Sex-Related Differences in Voluntary Alcohol Intake and mRNA Coding for Synucleins in the Brain of Adult Rats Prenatally Exposed to Alcohol
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Two-Bottle “10% Alcohol vs. Water” Choice Drinking Paradigm (Voluntary Alcohol Consumption)
2.3. Light-Dark Box
2.4. Tissue Collection
2.5. RNA Extraction, cDNA Synthesis and Quantitative RT-PCR
2.6. Data Analysis
3. Results
3.1. Observation of Pregnant Dam and Offspring
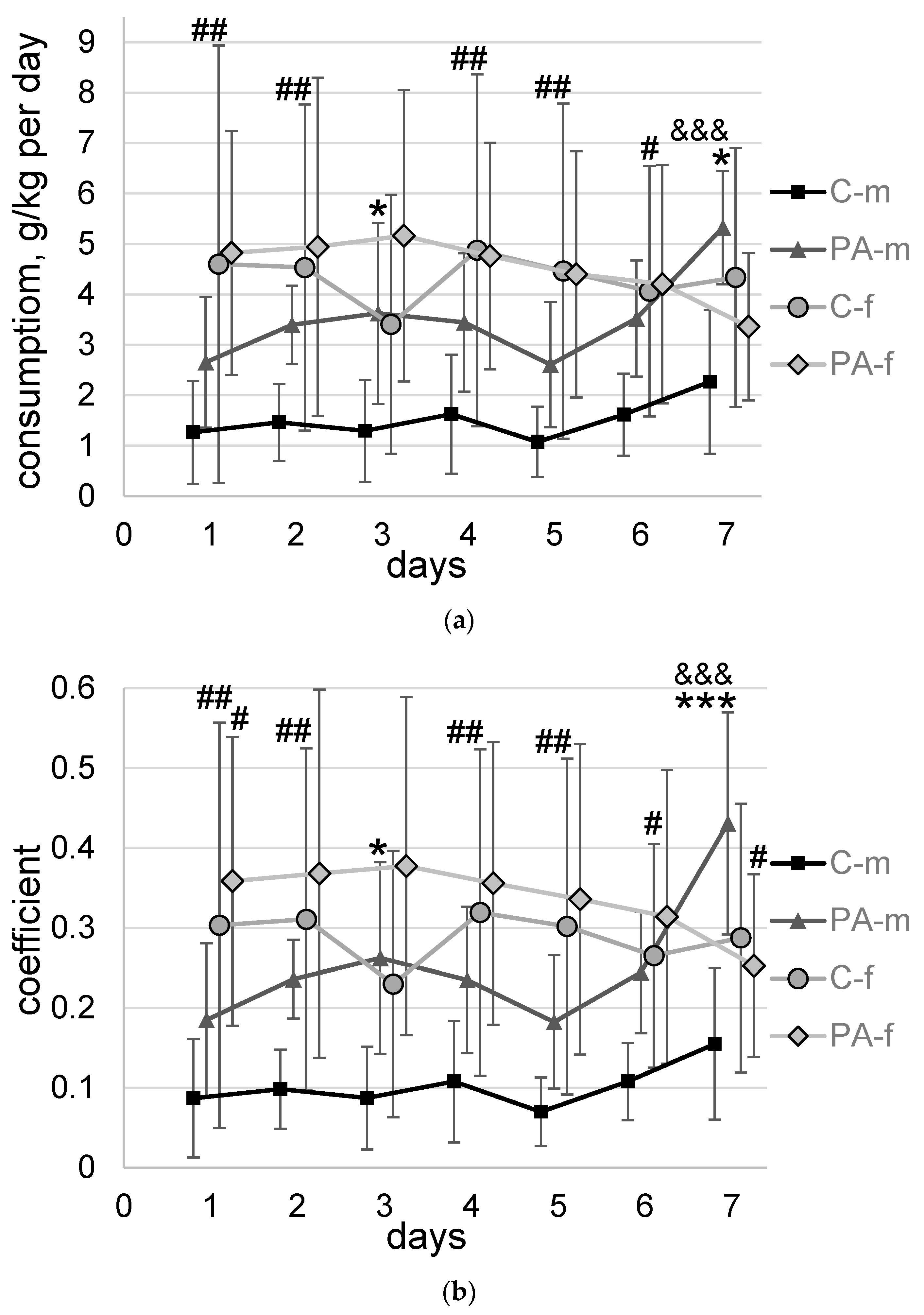
3.2. Voluntary Alcohol Consumption
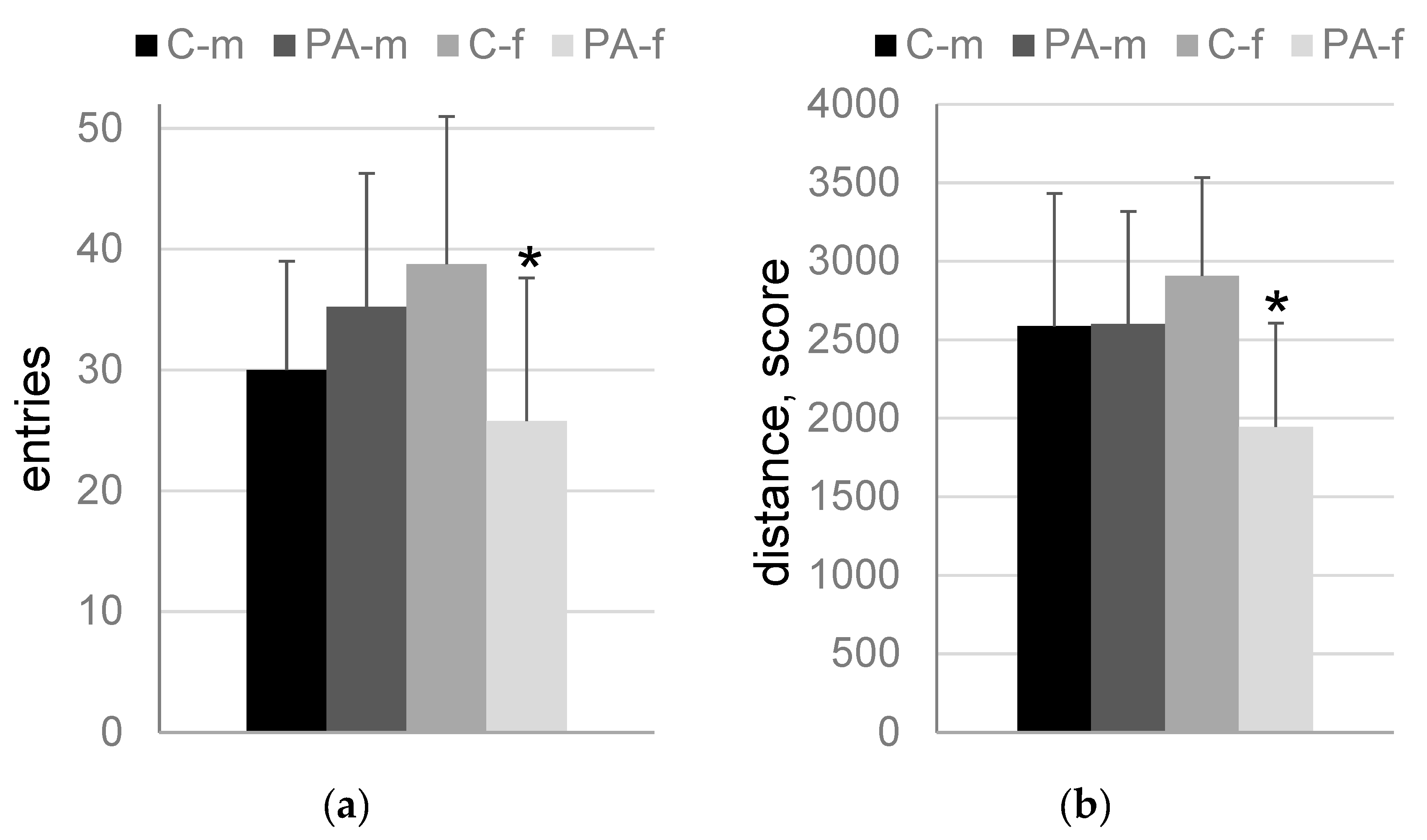
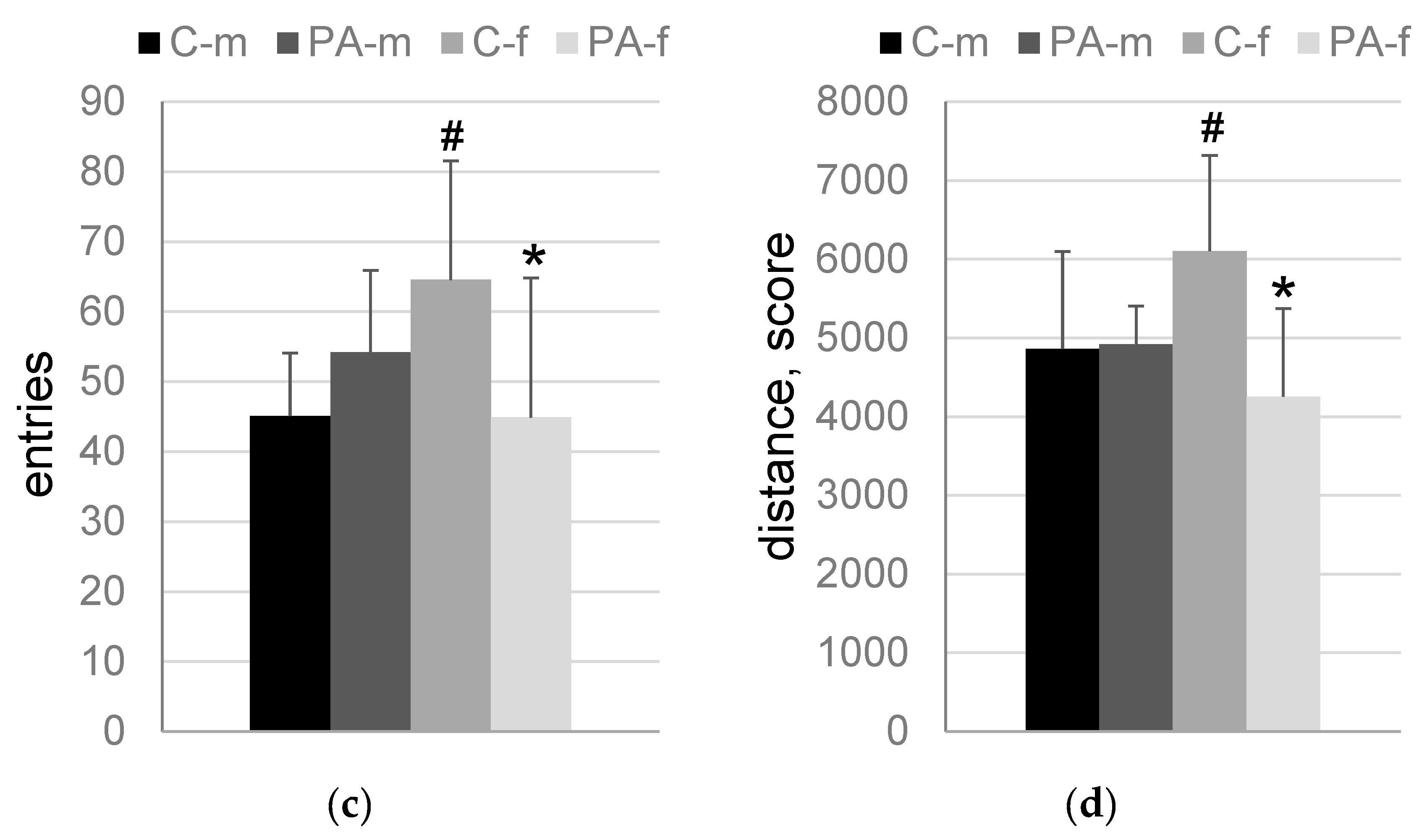
3.3. The “Light-Dark Box” Test
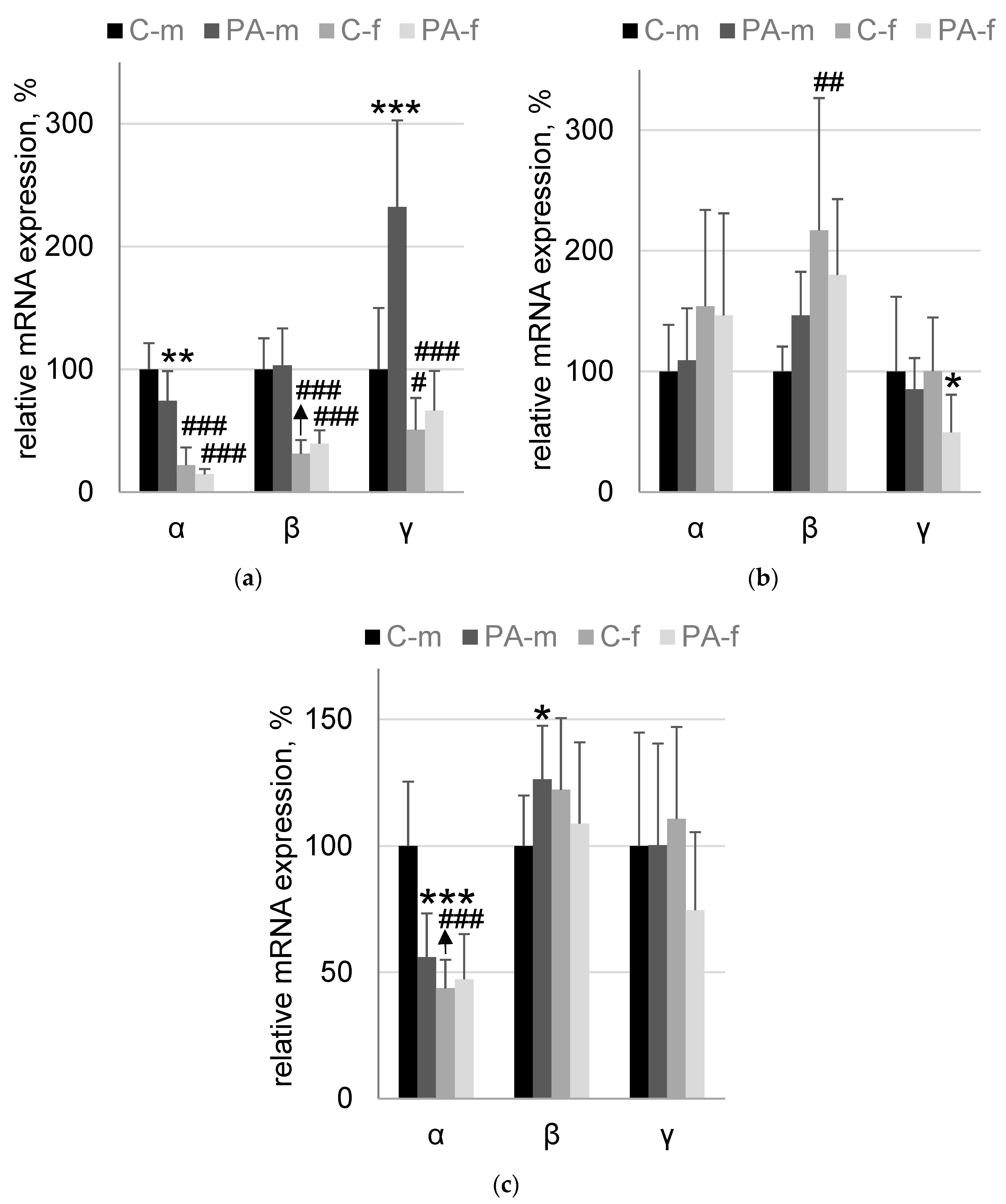
3.4. Synucleins mRNA Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, I.; Dandavate, R.; Gupta, P.; Agrawal, V.; Kapoor, M. Recent advances in genetic studies of alcohol use disorders. Curr. Genet. Med. Rep. 2020, 8, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Longley, M.J.; Lee, J.; Jung, J.; Lohoff, F.W. Epigenetics of alcohol use disorder-A review of recent advances in DNA methylation profiling. Addict. Biol. 2021, 26, e13006. [Google Scholar] [CrossRef] [PubMed]
- Gaztanaga, M.; Angulo-Alcalde, A.; Chotro, M.G. Prenatal Alcohol Exposure as a Case of Involuntary Early Onset of Alcohol Use: Consequences and Proposed Mechanisms From Animal Studies. Front. Behav. Neurosci. 2020, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.L.; Akkaya-Hocagil, T.; Ryan, L.M.; Dodge, N.C.; Richardson, G.A.; Olson, H.C.; Coles, C.D.; Day, N.L.; Cook, R.J.; Jacobson, S.W. Effects of prenatal alcohol exposure on cognitive and behavioral development: Findings from a hierarchical meta-analysis of data from six prospective longitudinal U.S. cohorts. Alcohol. Clin. Exp. Res. 2021, 45, 2040–2058. [Google Scholar] [CrossRef]
- Charness, M.E. Fetal Alcohol Spectrum Disorders: Awareness to Insight in Just 50 Years. Alcohol Res. 2022, 42, 05. [Google Scholar] [CrossRef]
- Das, J. SNARE Complex-Associated Proteins and Alcohol. Alcohol. Clin. Exp. Res. 2020, 44, 7–18. [Google Scholar] [CrossRef]
- Nemani, V.M.; Lu, W.; Berge, V.; Nakamura, K.; Onoa, B.; Lee, M.K.; Chaudhry, F.A.; Nicoll, R.A.; Edwards, R.H. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 2010, 65, 66–79. [Google Scholar] [CrossRef]
- Cahill, C.M.; Aleyadeh, R.; Gao, J.; Wang, C.; Rogers, J.T. Alpha-Synuclein in Alcohol Use Disorder, Connections with Parkinson’s Disease and Potential Therapeutic Role of 5’ Untranslated Region-Directed Small Molecules. Biomolecules 2020, 10, 1465. [Google Scholar] [CrossRef]
- Levey, D.F.; Le-Niculescu, H.; Frank, J.; Ayalew, M.; Jain, N.; Kirlin, B.; Learman, R.; Winiger, E.; Rodd, Z.; Shekhar, A.; et al. Genetic risk prediction and neurobiological understanding of alcoholism. Transl. Psychiatry 2014, 4, e391. [Google Scholar] [CrossRef]
- Janeczek, P.; MacKay, R.K.; Lea, R.A.; Dodd, P.R.; Lewohl, J.M. Reduced expression of alpha-synuclein in alcoholic brain: Influence of SNCA-Rep1 genotype. Addict. Biol. 2014, 19, 509–515. [Google Scholar] [CrossRef]
- Bonsch, D.; Reulbach, U.; Bayerlein, K.; Hillemacher, T.; Kornhuber, J.; Bleich, S. Elevated alpha synuclein mRNA levels are associated with craving in patients with alcoholism. Biol. Psychiatry 2004, 56, 984–986. [Google Scholar] [CrossRef] [PubMed]
- Foroud, T.; Wetherill, L.F.; Liang, T.; Dick, D.M.; Hesselbrock, V.; Kramer, J.; Nurnberger, J.; Schuckit, M.; Carr, L.; Porjesz, B.; et al. Association of alcohol craving with alpha-synuclein (SNCA). Alcohol. Clin. Exp. Res. 2007, 31, 537–545. [Google Scholar] [CrossRef]
- Rotermund, C.; Reolon, G.K.; Leixner, S.; Boden, C.; Bilbao, A.; Kahle, P.J. Enhanced motivation to alcohol in transgenic mice expressing human alpha-synuclein. J. Neurochem. 2017, 143, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Kimpel, M.W.; McClintick, J.N.; Skillman, A.R.; McCall, K.; Edenberg, H.J.; Carr, L.G. Candidate genes for alcohol preference identified by expression profiling in alcohol-preferring and -nonpreferring reciprocal congenic rats. Genome Biol. 2010, 11, R11. [Google Scholar] [CrossRef] [PubMed]
- Janowska, M.K.; Wu, K.P.; Baum, J. Unveiling transient protein-protein interactions that modulate inhibition of alpha-synuclein aggregation by beta-synuclein, a pre-synaptic protein that co-localizes with alpha-synuclein. Sci. Rep. 2015, 5, 15164. [Google Scholar] [CrossRef]
- Buchman, V.L.; Hunter, H.J.; Pinon, L.G.; Thompson, J.; Privalova, E.M.; Ninkina, N.N.; Davies, A.M. Persyn, a member of the synuclein family, has a distinct pattern of expression in the developing nervous system. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 9335–9341. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Kokhan, T.Y.G.; Samsonova, A.N.; Fisenko, V.P.; Ustyugov, A.A.; Aliev, G. The Dopaminergic Dysfunction and Altered Working Memory Performance of Aging Mice Lacking Gamma-synuclein Gene. CNS Neurol. Disord. Drug Targets 2018, 17, 604–607. [Google Scholar] [CrossRef]
- Senior, S.L.; Ninkina, N.; Deacon, R.; Bannerman, D.; Buchman, V.L.; Cragg, S.J.; Wade-Martins, R. Increased striatal dopamine release and hyperdopaminergic-like behaviour in mice lacking both alpha-synuclein and gamma-synuclein. Eur. J. Neurosci. 2008, 27, 947–957. [Google Scholar] [CrossRef]
- Wise, R.A.; Jordan, C.J. Dopamine, behavior, and addiction. J. Biomed. Sci. 2021, 28, 83. [Google Scholar] [CrossRef]
- Nutt, D.; Hayes, A.; Fonville, L.; Zafar, R.; Palmer, E.O.C.; Paterson, L.; Lingford-Hughes, A. Alcohol and the Brain. Nutrients 2021, 13, 3938. [Google Scholar] [CrossRef]
- White, A.M. Gender Differences in the Epidemiology of Alcohol Use and Related Harms in the United States. Alcohol Res. 2020, 40, 01. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, M.G.; Patalay, P.; Mackey, S.; Allen, N.B.; Batalla, A.; Bellani, M.; Chye, Y.; Cousijn, J.; Goudriaan, A.E.; Hester, R.; et al. Gender-related neuroanatomical differences in alcohol dependence: Findings from the ENIGMA Addiction Working Group. Neuroimage Clin. 2021, 30, 102636. [Google Scholar] [CrossRef] [PubMed]
- Flores-Bonilla, A.; De Oliveira, B.; Silva-Gotay, A.; Lucier, K.W.; Richardson, H.N. Shortening time for access to alcohol drives up front-loading behavior, bringing consumption in male rats to the level of females. Biol. Sex Differ. 2021, 12, 51. [Google Scholar] [CrossRef]
- Datta, U.; Schoenrock, S.E.; Bubier, J.A.; Bogue, M.A.; Jentsch, J.D.; Logan, R.W.; Tarantino, L.M.; Chesler, E.J. Prospects for finding the mechanisms of sex differences in addiction with human and model organism genetic analysis. Genes Brain Behav. 2020, 19, e12645. [Google Scholar] [CrossRef]
- Patten, A.R.; Fontaine, C.J.; Christie, B.R. A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Front. Pediatr. 2014, 2, 93. [Google Scholar] [CrossRef]
- West, J.R. Fetal alcohol-induced brain damage and the problem of determining temporal vulnerability: A review. Alcohol Drug Res. 1987, 7, 423–441. [Google Scholar]
- Quinn, R. Comparing rat’s to human’s age: How old is my rat in people years? Nutrition 2005, 21, 775–777. [Google Scholar] [CrossRef]
- Baer, J.S.; Sampson, P.D.; Barr, H.M.; Connor, P.D.; Streissguth, A.P. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch. Gen. Psychiatry 2003, 60, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.; Andreu-Fernandez, V.; Navarro-Tapia, E.; Aras-Lopez, R.; Serra-Delgado, M.; Martinez, L.; Garcia-Algar, O.; Gomez-Roig, M.D. Murine Models for the Study of Fetal Alcohol Spectrum Disorders: An Overview. Front. Pediatr. 2020, 8, 359. [Google Scholar] [CrossRef]
- Youngentob, S.L.; Glendinning, J.I. Fetal ethanol exposure increases ethanol intake by making it smell and taste better. Proc. Natl. Acad. Sci. USA 2009, 106, 5359–5364. [Google Scholar] [CrossRef]
- Glendinning, J.I.; Simons, Y.M.; Youngentob, L.; Youngentob, S.L. Fetal ethanol exposure attenuates aversive oral effects of TrpV1, but not TrpA1 agonists in rats. Exp. Biol. Med. 2012, 237, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, P.; Han, X.; Zuo, W.; Mei, Q.; Bian, E.Y.; Umeugo, J.; Ye, J. Differences between male and female rats in alcohol drinking, negative affects and neuronal activity after acute and prolonged abstinence. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 163–176. [Google Scholar]
- Blanchard, B.A.; Glick, S.D. Sex differences in mesolimbic dopamine responses to ethanol and relationship to ethanol intake in rats. Recent Dev. Alcohol. 1995, 12, 231–241. [Google Scholar] [CrossRef]
- Spence, J.P.; Reiter, J.L.; Qiu, B.; Gu, H.; Garcia, D.K.; Zhang, L.; Graves, T.; Williams, K.E.; Bice, P.J.; Zou, Y.; et al. Estrogen-Dependent Upregulation of Adcyap1r1 Expression in Nucleus Accumbens Is Associated With Genetic Predisposition of Sex-Specific QTL for Alcohol Consumption on Rat Chromosome 4. Front. Genet. 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, M.A.S.; Pinho, R.; Paiva, I.; Outeiro, T.F. The yin and yang of alpha-synuclein-associated epigenetics in Parkinson’s disease. Brain A J. Neurol. 2017, 140, 878–886. [Google Scholar] [CrossRef]
- Desplats, P.; Spencer, B.; Coffee, E.; Patel, P.; Michael, S.; Patrick, C.; Adame, A.; Rockenstein, E.; Masliah, E. Alpha-synuclein sequesters Dnmt1 from the nucleus: A novel mechanism for epigenetic alterations in Lewy body diseases. J. Biol. Chem. 2011, 286, 9031–9037. [Google Scholar] [CrossRef] [PubMed]
- Razumkina, E.; Anokhin, P.; Sarycheva, N.; Shamakina, I. Prenatal alcohol exposure increases DNA-methyltransferases 1 and 3a its mRNA levels in the rat mesolimbic brain areas. Eur. Neuropsychopharmacol. 2019, 29, S312–S313. [Google Scholar] [CrossRef]
- Gorbatyuk, O.S.; Li, S.; Nash, K.; Gorbatyuk, M.; Lewin, A.S.; Sullivan, L.F.; Mandel, R.J.; Chen, W.; Meyers, C.; Manfredsson, F.P.; et al. In vivo RNAi-mediated alpha-synuclein silencing induces nigrostriatal degeneration. Mol. Ther. J. Am. Soc. Gene Ther. 2010, 18, 1450–1457. [Google Scholar] [CrossRef]
- McCormack, A.L.; Mak, S.K.; Henderson, J.M.; Bumcrot, D.; Farrer, M.J.; Di Monte, D.A. Alpha-synuclein suppression by targeted small interfering RNA in the primate substantia nigra. PLoS ONE 2010, 5, e12122. [Google Scholar] [CrossRef]
- Polissidis, A.; Koronaiou, M.; Kollia, V.; Koronaiou, E.; Nakos-Bimpos, M.; Bogiongko, M.; Vrettou, S.; Karali, K.; Casadei, N.; Riess, O.; et al. Psychosis-Like Behavior and Hyperdopaminergic Dysregulation in Human alpha-Synuclein BAC Transgenic Rats. Mov. Disord. 2021, 36, 716–728. [Google Scholar] [CrossRef]
- Hirth, N.; Meinhardt, M.W.; Noori, H.R.; Salgado, H.; Torres-Ramirez, O.; Uhrig, S.; Broccoli, L.; Vengeliene, V.; Rossmanith, M.; Perreau-Lenz, S.; et al. Convergent evidence from alcohol-dependent humans and rats for a hyperdopaminergic state in protracted abstinence. Proc. Natl. Acad. Sci. USA 2016, 113, 3024–3029. [Google Scholar] [CrossRef] [PubMed]
- Hansson, A.C.; Grunder, G.; Hirth, N.; Noori, H.R.; Spanagel, R.; Sommer, W.H. Dopamine and opioid systems adaptation in alcoholism revisited: Convergent evidence from positron emission tomography and postmortem studies. Neurosci. Biobehav. Rev. 2019, 106, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.R.; Mulholland, P.J.; Truitt, W.A.; Waeiss, R.A.; Engleman, E.A.; Bell, R.L.; Rodd, Z.A. Adolescent Intermittent Ethanol (AIE) Enhances the Dopaminergic Response to Ethanol within the Mesolimbic Pathway during Adulthood: Alterations in Cholinergic/Dopaminergic Genes Expression in the Nucleus Accumbens Shell. Int. J. Mol. Sci. 2021, 22, 11733. [Google Scholar] [CrossRef]
- Santangelo, V.; Cavallina, C.; Colucci, P.; Santori, A.; Macri, S.; McGaugh, J.L.; Campolongo, P. Enhanced brain activity associated with memory access in highly superior autobiographical memory. Proc. Natl. Acad. Sci. USA 2018, 115, 7795–7800. [Google Scholar] [CrossRef] [PubMed]
- Wilhoit, L.F.; Scott, D.A.; Simecka, B.A. Fetal Alcohol Spectrum Disorders: Characteristics, Complications, and Treatment. Community Ment Health J. 2017, 53, 711–718. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Zhang, T. Spatial cognition and sexually dimorphic synaptic plasticity balance impairment in rats with chronic prenatal ethanol exposure. Behav. Brain Res. 2013, 256, 564–574. [Google Scholar] [CrossRef]
- An, L.; Zhang, T. Prenatal ethanol exposure impairs spatial cognition and synaptic plasticity in female rats. Alcohol 2015, 49, 581–588. [Google Scholar] [CrossRef]
- Guerri, C.; Bazinet, A.; Riley, E.P. Foetal Alcohol Spectrum Disorders and alterations in brain and behaviour. Alcohol Alcohol. 2009, 44, 108–114. [Google Scholar] [CrossRef]
- Bon, L.I.; Zimatkin, S.M. Disruption of synaptogenesis in the rats brain cortex after antenatal alcoholisation. J. Grodno State Med. Univ. 2017, 15, 538–543. [Google Scholar] [CrossRef]
- Sadrian, B.; Lopez-Guzman, M.; Wilson, D.A.; Saito, M. Distinct neurobehavioral dysfunction based on the timing of developmental binge-like alcohol exposure. Neuroscience 2014, 280, 204–219. [Google Scholar] [CrossRef]
- Hsu, L.J.; Mallory, M.; Xia, Y.; Veinbergs, I.; Hashimoto, M.; Yoshimoto, M.; Thal, L.J.; Saitoh, T.; Masliah, E. Expression pattern of synucleins (non-Abeta component of Alzheimer’s disease amyloid precursor protein/alpha-synuclein) during murine brain development. J. Neurochem. 1998, 71, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.D.; Rueter, S.M.; Trojanowski, J.Q.; Lee, V.M. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 2000, 20, 3214–3220. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Watanabe, Y.; Tsujimura, A.; Tatebe, H.; Miyata, S.; Tokuda, T.; Mizuno, T.; Tanaka, M. Differential expression of alpha-synuclein in hippocampal neurons. PLoS ONE 2014, 9, e89327. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Watanabe, Y.; Tsujimura, A.; Tanaka, M. Expression of alpha-synuclein is regulated in a neuronal cell type-dependent manner. Anat. Sci. Int. 2019, 94, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Gureviciene, I.; Gurevicius, K.; Tanila, H. Role of alpha-synuclein in synaptic glutamate release. Neurobiol. Dis. 2007, 28, 83–89. [Google Scholar] [CrossRef]
- Cheng, H.; Kellar, D.; Lake, A.; Finn, P.; Rebec, G.V.; Dharmadhikari, S.; Dydak, U.; Newman, S. Effects of Alcohol Cues on MRS Glutamate Levels in the Anterior Cingulate. Alcohol Alcohol. 2018, 53, 209–215. [Google Scholar] [CrossRef]
- Ceccarini, J.; Leurquin-Sterk, G.; Crunelle, C.L.; de Laat, B.; Bormans, G.; Peuskens, H.; Van Laere, K. Recovery of Decreased Metabotropic Glutamate Receptor 5 Availability in Abstinent Alcohol-Dependent Patients. J. Nucl. Med. 2020, 61, 256–262. [Google Scholar] [CrossRef]
- Burnette, E.M.; Nieto, S.J.; Grodin, E.N.; Meredith, L.R.; Hurley, B.; Miotto, K.; Gillis, A.J.; Ray, L.A. Novel Agents for the Pharmacological Treatment of Alcohol Use Disorder. Drugs 2022, 82, 251–274. [Google Scholar] [CrossRef]
- Gerace, E.; Landucci, E.; Bani, D.; Moroni, F.; Mannaioni, G.; Pellegrini-Giampietro, D.E. Glutamate Receptor-Mediated Neurotoxicity in a Model of Ethanol Dependence and Withdrawal in Rat Organotypic Hippocampal Slice Cultures. Front. Neurosci. 2018, 12, 1053. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Afanasyeva, M.A.; Van’kin, G.I. alpha-Synuclein knockout mice have cognitive impairments. Behav. Brain Res. 2012, 231, 226–230. [Google Scholar] [CrossRef]
- Mattson, S.N.; Crocker, N.; Nguyen, T.T. Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychol. Rev. 2011, 21, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Olguin, S.L.; Thompson, S.M.; Young, J.W.; Brigman, J.L. Moderate prenatal alcohol exposure impairs cognitive control, but not attention, on a rodent touchscreen continuous performance task. Genes Brain Behav. 2021, 20, e12652. [Google Scholar] [CrossRef] [PubMed]
- Anokhin, P.K.; Shamakina, I.Y.; Ustyugov, A.A.; Bachurin, S.O.; Proskuryakova, T.V. A comparison of the expression of α-synuclein mRNA in the brain of rats with different levels of alcohol consumption. Neurochem. J. 2016, 10, 294–299. [Google Scholar] [CrossRef]
- Tehranian, R.; Montoya, S.E.; Van Laar, A.D.; Hastings, T.G.; Perez, R.G. Alpha-synuclein inhibits aromatic amino acid decarboxylase activity in dopaminergic cells. J. Neurochem. 2006, 99, 1188–1196. [Google Scholar] [CrossRef]
- Hausknecht, K.A.; Acheson, A.; Farrar, A.M.; Kieres, A.K.; Shen, R.Y.; Richards, J.B.; Sabol, K.E. Prenatal alcohol exposure causes attention deficits in male rats. Behav. Neurosci. 2005, 119, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Hellemans, K.G.; Verma, P.; Yoon, E.; Yu, W.; Weinberg, J. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann. N. Y. Acad. Sci. 2008, 1144, 154–175. [Google Scholar] [CrossRef]
- Chiavegatto, S.; Izidio, G.S.; Mendes-Lana, A.; Aneas, I.; Freitas, T.A.; Torrao, A.S.; Conceicao, I.M.; Britto, L.R.; Ramos, A. Expression of alpha-synuclein is increased in the hippocampus of rats with high levels of innate anxiety. Mol. Psychiatry 2009, 14, 894–905. [Google Scholar] [CrossRef]
- Pena-Oliver, Y.; Buchman, V.L.; Stephens, D.N. Lack of involvement of alpha-synuclein in unconditioned anxiety in mice. Behav. Brain Res. 2010, 209, 234–240. [Google Scholar] [CrossRef][Green Version]
- Kokhan, V.S.; Van’kin, G.I.; Bachurin, S.O.; Shamakina, I.Y. Differential involvement of the gamma-synuclein in cognitive abilities on the model of knockout mice. BMC Neurosci. 2013, 14, 53. [Google Scholar] [CrossRef][Green Version]
- Chandra, S.; Fornai, F.; Kwon, H.B.; Yazdani, U.; Atasoy, D.; Liu, X.; Hammer, R.E.; Battaglia, G.; German, D.C.; Castillo, P.E.; et al. Double-knockout mice for alpha- and beta-synucleins: Effect on synaptic functions. Proc. Natl. Acad. Sci. USA 2004, 101, 14966–14971. [Google Scholar] [CrossRef]
- Connor-Robson, N.; Peters, O.M.; Millership, S.; Ninkina, N.; Buchman, V.L. Combinational losses of synucleins reveal their differential requirements for compensating age-dependent alterations in motor behavior and dopamine metabolism. Neurobiol. Aging 2016, 46, 107–112. [Google Scholar] [CrossRef]
- Carnazza, K.E.; Komer, L.E.; Xie, Y.X.; Pineda, A.; Briano, J.A.; Gao, V.; Na, Y.; Ramlall, T.; Buchman, V.L.; Eliezer, D.; et al. Synaptic vesicle binding of alpha-synuclein is modulated by beta- and gamma-synucleins. Cell Rep. 2022, 39, 110675. [Google Scholar] [CrossRef] [PubMed]
- Mak, S.K.; McCormack, A.L.; Langston, J.W.; Kordower, J.H.; Di Monte, D.A. Decreased alpha-synuclein expression in the aging mouse substantia nigra. Exp. Neurol. 2009, 220, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Pavia-Collado, R.; Rodriguez-Aller, R.; Alarcon-Aris, D.; Miquel-Rio, L.; Ruiz-Bronchal, E.; Paz, V.; Campa, L.; Galofre, M.; Sgambato, V.; Bortolozzi, A. Up and Down gamma-Synuclein Transcription in Dopamine Neurons Translates into Changes in Dopamine Neurotransmission and Behavioral Performance in Mice. Int. J. Mol. Sci. 2022, 23, 1807. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primers | |
|---|---|---|
| Forward | Reverse | |
| β-actin | 5′-cactgccg-catcctcttcct-3′ | 5′-aaccgctcatt-gccgatagtg-3′ |
| Snca (α-synuclein) | 5′-tgtcaagaaggaccagatg-3′ | 5′-caggctcatagtcttggtag-3′ |
| Sncb (β-synuclein) | 5′-agttccccacagacctgaag-3′ | 5′-ttacgcctctggctcgtattc-3′ |
| Sncg (γ-synuclein) | 5′-aaacatcgtggtcaccacc-3′ | 5′-tctagtctcctccactcttg-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokhan, V.S.; Chaprov, K.; Ninkina, N.N.; Anokhin, P.K.; Pakhlova, E.P.; Sarycheva, N.Y.; Shamakina, I.Y. Sex-Related Differences in Voluntary Alcohol Intake and mRNA Coding for Synucleins in the Brain of Adult Rats Prenatally Exposed to Alcohol. Biomedicines 2022, 10, 2163. https://doi.org/10.3390/biomedicines10092163
Kokhan VS, Chaprov K, Ninkina NN, Anokhin PK, Pakhlova EP, Sarycheva NY, Shamakina IY. Sex-Related Differences in Voluntary Alcohol Intake and mRNA Coding for Synucleins in the Brain of Adult Rats Prenatally Exposed to Alcohol. Biomedicines. 2022; 10(9):2163. https://doi.org/10.3390/biomedicines10092163
Chicago/Turabian StyleKokhan, Viktor S., Kirill Chaprov, Natalia N. Ninkina, Petr K. Anokhin, Ekaterina P. Pakhlova, Natalia Y. Sarycheva, and Inna Y. Shamakina. 2022. "Sex-Related Differences in Voluntary Alcohol Intake and mRNA Coding for Synucleins in the Brain of Adult Rats Prenatally Exposed to Alcohol" Biomedicines 10, no. 9: 2163. https://doi.org/10.3390/biomedicines10092163
APA StyleKokhan, V. S., Chaprov, K., Ninkina, N. N., Anokhin, P. K., Pakhlova, E. P., Sarycheva, N. Y., & Shamakina, I. Y. (2022). Sex-Related Differences in Voluntary Alcohol Intake and mRNA Coding for Synucleins in the Brain of Adult Rats Prenatally Exposed to Alcohol. Biomedicines, 10(9), 2163. https://doi.org/10.3390/biomedicines10092163






