Potential Role of Sleep Deficiency in Inducing Immune Dysfunction
Abstract
1. Introduction
2. The Interrelation between Sleep and Alterations in Inflammatory Markers Secretion
2.1. Inflammatory Markers in Sleep Deprivation Model: The Insight into Animal Studies
2.2. Interaction between Inflammation and Sleep Disturbances—Human Studies
2.3. Interaction of Sleep Medications with Inflammatory Mediators
2.4. Cell Mediated-Immunity Alterations in Sleep Deprivation or Natural Sleep Loss Observed in Sleep-Deprived Individuals
2.5. Summary
3. Sleep Deficiency and Its Influence on Autoimmunity
4. May Sleep Deficiency Affect Vaccination Effectiveness?
4.1. Vaccination Studies
4.2. Immune Memory Formation
4.3. Infectious Diseases Susceptibility
4.4. Summary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bathgate, C.J.; Edinger, J.D.; Wyatt, J.K.; Krystal, A.D. Objective but Not Subjective Short Sleep Duration Associated with Increased Risk for Hypertension in Individuals with Insomnia. Sleep 2016, 39, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Itani, O.; Jike, M.; Watanabe, N.; Kaneita, Y. Short Sleep Duration and Health Outcomes: A Systematic Review, Meta-Analysis, and Meta-Regression. Sleep Med. 2017, 32, 246–256. [Google Scholar] [CrossRef] [PubMed]
- AlDabal, L.; BaHammam, A.S. Metabolic, Endocrine, and Immune Consequences of Sleep Deprivation. Open Respir. Med. J. 2011, 5, 31–43. [Google Scholar] [CrossRef]
- Pigeon, W.R.; Bishop, T.M.; Krueger, K.M. Insomnia as a Precipitating Factor in New Onset Mental Illness: A Systematic Review of Recent Findings. Curr. Psychiatry Rep. 2017, 19, 44. [Google Scholar] [CrossRef]
- Katz, D.A.; McHorney, C.A. Clinical Correlates of Insomnia in Patients With Chronic Illness. Arch. Intern. Med. 1998, 158, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, S.-J.; Ma, M.-Y.; Bao, Y.-P.; Han, Y.; Wang, Y.-M.; Shi, J.; Vitiello, M.V.; Lu, L. Sleep Disturbances Increase the Risk of Dementia: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2018, 40, 4–16. [Google Scholar] [CrossRef]
- Irwin, M.R.; Vitiello, M.V. Implications of Sleep Disturbance and Inflammation for Alzheimer’s Disease Dementia. Lancet Neurol. 2019, 18, 296–306. [Google Scholar] [CrossRef]
- Taylor, D.J.; Kelly, K.; Kohut, M.L.; Song, K.-S. Is Insomnia a Risk Factor for Decreased Influenza Vaccine Response? Behav. Sleep Med. 2017, 15, 270–287. [Google Scholar] [CrossRef]
- Hsiao, Y.-H.; Chen, Y.-T.; Tseng, C.-M.; Wu, L.-A.; Lin, W.-C.; Su, V.Y.-F.; Perng, D.-W.; Chang, S.-C.; Chen, Y.-M.; Chen, T.-J.; et al. Sleep Disorders and Increased Risk of Autoimmune Diseases in Individuals without Sleep Apnea. Sleep 2015, 38, 581–586. [Google Scholar] [CrossRef]
- Sochal, M.; Małecka-Panas, E.; Gabryelska, A.; Talar-Wojnarowska, R.; Szmyd, B.; Krzywdzińska, M.; Białasiewicz, P. Determinants of Sleep Quality in Inflammatory Bowel Diseases. J. Clin. Med. 2020, 9, 2921. [Google Scholar] [CrossRef]
- Sochal, M.; Małecka-Panas, E.; Gabryelska, A.; Fichna, J.; Talar-Wojnarowska, R.; Szmyd, B.; Białasiewicz, P. Brain-Derived Neurotrophic Factor Is Elevated in the Blood Serum of Crohn’s Disease Patients, but Is Not Influenced by Anti-TNF-α Treatment-A Pilot Study. Neurogastroenterol. Motil. 2021, 33, e13978. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, B.; Lallukka, T.; Salo, P.; Pallesen, S.; Hysing, M.; Krokstad, S.; Øverland, S. Insomnia as a Risk Factor for Ill Health: Results from the Large Population-Based Prospective HUNT Study in Norway. J. Sleep Res. 2014, 23, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; ISBN 978-0-323-75750-8. [Google Scholar]
- Ordovas-Montanes, J.; Rakoff-Nahoum, S.; Huang, S.; Riol-Blanco, L.; Barreiro, O.; Andrian, U.H. von The Regulation of Immunological Processes by Peripheral Neurons in Homeostasis and Disease. Trends Immunol. 2015, 36, 578–604. [Google Scholar] [CrossRef]
- Flierl, M.A.; Rittirsch, D.; Nadeau, B.A.; Chen, A.J.; Sarma, J.V.; Zetoune, F.S.; McGuire, S.R.; List, R.P.; Day, D.E.; Hoesel, L.M.; et al. Phagocyte-Derived Catecholamines Enhance Acute Inflammatory Injury. Nature 2007, 449, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Pert, C.B.; Ruff, M.R.; Weber, R.J.; Herkenham, M. Neuropeptides and Their Receptors: A Psychosomatic Network. J. Immunol. 1985, 135, 820s–826s. [Google Scholar] [PubMed]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiological Reviews 2019, 99, 1325–1380. [Google Scholar] [CrossRef]
- Karatas, G.; Bal, A.; Yuceege, M.; Yalcin, E.; Firat, H.; Dulgeroglu, D.; Karataş, F.; Sahin, S.; Cakci, A.; Ardic, S. The Evaluation of Sleep Quality and Response to Anti-Tumor Necrosis Factor α Therapy in Rheumatoid Arthritis Patients. Clin. Rheumatol. 2017, 36, 45–50. [Google Scholar] [CrossRef]
- Zamarrón, C.; Maceiras, F.; Mera, A.; Gómez-Reino, J.J. Effect of the First Infliximab Infusion on Sleep and Alertness in Patients with Active Rheumatoid Arthritis. Ann. Rheum. Dis. 2004, 63, 88–90. [Google Scholar] [CrossRef]
- Penner, I.-K.; Sivertsdotter, E.C.; Celius, E.G.; Fuchs, S.; Schreiber, K.; Berkö, S.; Svenningsson, A.; for the TYNERGY trial investigators. Improvement in Fatigue during Natalizumab Treatment Is Linked to Improvement in Depression and Day-Time Sleepiness. Front. Neurol. 2015, 6, 18. [Google Scholar] [CrossRef]
- Fan, T.-T.; Chen, W.-H.; Shi, L.; Lin, X.; Tabarak, S.; Chen, S.-J.; Que, J.-Y.; Bao, Y.; Tang, X.-D.; Shi, J.; et al. Objective Sleep Duration Is Associated with Cognitive Deficits in Primary Insomnia: BDNF May Play a Role. Sleep 2019, 42, zsy192. [Google Scholar] [CrossRef]
- Fernandez-Mendoza, J.; Baker, J.H.; Vgontzas, A.N.; Gaines, J.; Liao, D.; Bixler, E.O. Insomnia Symptoms with Objective Short Sleep Duration Are Associated with Systemic Inflammation in Adolescents. Brain Behav. Immun. 2017, 61, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Coronado, E.G.; Pantaleón-Martínez, A.M.; Velazquéz-Moctezuma, J.; Prospéro-García, O.; Méndez-Díaz, M.; Pérez-Tapia, M.; Pavón, L.; Morales-Montor, J. The Bidirectional Relationship between Sleep and Immunity against Infections. J. Immunol. Res. 2015, 2015, 678164. [Google Scholar] [CrossRef] [PubMed]
- Venancio, D.P.; Suchecki, D. Prolonged REM Sleep Restriction Induces Metabolic Syndrome-Related Changes: Mediation by pro-Inflammatory Cytokines. Brain Behav. Immun. 2015, 47, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, S.; Sredni, B.; Carasso, R.L.; Kenigsbuch-Sredni, D. REM Sleep Deprivation in Rats Results in Inflammation and Interleukin-17 Elevation. J. Interferon. Cytokine Res. 2009, 29, 393–398. [Google Scholar] [CrossRef]
- Baracchi, F.; Opp, M.R. Sleep-Wake Behavior and Responses to Sleep Deprivation of Mice Lacking Both Interleukin-1 Beta Receptor 1 and Tumor Necrosis Factor-Alpha Receptor 1. Brain Behav. Immun. 2008, 22, 982–993. [Google Scholar] [CrossRef]
- Deboer, T.; Fontana, A.; Tobler, I. Tumor Necrosis Factor (TNF) Ligand and TNF Receptor Deficiency Affects Sleep and the Sleep EEG. J. Neurophysiol. 2002, 88, 839–846. [Google Scholar] [CrossRef][Green Version]
- Burgos, I.; Richter, L.; Klein, T.; Fiebich, B.; Feige, B.; Lieb, K.; Voderholzer, U.; Riemann, D. Increased Nocturnal Interleukin-6 Excretion in Patients with Primary Insomnia: A Pilot Study. Brain Behav. Immun. 2006, 20, 246–253. [Google Scholar] [CrossRef]
- Carroll, J.E.; Carrillo, C.; Olmstead, R.; Witarama, T.; Breen, E.C.; Yokomizo, M.; Seeman, T.; Irwin, M.R. Sleep Deprivation and Divergent Toll-like Receptor-4 Activation of Cellular Inflammation in Aging. Sleep 2015, 38, 205–211. [Google Scholar] [CrossRef]
- Jewett, K.A.; Krueger, J.M. Humoral Sleep Regulation; Interleukin-1 and Tumor Necrosis Factor. Vitam. Horm. 2012, 89, 241–257. [Google Scholar] [CrossRef]
- Krueger, J.M.; Clinton, J.M.; Winters, B.D.; Zielinski, M.R.; Taishi, P.; Jewett, K.A.; Davis, C.J. Involvement of Cytokines in Slow Wave Sleep. Prog. Brain Res. 2011, 193, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.-F.; Yeung, W.-F.; Ho, F.Y.-Y.; Yung, K.-P.; Yu, Y.-M.; Kwok, C.-W. Cross-Cultural and Comparative Epidemiology of Insomnia: The Diagnostic and Statistical Manual (DSM), International Classification of Diseases (ICD) and International Classification of Sleep Disorders (ICSD). Sleep Med. 2015, 16, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Slavish, D.C.; Graham-Engeland, J.E.; Engeland, C.G.; Taylor, D.J.; Buxton, O.M. Insomnia Symptoms Are Associated with Elevated C-Reactive Protein in Young Adults. Psychol. Health 2018, 33, 1396–1415. [Google Scholar] [CrossRef] [PubMed]
- Prather, A.A.; Vogelzangs, N.; Penninx, B.W.J.H. Sleep Duration, Insomnia, and Markers of Systemic Inflammation: Results from the Netherlands Study of Depression and Anxiety (NESDA). J. Psychiatr. Res. 2015, 60, 95–102. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Fernandez-Mendoza, J.; Liao, D.; Bixler, E.O. Insomnia with Objective Short Sleep Duration: The Most Biologically Severe Phenotype of the Disorder. Sleep Med. Rev. 2013, 17, 241–254. [Google Scholar] [CrossRef]
- Watson, N.F.; Buchwald, D.; Delrow, J.J.; Altemeier, W.A.; Vitiello, M.V.; Pack, A.I.; Bamshad, M.; Noonan, C.; Gharib, S.A. Transcriptional Signatures of Sleep Duration Discordance in Monozygotic Twins. Sleep 2017, 40, zsw019. [Google Scholar] [CrossRef]
- Jaehne, E.J.; Corrigan, F.; Toben, C.; Jawahar, M.C.; Baune, B.T. The Effect of the Antipsychotic Drug Quetiapine and Its Metabolite Norquetiapine on Acute Inflammation, Memory and Anhedonia. Pharmacol. Biochem. Behav. 2015, 135, 136–144. [Google Scholar] [CrossRef]
- Turra, B.O.; Barbisan, F.; Azzolin, V.F.; Teixeira, C.F.; Flores, T.; Braun, L.E.; de Oliveira Nerys, D.A.; Rissi, V.B.; de Oliveira Alves, A.; Assmann, C.E.; et al. Unmetabolized Quetiapine Exerts an in Vitro Effect on Innate Immune Cells by Modulating Inflammatory Response and Neutrophil Extracellular Trap Formation. Biomed Pharm. 2020, 131, 110497. [Google Scholar] [CrossRef]
- Daniele, S.; Da Pozzo, E.; Zappelli, E.; Martini, C. Trazodone Treatment Protects Neuronal-like Cells from Inflammatory Insult by Inhibiting NF-ΚB, P38 and JNK. Cell. Signal. 2015, 27, 1609–1629. [Google Scholar] [CrossRef]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a Master Regulator of Cell Death and Inflammation: Molecular Mechanisms and Clinical Implications for Newborn Care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and Inflammation—Story of a Double-Edged Blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef] [PubMed]
- Rockstrom, M.D.; Chen, L.; Taishi, P.; Nguyen, J.T.; Gibbons, C.M.; Veasey, S.C.; Krueger, J.M. Tumor Necrosis Factor Alpha in Sleep Regulation. Sleep Med. Rev. 2018, 40, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, J.F.; Raison, C.L.; Rye, D.B.; Montague, A.R.; Woolwine, B.J.; Felger, J.C.; Haroon, E.; Miller, A.H. Inhibition of Tumor Necrosis Factor Improves Sleep Continuity in Patients with Treatment Resistant Depression and High Inflammation. Brain Behav. Immun. 2015, 47, 193–200. [Google Scholar] [CrossRef]
- Wolfe, F.; Michaud, K.; Li, T. Sleep Disturbance in Patients with Rheumatoid Arthritis: Evaluation by Medical Outcomes Study and Visual Analog Sleep Scales. J. Rheumatol. 2006, 33, 1942–1951. [Google Scholar] [PubMed]
- Karatas, G.; Bal, A.; Yuceege, M.; Firat, H.; Gurcay, E.; Ardic, S.; Cakci, F.A. Evaluation of Sleep Quality in Patients with Ankylosing Spondylitis and Efficacy of Anti-TNF-α Therapy on Sleep Problems: A Polisomnographic Study. Int. J. Rheum. Dis. 2018, 21, 1263–1269. [Google Scholar] [CrossRef]
- Fragiadaki, K.; Tektonidou, M.G.; Konsta, M.; Chrousos, G.P.; Sfikakis, P.P. Sleep Disturbances and Interleukin 6 Receptor Inhibition in Rheumatoid Arthritis. J. Rheumatol. 2012, 39, 60–62. [Google Scholar] [CrossRef]
- Savard, J.; Laroche, L.; Simard, S.; Ivers, H.; Morin, C.M. Chronic Insomnia and Immune Functioning. Psychosom. Med. 2003, 65, 211–221. [Google Scholar] [CrossRef]
- Lange, T.; Dimitrov, S.; Fehm, H.-L.; Born, J. Sleep-like Concentrations of Growth Hormone and Cortisol Modulate Type1 and Type2 in-Vitro Cytokine Production in Human T Cells. Int. Immunopharmacol. 2006, 6, 216–225. [Google Scholar] [CrossRef]
- Fondell, E.; Axelsson, J.; Franck, K.; Ploner, A.; Lekander, M.; Bälter, K.; Gaines, H. Short Natural Sleep Is Associated with Higher T Cell and Lower NK Cell Activities. Brain Behav. Immun. 2011, 25, 1367–1375. [Google Scholar] [CrossRef]
- Lasselin, J.; Rehman, J.; Åkerstedt, T.; Lekander, M.; Axelsson, J. Effect of Long-Term Sleep Restriction and Subsequent Recovery Sleep on the Diurnal Rhythms of White Blood Cell Subpopulations. Brain Behav. Immun. 2015, 47, 93–99. [Google Scholar] [CrossRef]
- Seo, H.-M.; Kim, T.L.; Kim, J.S. The Risk of Alopecia Areata and Other Related Autoimmune Diseases in Patients with Sleep Disorders: A Korean Population-Based Retrospective Cohort Study. Sleep 2018, 41, zsy111. [Google Scholar] [CrossRef] [PubMed]
- Young, K.A.; Munroe, M.E.; Harley, J.B.; Guthridge, J.M.; Kamen, D.L.; Gilkensen, G.S.; Weisman, M.H.; Karp, D.R.; Wallace, D.J.; James, J.A.; et al. Less than 7 Hours of Sleep per Night Is Associated with Transitioning to Systemic Lupus Erythematosus. Lupus 2018, 27, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Palma, B.D.; Gabriel, A.; Colugnati, F.A.B.; Tufik, S. Effects of Sleep Deprivation on the Development of Autoimmune Disease in an Experimental Model of Systemic Lupus Erythematosus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1527–R1532. [Google Scholar] [CrossRef]
- Sahraian, M.A.; Rezaali, S.; Hosseiny, M.; Doosti, R.; Tajik, A.; Naser Moghadasi, A. Sleep Disorder as a Triggering Factor for Relapse in Multiple Sclerosis. Eur. Neurol. 2017, 77, 258–261. [Google Scholar] [CrossRef]
- Caloz, E.; Vullièmoz, M.; Haba Rubio, J.; Pittet, V.; Mamadou Barry, P.; Michetti, P.; Heinzer, R.; Maillard, M.H. P808 Prevalence and Factors Associated with Sleep Disturbances in Inflammatory Bowel Disease Patients Compared with Normal Controls. J. Crohn’s Colitis 2020, 14, S631–S632. [Google Scholar] [CrossRef]
- kotb, H.A.; Rady, H.M.; Ghanim, D.H. Sleep Disturbance in Female Patients with Systemic Lupus Erythematosus and Its Relation to Disease Parameters. Egypt. Rheumatol. 2013, 35, 127–132. [Google Scholar] [CrossRef]
- Guo, G.; Fu, T.; Yin, R.; Zhang, L.; Zhang, Q.; Xia, Y.; Li, L.; Gu, Z. Sleep Quality in Chinese Patients with Rheumatoid Arthritis: Contributing Factors and Effects on Health-Related Quality of Life. Health Qual. Life Outcomes 2016, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Saçmacı, H.; Gürel, G. Sleep Disorders in Patients with Psoriasis: A Cross-Sectional Study Using Non-Polysomnographical Methods. Sleep Breath 2019, 23, 893–898. [Google Scholar] [CrossRef]
- Boeschoten, R.E.; Braamse, A.M.J.; Beekman, A.T.F.; Cuijpers, P.; van Oppen, P.; Dekker, J.; Uitdehaag, B.M.J. Prevalence of Depression and Anxiety in Multiple Sclerosis: A Systematic Review and Meta-Analysis. J. Neurol. Sci. 2017, 372, 331–341. [Google Scholar] [CrossRef]
- Viana, P.; Rodrigues, E.; Fernandes, C.; Matas, A.; Barreto, R.; Mendonça, M.; Peralta, R.; Geraldes, R. InMS: Chronic Insomnia Disorder in Multiple Sclerosis-a Portuguese Multicentre Study on Prevalence, Subtypes, Associated Factors and Impact on Quality of Life. Mult. Scler. Relat. Disord. 2015, 4, 477–483. [Google Scholar] [CrossRef]
- Bamer, A.; Johnson, K.; Amtmann, D.; Kraft, G. Prevalence of Sleep Problems in Individuals with Multiple Sclerosis. Mult. Scler. 2008, 14, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Ditmer, M.; Gabryelska, A.; Turkiewicz, S.; Białasiewicz, P.; Małecka-Wojciesko, E.; Sochal, M. Sleep Problems in Chronic Inflammatory Diseases: Prevalence, Treatment, and New Perspectives: A Narrative Review. J. Clin. Med. 2022, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Kinnucan, J.A.; Rubin, D.T.; Ali, T. Sleep and Inflammatory Bowel Disease: Exploring the Relationship Between Sleep Disturbances and Inflammation. Gastroenterol. Hepatol. 2013, 9, 718–727. [Google Scholar]
- Vaccaro, A.; Dor, Y.K.; Nambara, K.; Pollina, E.A.; Lin, C.; Greenberg, M.E.; Rogulja, D. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell 2020, 181, 1307–1328.e15. [Google Scholar] [CrossRef]
- Scavuzzi, B.M.; Simão, A.N.C.; Iriyoda, T.M.V.; Lozovoy, M.A.B.; Stadtlober, N.P.; Franchi Santos, L.F.D.R.; Flauzino, T.; de Medeiros, F.A.; de Sá, M.C.; Consentin, L.; et al. Increased Lipid and Protein Oxidation and Lowered Anti-Oxidant Defenses in Systemic Lupus Erythematosus Are Associated with Severity of Illness, Autoimmunity, Increased Adhesion Molecules, and Th1 and Th17 Immune Shift. Immunol. Res. 2018, 66, 158–171. [Google Scholar] [CrossRef]
- Teixeira, K.R.C.; dos Santos, C.P.; de Medeiros, L.A.; Mendes, J.A.; Cunha, T.M.; De Angelis, K.; Penha-Silva, N.; de Oliveira, E.P.; Crispim, C.A. Night Workers Have Lower Levels of Antioxidant Defenses and Higher Levels of Oxidative Stress Damage When Compared to Day Workers. Sci. Rep. 2019, 9, 4455. [Google Scholar] [CrossRef]
- Bertolotti, M.; Yim, S.H.; Garcia-Manteiga, J.M.; Masciarelli, S.; Kim, Y.-J.; Kang, M.-H.; Iuchi, Y.; Fujii, J.; Vené, R.; Rubartelli, A.; et al. B- to Plasma-Cell Terminal Differentiation Entails Oxidative Stress and Profound Reshaping of the Antioxidant Responses. Antioxid. Redox Signal. 2010, 13, 1133–1144. [Google Scholar] [CrossRef]
- Hurtado-Alvarado, G.; Pavón, L.; Castillo-García, S.A.; Hernández, M.E.; Domínguez-Salazar, E.; Velázquez-Moctezuma, J.; Gómez-González, B. Sleep Loss as a Factor to Induce Cellular and Molecular Inflammatory Variations. Clin. Dev. Immunol. 2013, 2013, 801341. [Google Scholar] [CrossRef]
- Voderholzer, U.; Fiebich, B.L.; Dersch, R.; Feige, B.; Piosczyk, H.; Kopasz, M.; Riemann, D.; Lieb, K. Effects of Sleep Deprivation on Nocturnal Cytokine Concentrations in Depressed Patients and Healthy Control Subjects. JNP 2012, 24, 354–366. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Long, M.D.; Martin, C.F.; Sandler, R.S.; Kappelman, M.D. Sleep Disturbance and Risk of Active Disease in Patients with Crohn’s Disease and Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2013, 11, 965–971. [Google Scholar] [CrossRef]
- Prather, A.A.; Hall, M.; Fury, J.M.; Ross, D.C.; Muldoon, M.F.; Cohen, S.; Marsland, A.L. Sleep and Antibody Response to Hepatitis B Vaccination. Sleep 2012, 35, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Everson, C.A. Sustained Sleep Deprivation Impairs Host Defense. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 1993, 265, R1148–R1154. [Google Scholar] [CrossRef] [PubMed]
- Prather, A.A.; Janicki-Deverts, D.; Hall, M.H.; Cohen, S. Behaviorally Assessed Sleep and Susceptibility to the Common Cold. Sleep 2015, 38, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Niu, Y.; Zhao, W.; Bao, P.; Li, D. Reduced Sleep in the Week Prior to Diagnosis of COVID-19 Is Associated with the Severity of COVID-19. Nat. Sci. Sleep 2020, 12, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Lange, T.; Perras, B.; Fehm, H.L.; Born, J. Sleep Enhances the Human Antibody Response to Hepatitis A Vaccination. Psychosom. Med. 2003, 65, 831–835. [Google Scholar] [CrossRef]
- Lange, T.; Born, J.; Westermann, J. Sleep Matters: CD4+ T Cell Memory Formation and the Central Nervous System. Trends Immunol. 2019, 40, 674–686. [Google Scholar] [CrossRef]
- CDC-Data and Statistics-Sleep and Sleep Disorders. Available online: https://www.cdc.gov/sleep/data_statistics.html (accessed on 18 August 2022).
- Prather, A.A.; Pressman, S.D.; Miller, G.E.; Cohen, S. Temporal Links Between Self-Reported Sleep and Antibody Responses to the Influenza Vaccine. Int. J. Behav. Med. 2021, 28, 151–158. [Google Scholar] [CrossRef]
- Sharpley, A.L.; Cooper, C.M.; Williams, C.; Godlewska, B.R.; Cowen, P.J. Effects of Typhoid Vaccine on Inflammation and Sleep in Healthy Participants: A Double-Blind, Placebo-Controlled, Crossover Study. Psychopharmacology 2016, 233, 3429–3435. [Google Scholar] [CrossRef]
- Benedict, C.; Scheller, J.; Rose-John, S.; Born, J.; Marshall, L. Enhancing Influence of Intranasal Interleukin-6 on Slow-Wave Activity and Memory Consolidation during Sleep. FASEB J. 2009, 23, 3629–3636. [Google Scholar] [CrossRef]
- Born, J.; Lange, T.; Hansen, K.; Mölle, M.; Fehm, H.L. Effects of Sleep and Circadian Rhythm on Human Circulating Immune Cells. J. Immunol. 1997, 158, 4454–4464. [Google Scholar]
- Dimitrov, S.; Lange, T.; Nohroudi, K.; Born, J. Number and Function of Circulating Human Antigen Presenting Cells Regulated by Sleep. Sleep 2007, 30, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Sakami, S.; Ishikawa, T.; Kawakami, N.; Haratani, T.; Fukui, A.; Kobayashi, F.; Fujita, O.; Araki, S.; Kawamura, N. Coemergence of Insomnia and a Shift in the Th1/Th2 Balance toward Th2 Dominance. NIM 2002, 10, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Orzech, K.M.; Acebo, C.; Seifer, R.; Barker, D.; Carskadon, M.A. Sleep Patterns Are Associated with Common Illness in Adolescents. J. Sleep Res. 2014, 23, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Mello, M.T.D.; Silva, A.; Guerreiro, R.D.C.; da-Silva, F.R.; Esteves, A.M.; Poyares, D.; Piovezan, R.; Treptow, E.; Starling, M.; Rosa, D.S.; et al. Sleep and COVID-19: Considerations about Immunity, Pathophysiology, and Treatment. Sleep Sci. 2020, 13, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long COVID—Mechanisms, Risk Factors, and Management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef] [PubMed]
- Tesoriero, C.; Del Gallo, F.; Bentivoglio, M. Sleep and Brain Infections. Brain Res. Bull. 2019, 145, 59–74. [Google Scholar] [CrossRef]
- Najafi, A.; Sadeghniiat-Haghighi, K.; Alemohammad, Z.B.; Akbarpour, S. COVID-19: Sleep Research Perspectives. Sleep Sci. 2020, 13, 184–185. [Google Scholar] [CrossRef]
- Szmyd, B.; Bartoszek, A.; Karuga, F.F.; Staniecka, K.; Błaszczyk, M.; Radek, M. Medical Students and SARS-CoV-2 Vaccination: Attitude and Behaviors. Vaccines 2021, 9, 128. [Google Scholar] [CrossRef]
- Szmyd, B.; Karuga, F.F.; Bartoszek, A.; Staniecka, K.; Siwecka, N.; Bartoszek, A.; Błaszczyk, M.; Radek, M. Attitude and Behaviors towards SARS-CoV-2 Vaccination among Healthcare Workers: A Cross-Sectional Study from Poland. Vaccines 2021, 9, 218. [Google Scholar] [CrossRef]
- Silva, F.R.D.; Guerreiro, R.D.C.; Andrade, H.D.A.; Stieler, E.; Silva, A.; de Mello, M.T. Does the Compromised Sleep and Circadian Disruption of Night and Shiftworkers Make Them Highly Vulnerable to 2019 Coronavirus Disease (COVID-19)? Chronobiol. Int. 2020, 37, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-Month Consequences of COVID-19 in Patients Discharged from Hospital: A Cohort Study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Carod-Artal, F.J. Post-COVID-19 Syndrome: Epidemiology, Diagnostic Criteria and Pathogenic Mechanisms Involved. Rev. Neurol. 2021, 72, 384–396. [Google Scholar] [CrossRef] [PubMed]
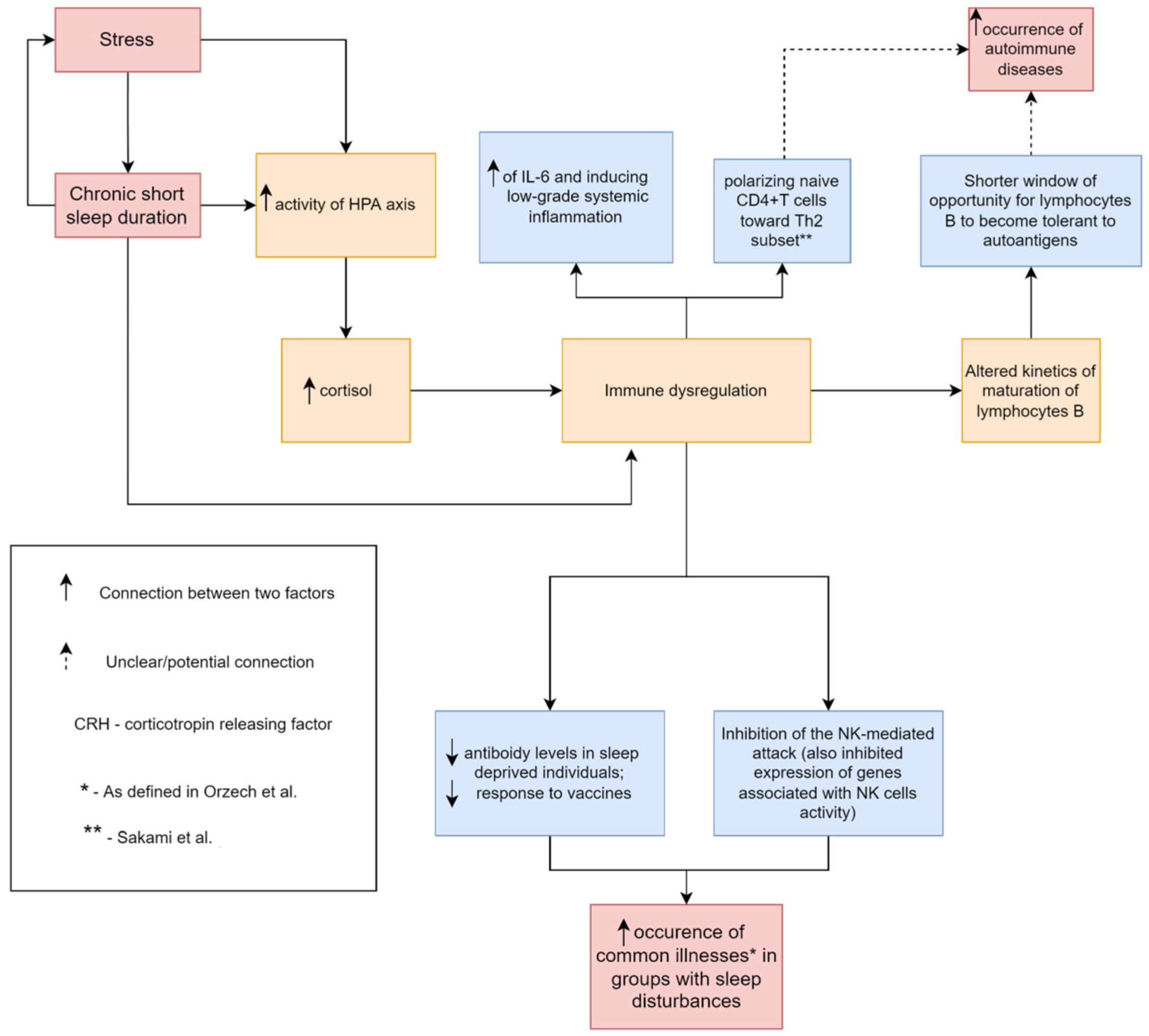
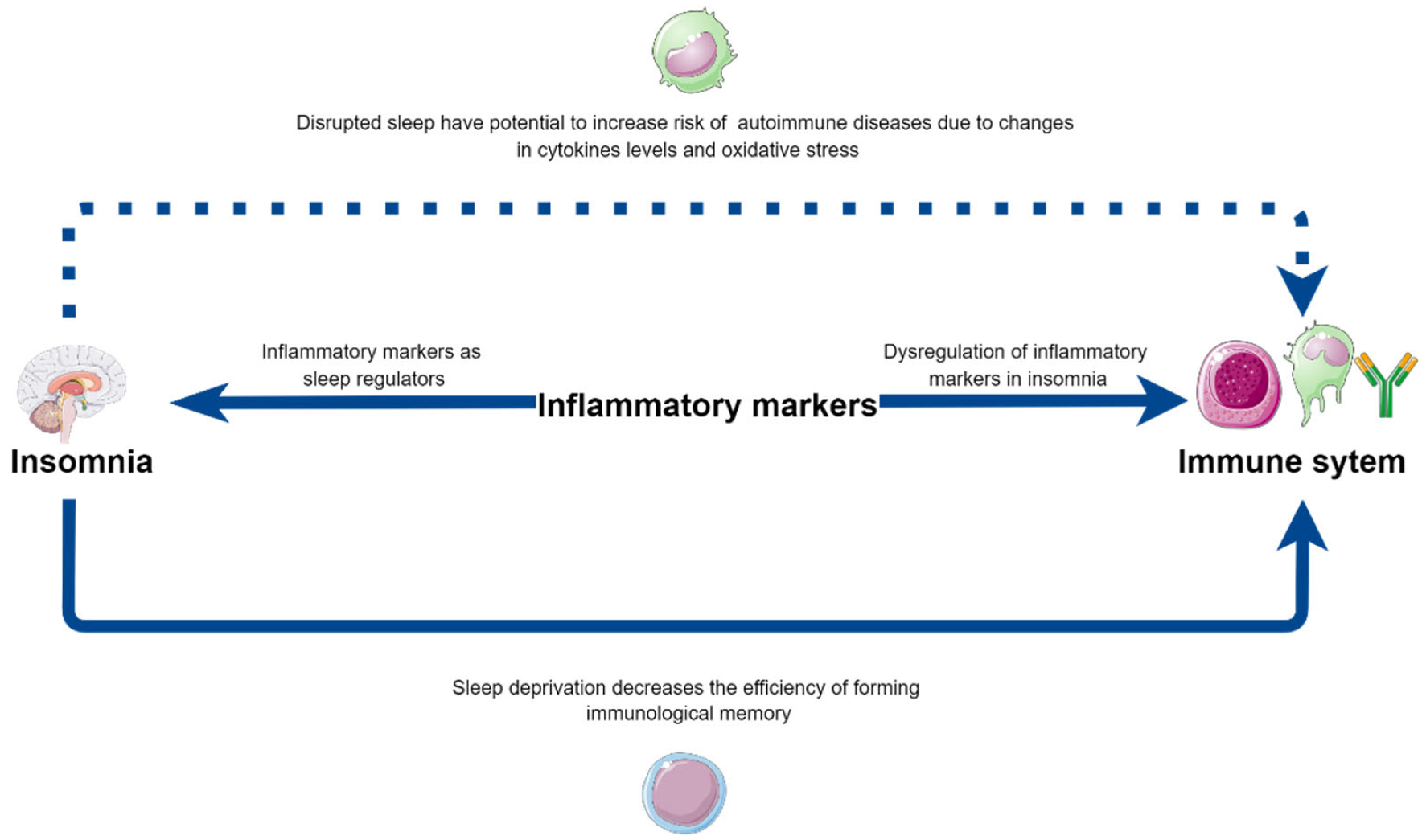
| J. Fernandez-Mendoza et al. [22] | M.R. Irwin [23] | D.P. Venancio, D. Suchecki [25] | S. Yehuda et al. [26] | I. Burgos et al. [29] | |
|---|---|---|---|---|---|
| Year | 2017 | 2016 | 2015 | 2009 | 2006 |
| Type of Study | Cohort study | Meta-analysis | Comparative experimental | Comparative experimental | Controlled clinical trial |
| Patients/Animal Models | adolescents from Penn State Child Cohort | patients | male rats | Male Long Evans hooded rats | Patients |
| Assessment of Sleep Quality | polysomnography | various | - | - | polysomnography, PSQI, SF-A |
| Samples/Patients | 378 | >50,000 | 34 | 48 | 22 |
| Sleep Disturbance | self-reported insomnia symptoms | varied among considered studies | sleep deprivation 18 h/day for 21 days | 72 h of REM deprivation | primary insomnia |
| Results | Adolescents with insomnia symptoms and objective short sleep duration had ↑CRP. | Sleep disturbances associated with ↑CRP and IL-6; insomnia symptoms with ↑IL-6; worse sleep questionnaires scores with ↑IL-6 and CRP; long sleep duration (>8 h) with ↑CRP and IL-6. | ↑ plasma TNF-α and IL-6 compared to the control group. ↑ pNFκB in the RPAT of SR21 rats. ↑levels of plasma endotoxin in SR 21 rats. | ↑ IL-1α, IL-1β, IL-6, homocysteine, corticosterone, and hyperthermia immediately after 72 h of sleep deprivation. After 7 days of recovery, ↑TNF-α and IL-17A. elevated. | The secretion of IL-6 had a biphasic rhythm in both groups. In insomniacs ↑IL-6 levels during the second half of the night. |
| YH Hsiao et al. [9] | B. Sivertsen et al. [12] | HM Seo et al. [52] | KA Young et al. [53] | |
|---|---|---|---|---|
| Year | 2015 | 2014 | 2018 | 2018 |
| Type of Study | Prospective study | Cohort study | Retrospective cohort analysis | Cohort study |
| Sample Size | 24,715 | 84,996 | 154,800 | 436 |
| Sleep Disturbance | Insomnia | Non-apnea sleep disorder | Diagnosed non-organic sleep disorders (F51) or disorders of initiating and maintaining sleep (G47) according to ICD-10 criteria | Self-reported sleep duration lower than 7 h per night |
| Results | People afflicted with insomnia are at ↑ risk of developing RA (OR: 1.87, 95% CI 1.29–2.52). | Non-sleep apnea sleep disorders ↑ the risk of SLE (aHR 1.81, 95% CI 1.50–2.18), RA (aHR 1.45, 95% CI 1.36–1.54), AS (aHR 1.53, 95% CI 1.38–1.70), SS (aHR 1.51, 95% CI 1.43–1.60). | Sleep disorders ↑ risk of alopecia areata (OR: 1.913, 95% CI 1.717–2.171), vitiligo (OR: 1.539, 95% CI 1.236–1.917), Graves’ disease (OR: 1.717, 95% CI 1.562–1.886) and Hashimoto disease (OR: 1.641, 95% CI 1.413–1.905). | Relatives of individuals afflicted with SLE who sleep less than 7 h per night have a ↑ risk of developing SLE (aOR 2.8, 95% CI 1.6–5.1). |
| S. Taylor et al. [8] | Prather et al. [74] | Sharpley et al. [80] | Orzech et al. [85] | Mello et al. [86] | |
|---|---|---|---|---|---|
| Year | 2017 | 2015 | 2016 | 2014 | 2020 |
| Type of study | Controlled clinical trial | Controlled clinical trial | Double-blind placebo controlled study | Field-based study | Narrative review |
| Patients | Young adult college students with Insomnia (65) or No Insomnia (68) | 94 men and 70 women aged 18– 55 years | 16 healthy male and female adult participants | 56 adolescents aged 14–19 years | - |
| Assessment of Sleep Quality | Questionnaires and self-reported sleep diaries | Actigraphy and self-reported sleep diaries | PSG | Actigraphy and In-Person interviews | - |
| Measures | HI assay for detecting the presence of anti-influenza antibodies in serum | Viral-specific antibody levels to RV and numerous variables previously associated with susceptibility to the common cold | IL-6 serum levels | Number of illness bouts, illness duration, and absences from school | A thorough investigation of various sleep disturbances treatment and maintenance during the COVID-19 pandemic. |
| Results | The insomnia group had ↓ baseline antibody levels and remained with ↓ levels four weeks post-vaccination. | Shorter sleep duration was associated with ↑ risk for the development of the cold. | ↓ TST and SE% ↑ WASO, total wake, sleep stage transitions, number of awakenings, and awakening index. | A trend for shorter TST in the 6-day window before the illness was found. | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuna, K.; Szewczyk, K.; Gabryelska, A.; Białasiewicz, P.; Ditmer, M.; Strzelecki, D.; Sochal, M. Potential Role of Sleep Deficiency in Inducing Immune Dysfunction. Biomedicines 2022, 10, 2159. https://doi.org/10.3390/biomedicines10092159
Kuna K, Szewczyk K, Gabryelska A, Białasiewicz P, Ditmer M, Strzelecki D, Sochal M. Potential Role of Sleep Deficiency in Inducing Immune Dysfunction. Biomedicines. 2022; 10(9):2159. https://doi.org/10.3390/biomedicines10092159
Chicago/Turabian StyleKuna, Kasper, Krzysztof Szewczyk, Agata Gabryelska, Piotr Białasiewicz, Marta Ditmer, Dominik Strzelecki, and Marcin Sochal. 2022. "Potential Role of Sleep Deficiency in Inducing Immune Dysfunction" Biomedicines 10, no. 9: 2159. https://doi.org/10.3390/biomedicines10092159
APA StyleKuna, K., Szewczyk, K., Gabryelska, A., Białasiewicz, P., Ditmer, M., Strzelecki, D., & Sochal, M. (2022). Potential Role of Sleep Deficiency in Inducing Immune Dysfunction. Biomedicines, 10(9), 2159. https://doi.org/10.3390/biomedicines10092159






