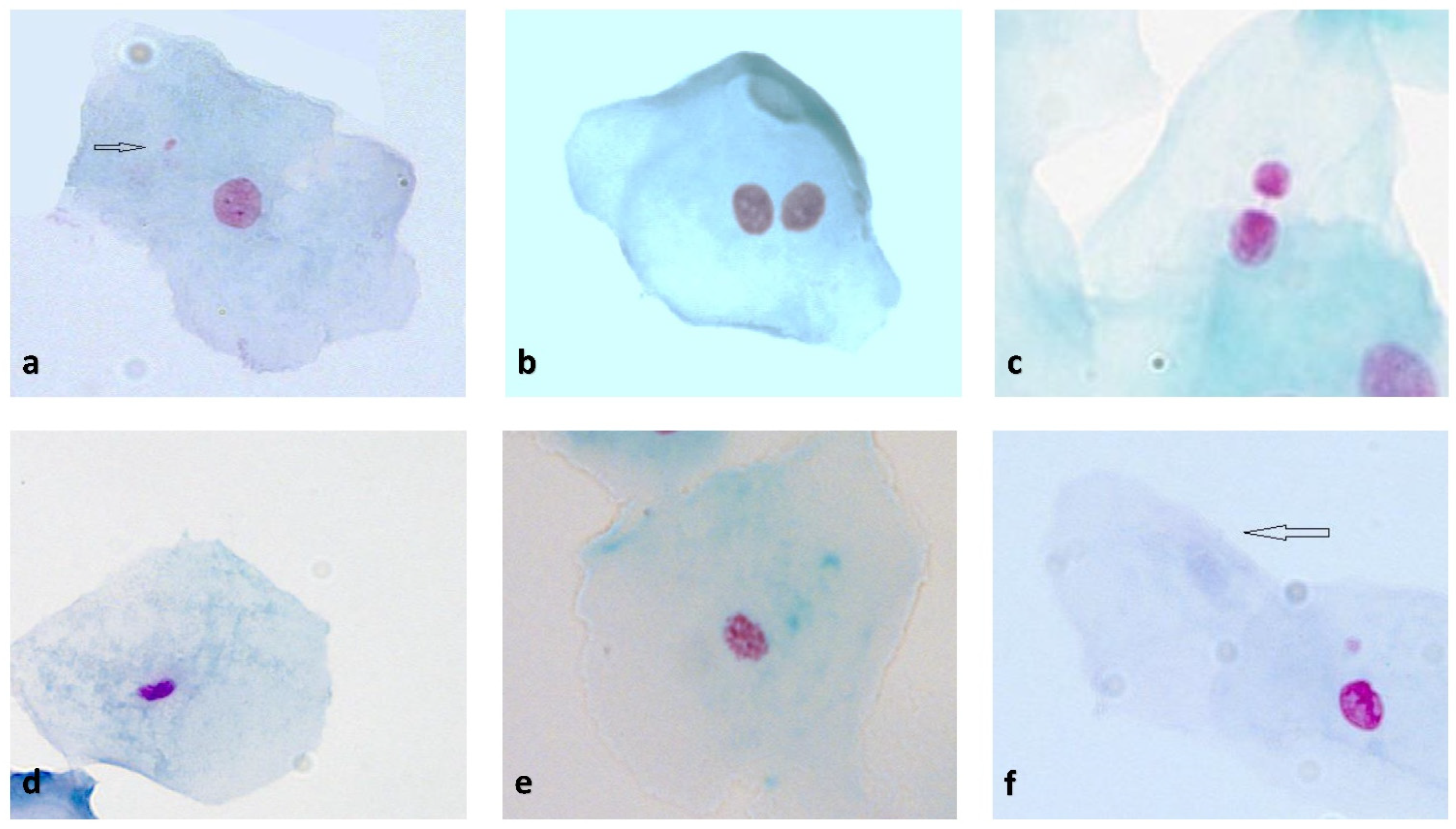
2.4. Buccal Mucosal Cell Sampling and Buccal Micronucleus Cytome Assay (BMCyt Assay)
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tadin, A.; Gavic, L.; Govic, T.; Galic, N.; Zorica Vladislavic, N.; Zeljezic, D. In vivo evaluation of fluoride and sodium lauryl sulphate in toothpaste on buccal epithelial cells toxicity. Acta Odontol. Scand. 2019, 77, 386–393. [Google Scholar] [CrossRef]
- Aoba, T.; Fejerskov, O. Dental fluorosis: Chemistry and biology. Crit. Rev. Oral Biol. Med. 2002, 13, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Jung, J.-Y.; Jeong, Y.-J.; Park, J.-H.; Yang, K.-H.; Choi, N.-K.; Kim, S.-H.; Kim, W.-J. Involvement of both mitochondrial- and death receptor-dependent apoptotic pathways regulated by Bcl-2 family in sodium fluoride-induced apoptosis of the human gingival fibroblasts. Toxicology 2008, 243, 340–347. [Google Scholar] [CrossRef]
- Dey Bhowmik, A.; Chattopadhyay, A. A review on fluoride induced organotoxicity and genotoxicity in mammals and zebrafish. Nucleus 2019, 62, 177–185. [Google Scholar] [CrossRef]
- Davies, R.M.; Ellwood, R.P.; Davies, G.M. The rational use of fluoride toothpaste. Int. J. Dent. Hyg. 2003, 1, 3–8. [Google Scholar] [CrossRef]
- Li, Y. Fluoride: Safety issues. J. Indiana Dent. Assoc. 1993, 72, 22–26. [Google Scholar] [PubMed]
- Kleinsasser, N.H.; Weissacher, H.; Wallner, B.C.; Kastenbauer, E.R.; Harréus, U.A. Assessment of Cytotoxicity and Genotoxicity of Fluorides in Human Mucosa and Lymphocytes. Laryngo-Rhino-Otol. 2001, 80, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Refsnes, M.; Becher, R.; Låg, M.; Skuland, T.; Schwarze, P.E. Fluoride-induced interleukin-6 and interleukin-8 synthesis in human epithelial lung cells. Hum. Exp. Toxicol. 1999, 18, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Correia, D.; Souza, C.; Maltz, M.; Hashizume, L.N. Fluoride retention in saliva and in dental biofilm after different home-use fluoride treatments. Braz. Oral Res. 2014, 28, 1–5. [Google Scholar] [CrossRef]
- Thomas, P.; Fenech, M. Buccal Micronucleus Cytome Assay. In DNA Damage Detection In Situ, Ex Vivo, and In Vivo: Methods and Protocols, 1st ed.; Didenko, V.V., Ed.; Humana Press: New York, NY, USA, 2011; Volume 682, pp. 235–248. ISBN 978-1-60327-409-8. [Google Scholar]
- Ceppi, M.; Biasotti, B.; Fenech, M.; Bonassi, S. Human population studies with the exfoliated buccal micronucleus assay: Statistical and epidemiological issues. Mutat. Res. Rev. Mutat. Res. 2010, 705, 11–19. [Google Scholar] [CrossRef]
- Bonassi, S.; Znaor, A.; Ceppi, M.; Lando, C.; Chang, W.P.; Holland, N.; Kirsch-Volders, M.; Zeiger, E.; Ban, S.; Barale, R.; et al. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis 2006, 28, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.A.; Yujra, V.Q.; da Silva, V.H.P.; Claudio, S.R.; Estadella, D.; de Barros Viana, M.; Oshima, C.T.F. Putative mechanisms of genotoxicity induced by fluoride: A comprehensive review. Environ. Sci. Pollut. Res. 2017, 24, 15254–15259. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, A.H. Buccal mucosa as a route for systemic drug delivery: A review. J. Pharm. Pharm. Sci. 1998, 1, 15–30. [Google Scholar]
- Surrallés, J.; Autio, K.; Nylund, L.; Järventaus, H.; Norppa, H.; Veidebaum, T.; Sorsa, M.; Peltonen, K. Molecular cytogenetic analysis of buccal cells and lymphocytes from benzene-exposed workers. Carcinogenesis 1997, 18, 817–823. [Google Scholar] [CrossRef][Green Version]
- Galey, W.R.; Lonsdale, H.K.; Nacht, S. The In Vitro Permeability Of Skin And Buccal Mucosa To Selected Drugs And Tritiated Water. J. Investig. Dermatol. 1976, 67, 713–717. [Google Scholar] [CrossRef]
- Bolognesi, C.; Roggieri, P.; Ropolo, M.; Thomas, P.; Hor, M.; Fenech, M.; Nersesyan, A.; Knasmueller, S. Buccal micronucleus cytome assay: Results of an intra- and inter-laboratory scoring comparison. Mutagenesis 2015, 30, 545–555. [Google Scholar] [CrossRef]
- Staun Larsen, L.; Baelum, V.; Richards, A.; Nyvad, B. Fluoride in Saliva and Oral Mucosa after Brushing with 1450 or 5000 ppm Fluoride Toothpaste. Caries Res. 2019, 53, 675–681. [Google Scholar] [CrossRef]
- Rose, R.K.; Shellis, R.P.; Lee, A.R. The Role of Cation Bridging in Microbial Fluoride Binding. Caries Res. 1996, 30, 458–464. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Marques, M.E.A.; de Assis, G.F.; Anzai, A.; Poleti, M.L.; Salvadori, D.M.F. No Relationship between Subchronic Fluoride Intake and DNA Damage in Wistar Rats. Caries Res. 2004, 38, 576–579. [Google Scholar] [CrossRef]
- Ribeiro, D.A. Fluoride Induces Apoptosis in Mammalian Cells: In Vitro and In Vivo Studies. Anticancer Res. 2017, 37, 4767–4777. [Google Scholar] [CrossRef]
- Tadin, A.; Gavic, L.; Zeravica, A.; Ugrin, K.; Galic, N.; Zeljezic, D. Assessment of cytotoxic and genotoxic effects of conventional and whitening kinds of toothpaste on oral mucosa cells. Acta Odontol. Scand. 2018, 76, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Apiwantanakul, N.; Chantarawaratit, P. Cytotoxicity, genotoxicity, and cellular metal accumulation caused by professionally applied fluoride products in patients with fixed orthodontic appliances: A randomized clinical trial. J. World Fed. Orthod 2021, 10, 98–104. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, A.; Verma, K.; Paliwal, S.; Sharma, S.; Dwivedi, J. Fluoride: A review of pre-clinical and clinical studies. Environ. Toxicol. Pharmacol. 2017, 56, 297–313. [Google Scholar] [CrossRef]
- Amaechi, B.T.; van Loveren, C. Fluorides and Non-Fluoride Remineralization Systems. In Toothpastes, 1st ed.; van Loveren, C., Ed.; Karger Publisher: Basel, Switzerland, 2013; Volumen 23, pp. 15–26. ISBN 978-3-318-02206-3. [Google Scholar]
- Duckworth, R.M.; Morgan, S.N. Oral Fluoride Retention after Use of Fluoride Dentifrices. Caries Res. 1991, 25, 123–129. [Google Scholar] [CrossRef]
- Cuschieri, S. The CONSORT statement. Saudi J. Anaesth. 2019, 13, S27–S30. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J. Hum. Reprod Sci. 2011, 4, 8–11. [Google Scholar] [CrossRef]
- Bolognesi, C.; Knasmueller, S.; Nersesyan, A.; Thomas, P.; Fenech, M. The HUMNxl scoring criteria for different cell types and nuclear anomalies in the buccal micronucleus cytome assay–An update and expanded photogallery. Mutat. Res. Rev. Mutat. Res. 2013, 753, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, P.E.; Shy, C.M.; Allen, J.W. Micronuclei and other nuclear anomalies in buccal smears: Methods development. Mutat. Res. Genet. Toxicol. Environ. Mutagen 1992, 271, 69–77. [Google Scholar] [CrossRef]
- Johnston, N.R.; Strobel, S.A. Principles of fluoride toxicity and the cellular response: A review. Arch Toxicol. 2020, 94, 1051–1069. [Google Scholar] [CrossRef]
- Thomas, P.; Holland, N.; Bolognesi, C.; Kirsch-Volders, M.; Bonassi, S.; Zeiger, E.; Knasmueller, S.; Fenech, M. Buccal micronucleus cytome assay. Nat. Protoc. 2009, 4, 825–837. [Google Scholar] [CrossRef]
- Nersesyan, A.; Kundi, M.; Atefie, K.; Schulte-Hermann, R.; Knasmüller, S. Effect of staining procedures on the results of micronucleus assays with exfoliated oral mucosa cells. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Nersesyan, A.; Kundi, M.; Fenech, M.; Stopper, H.; da Silva, J.; Bolognesi, C.; Mišík, M.; Knasmueller, S. Recommendations and quality criteria for micronucleus studies with humans. Mutat. Res. Rev. Mutat. Res. 2022, 789, 108410. [Google Scholar] [CrossRef] [PubMed]
- Chitra, P.; Prashantha, G.; Rao, A.; Jois, H.S. In Vivo Evaluation of Micronucleus Frequencies in Buccal Mucosal Cells of Orthodontic Patients with and Without Fluoride Use. J. Indian Orthod. Soc. 2021, 9, 274–281. [Google Scholar] [CrossRef]
- Jeng, J.H.; Hsieh, C.C.; Lan, W.H.; Chang, M.C.; Lin, S.K.; Hahn, L.J.; Kuo, M. Cytotoxicity of sodium fluoride on human oral mucosal fibroblasts and its mechanisms. Cell Biol. Toxicol. 1998, 14, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Gabler, W.L. Absorption of fluoride through the oral mucosa of rats. Arch. Oral Biol. 1968, 13, 619–623. [Google Scholar] [CrossRef]
- He, L.-F. DNA damage, apoptosis and cell cycle changes induced by fluoride in rat oral mucosal cells and hepatocytes. World J. Gastroenterol. 2006, 12, 1144. [Google Scholar] [CrossRef]
- Susik, M.S.S. Effects of Different Concentrations of Fluoride in Oral Mucosal Cells in Albino Rats. J Clin. Diagn. Res. 2015, 9, ZF01–ZF04. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Scolastici, C.; Marques, M.E.A.; Salvadori, D.M.F. Fluoride does not induce DNA breakage in Chinese hamster ovary cells in vitro. Braz. Oral Res. 2004, 18, 192–196. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Salvadori, D.M.F.; Assis, G.F.; Marques, M.E.A. Does fluoride cause DNA damage? Vitr. Eval. Using Rats Oral Mucosa Cells. Braz. J. Oral Sci. 2015, 2, 268–271. [Google Scholar]
- Escobar-García, D.M.; Puente-Amaro, J.; Rosales-Berber, M.; Pozos-Guillén, A.; Ruiz-Rodríguez, S.; Garrocho-Rangel, A. Biological effects of sodium fluoride varnishes used in remineralisation of enamel: An in vitro study. Eur. J. Paediatr. Dent. 2021, 22, 107–113. [Google Scholar] [CrossRef]
- Rohr, P.; da Silva, G.F.; Vicentini, V.E.P.; de Almeida, I.V.; dos Santos, R.A.; Takahashi, C.S.; Goulart, M.O.; da Silva, G.N.; de Oliveira, L.B.; Grisolia, C.K.; et al. Buccal micronucleus cytome assay: Inter-laboratory scoring exercise and micronucleus and nuclear abnormalities frequencies in different populations from Brazil. Toxicol. Lett. 2020, 333, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Satoh, R.; Kishino, K.; Morshed, S.R.; Takayama, F.; Otsuki, S.; Suzuki, F.; Hashimoto, K.; Kikuchi, H.; Nishikawa, H.; Yasui, T.; et al. Changes in fluoride sensitivity during in vitro senescence of normal human oral cells. Anticancer Res. 2005, 25, 2085–2090. [Google Scholar]
- Fenech, M.; Bonassi, S. The effect of age, gender, diet and lifestyle on DNA damage measured using micronucleus frequency in human peripheral blood lymphocytes. Mutagenesis 2011, 26, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, G.; Butryee, C.; Stacewicz-Sapuntzakis, M.; Bowen, P.E. Exfoliated Buccal Mucosa Cells as a Source of DNA to Study Oxidative Stress. Cancer Epidemiol. Biomark. Prev. 2008, 17, 212–219. [Google Scholar] [CrossRef]
- Thomas, P.; Harvey, S.; Gruner, T.; Fenech, M. The buccal cytome and micronucleus frequency is substantially altered in Down’s syndrome and normal ageing compared to young healthy controls. Mutat. Res.-Fundam. Mol. Mech. Mutagen 2008, 638, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Simmer, J.P.; Hardy, N.C.; Chinoy, A.F.; Bartlett, J.D.; Hu, J.C. How Fluoride Protects Dental Enamel from Demineralization. J. Int. Soc. Prev. Community Dent. 2020, 10, 134–141. [Google Scholar] [CrossRef]
- Kanduti, D.; Sterbenk, P.; Artnik, B. Fluoride: A review of use and effects on health. Mater. Sociomed. 2016, 28, 133–137. [Google Scholar] [CrossRef]
| Oral Hygiene Product | Manufacturer | Ingredients |
|---|---|---|
| Biomed Calcimax | Splat, Moscow, Russia | Water, hydrogenated starch hydrolysate, dicalcium phosphate dihydrate, hydrated silica, glycerin, sodium coco-sulfate, cellulose gum, sodium chloride, aroma, zinc citrate, sodium bicarbonate, benzyl alcohol, tetrasodium glutamate diacetate, Laminaria digitata extract, Fucus vesiculosus extract, Spirulina maxima powder extract, kaolin, xanthan gum, menthol, hydroxyapatite, arginine, menthyl lactate, Betula verrucosa leaf extract, Plantago major extract, Thymus serpyllum extract, Stevia rebaudiana extract, sodium benzoate, potassium sorbate, geraniol, linalool, d-limonene |
| Toothpaste 1–0 ppm F | Pharmagal, Split, Croatia | Water, hypermelosis, calcium carbonate, cocamidopropyl betaine, glycerol, sodium saccharin, peppermint essential oil |
| Toothpaste 2–1050 ppm F | Pharmagal, Split, Croatia | Water, hypermelosis, calcium carbonate, cocamidopropyl betaine, glycerol, sodium saccharin, peppermint essential oil, sodium fluoride (1050 ppm) |
| Toothpaste 3–1450 ppm F | Pharmagal, Split, Croatia | Water, hypermelosis, calcium carbonate, cocamidopropyl betaine, glycerol, sodium saccharin, peppermint essential oil, sodium fluoride (1450 ppm) |
| Mouthwash A–0 ppm F | Pharmagal, Split, Croatia | Water, sorbitol, glycerol, Cremophor RH 40, sodium citrate, peppermint essential oil, Cl 42090 |
| Mouthwash B–450 ppm F | Pharmagal, Split, Croatia | Water, sorbitol, glycerol, Cremophor RH 40, sodium citrate, peppermint essential oil, Cl 42090, sodium fluoride (450 ppm) |
| Characteristics | Group A (Mouthwash with 0 ppm F) (n = 20) | Group B (Mouthwash with 450 ppm F) (n = 21) | p-Value | |
|---|---|---|---|---|
| Age | 34.35 ± 11.61 | 35.67 ± 12.19 | 0.675 | |
| Weight | 73.63 ± 13.16 | 77.06 ± 15.01 | 0.497 | |
| Height | 176.35 ± 7.48 | 177.89 ± 7.52 | 0.557 | |
| BMI | 23.44 ± 3.24 | 24.21 ± 3.39 | 0.684 | |
| Composite fillings | 4.35 ± 3.33 | 6.24 ± 3.60 | 0.097 | |
| Gender | Female | 13 (65.0%) | 11 (52.3%) | 0.412 |
| Male | 7 (35.0%) | 10 (47.6%) | ||
| Group A (Mouthwash with 0 ppm F) (n = 20) | Group B (Mouthwash with 450 ppm F) (n = 21) | |||
|---|---|---|---|---|
| Time Point | Median (IQR) | Median (IQR) | p-Value | |
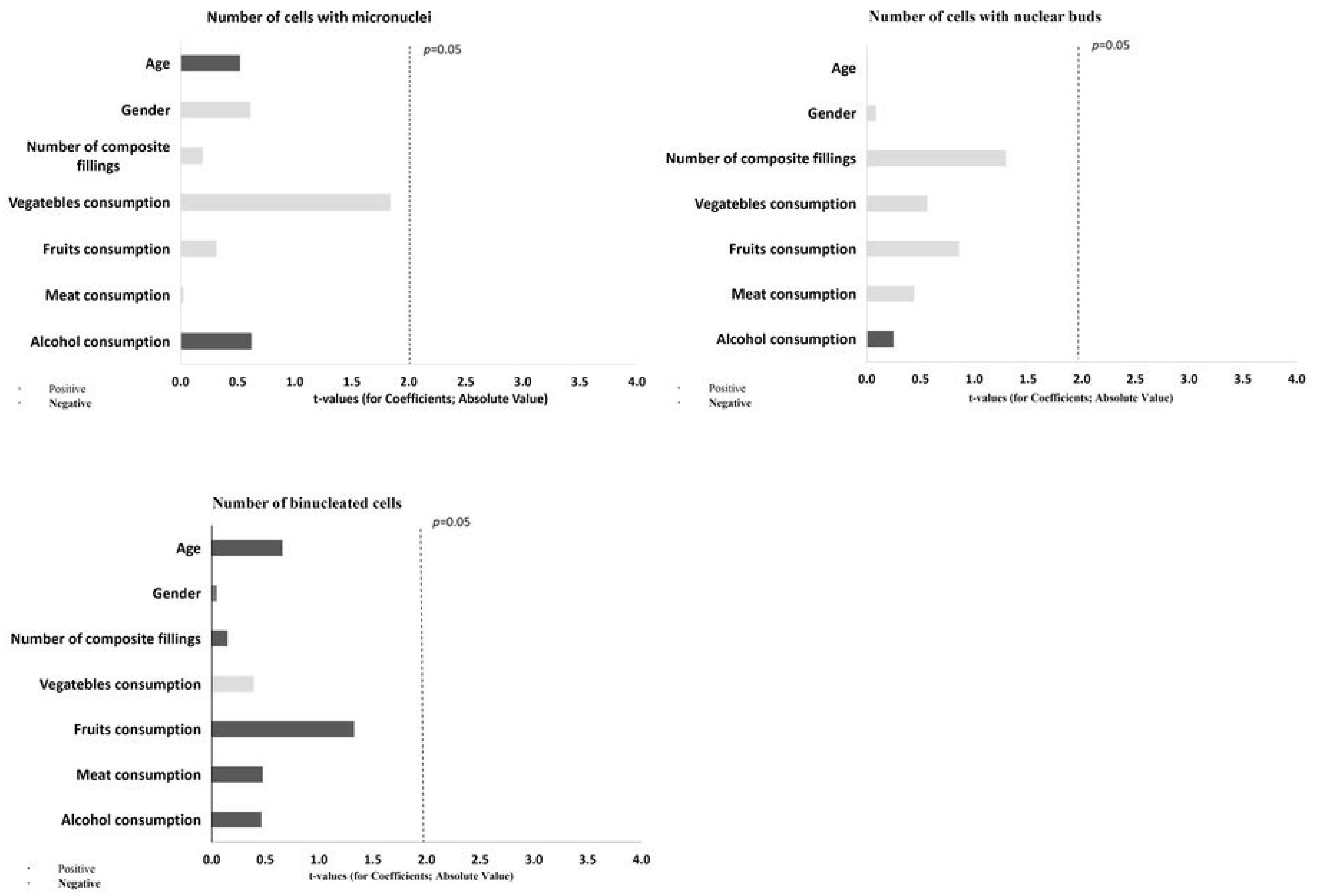
| Micronucleus | T0—baseline (after usage of Biomed Calcimax) | 0.5 (1) | 0 (1) | 0.880 |
| T1—4 weeks (after usage of Toothpaste 1 with 0 ppm F) | 0 (1) | 0 (1) | 0.436 | |
| T2—8 weeks (after usage of Toothpaste 2 with 1050 ppm F) | 0 (1) | 1 (1) | 0.066 | |
| T3—12 weeks (after usage of Toothpaste 3 with 1450 ppm F) | 0 (1) | 0 (1) | 0.354 | |
| p-value | 0.888 | 0.471 | ||
| Nuclear buds | T0—baseline (after usage of Biomed Calcimax) | 1 (1) a | 1 (2) b,c,d | 0672 |
| T1—4 weeks (after usage of Toothpaste 1 with 0 ppm F) | 2 (2) A | 3 (7) A,b | 0.048 * | |
| T2—8 weeks (after usage of Toothpaste 2 with 1050 ppm F) | 4.5 (5.5) | 3 (3.5) c | 0.813 | |
| T3—12 weeks (after usage of Toothpaste 3 with 1450 ppm F) | 5.5 (6) a | 3 (4) d | 0.733 | |
| p-value | ≤0.001 * | ≤0.001 * | ||
| Binucleated cells | T0—baseline (after usage of Biomed Calcimax) | 12 (5) | 12 (9) f | 0.844 |
| T1—4 weeks (after usage of Toothpaste 1 with 0 ppm F) | 12 (5) | 7 (10.5) | 0.210 | |
| T2—8 weeks (after usage of Toothpaste 2 with 1050 ppm F) | 14 (12.75) e | 10 (8.5) g | 0.084 | |
| T3—12 weeks (after usage of Toothpaste 3 with 1450 ppm F) | 6 (7) e | 6 (9.5) f,g | 0.813 | |
| p-value | 0.016 * | 0.014 * |
| Group A (Mouthwashwith 0 ppm F) (n = 20) | Group B (Mouthwashwith 450 ppm F) (n = 21) | |||
|---|---|---|---|---|
| Time Point | Median (IQR) | Median (IQR) | p-Value | |
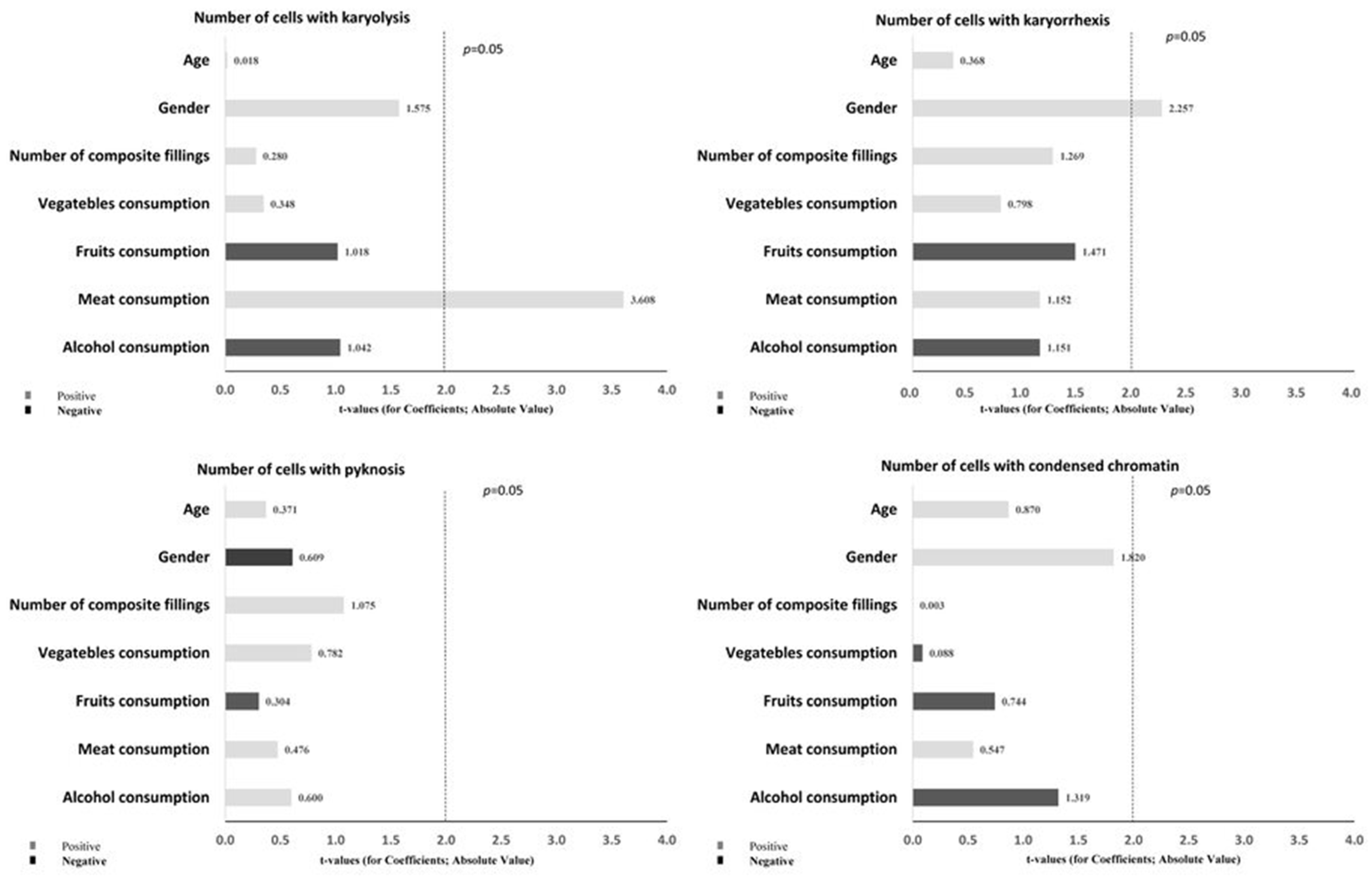
| Karyolysis | T0—baseline (after usage of Biomed Calcimax) | 180 (125) | 184 (87) | 1.000 |
| T1—4 weeks (after usage of Toothpaste 1 with 0 ppm F) | 186 (86) | 191 (73) | 0.855 | |
| T2—8 weeks (after usage of Toothpaste 2 with 1050 ppm F) | 140 (83.25) | 204 (134) | 0.225 | |
| T3—12 weeks (after usage of Toothpaste 3 with 1450 ppm F) | 200 (139.75) | 210 (131) | 0.382 | |
| p-value | 0.318 | 0.088 | ||
| Karyorrhexis | T0—baseline (after usage of Biomed Calcimax) | 43 (68) a | 40 (52) b,c,d | 0.990 |
| T1—4 weeks (after usage of Toothpaste 1 with 0 ppm F) | 62.5 (61.75) A | 99 (56) A,b | 0.020 * | |
| T2—8 weeks (after usage of Toothpaste 2 with 1050 ppm F) | 67 (66.25) | 89 (53.5) c | 0.285 | |
| T3—12 weeks (after usage of Toothpaste 3 with 1450 ppm F) | 68 (32.25) B,a | 100 (50.5) B,d | 0.003 * | |
| p-value | 0.017 * | ≤0.001 * | ||
| Pyknosis | T0—baseline (after usage of Biomed Calcimax) | 3 (2) | 2 (3) | 0.926 |
| T1—4 weeks (after usage of Toothpaste 1 with 0 ppm F) | 3.5 (2.75) | 4 (2.5) | 0.958 | |
| T2—8 weeks (after usage of Toothpaste 2 with 1050 ppm F) | 3 (2.5) | 3 (1.5) | 1.000 | |
| T3—12 weeks (after usage of Toothpaste 3 with 1450 ppm F) | 3.5 (2.75) | 3 (3.5) | 0.063 | |
| p-value | 0.182 | 0.402 | ||
| Condensed chromatin | T0—baseline (after usage of Biomed Calcimax) | 20 (5.5) e | 20 (9.5) g,h | 0.927 |
| T1—4 weeks (after usage of Toothpaste 1 with 0 ppm F) | 18 (4.75) f | 15 (16.5) i | 0.218 | |
| T2—8 weeks (after usage of Toothpaste 2 with 1050 ppm F) | 11 (10.75) | 9 (4) g | 0.378 | |
| T3—12 weeks (after usage of Toothpaste 3 with 1450 ppm F) | 10 (10.74) e,f | 8 (4) h,i | 0.464 | |
| p-value | ≤0.001 * | ≤0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puizina Mladinic, E.; Puizina, J.; Gavic, L.; Tadin, A. Clinical Prospective Assessment of Genotoxic and Cytotoxic Effects of Fluoride Toothpaste and Mouthwash in Buccal Mucosal Cells. Biomedicines 2022, 10, 2206. https://doi.org/10.3390/biomedicines10092206
Puizina Mladinic E, Puizina J, Gavic L, Tadin A. Clinical Prospective Assessment of Genotoxic and Cytotoxic Effects of Fluoride Toothpaste and Mouthwash in Buccal Mucosal Cells. Biomedicines. 2022; 10(9):2206. https://doi.org/10.3390/biomedicines10092206
Chicago/Turabian StylePuizina Mladinic, Ema, Jasna Puizina, Lidia Gavic, and Antonija Tadin. 2022. "Clinical Prospective Assessment of Genotoxic and Cytotoxic Effects of Fluoride Toothpaste and Mouthwash in Buccal Mucosal Cells" Biomedicines 10, no. 9: 2206. https://doi.org/10.3390/biomedicines10092206
APA StylePuizina Mladinic, E., Puizina, J., Gavic, L., & Tadin, A. (2022). Clinical Prospective Assessment of Genotoxic and Cytotoxic Effects of Fluoride Toothpaste and Mouthwash in Buccal Mucosal Cells. Biomedicines, 10(9), 2206. https://doi.org/10.3390/biomedicines10092206






