Biomarkers for Immunotherapy in Poorly Differentiated Sinonasal Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Immunohistochemical Analysis
2.3. Statistical Analysis
3. Results
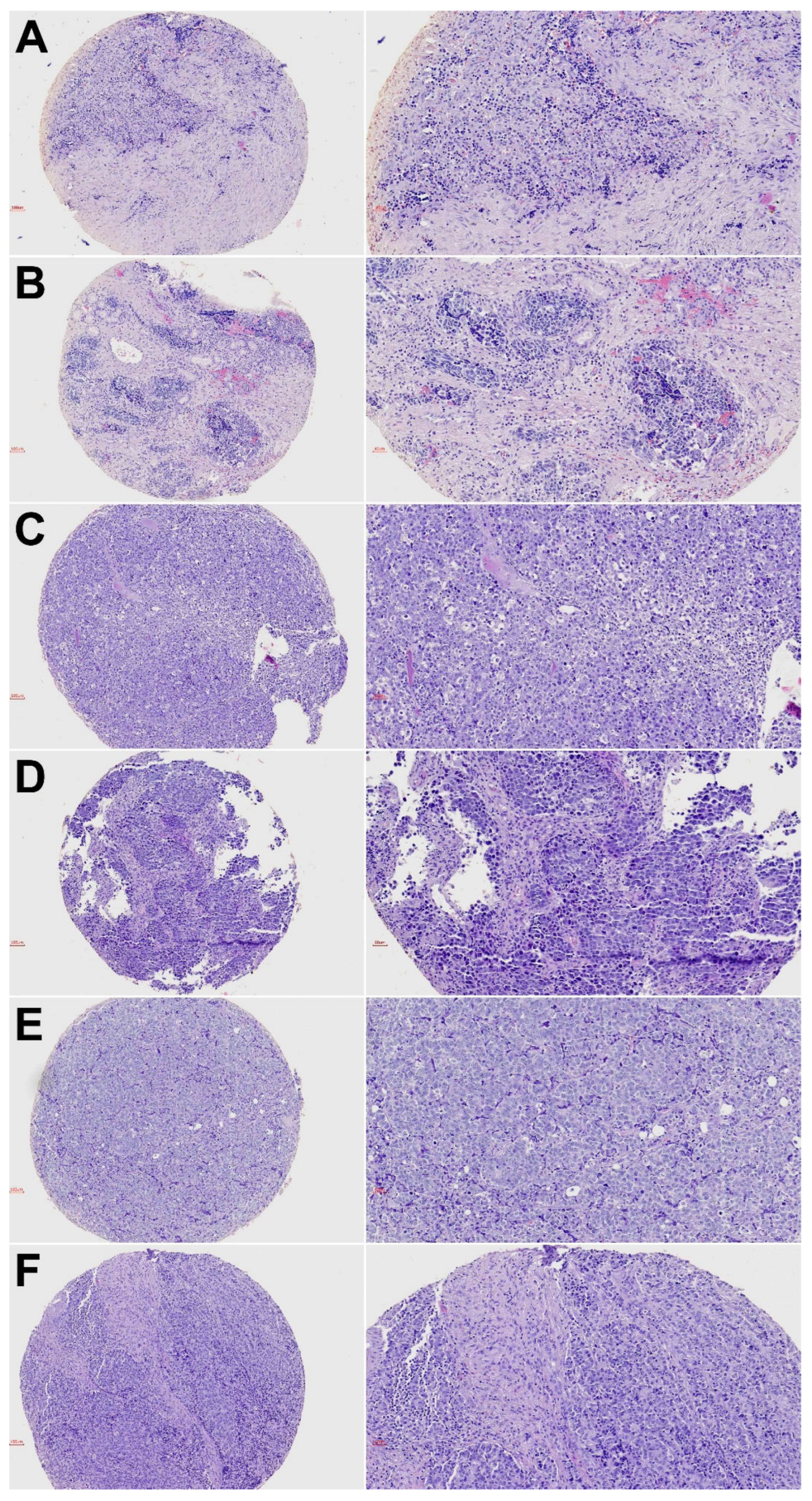
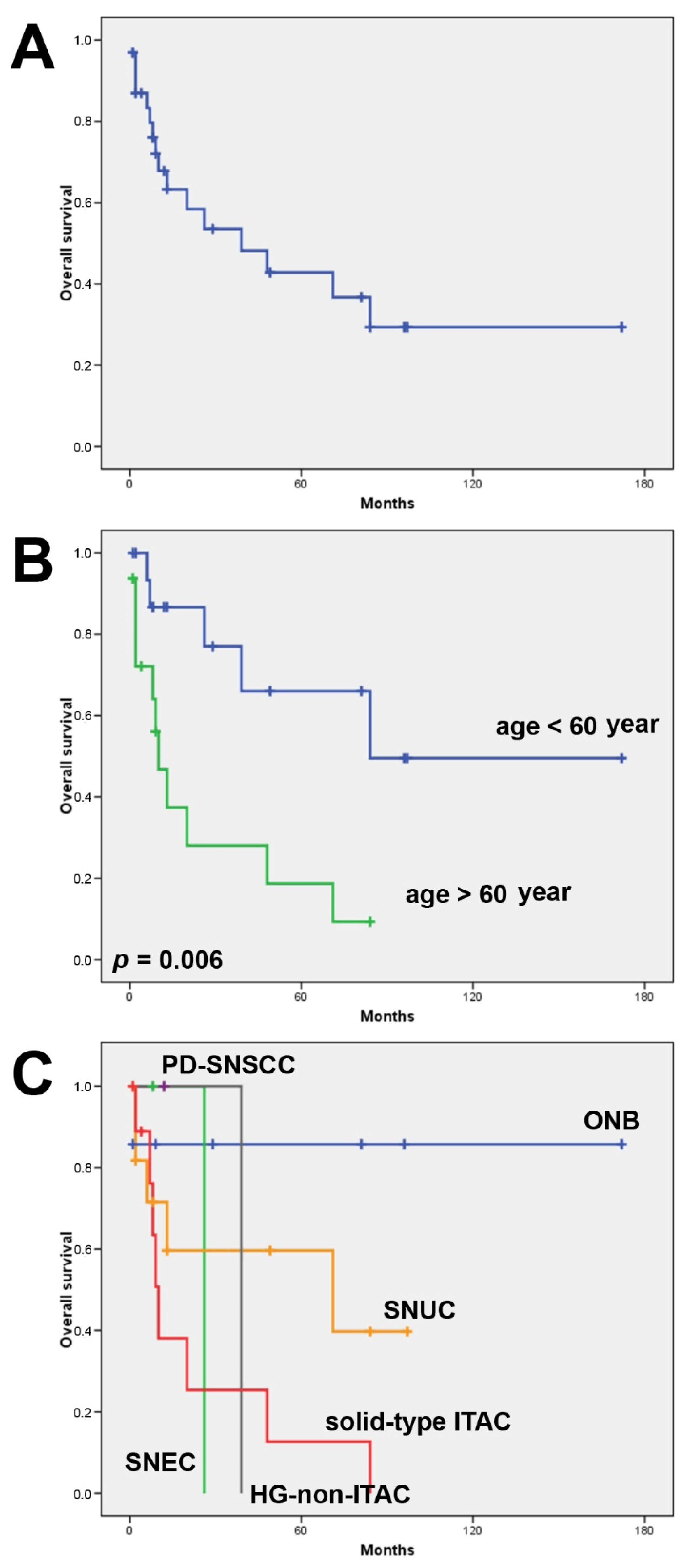
3.1. Clinical Features and Follow-Up
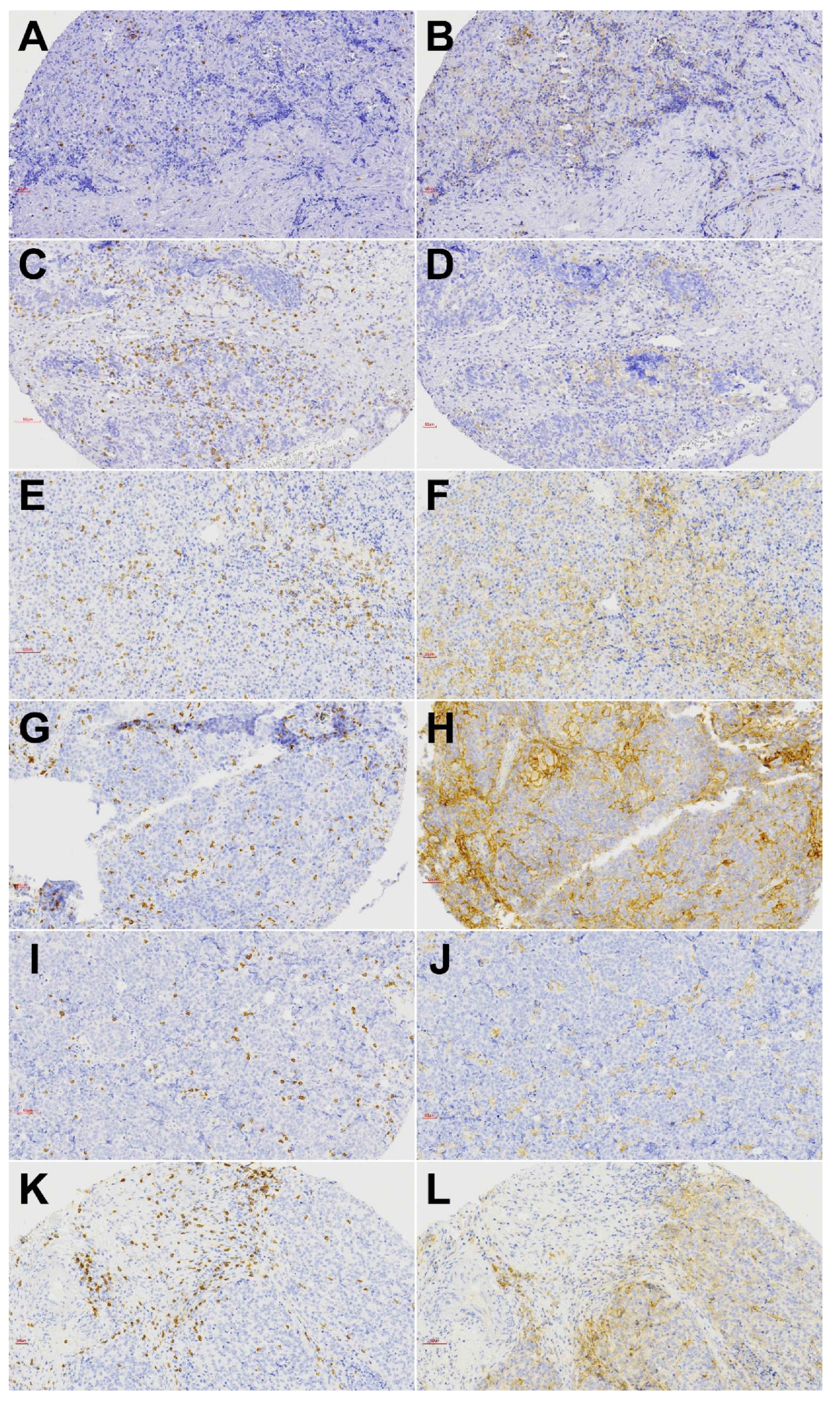
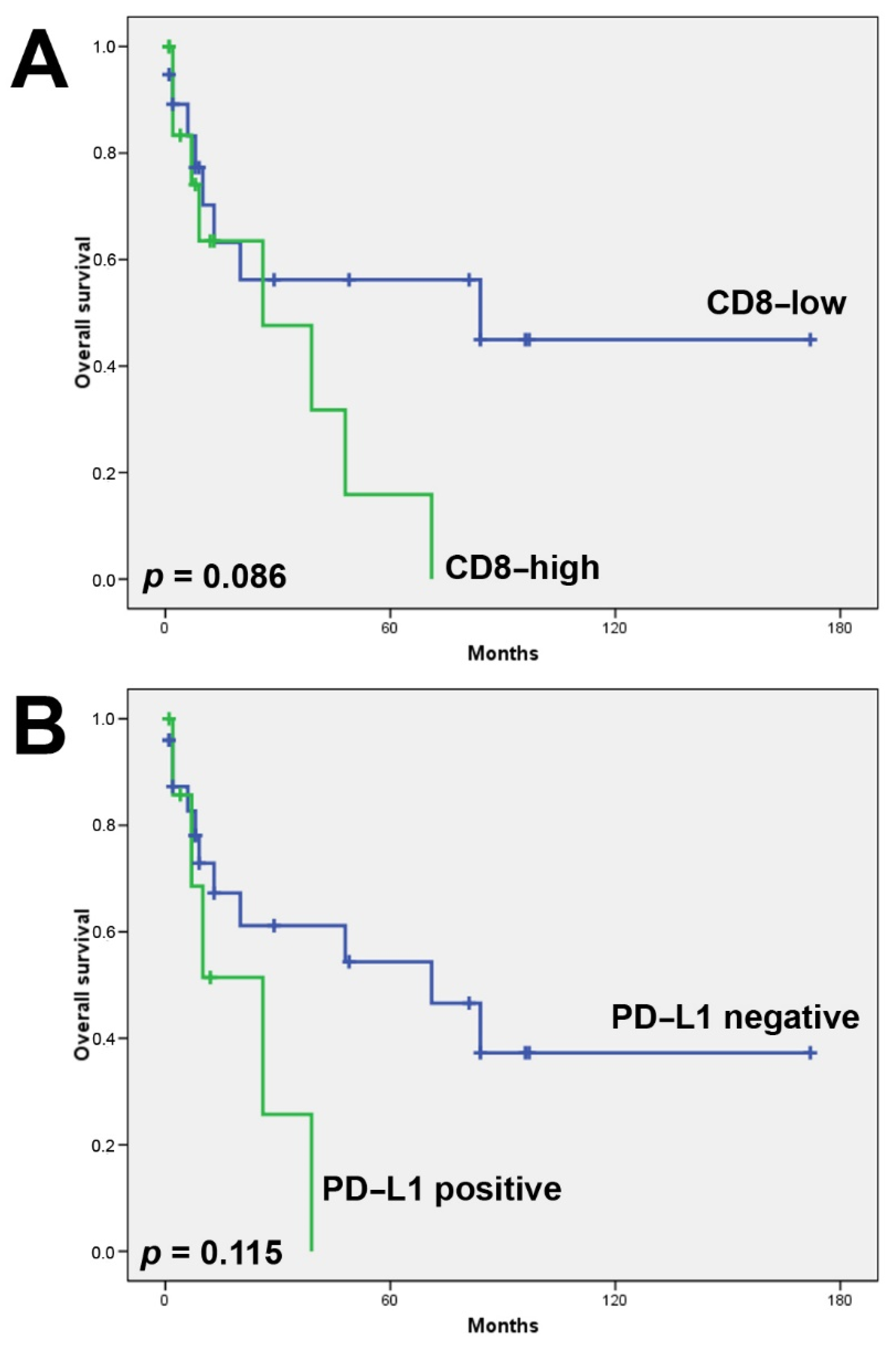
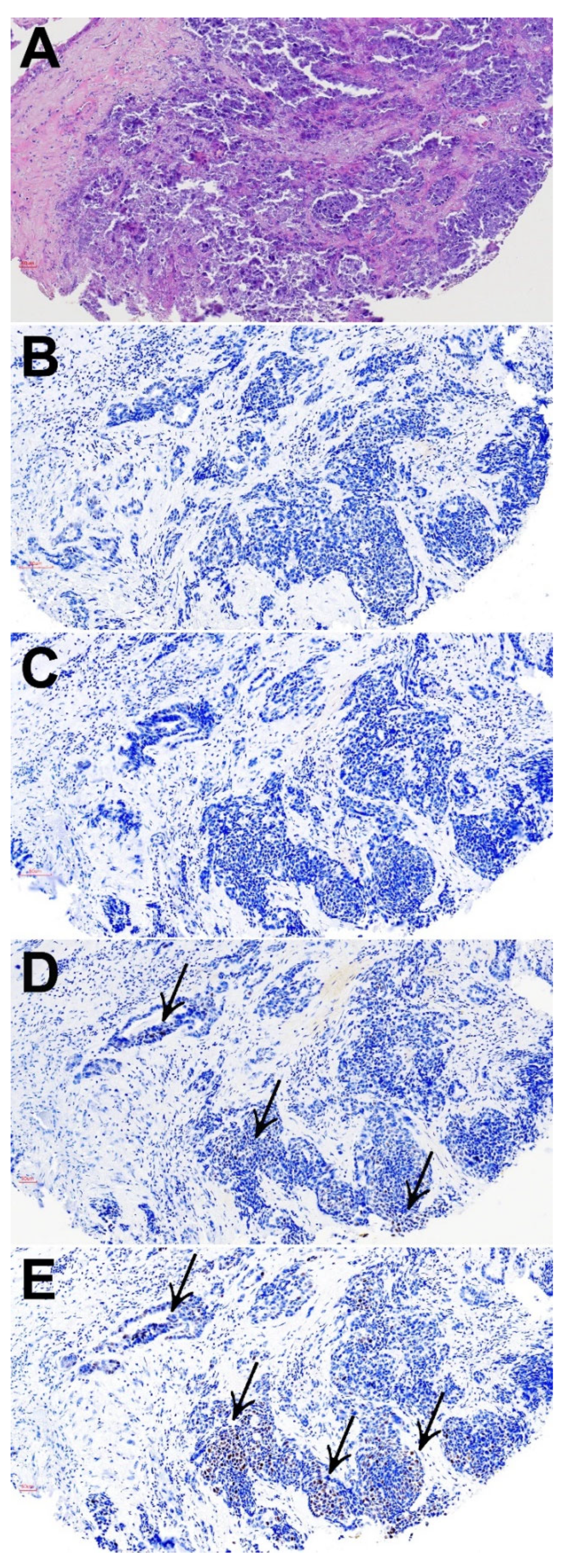
3.2. CD8+ Tumor Infiltrating Lymphocytes
3.3. PD-L1 Expression
3.4. Microsatellite Instability
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. World Health Organization Classification of Head and Neck Tumours, 4th ed.; IARC Press: Lyon, France, 2017; pp. 18–21. [Google Scholar]
- Llorente, J.L.; López, F.; Suárez, C.; Hermsen, M.A. Sinonasal carcinoma: Clinical, pathological, genetic and therapeutic advances. Nat. Rev. Clin. Oncol. 2014, 11, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Hanna, E.Y.; Weber, R.S.; Demonte, F.; Triantafyllou, A.; Lewis, J.S.; Cardesa, A.; Slootweg, P.J.; Stenman, G.; Gnepp, D.R.; et al. Neuroendocrine neoplasms of the sinonasal region. Head Neck 2015, 38, E2259–E2266. [Google Scholar] [CrossRef]
- Bell, D.; Hanna, E.Y. Sinonasal undifferentiated carcinoma: Morphological heterogeneity, diagnosis, management and biological markers. Expert Rev. Anticancer Ther. 2013, 13, 285–296. [Google Scholar] [CrossRef]
- Thompson, L.D.R.; Franchi, A. New tumor entities in the 4th edition of the World Health Organization classification of head and neck tumors: Nasal cavity, paranasal sinuses and skull base. Virchows Arch. 2017, 472, 315–330. [Google Scholar] [CrossRef]
- Taverna, C.; Agaimy, A.; Franchi, A. Towards a Molecular Classification of Sinonasal Carcinomas: Clinical Implications and Opportunities. Cancers 2022, 14, 1463. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.A. Recently described neoplasms of the sinonasal tract. Semin. Diagn. Pathol. 2016, 33, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Stelow, E.B.; Bishop, J.A. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Tumours of the Nasal Cavity, Paranasal Sinuses and Skull Base. Head Neck Pathol. 2017, 11, 3–15. [Google Scholar] [CrossRef]
- Franchi, A. An Update on Sinonasal Round Cell Undifferentiated Tumors. Head Neck Pathol. 2016, 10, 75–84. [Google Scholar] [CrossRef]
- Thompson, L. Small round blue cell tumors of the sinonasal tract: A differential diagnosis approach. Mod. Pathol. 2017, 30, S1–S26. [Google Scholar] [CrossRef] [PubMed]
- Jo, V.Y.; Chau, N.G.; Hornick, J.; Krane, J.F.; Sholl, L.M. Recurrent IDH2 R172X mutations in sinonasal undifferentiated carcinoma. Mod. Pathol. 2017, 30, 650–659. [Google Scholar] [CrossRef]
- Dogan, S.; Chute, D.J.; Xu, B.; Ptashkin, R.N.; Chandramohan, R.; Casanova-Murphy, J.; Nafa, K.; A Bishop, J.; I Chiosea, S.; Stelow, E.B.; et al. Frequent IDH2 R172 mutations in undifferentiated and poorly-differentiated sinonasal carcinomas. J. Pathol. 2017, 242, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.D.; Vazquez, A.; Dubal, P.M.; Baredes, S.; Liu, J.K.; Eloy, J.A. Sinonasal neuroendocrine carcinoma: A population-based analysis of incidence and survival. Int. Forum Allergy Rhinol. 2015, 5, 448–453. [Google Scholar] [CrossRef]
- Wenig, B.M. Undifferentiated Malignant Neoplasms of the Sinonasal Tract. Arch. Pathol. Lab. Med. 2009, 133, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.; Manes, R.P.; Schwam, Z.G.; Judson, B.L. Survival Outcomes for Combined Modality Therapy for Sinonasal Undifferentiated Carcinoma. Otolaryngol. Neck Surg. 2016, 156, 132–136. [Google Scholar] [CrossRef]
- López, F.; Suárez, V.; Vivanco, B.; Suárez, C.; Llorente, J.L. Current management of sinonasal undifferentiated carcinoma. Rhinology 2015, 53, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, E.; Cavalieri, S.; Granata, R.; Nicolai, P.; Castelnuovo, P.; Piazza, C.; Schreiber, A.; Turri-Zanoni, M.; Quattrone, P.; Miceli, R.; et al. Locally advanced epithelial sinonasal tumors: The impact of multimodal approach. Laryngoscope 2019, 130, 857–865. [Google Scholar] [CrossRef]
- Ramaekers, B.L.; Pijls-Johannesma, M.; Joore, M.; Ende, P.V.D.; Langendijk, J.A.; Lambin, P.; Kessels, A.G.; Grutters, J. Systematic review and meta-analysis of radiotherapy in various head and neck cancers: Comparing photons, carbon-ions and protons. Cancer Treat. Rev. 2011, 37, 185–201. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Bell, R.B.; Bifulco, C.B.; Burtness, B.; Gillison, M.L.; Harrington, K.J.; Le, Q.T.; Lee, N.Y.; Leidner, R.; Lewis, R.L.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer 2019, 7, 184. [Google Scholar] [CrossRef]
- Antonia, S.; Goldberg, S.B.; Balmanoukian, A.; Chaft, J.E.; Sanborn, R.E.; Gupta, A.; Narwal, R.; Steele, K.; Gu, Y.; Karakunnel, J.J.; et al. Safety and antitumor activity of durvalumab plus tremelimumab in non-small cell lung cancer: A multicentre, phase 1b study. Lancet Oncol. 2016, 17, 299–308. [Google Scholar] [CrossRef]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.-Y.; Chin, K.; Kadowaki, S.; Ahn, M.-J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517. [Google Scholar] [CrossRef]
- García-Marín, R.; Reda, S.; Riobello, C.; Cabal, V.N.; Suárez-Fernández, L.; Vivanco, B.; López, F.; Llorente, J.L.; Hermsen, M.A. CD8+ Tumor-Infiltrating Lymphocytes and Tumor Microenvironment Immune Types as Biomarkers for Immunotherapy in Sinonasal Intestinal-Type Adenocarcinoma. Vaccines 2020, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Riobello, C.; Vivanco, B.; Reda, S.; López-Hernández, A.; García-Inclán, C.; Potes-Ares, S.; Cabal, V.N.; López, F.; Llorente, J.L.; Hermsen, M.A. Programmed death ligand-1 expression as immunotherapeutic target in sinonasal cancer. Head Neck 2018, 40, 818–827. [Google Scholar] [CrossRef]
- Quan, H.; Yan, L.; Wang, S.; Wang, S. Clinical relevance and significance of programmed death-ligand 1 expression, tumor-infiltrating lymphocytes, and p16 status in sinonasal squamous cell carcinoma. Cancer Manag. Res. 2019, 11, 4335–4345. [Google Scholar] [CrossRef] [PubMed]
- García-Marín, R.; Reda, S.; Riobello, C.; Cabal, V.; Suárez-Fernández, L.; Vivanco, B.; Álvarez-Marcos, C.; López, F.; Llorente, J.; Hermsen, M. Prognostic and Therapeutic Implications of Immune Classification by CD8+ Tumor-Infiltrating Lymphocytes and PD-L1 Expression in Sinonasal Squamous Cell Carcinoma. Int. J. Mol. Sci. 2021, 22, 6926. [Google Scholar] [CrossRef]
- Hongo, T.; Yamamoto, H.; Jiromaru, R.; Yasumatsu, R.; Kuga, R.; Nozaki, Y.; Hashimoto, K.; Matsuo, M.; Wakasaki, T.; Tamae, A.; et al. PD-L1 expression, tumor-infiltrating lymphocytes, mismatch repair deficiency, EGFR alteration and HPV infection in sinonasal squamous cell carcinoma. Mod. Pathol. 2021, 34, 1966–1978. [Google Scholar] [CrossRef]
- Classe, M.; Burgess, A.; El Zein, S.; Wassef, M.; Herman, P.; Mortuaire, G.; Leroy, X.; Malouf, G.G.; Verillaud, B. Evaluating the prognostic potential of the Ki67 proliferation index and tumor-infiltrating lymphocytes in olfactory neuroblastoma. Histopathology 2019, 75, 853–864. [Google Scholar] [CrossRef] [PubMed]
- London, N.R., Jr.; Rooper, L.M.; Bishop, J.A.; Xu, H.; Bernhardt, L.J.; Ishii, M.; Hann, C.L.; Taube, J.M.; Izumchenko, E.; Gaykalova, D.A.; et al. Expression of Programmed Cell Death Ligand 1 and Associated Lymphocyte Infiltration in Olfactory Neuroblastoma. World Neurosurg. 2020, 135, e187–e193. [Google Scholar] [CrossRef]
- Hieggelke, L.; Heydt, C.; Castiglione, R.; Rehker, J.; Merkelbach-Bruse, S.; Riobello, C.; Llorente, J.L.; Hermsen, M.A.; Buettner, R. Mismatch Repair Deficiency and Somatic Mutations in Human Sinonasal Tumors. Cancers 2021, 13, 6081. [Google Scholar] [CrossRef]
- Martínez, J.G.; Pérez-Escuredo, J.; López, F.; Suárez, C.; Álvarez-Marcos, C.; Llorente, J.L.; Hermsen, M.A. Microsatellite instability analysis of sinonasal carcinomas. Otolaryngol. Neck Surg. 2009, 140, 55–60. [Google Scholar] [CrossRef]
- Riobello, C.; López-Hernández, A.; Cabal, V.N.; García-Marín, R.; Suárez-Fernández, L.; Sánchez-Fernández, P.; Vivanco, B.; Blanco, V.; López, F.; Franchi, A.; et al. IDH2 Mutation Analysis in Undifferentiated and Poorly Differentiated Sinonasal Carcinomas for Diagnosis and Clinical Management. Am. J. Surg. Pathol. 2019, 44, 396–405. [Google Scholar] [CrossRef]
- López-Hernández, A.; Vivanco, B.; Franchi, A.; Bloemena, E.; Cabal, V.N.; Potes, S.; Riobello, C.; García-Inclán, C.; López, F.; Llorente, J.L.; et al. Genetic profiling of poorly differentiated sinonasal tumours. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Fuchs, T.L.; Sioson, L.; Sheen, A.; Jafari-Nejad, K.; Renaud, C.J.; Andrici, J.; Ahadi, M.; Chou, A.; Gill, A.J. Assessment of Tumor-infiltrating Lymphocytes Using International TILs Working Group (ITWG) System Is a Strong Predictor of Overall Survival in Colorectal Carcinoma: A Study of 1034 Patients. Am. J. Surg. Pathol. 2020, 44, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M.; Haddad, R.; Gupta, S.; Mahipal, A.; Mehra, R.; Tahara, M.; Berger, R.; Eder, J.P.; Burtness, B.; Lee, S.-H.; et al. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. J. Clin. Oncol. 2016, 34, 3838–3845. [Google Scholar] [CrossRef]
- Bell, D.; Bell, A.; Ferrarotto, R.; Glisson, B.; Takahashi, Y.; Fuller, G.; Weber, R.; Hanna, E. High-grade sinonasal carcinomas and surveillance of differential expression in immune related transcriptome. Ann. Diagn. Pathol. 2020, 49, 151622. [Google Scholar] [CrossRef]
- De Cecco, L.; Serafini, M.S.; Facco, C.; Granata, R.; Orlandi, E.; Fallai, C.; Licitra, L.; Marchesi, E.; Perrone, F.; Pilotti, S.; et al. A functional gene expression analysis in epithelial sinonasal cancer: Biology and clinical relevance behind three histological subtypes. Oral Oncol. 2019, 90, 94–101. [Google Scholar] [CrossRef]
- Nonomura, Y.; Nakayama, K.; Nakamura, K.; Razia, S.; Yamashita, H.; Ishibashi, T.; Ishikawa, M.; Sato, S.; Nakayama, S.; Otsuki, Y.; et al. Ovarian Endometrioid and Clear Cell Carcinomas with Low Prevalence of Microsatellite Instability: A Unique Subset of Ovarian Carcinomas Could Benefit from Combination Therapy with Immune Checkpoint Inhibitors and Other Anticancer Agents. Healthcare 2022, 10, 694. [Google Scholar] [CrossRef]
- Dislich, B.; Mertz, K.D.; Gloor, B.; Langer, R. Interspatial Distribution of Tumor and Immune Cells in Correlation with PD-L1 in Molecular Subtypes of Gastric Cancers. Cancers 2022, 14, 1736. [Google Scholar] [CrossRef]
- Liu, X.; Choi, M.G.; Kim, K.; Kim, K.-M.; Kim, S.T.; Park, S.H.; Cristescu, R.; Peter, S.; Lee, J. High PD-L1 expression in gastric cancer (GC) patients and correlation with molecular features. Pathol.-Res. Pract. 2020, 216, 152881. [Google Scholar] [CrossRef]
- Lou, H.; Fang, J.; Li, P.; Zhou, W.; Wang, Y.; Fan, E.; Li, Y.; Wang, H.; Liu, Z.; Xiao, L.; et al. Frequency, Suppressive Capacity, Recruitment and Induction Mechanisms of Regulatory T Cells in Sinonasal Squamous Cell Carcinoma and Nasal Inverted Papilloma. PLoS ONE 2015, 10, e0126463. [Google Scholar] [CrossRef]
- Takahashi, Y.; Amit, M.; Gleber-Netto, F.O.; Silverman, D.; Bell, D.; Xie, T.-X.; Roberts, D.; Myers, J.N.; Hanna, E.Y. Evaluation of the Immune Microenvironment in Sinonasal Squamous Cell Carcinoma and Its Association with Patients’ Survival. J. Neurol. Surg. Part B Skull Base 2021, 82, S022. [Google Scholar] [CrossRef]
- Gu, J.T.; Ma, N.C.; Betts, C.; Ms, S.S.; Geltzeiler, M.; Pucci, F. Characterization of the tumor immune microenvironment of sinonasal squamous-cell carcinoma. Int. Forum Allergy Rhinol. 2021, 12, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Ock, C.-Y.; Keam, B.; Kim, S.; Lee, J.-S.; Kim, M.; Kim, T.M.; Jeon, Y.K.; Kim, D.-W.; Chung, D.H.; Heo, D.S. Pan-Cancer Immunogenomic Perspective on the Tumor Microenvironment Based on PD-L1 and CD8 T-Cell Infiltration. Clin. Cancer Res. 2016, 22, 2261–2270. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, O.; Yamazaki, K.; Oizumi, S.; Hommura, F.; Kinoshita, I.; Ogura, S.; Dosaka-Akita, H.; Nishimura, M. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003, 94, 1003–1009. [Google Scholar] [CrossRef]
- Kinoshita, T.; Kudo-Saito, C.; Muramatsu, R.; Fujita, T.; Saito, M.; Nagumo, H.; Sakurai, T.; Noji, S.; Takahata, E.; Yaguchi, T.; et al. Determination of poor prognostic immune features of tumour microenvironment in non-smoking patients with lung adenocarcinoma. Eur. J. Cancer 2017, 86, 15–27. [Google Scholar] [CrossRef]
- Lin, Y.M.; Sung, W.W.; Hsieh, M.J.; Tsai, S.C.; Lai, H.W.; Yang, S.M.; Shen, K.H.; Chen, M.K.; Lee, H.; Yeh, K.T.; et al. High PD-L1 expression correlates with metastatic and poor prognosis in oral squamous cell carcinoma. PLoS ONE 2015, 10, e0142656. [Google Scholar] [CrossRef]
- Parra, E.R.; Behrens, C.; Rodriguez-Canales, J.; Lin, H.; Mino, B.; Blando, J.; Zhang, J.; Gibbons, D.L.; Heymach, J.V.; Sepesi, B.; et al. Image Analysis–based Assessment of PD-L1 and Tumor-Associated Immune Cells Density Supports Distinct Intratumoral Microenvironment Groups in Non–small Cell Lung Carcinoma Patients. Clin. Cancer Res. 2016, 22, 6278–6289. [Google Scholar] [CrossRef]
- Vassilakopoulou, M.; Avgeris, M.; Velcheti, V.; Kotoula, V.; Rampias, T.; Chatzopoulos, K.; Perisanidis, C.; Kontos, C.K.; Giotakis, A.I.; Scorilas, A.; et al. Evaluation of PD-L1 Expression and Associated Tumor-Infiltrating Lymphocytes in Laryngeal Squamous Cell Carcinoma. Clin. Cancer Res. 2016, 22, 704–713. [Google Scholar] [CrossRef]
- Velcheti, V.; Schalper, K.A.; Carvajal, D.E.; Anagnostou, V.K.; Syrigos, K.N.; Sznol, M.; Herbst, R.S.; Gettinger, S.N.; Chen, L.; Rimm, D.L. Programmed death ligand-1 expression in non-small cell lung cancer. Lab. Investig. 2014, 94, 107–116. [Google Scholar] [CrossRef]
- Tsao, M.; Le Teuff, G.; Shepherd, F.A.; Landais, C.; Hainaut, P.; Filipits, M.; Pirker, R.; Le Chevalier, T.; Graziano, S.; Kratze, R.; et al. PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected non-small cell lung cancer. Ann. Oncol. 2017, 28, 882–889. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Hanna, G.J.; Lizotte, P.; Cavanaugh, M.; Kuo, F.C.; Shivdasani, P.; Frieden, A.; Chau, N.G.; Schoenfeld, J.D.; Lorch, J.H.; Uppaluri, R.; et al. Frameshift events predict anti–PD-1/L1 response in head and neck cancer. JCI Insight 2018, 3, e98811. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Faquin, W.C.; Durbeck, J.; Faden, D.L. Immune checkpoint inhibitors in sinonasal squamous cell carcinoma. Oral Oncol. 2020, 109, 104776. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Okano, S.; Enokida, T.; Fujisawa, T.; Ito, K.; Sato, M.; Tanaka, H.; Wada, A.; Tahara, M. Nivolumab for recurrent or metastatic head and neck cancer patients with non-squamous cell carcinoma and/or a primary subsite excluded from CheckMate141, a retrospective study. Oral Oncol. 2022, 130, 105932. [Google Scholar] [CrossRef] [PubMed]
- Colevas, A.; Bahleda, R.; Braiteh, F.; Balmanoukian, A.; Brana, I.; Chau, N.; Sarkar, I.; Molinero, L.; Grossman, W.; Kabbinavar, F.; et al. Safety and clinical activity of atezolizumab in head and neck cancer: Results from a phase I trial. Ann. Oncol. 2018, 29, 2247–2253. [Google Scholar] [CrossRef]
- Blank, C.U.; Haanen, J.B.; Ribas, A.; Schumacher, T.N. CANCER IMMUNOLOGY. The “cancer immunogram”. Science 2016, 352, 658–660. [Google Scholar] [CrossRef]
- Chabanon, R.M.; Morel, D.; Postel-Vinay, S. Exploiting epigenetic vulnerabilities in solid tumors: Novel therapeutic opportunities in the treatment of SWI/SNF-defective cancers. Semin. Cancer Biol. 2019, 61, 180–198. [Google Scholar] [CrossRef]
- Jelinic, P.; Ricca, J.; Van Oudenhove, E.; Olvera, N.; Merghoub, T.; A Levine, D.; Zamarin, D. Immune-Active Microenvironment in Small Cell Carcinoma of the Ovary, Hypercalcemic Type: Rationale for Immune Checkpoint Blockade. JNCI J. Natl. Cancer Inst. 2018, 110, 787–790. [Google Scholar] [CrossRef]
- Ngo, C.; Postel-Vinay, S. Immunotherapy for SMARCB1-Deficient Sarcomas: Current Evidence and Future Developments. Biomedicines 2022, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Botta, G.P.; Kato, S.; Patel, H.; Fanta, P.; Lee, S.; Okamura, R.; Kurzrock, R. SWI/SNF complex alterations as a biomarker of immunotherapy efficacy in pancreatic cancer. JCI Insight 2021, 6, e150453. [Google Scholar] [CrossRef]
- Li, X.; Shi, H.; Zhang, W.; Bai, C.; He, M.; Ta, N.; Huang, H.; Ning, Y.; Fang, C.; Qin, H.; et al. Immunotherapy and Targeting the Tumor Microenvironment: Current Place and New Insights in Primary Pulmonary NUT Carcinoma. Front. Oncol. 2021, 11, 690115. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | All | ONB | SNEC | SNUC | PD-SNSCC | HG-Non-ITAC | Solid-Type ITAC | SmarcB1-Def Carcinoma | SmarcA4-Def Carcinoma | NUT Carcinoma |
|---|---|---|---|---|---|---|---|---|---|---|
| All | 69 | 14 | 6 | 25 | 6 | 5 | 10 | 1 | 1 | 1 |
| Age * | 56 (20–83) | 47 (20–69) | 60 (49–77) | 56 (34–83) | 67 (57–77) | 37 (29–47) | 67 (49–82) | 59 | NA | 40 |
| Sex | ||||||||||
| Male | 35/65 (54) | 3/13 (23) | 2/6 (33) | 13/27 (52) | 4/5 (80) | 3/4 (75) | 9/10 (90) | 1 (100) | NA | 0 (0) |
| Female | 30/65 (46) | 10/13 (77) | 4/6 (67) | 12/27 (48) | 1/5 (20) | 1/4 (25) | 1/10 (10) | 0 (0) | NA | 1 (100) |
| Disease stage | ||||||||||
| I | 8/64 (12) | 0/13 (0) | 0/6 (0) | 0/27 (0) | 4/5 (80) | 2/3 (67) | 2/10 (20) | 0 (0) | NA | 0 (0) |
| II | 24/64 (38) | 7/13 (54) | 4/6 (67) | 10/27 (40) | 1/5 (20) | 1/3 (33) | 0/10 (0) | 0 (0) | NA | 1 (100) |
| III | 8/64 (12) | 2/13 (15) | 1/6 (17) | 2/27 (8) | 0/5 (0) | 0/3 (0) | 3/10 (30) | 0 (0) | NA | 0 (0) |
| IVa | 20/64 (31) | 4/13 (31) | 0/6 (0) | 13/27 (52) | 0/5 (0) | 0/3 (0) | 2/10 (20) | 1 (100) | NA | 0 (0) |
| IVb | 4/64 (6) | 0/13 (0) | 1/6 (17) | 0/27 (0) | 0/5 (0) | 0/3 (0) | 3/10 (30) | 0 (0) | NA | 0 (0) |
| Recurrence | 19/32 (59) | 5/7 (71) | 2/2 (100) | 4/12 (33) | NA | 1/1 (100) | 7/10 (70) | NA | NA | NA |
| Follow up * | 31 (1–172) | 56 (1–172) | 17 (8–26) | 29 (1–97) | 12 (12–12) | 39 (39–39) | 19 (1–84) | NA | NA | NA |
| Patient status | ||||||||||
| Alive | 17/33 (52) | 6/7 (86) | 1/2 (50) | 7/12 (58) | 1/1 (100) | 0/1 (0) | 2/10 (20) | NA | NA | NA |
| DOD | 13/33 (39) | 1/7 (14) | 1/2 (50) | 3/12 (25) | 0/1 (0) | 1/1 (100) | 7/10 (70) | NA | NA | NA |
| DOC | 3/33 (9) | 0/7 (0) | 0/2 (0) | 2/12 (17) | 0/1 (0) | 0/1 (0) | 1/10 (10) | NA | NA | NA |
| CD8+ TILs > 10% | PD-L1 > 5% | MSI-Positive | |
|---|---|---|---|
| ONB | 3/14 (21%) | 2/14 (14%) | 0/14 (0%) |
| SNEC | 1/6 (17%) | 2/6 (33%) | 2/6 (33%) |
| SNUC | 8/25 (32%) | 4/25 (16%) | 2/25 (8%) |
| PD-SNSCC | 2/6 (33%) | 5/6 (83%) | 0/6 (0%) |
| HG-non-ITAC | 3/5 (60%) | 4/5 (80%) | 1/5 (20%) |
| Solid-type ITAC | 6/10 (60%) | 5/10 (50%) | 0/10 (0%) |
| SmarcB1-def carcinoma | 0/1 (0%) | 1/1 (100%) | 0/1 (0%) |
| SmarcA4-def carcinoma | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) |
| NUT carcinoma | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) |
| All | 23/69 (33%) | 23/69 (33%) | 5/69 (7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villanueva-Fernández, E.; Hermsen, M.A.; Suárez-Fernández, L.; Vivanco, B.; Franchi, A.; García-Marín, R.; Cabal, V.N.; Codina-Martínez, H.; Lorenzo-Guerra, S.L.; Llorente, J.L.; et al. Biomarkers for Immunotherapy in Poorly Differentiated Sinonasal Tumors. Biomedicines 2022, 10, 2205. https://doi.org/10.3390/biomedicines10092205
Villanueva-Fernández E, Hermsen MA, Suárez-Fernández L, Vivanco B, Franchi A, García-Marín R, Cabal VN, Codina-Martínez H, Lorenzo-Guerra SL, Llorente JL, et al. Biomarkers for Immunotherapy in Poorly Differentiated Sinonasal Tumors. Biomedicines. 2022; 10(9):2205. https://doi.org/10.3390/biomedicines10092205
Chicago/Turabian StyleVillanueva-Fernández, Eva, Mario A. Hermsen, Laura Suárez-Fernández, Blanca Vivanco, Alessandro Franchi, Rocío García-Marín, Virginia N. Cabal, Helena Codina-Martínez, Sara Lucila Lorenzo-Guerra, José L. Llorente, and et al. 2022. "Biomarkers for Immunotherapy in Poorly Differentiated Sinonasal Tumors" Biomedicines 10, no. 9: 2205. https://doi.org/10.3390/biomedicines10092205
APA StyleVillanueva-Fernández, E., Hermsen, M. A., Suárez-Fernández, L., Vivanco, B., Franchi, A., García-Marín, R., Cabal, V. N., Codina-Martínez, H., Lorenzo-Guerra, S. L., Llorente, J. L., & López, F. (2022). Biomarkers for Immunotherapy in Poorly Differentiated Sinonasal Tumors. Biomedicines, 10(9), 2205. https://doi.org/10.3390/biomedicines10092205









