The Potential of Antibody Technology and Silver Nanoparticles for Enhancing Photodynamic Therapy for Melanoma
Abstract
1. Melanoma
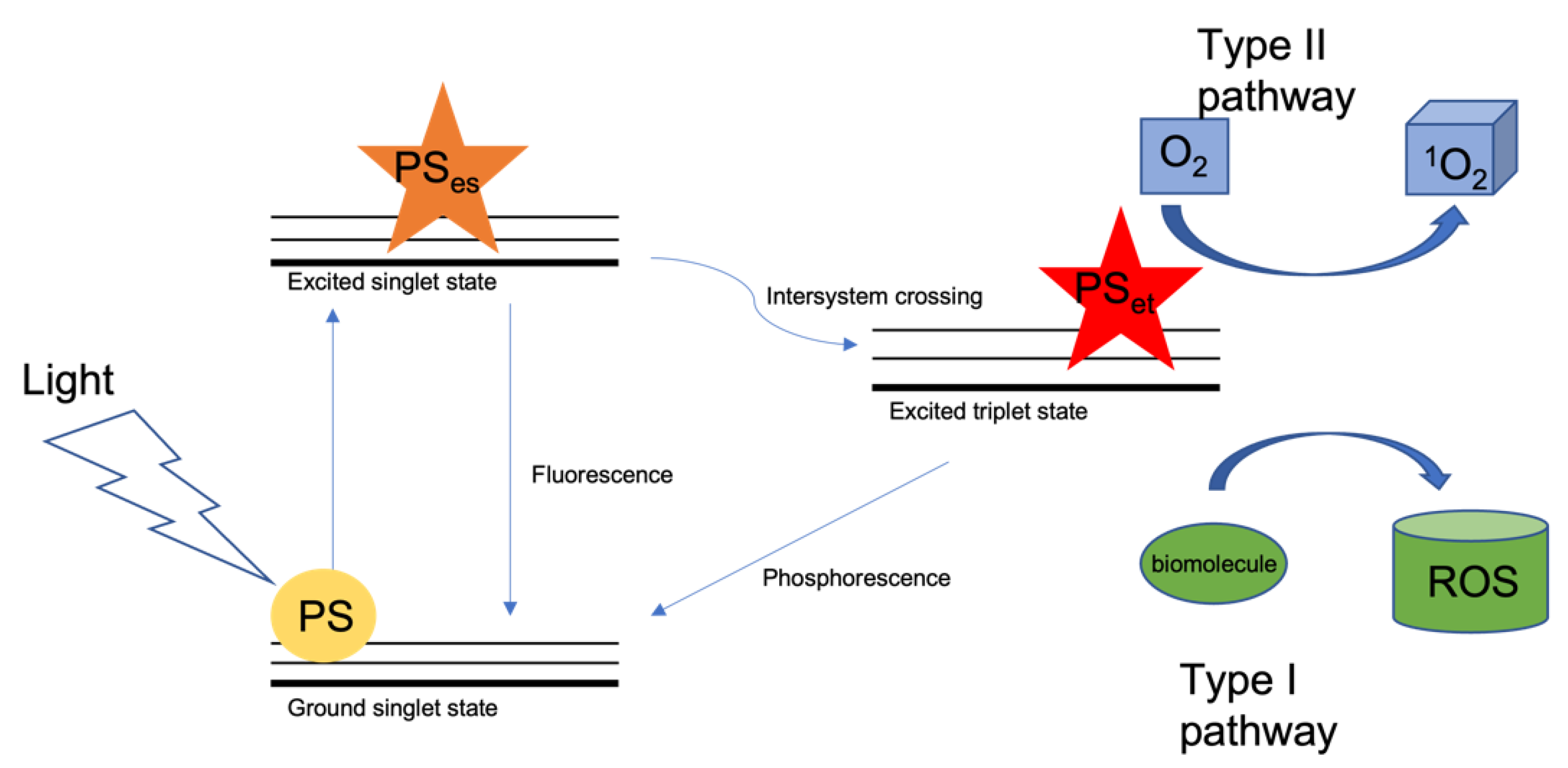
2. Photodynamic Therapy
3. Nanoparticles
4. Targeted Delivery
4.1. Monoclonal Antibodies and Antibody–Drug Conjugates
4.2. Silver Nanobioconjugates in PDT
4.3. ADC Limitations
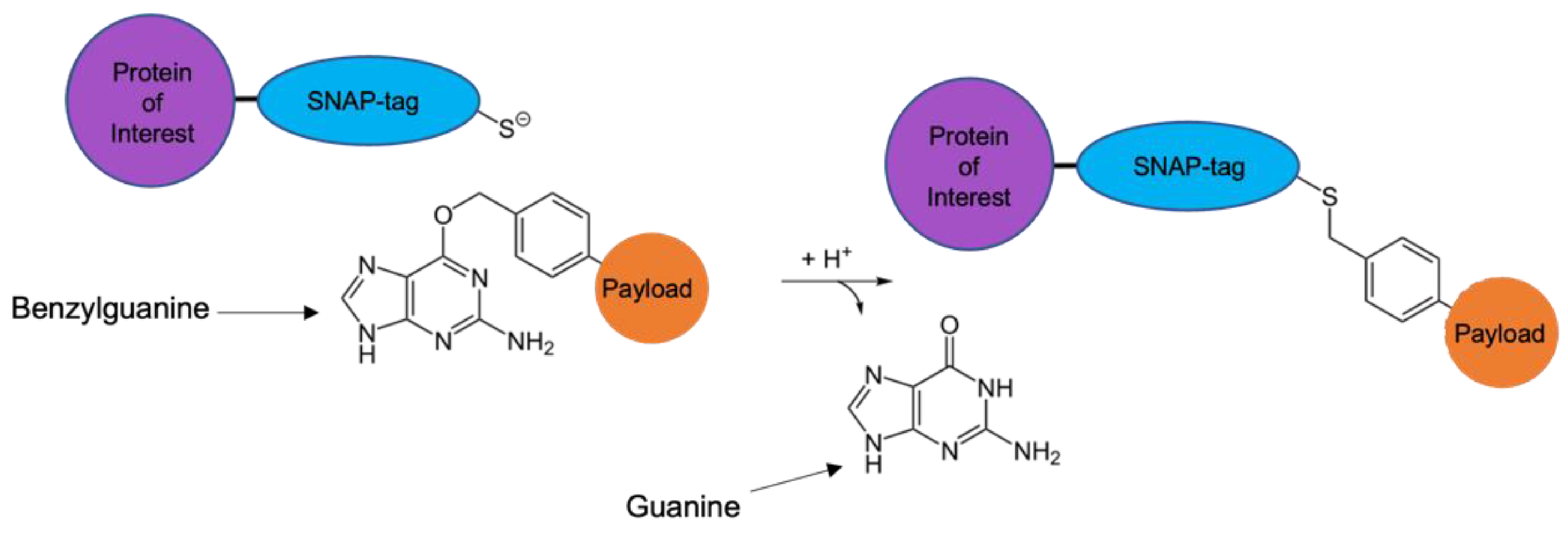
5. SNAP-TAG Technology
6. TAA Target Selection
7. Remaining Challenges
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dratkiewicz, E.; Simiczyjew, A.; Mazurkiewicz, J.; Ziętek, M.; Matkowski, R.; Nowak, D. Hypoxia and Extracellular Acidification as Drivers of Melanoma Progression and Drug Resistance. Cells 2021, 10, 862. [Google Scholar] [CrossRef] [PubMed]
- Bertolotto, C. Melanoma: From melanocyte to genetic alterations and clinical options. Scientifica 2013, 2013, 635203. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Zdzienicki, M.; Nowecki, Z.I.; Van Akkooi, A.C. Surgery of primary melanomas. Cancers 2010, 2, 824. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Castañeda, L.D.; Nova, J.A.; Tovar-Parra, J.D. Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: A systemic review. Melanoma Res. 2020, 30, 62. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.J.; Flaherty, K. MAP kinase signaling and inhibition in melanoma. Oncogene 2013, 32, 2373–2379. [Google Scholar] [CrossRef]
- Amaral, T.; Sinnberg, T.; Meier, F.; Krepler, C.; Levesque, M.; Niessner, H.; Garbe, C. The mitogen-activated protein kinase pathway in melanoma part I—Activation and primary resistance mechanisms to BRAF inhibition. Eur. J. Cancer 2017, 73, 85–92. [Google Scholar] [CrossRef]
- Takeda, T.; Tsubaki, M.; Sakamoto, K.; Ichimura, E.; Enomoto, A.; Suzuki, Y.; Itoh, T.; Imano, M.; Tanabe, G.; Muraoka, O.; et al. Mangiferin, a novel nuclear factor kappa B-inducing kinase inhibitor, suppresses metastasis and tumor growth in a mouse metastatic melanoma model. Toxicol. Appl. Pharmacol. 2016, 306, 105–112. [Google Scholar] [CrossRef]
- Madonna, G.; Ullman, C.D.; Gentilcore, G.; Palmieri, G.; Ascierto, P.A. NF-κB as potential target in the treatment of melanoma. J. Transl. Med. 2012, 10, 53. [Google Scholar] [CrossRef]
- Ambrosini, G.; Do, C.; Tycko, B.; Realubit, R.B.; Karan, C.; Musi, E.; Carvajal, R.D.; Chua, V.; Aplin, A.E.; Schwartz, G.K. Inhibition of NF-κB-dependent signaling enhances sensitivity and overcomes resistance to bet inhibition in uveal melanoma. Cancer Res. 2019, 79, 2415–2425. [Google Scholar] [CrossRef]
- Takeda, T.; Tsubaki, M.; Asano, R.; Itoh, T.; Imano, M.; Satou, T.; Nishida, S. Dimethyl fumarate suppresses metastasis and growth of melanoma cells by inhibiting the nuclear translocation of NF-κB. J. Dermatol. Sci. 2020, 99, 168–176. [Google Scholar] [CrossRef]
- Michaelis, M.; Rothweiler, F.; Nerreter, T.; van Rikxoort, M.; Zehner, R.; Dirks, W.G.; Wiese, M.; Cinatl, J. Association between acquired resistance to PLX4032 (vemurafenib) and ATP-binding cassette transporter expression. BMC Res. Notes 2014, 7, 710. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Si, X.; Gao, Z.; Xu, F.; Zheng, Y. SOX2 upregulates side population cells and enhances their chemoresistant ability by transactivating ABCC1 expression contributing to intrinsic resistance to paclitaxel in melanoma. Mol. Carcinog. 2020, 59, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Qian, Y.; Mathes, S.; Terry, J.; Arnett, N.; Riddell, T.; Stevens, A.; Funk, K.; Bell, S.; Bokal, Z.; et al. Growth hormone receptor antagonism downregulates ATP-binding cassette transporters contributing to improved drug efficacy against melanoma and hepatocarcinoma in vivo. Front. Oncol. 2022, 12, 936145. [Google Scholar] [CrossRef]
- Biteghe, F.A.N.; Padayachee, E.; Davids, L.M.; Chalomie, N.E.T.; Ndong, J.C.; Barth, S. Desensitization of metastatic melanoma cells to therapeutic treatment through repeated exposure to dacarbazine. J. Photochem. Photobiol. B 2020, 211, 111982. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Liu-Smith, F.; Dellinger, R.W.; Salvador, R.; Meysken, F.L.; Estrela, J.M. Oxidative stress and antioxidants in the pathophysiology of malignant melanoma. Biol. Chem. 2019, 400, 589–612. [Google Scholar] [CrossRef]
- Hansda, S.; Ghosh, R. Bystander effect of ultraviolet A radiation protects A375 melanoma cells by induction of antioxidant defense. J. Environ. Sci. Health C Toxicol. Carcinog. 2021, 40, 46–67. [Google Scholar] [CrossRef]
- Blázquez-Castro, A.; Stockert, J.C. Biomedical overview of melanin. 1. Updating melanin biology and chemistry, physico-chemical properties, melanoma tumors, and photothermal therapy. Biocell 2021, 45, 849–862. [Google Scholar] [CrossRef]
- Melanoma: Statistics|Cancer.Net. Available online: https://www.cancer.net/cancer-types/melanoma/statistics (accessed on 28 February 2022).
- Dąbrowski, J.M. Reactive Oxygen Species in Photodynamic Therapy: Mechanisms of Their Generation and Potentiation. Adv. Inorg. Chem. 2017, 70, 343–394. [Google Scholar]
- Sharma, K.V.; Bowers, N.; Davids, L.M. Photodynamic therapy-induced killing is enhanced in depigmented metastatic melanoma cells. Cell Biol. Int. 2011, 35, 939–944. [Google Scholar] [CrossRef]
- Davids, L.M.; Kleemann, B.; Cooper, S.; Kidson, S.H. Melanomas display increased cytoprotection to hypericin-mediated cytotoxicity through the induction of autophagy. Cell Biol. Int. 2009, 33, 1065–1072. [Google Scholar] [CrossRef]
- Turubanova, V.D.; Balalaeva, I.V.; Mishchenko, T.A.; Catanzaro, E.; Alzeibak, R.; Peskova, N.N.; Efimova, I.; Bachert, C.; Mitroshina, E.V.; Krysko, O.; et al. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J. ImmunoTher. Cancer 2019, 7, 350. [Google Scholar] [CrossRef]
- Naidoo, C.; Kruger, C.A.; Abrahamse, H. Simultaneous photodiagnosis and photodynamic treatment of metastatic melanoma. Molecules 2019, 24, 3153. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Luksiene, Z. Photodynamic therapy: Mechanism of action and ways to improve the efficiency of treatment. Medicina 2003, 39, 1137–1150. [Google Scholar]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Gilaberte, Y.; Milla, L.; Salazar, N.; Vera-Alvarez, J.; Kourani, O.; Damian, A.; Rivarola, V.; Roca, M.J.; Espada, J.; González, S.; et al. Cellular intrinsic factors involved in the resistance of squamous cell carcinoma to photodynamic therapy. J. Investig. Dermatol. 2014, 134, 2428–2437. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, Z.; Zhang, J.; Yang, S. Photodynamic therapy enhances skin cancer chemotherapy effects through autophagy regulation. Photodiagnosis Photodyn. Ther. 2019, 28, 159–165. [Google Scholar] [CrossRef]
- Photodynamic Therapy to Treat Cancer. National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/treatment/types/photodynamic-therapy (accessed on 20 August 2022).
- Niculescu, A.G.; Mihai Grumezescu, A.; Photodynamic, A. Photodynamic Therapy-An Up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer–A Review of the Current Clinical Status. Front. Chem. 2021, 9, 608. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Photodynamic Therapy for Cancer: What’s Past is Prologue. Photochem. Photobiol. 2020, 96, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Hadjur, C.; Richard, M.J.; Parat, M.O.; Jardon, P.; Favier, A. Photodynamic effects of hypericin on lipid peroxidation and antioxidant status in melanoma cells. Photochem. Photobiol. 1996, 64, 375–381. [Google Scholar] [CrossRef]
- Kleemann, B.; Loos, B.; Scriba, T.J.; Lang, D.; Davids, L.M. St John’s Wort (Hypericum perforatum L.) photomedicine: Hypericin-photodynamic therapy induces metastatic melanoma cell death. PLoS ONE 2014, 9, e103762. [Google Scholar]
- Davids, L.M.; Kleemann, B.; Kacerovská, D.; Pizinger, K.; Kidson, S.H. Hypericin phototoxicity induces different modes of cell death in melanoma and human skin cells. J. Photochem. Photobiol. B Biol. 2008, 91, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.F.; Liu, Y.R.; Huang, C.C.; Ueng, Y.F. The time-dependent effects of St John’s wort on cytochrome P450, uridine diphosphate-glucuronosyltransferase, glutathione S-transferase, and NAD(P)H-quinone oxidoreductase in mice. J. Food Drug Anal. 2018, 26, 422–431. [Google Scholar] [CrossRef]
- Nicolussi, S.; Drewe, J.; Butterweck, V.; Meyer zu Schwabedissen, H.E. Clinical relevance of St. John’s wort drug interactions revisited. Bri. J. Pharmacol. 2020, 177, 1212–1226. [Google Scholar] [CrossRef]
- Scholz, I.; Liakoni, E.; Hammann, F.; Grafinger, K.E.; Duthaler, U.; Nagler, M.; Krähenbühl, S.; Haschke, M. Effects of Hypericum perforatum (St John’s wort) on the pharmacokinetics and pharmacodynamics of rivaroxaban in humans. Br. J. Clin. Pharmacol. 2021, 87, 1466–1474. [Google Scholar] [CrossRef]
- Colebatch, A.J.; Scolyer, R.A. Trajectories of premalignancy during the journey from melanocyte to melanoma. Pathology 2018, 50, 16–23. [Google Scholar] [CrossRef]
- Faber, D.J.; Mik, E.G.; Aalders, M.C.G.; van Leeuwen, T.G. Light absorption of (oxy-)hemoglobin assessed by spectroscopic optical coherence tomography. Opt. Lett. 2003, 28, 1436–1438. [Google Scholar] [CrossRef]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Cao, Q.; Zhegalova, N.G.; Wang, S.T.; Akers, W.J.; Berezin, M.Y. Multispectral imaging in the extended near-infrared window based on endogenous chromophores. J. Biomed. Opt. 2022, 18, 101318. [Google Scholar] [CrossRef] [PubMed]
- Cheng-Che, L.E. Effects and interactions of increased environmental temperature and UV radiation on photoageing and photocarcinogenesis of the skin. Exp. Dermatol. 2019, 28, 23–27. [Google Scholar]
- Yanine Neira, Z.; Dicker Jiménez, V.; Ortegón Pulido, L.F.; Rueda Rugeles, A.J.; Buitrago-Medina, D.A. Photoaging factors in patients from two healthcare centers in Colombia. J. Cosmet. Dermatol. 2022, 21, 2984–2994. [Google Scholar] [CrossRef]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Sordillo, L.A.; Pratavieira, S.; Pu, Y.; Salas-Ramirez, K.; Shi, L.; Zhang, L.; Budansky, Y.; Alfano, R.R. Third therapeutic spectral window for deep tissue imaging. Proc. SPIE 2014, 8940, 128–134. [Google Scholar]
- Qian, X.; Peng, X.-H.; Ansari, D.O.; Yin-Goen, Q.; Chen, G.Z.; Shin, D.M.; Yang, L.; Young, A.N.; Wang, M.D.; Nie, S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2008, 26, 83–90. [Google Scholar] [CrossRef]
- Lane, L.A.; Xue, R.; Nie, S. Emergence of two near.r.r-infrared windows for in vivo and intraoperative SERS. Curr. Opin. Chem. Biol. 2018, 45, 95–103. [Google Scholar] [CrossRef]
- Nagaya, T.; Nakamura, Y.; Sato, K.; Harada, T.; Choyke, P.L.; Hodge, J.W.; Schlom, J.; Kobayashi, H. Near infrared photoimmunotherapy with avelumab, an anti-programmed death-ligand 1 (PD-L1) antibody. Oncotarget 2017, 8, 8807. [Google Scholar] [CrossRef]
- Nagaya, T.; Nakamura, Y.; Sato, K.; Zhang, Y.-F.; Ni, M.; Choyke, P.L.; Ho, M.; Kobayashi, H. Near infrared photoimmunotherapy with an anti-mesothelin antibody. Oncotarget 2016, 7, 23361–23369. [Google Scholar] [CrossRef]
- Mitsunaga, M.; Nakajima, T.; Sano, K.; Choyke, P.L.; Kobayashi, H. Near Infrared Theranostic Photoimmunotherapy (PIT): Repeated Exposure of Light Enhances the Effect of Immunoconjugate. Bioconjug. Chem. 2012, 23, 604. [Google Scholar] [CrossRef] [PubMed]
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer Cell-Selective In Vivo Near Infrared Photoimmunotherapy Targeting Specific Membrane Molecules. Nat. Med. 2011, 17, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Cognetti, D.M.; Johnson, J.M.; Curry, J.M.; Kochuparambil, S.T.; McDonald, D.; Mott, F.; Fidler, M.J.; Stenson, K.; Vasan, N.R.; Razaq, M.A.; et al. Phase 1/2a, open-label, multicenter study of RM-1929 photoimmunotherapy in patients with locoregional, recurrent head and neck squamous cell carcinoma. Head Neck 2021, 43, 3875–3887. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, R.; Rocha, L.B.; Dąbrowski, J.M.; Arnaut, L.G. Modulation of biodistribution, pharmacokinetics, and photosensitivity with the delivery vehicle of a bacteriochlorin photosensitizer for photodynamic therapy. ChemMedChem 2014, 9, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Kruger, C.; Abrahamse, H. Utilisation of Targeted Nanoparticle Photosensitiser Drug Delivery Systems for the Enhancement of Photodynamic Therapy. Molecules 2018, 23, 2628. [Google Scholar] [CrossRef]
- Mohanraj, V.J.; Chen, Y. Nanoparticles—A Review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef]
- Lim, C.-K.; Heo, J.; Shin, S.; Jeong, K.; Seo, Y.H.; Jang, W.-D.; Park, C.R.; Park, S.Y.; Kim, S.; Kwon, I.C. Nanophotosensitizers toward advanced photodynamic therapy of Cancer. Cancer Lett. 2013, 334, 176–187. [Google Scholar] [CrossRef]
- Master, A.; Livingston, M.; sen Gupta, A. Photodynamic nanomedicine in the treatment of solid tumors: Perspectives and challenges. J. Control Release 2013, 168, 88–102. [Google Scholar] [CrossRef]
- Crous, A.; Abrahamse, H. Effective gold nanoparticle-antibody-mediated drug delivery for photodynamic therapy of lung cancer stem cells. Int. J. Mol. Sci. 2020, 21, 3742. [Google Scholar] [CrossRef]
- Zolnik, B.S.; González-Fernández, Á.; Sadrieh, N.; Dobrovolskaia, M.A. Minireview: Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Shurin, M.; Shvedova, A.A. Current understanding of interactions between nanoparticles and the immune system. Toxicol. Appl. Pharmacol. 2015, 299, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Pattadar, D.K.; Mainali, B.P.; Zamborini, F.P. Size Determination of Metal Nanoparticles Based on Electrochemically Measured Surface-Area-to-Volume Ratios. Anal. Chem. 2018, 90, 9308–9314. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C.X. Development of High-Drug-Loading Nanoparticles. ChemPlusChem 2020, 85, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Nanocarriers in photodynamic therapy—in vitro and in vivo studies. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1509. [Google Scholar] [CrossRef] [PubMed]
- Nicol, J.R.; Dixon, D.; Coulter, J.A. Gold nanoparticle surface functionalization: A necessary requirement in the development of novel nanotherapeutics. Nanomedicine 2015, 10, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Mfouo-Tynga, I.; El-Hussein, A.; Abdel-Harith, M.; Abrahamse, H. Photodynamic ability of silver nanoparticles in inducing cytotoxic effects in breast and lung cancer cell lines. Int. J. Nanomed. 2014, 9, 3771–3780. [Google Scholar]
- Kumar, S.S.D.; Houreld, N.N.; Kroukamp, E.M.; Abrahamse, H. Cellular imaging and bactericidal mechanism of green-synthesized silver nanoparticles against human pathogenic bacteria. J. Photochem. Photobiol. B 2018, 178, 259–269. [Google Scholar] [CrossRef]
- Kumar, S.S.D.; Rajendran, N.K.; Houreld, N.N.; Abrahamse, H. Recent advances on silver nanoparticle and biopolymer-based biomaterials for wound healing applications. Int. J. Biol. Macromol. 2018, 115, 165–175. [Google Scholar] [CrossRef]
- Pradeep Chandran, C.; Mani Rahulan, K.; Ganesan, S. Synthesis and study of photodynamic activity of silver nanoparticles. In Proceedings of the Photonics 2010: 10th International Conference on Fiber Optics & Photonics, Guwahati, India, 11–15 December 2010. [Google Scholar]
- Erdogan, O.; Abbak, M.; Demirbolat, G.M.; Birtekocak, F.; Aksel, M.; Pasa, S.; Cevik, O. Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: The characterization, anticancer potential with photodynamic therapy in MCF7 cells. PLoS ONE 2019, 14, e0216496. [Google Scholar] [CrossRef]
- Srinivasan, S.; Bhardwaj, V.; Nagasetti, A.; Fernandez-Fernandez, A.; McGoron, A.J. Multifunctional surface-enhanced raman spectroscopy-detectable silver nanoparticles for combined photodynamic therapy and pH-triggered chemotherapy. J. Biomed. Nanotechnol. 2016, 12, 2202–2219. [Google Scholar] [CrossRef]
- Aiello, M.B.R.; Castrogiovanni, D.; Parisi, J.; Azcárate, J.C.; Einschlag, F.S.G.; Gensch, T.; Bosio, G.N.; Mártire, D. Photodynamic Therapy in HeLa Cells Incubated with Riboflavin and Pectin-coated Silver Nanoparticles. Photochem. Photobiol. 2018, 94, 1159–1166. [Google Scholar] [CrossRef]
- Mahajan, P.G.; Dige, N.C.; Vanjare, B.D.; Eo, S.-H.; Seo, S.-Y.; Kim, S.J.; Hong, S.-K.; Choi, C.-S.; Lee, K.H. A potential mediator for photodynamic therapy based on silver nanoparticles functionalized with porphyrin. J. Photochem. Photobiol. A Chem. 2019, 377, 26–35. [Google Scholar] [CrossRef]
- Liu, R.; Yang, Z.; Zhang, L.; Zhao, J.; Hou, C.; Zhao, S. A near infrared dye-coated silver nanoparticle/carbon dot nanocomposite for targeted tumor imaging and enhanced photodynamic therapy. Nanoscale Adv. 2020, 2, 489–494. [Google Scholar] [CrossRef]
- Aghajanzadeh, M.; Zamani, M.; Kouchi, F.R.; Eixenberger, J.; Shirini, D.; Estrada, D.; Shirini, F. Synergic Antitumor Effect of Photodynamic Therapy and Chemotherapy Mediated by Nano Drug Delivery Systems. Pharmaceutic 2022, 14, 322. [Google Scholar] [CrossRef] [PubMed]
- El-Hussein, A.; Mfouo-Tynga, I.; Abdel-Harith, M.; Abrahamse, H. Comparative study between the photodynamic ability of gold and silver nanoparticles in mediating cell death in breast and lung cancer cell lines. J. Photochem. Photobiol. B 2015, 153, 67–75. [Google Scholar] [CrossRef]
- García Calavia, P.; Bruce, G.; Pérez-García, L.; Russell, D.A. Photosensitiser-gold nanoparticle conjugates for photodynamic therapy of cancer. Photochem. Photobiol. Sci. 2018, 17, 1534–1552. [Google Scholar] [CrossRef]
- Caro, C.M.P.; Klippstein, R.; Pozo, D.P.A. Silver Nanoparticles: Sensing and Imaging Applications. In Silver Nanoparticles; InTech: London, UK, 2010. [Google Scholar]
- Guerrero, A.R.; Hassan, N.; Escobar, C.A.; Albericio, F.; Kogan, M.J.; Araya, E. Gold nanoparticles for photothermally controlled drug release. Nanomedicine 2014, 9, 2023–2039. [Google Scholar] [CrossRef]
- Di Corato, R.; Palumberi, D.; Marotta, R.; Scotto, M.; Carregal-Romero, S.; Gil, P.R.; Parak, W.J.; Pellegrino, T. Magnetic nanobeads decorated with silver nanoparticles as cytotoxic agents and photothermal probes. Small 2012, 8, 2731–2742. [Google Scholar] [CrossRef]
- El-Hussein, A. Study DNA Damage after Photodynamic Therapy Using Silver Nanoparticles with A549 Cell Line. J. Mol. Nanotechnol. Nanomed. 2016, 7, 2–7. [Google Scholar]
- Emens, L. Breast cancer immunobiology driving immunotherapy: Vaccines and immune checkpoint blockade. Expert Rev. Anticancer. Ther. 2012, 12, 1597–1611. [Google Scholar] [CrossRef]
- Pettinato, M.C. Introduction to Antibody-Drug Conjugates. Antibodies 2021, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; AlDeghaither, D.; O’Connell, A.; Weiner, L.M. Enhancing antibody-dependent cell-mediated cytotoxicity: A strategy for improving antibody-based immunotherapy. Antib. Ther. 2018, 1, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, C.; Jin, X.; Du, Q.; Wu, H.; Dall’Acqua, W.; Mazor, Y. Regulation of antibody-mediated complement-dependent cytotoxicity by modulating the intrinsic affinity and binding valency of IgG for target antigen. MAbs 2020, 12, 1690959. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I.J.N. Elements of cancer immunity and the cancer–immune set point. Nature 2017, 541, 321. [Google Scholar] [CrossRef] [PubMed]
- Juneja, V.R.; McGuire, K.A.; Manguso, R.T.; LaFleur, M.W.; Collins, N.; Haining, W.N.; Freeman, G.J.; Sharpe, A.H. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J. Exp. Med. 2017, 214, 895–904. [Google Scholar] [CrossRef]
- Tauriello, D.V.F.; Sancho, E.; Batlle, E. Overcoming TGFβ-mediated immune evasion in cancer. Nat. Rev. Cancer 2021, 22, 25–44. [Google Scholar] [CrossRef]
- Daassi, D.; Mahoney, K.M.; Freeman, G.J. The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. 2020, 20, 209–215. [Google Scholar] [CrossRef]
- Restifo, N.P.; Smyth, M.J.; Snyder, A. Acquired resistance to immunotherapy and future challenges. Nat. Rev. Cancer 2016, 16, 121. [Google Scholar] [CrossRef]
- Thery, J.-C.; Spano, J.-P.; Azria, D.; Raymond, E.; Penault Llorca, F. Resistance to human epidermal growth factor receptor type 2-targeted therapies. Eur. J. Cancer 2014, 50, 892–901. [Google Scholar] [CrossRef]
- Shapiro, C.L. Highlights of Recent Findings on Quality-of-Life Management for Patients with Cancer and Their Survivors. JAMA Oncol. 2016, 2, 1401–1402. [Google Scholar] [CrossRef]
- Alley, S.C.; Okeley, N.M.; Senter, P.D. Antibody–drug conjugates: Targeted drug delivery for cancer. Curr. Opin. Chem. Biol. 2010, 14, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Pohlit, H.; Bellinghausen, I.; Schoömer, M.; Heydenreich, B.R.; Saloga, J.; Frey, H.J.B. Biodegradable pH-sensitive poly (ethylene glycol) nanocarriers for allergen encapsulation and controlled release. Biomacromolecules 2015, 16, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- von Felbert, V.; Bauerschlag, D.; Maass, N.; Bräutigam, K.; Meinhold-Heerlein, I.; Woitok, M.; Barth, S.; Hussain, A.F. A specific photoimmunotheranostics agent to detect and eliminate skin cancer cells expressing EGFR. J. Cancer Res. Clin. Oncol. 2016, 142, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Leong, A.S.Y.; Zhuang, Z. The Changing Role of Pathology in Breast Cancer Diagnosis and Treatment. Pathobiology 2011, 78, 99–114. [Google Scholar] [CrossRef]
- Panieri, E. Breast cancer screening in developing countries. Best Pr. Res. Clin. Obstet. Gynaecol. 2012, 26, 283–290. [Google Scholar] [CrossRef]
- Mohd-Zahid, M.H.; Mohamud, R.; Abdullah, C.A.C.; Lim, J.; Alem, H.; Hanaffi, W.N.W.; Iskandar, Z.A. Colorectal cancer stem cells: A review of targeted drug delivery by gold nanoparticles. RSC Adv. 2020, 10, 973–985. [Google Scholar] [CrossRef]
- Crous, A.; Abrahamse, H. Photodynamic Therapy and Lung Cancer Stem Cells—The effects of AlPcS4Cl on Isolated Lung Cancer Stem Cells. Food Sci. Hum. Wellness 2019, 8, 73–81. [Google Scholar]
- Ning, S.-T.; Lee, S.-Y.; Wei, M.-F.; Peng, C.-L.; Lin, S.Y.-F.; Tsai, M.-H.; Lee, P.-C.; Shih, Y.-H.; Lin, C.-Y.; Luo, T.-Y.; et al. Targeting Colorectal Cancer Stem-Like Cells with Anti-CD133 Antibody-Conjugated SN-38 Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 17793–17804. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, C.; Kruger, C.A.; Abrahamse, H. Targeted photodynamic therapy treatment of in vitro A375 metastatic melanoma cells. Oncotarget 2019, 10, 6079–6095. [Google Scholar] [CrossRef]
- Simelane, N.W.N.; Kruger, C.A.; Abrahamse, H. Targeted nanoparticle photodynamic diagnosis and therapy of colorectal cancer. Int. J. Mol. Sci. 2021, 22, 9779. [Google Scholar] [CrossRef]
- Tai, S.-P.; Wu, Y.; Shieh, D.-B.; Chen, L.-J.; Lin, K.-J.; Yu, C.-H.; Chu, S.-W.; Chang, C.-H.; Shi, X.-Y.; Wen, Y.-C.; et al. Molecular imaging of cancer cells using plasmon-resonant-enhanced third-harmonic-generation in silver nanoparticles. Adv. Mater. 2007, 19, 4520–4523. [Google Scholar] [CrossRef]
- Khristunova, Y.; Korotkova, E.; Kratochvil, B.; Barek, J.; Dorozhko, E.; Vyskocil, V.; Plotnikov, E.; Voronova, O.; Sidelnikov, V. Preparation and Investigation of Silver Nanoparticle-Antibody Bioconjugates for Electrochemical Immunoassay of Tick-Borne Encephalitis. Sensors 2019, 19, 2103. [Google Scholar] [CrossRef]
- Szymanski, M.S.; Porter, R.A. Preparation and quality control of silver nanoparticle-antibody conjugate for use in electrochemical immunoassays. J. Immunol. Methods 2013, 387, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Pollok, N.E.; Rabin, C.; Smith, L.; Crooks, R.M. Orientation-Controlled Bioconjugation of Antibodies to Silver Nanoparticles. Conjug. Chem 2019, 30, 3078–3086. [Google Scholar] [CrossRef] [PubMed]
- Nima, Z.A.; Alwbari, A.M.; Dantuluri, V.; Hamzah, R.N.; Sra, N.; Motwani, P.; Arnaoutakis, K.; Levy, R.A.; Bohliqa, A.F.; Nedosekin, D.; et al. Targeting nano drug delivery to cancer cells using tunable, multi-layer, silver-decorated gold nanorods. J. Appl. Toxicol. 2017, 37, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Bösmüller, H.; Fischer, A.; Pham, D.L.; Fehm, T.; Capper, D.; von Deimling, A.; Bonzheim, I.; Staebler, A.; Fend, F. Detection of the BRAF V600E mutation in serous ovarian tumors: A comparative analysis of immunohistochemistry with a mutation-specific monoclonal antibody and allele-specific PCR. Hum. Pathol. 2013, 44, 329–335. [Google Scholar] [CrossRef]
- Fitting, J.; Blume, T.; Ten Haaf, A.; Blau, W.; Gattenlöhner, S.; Tur, M.K.; Barth, S. Phage display-based generation of novel internalizing antibody fragments for immunotoxin-based treatment of acute myeloid leukemia. MAbs 2015, 7, 390–402. [Google Scholar] [CrossRef]
- Axup, J.Y.; Bajjuri, K.M.; Ritland, M.; Hutchins, B.M.; Kim, C.H.; Kazane, S.A.; Halder, R.; Forsyth, J.S.; Santidrian, A.F.; Stafin, K.; et al. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc. Natl. Acad. Sci. USA 2012, 109, 16101–16106. [Google Scholar] [CrossRef]
- Liu, J.; Dang, H.; Wang, X.W. The significance of intertumor and intratumor heterogeneity in liver cancer. Exp. Mol. Med. 2018, 50, e416. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Paik, J. Nivolumab Plus Relatlimab: First Approval. Drugs 2022, 82, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Milton Harris, J.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Hamblett, K.J.; Senter, P.D.; Chace, D.F.; Sun, M.M.C.; Lenox, J.; Cerveny, C.G.; Kissler, K.M.; Bernhardt, S.X.; Kopcha, A.K.; Zabinski, R.F.; et al. Effects of Drug Loading on the Antitumor Activity of a Monoclonal Antibody Drug Conjugate. Proc. Am. Assoc. Cancer Res. 2004, 10, 7063–7070. [Google Scholar] [CrossRef]
- Lyon, R.P.; Bovee, T.D.; Doronina, S.O.; Burke, P.J.; Hunter, J.H.; Neff-LaFord, H.D.; Jonas, M.; Anderson, M.E.; Setter, J.R.; Senter, P.D. Reducing hydrophobicity of homogeneous antibody-drug conjugates improves pharmacokinetics and therapeutic index. Nat. Biotechnol. 2015, 33, 733–735. [Google Scholar] [CrossRef]
- Simmons, J.K.; Burke, P.J.; Cochran, J.H.; Pittman, P.G.; Lyon, R.P. Reducing the antigen-independent toxicity of antibody-drug conjugates by minimizing their non-specific clearance through PEGylation. Toxicol. Appl. Pharmacol. 2020, 392, 114932. [Google Scholar] [CrossRef]
- Oliveira, S.; Van Dongen, G.A.M.S.; Stigter-Van Walsum, M.; Roovers, R.C.; Stam, J.C.; Mali, W.; Van Diest, P.J.; Van Bergen En Henegouwen, P.M.P. Rapid Visualization of Human Tumor Xenografts through Optical Imaging with a Near-infrared Fluorescent Anti—Epidermal Growth Factor Receptor Nanobody. Mol. Imaging 2012, 11, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Zangemeister-Wittke, U. Antibodies for targeted cancer therapy–technical aspects and clinical perspectives. Pathobiology 2005, 72, 279–286. [Google Scholar] [CrossRef]
- Dolan, M.E.; Moschel, R.C.; Pegg, A.E. Depletion of mammalian O6-alkylguanine-DNA alkyltransferase activity by O6-benzylguanine provides a means to evaluate the role of this protein in protection against carcinogenic and therapeutic alkylating agents. Proc. Natl. Acad. Sci. USA 1990, 87, 5368–5372. [Google Scholar] [CrossRef]
- Keppler, A.; Kindermann, M.; Gendreizig, S.; Pick, H.; Vogel, H.; Johnsson, K. Labeling of fusion proteins of O6-alkylguanine-DNA alkyltransferase with small molecules in vivo and in vitro. Methods 2004, 32, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E.; Dolan, M.E.; Moschel, R.C. Structure, function, and inhibition of O6-alkylguanine-DNA alkyltransferase. Prog. Nucleic Acid. Res. Mol. Biol. 1995, 51, 167–223. [Google Scholar] [PubMed]
- Kampmeier, F.; Ribbert, M.; Nachreiner, T.; Dembski, S.; Beaufils, F.; Brecht, A.; Barth, S. Site-specific, covalent labeling of recombinant antibody fragments via fusion to an engineered version of 6-O-alkylguanine DNA alkyltransferase. Bioconjug. Chem. 2009, 20, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. MAbs 2010, 2, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar] [CrossRef]
- Rodrigo, G.; Gruvegård, M.; van Alstine, J.M. Antibody Fragments and Their Purification by Protein L Affinity Chromatography. Antibodies 2015, 4, 259–277. [Google Scholar] [CrossRef]
- Puettmann, C.; Kolberg, K.; Hagen, S.; Schmies, S.; Fischer, R.; Naehring, J.; Barth, S. A monoclonal antibody for the detection of SNAP/CLIP-tagged proteins. Immunol. Lett. 2013, 150, 69–74. [Google Scholar] [CrossRef]
- Kampmeier, F.; Niesen, J.; Koers, A.; Ribbert, M.; Brecht, A.; Fischer, R.; Kießling, F.; Barth, S.; Thepen, T. Rapid optical imaging of EGF receptor expression with a single-chain antibody SNAP-tag fusion protein. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1926–1934. [Google Scholar] [CrossRef]
- Kijanka, M.; Warnders, F.J.; El Khattabi, M.; Lub-de Hooge, M.; van Dam, G.M.; Ntziachristos, V.; de Vries, L.; Oliveira, S.; van Bergen En Henegouwen, P.M. Rapid optical imaging of human breast tumour xenografts using anti-HER2 VHHs site-directly conjugated to IRDye 800CW for image-guided surgery. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1718–1729. [Google Scholar] [CrossRef]
- Gong, H.; Kovar, J.L.; Baker, B.; Zhang, A.; Cheung, L.; Draney, D.R.; Corrêa, I.R., Jr.; Xu, M.Q.; Olive, D.M. Near-Infrared Fluorescence Imaging of Mammalian Cells and Xenograft Tumors with SNAP-Tag. PLoS ONE 2012, 7, e34003. [Google Scholar] [CrossRef]
- Li, Q.; White, J.B.; Peterson, N.C.; Rickert, K.W.; Lloyd, C.O.; Allen, K.L.; Rosenthal, K.; Gao, X.; Wu, H.; Dall’Acqua, W.F.; et al. Tumor uptake of pegylated diabodies: Balancing systemic clearance and vascular transport. Journal of Controlled Release. J. Control. Release 2018, 279, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Liu, J.; Deng, W.; Xing, J.; Li, Q.; Wang, Z. Site-specific PEGylation of an anti-CEA/CD3 bispecific antibody improves its antitumor efficacy. Int. J. Nanomed. 2018, 13, 3189–3201. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.F.; Kampmeier, F.; von Felbert, V.; Merk, H.-F.; Tur, M.K.; Barth, S. SNAP-Tag Technology Mediates Site Specific Conjugation of Antibody Fragments with a Photosensitizer and Improves Target Specific Phototoxicity in Tumor Cells. Bioconjug. Chem. 2011, 22, 2487–2495. [Google Scholar] [CrossRef]
- Bauerschlag, D.; Meinhold-Heerlein, I.; Maass, N.; Bleilevens, A.; Bräutigam, K.; Al Rawashdeh, W.; Di Fiore, S.; Haugg, A.M.; Gremse, F.; Steitz, J.; et al. Detection and Specific Elimination of EGFR + Ovarian Cancer Cells Using a Near Infrared Photoimmunotheranostic Approach. Pharm. Res. 2017, 34, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Davids, L.M.; Biteghe, F.N.; Padayachee, E.; Barth, S. Targeted photodynamic therapy enhances the therapeutic efficacy of combination therapy (PDT and chemotherapy) on chemoresistant melanoma cells. Cancer Res. 2019, 79 (Suppl. S13), 3732. [Google Scholar] [CrossRef]
- Hussain, A.F.; Heppenstall, P.A.; Kampmeier, F.; Meinhold-Heerlein, I.; Barth, S. One-step site-specific antibody fragment auto-conjugation using SNAP-tag technology. Nat. Protoc. 2019, 14, 3101–3125. [Google Scholar] [CrossRef]
- Ritchie, M.; Tchistiakova, L.; Scott, N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. MAbs 2013, 5, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Almutairi, A.R.; McBride, A.; Slack, M.; Erstad, B.L.; Abraham, I. Potential Immune-Related Adverse Events Associated with Monotherapy and Combination Therapy of Ipilimumab, Nivolumab, and Pembrolizumab for Advanced Melanoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 91. [Google Scholar] [CrossRef]
- Muenst, S.; Schaerli, A.R.; Gao, F.; Däster, S.; Trella, E.; Droeser, R.A.; Muraro, M.G.; Zajac, P.; Zanetti, R.; Gillanders, W.E.; et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res. Treat. 2014, 146, 15–24. [Google Scholar] [CrossRef]
- Rose, A.A.N.; Armstrong, S.M.; Hogg, D.; Butler, M.O.; Saibil, S.D.; Arteaga, D.P.; Muniz, T.P.; Kelly, D.; Ghazarian, D.; King, I.; et al. Biologic subtypes of melanoma predict survival benefit of combination anti-PD1+anti-CTLA4 immune checkpoint inhibitors versus anti-PD1 monotherapy. J. Immunother. Cancer 2021, 9, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef]
- Kim, Y.J.; Won, C.H.; Lee, M.W.; Choi, J.H.; Chang, S.E.; Lee, W.J. Correlation Between Tumor-Associated Macrophage and Immune Checkpoint Molecule Expression and Its Prognostic Significance in Cutaneous Melanoma. J. Clin. Med. 2020, 9, 2500. [Google Scholar] [CrossRef]
- Tong, J.T.W.; Harris, P.W.R.; Brimble, M.A.; Kavianinia, I. An Insight into FDA Approved Antibody-Drug Conjugates for Cancer Therapy. Molecules 2021, 26, 5847. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.M.; Crescioli, S.; Mele, S.; Sachouli, E.; Cheung, A.; Chui, C.K.; Andriollo, P.; Jackson, P.J.M.; Lacy, K.E.; Spicer, J.F.; et al. A Novel Antibody-Drug Conjugate (ADC) Delivering a DNA Mono-Alkylating Payload to Chondroitin Sulfate Proteoglycan (CSPG4)-Expressing Melanoma. Cancers 2020, 12, 1029. [Google Scholar] [CrossRef] [PubMed]
- Price, M.A.; Wanshura, L.E.C.; Yang, J.; Carlson, J.; Xiang, B.; Li, G.; Ferrone, S.; Dudek, A.Z.; Turley, E.A.; McCarthy, J.B. CSPG4, a potential therapeutic target, facilitates malignant progression of melanoma. Pigment. Cell Melanoma Res. 2011, 24, 1148–1157. [Google Scholar] [CrossRef]
- Yang, J.; Price, M.A.; Neudauer, C.L.; Wilson, C.; Ferrone, S.; Xia, H.; Iida, J.; Simpson, M.A.; McCarthy, J.B. Melanoma chondroitin sulfate proteoglycan enhances FAK and ERK activation by distinct mechanisms. J. Cell Biol. 2004, 165, 881–891. [Google Scholar] [CrossRef]
- Svendsen, A.; Verhoeff, J.J.; Immervoll, H.; Brøgger, J.C.; Kmiecik, J.; Poli, A.; Netland, I.A.; Prestegarden, L.; Planaguma, J.; Torsvik, A.; et al. Expression of the progenitor marker NG2/CSPG4 predicts poor survival and resistance to ionising radiation in glioblastoma. Acta Neuropathol. 2011, 122, 495–510. [Google Scholar] [CrossRef]
- Uranowska, K.; Kalic, T.; Valtsanidis, V.; Kitzwögerer, M.; Breiteneder, H.; Hafner, C. Expression of chondroitin sulfate proteoglycan 4 (CSPG4) in melanoma cells is downregulated upon inhibition of BRAF. Oncol. Rep. 2021, 45, 14. [Google Scholar] [CrossRef]
- Ghosh, A.; Syed, S.; Kumar, M.; Carpenter, T.J.; Teixeira, J.M.; Houairia, N.; Negi, S.; Tanwar, P.S. In Vivo Cell Fate Tracing Provides No Evidence for Mesenchymal to Epithelial Transition in Adult Fallopian Tube and Uterus. Cell Rep. 2020, 31, 107631. [Google Scholar] [CrossRef]
- Yang, J.; Price, M.A.; Li, G.Y.; Bar-Eli, M.; Salgia, R.; Jagedeeswaran, R.; Carlson, J.H.; Ferrone, S.; Turley, E.A.; McCarthy, J.B. Melanoma proteoglycan modifies gene expression to stimulate tumor cell motility, growth, and epithelial-to-mesenchymal transition. Cancer Res. 2009, 69, 7538–7547. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Makagiansar, I.T.; Fukushi, J.-I.; Liu, F.-T.; Fukuda, M.N.; Stallcup, W.B. Molecular basis of interaction between NG2 proteoglycan and galectin-3. J. Cell. Biochem. 2006, 98, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Natali, P.; Bigotti, A.; Cavalieri, R.; Wakabayaski, S.; Taniguchi, M.; Ferrone, S. Distribution of a cross-species melanoma-associated antigen in normal and neoplastic human tissues. J. Investig. Dermatol 1985, 85, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Osada, T.; Wang, Y.; Yu, L.; Sakakura, K.; Katayama, A.; McCarthy, J.B.; Brufsky, A.; Chivukula, M.; Khoury, T.; et al. CSPG4 protein as a new target for the antibody-based immunotherapy of triple-negative breast cancer. Natl. Cancer Inst. 2010, 102, 1496–1512. [Google Scholar] [CrossRef] [PubMed]
- Bonhoure, A.; Henry, L.; Morille, M.; Aissaoui, N.; Bellot, G.; Stoebner, P.E.; Vidal, M. Melanotransferrin is efficiently sorted on the surface of exosomes secreted by melanoma cells. Melanoma Res. 2021, 31, 338–351. [Google Scholar] [CrossRef]
- Abrams, T.; Connor, A.; Fanton, C.; Cohen, S.B.; Huber, T.; Miller, K.; Hong, E.E.; Niu, X.; Kline, J.; Ison-Dugenny, M.; et al. Preclinical Antitumor Activity of a Novel Anti–c-KIT Antibody–Drug Conjugate against Mutant and Wild-type c-KIT–Positive Solid Tumors. Clin. Cancer Res. 2018, 24, 4297–4308. [Google Scholar] [CrossRef]
- Cardillo, T.M.; Govindan, S.V.; Zalath, M.B.; Rossi, D.L.; Wang, Y.; Chang, C.-H.; Goldenberg, D.M. IMMU-140, a novel SN-38 antibody-drug conjugate targeting HLA-DR, mediates dual cytotoxic effects in hematologic cancers and malignant melanoma. Mol. Cancer Ther. 2018, 17, 150–160. [Google Scholar] [CrossRef]
- Capone, E.; Lamolinara, A.; D’Agostino, D.; Rossi, C.; De Laurenzi, V.; Iezzi, M.; Iacobelli, S.; Sala, G. EV20-mediated delivery of cytotoxic auristatin MMAF exhibits potent therapeutic efficacy in cutaneous melanoma. J. Control. Release 2018, 277, 48–56. [Google Scholar] [CrossRef]
- Ott, P.A.; Pavlick, A.C.; Johnson, D.B.; Hart, L.L.; Infante, J.R.; Luke, J.J.; Lutzky, J.; Rothschild, N.E.; Spitler, L.E.; Cowey, C.L.; et al. A phase 2 study of glembatumumab vedotin, an antibody-drug conjugate targeting glycoprotein NMB, in patients with advanced melanoma. Cancer 2019, 125, 1113–1123. [Google Scholar] [CrossRef]
- Boshuizen, J.; Koopman, L.A.; Krijgsman, O.; Shahrabi, A.; van den Heuvel, E.G.; Ligtenberg, M.A.A.; Vredevoogd, D.W.; Kemper, K.; Kuilman, T.; Song, J.-Y.; et al. Cooperative targeting of melanoma heterogeneity with an AXL antibody-drug conjugate and BRAF/MEK inhibitors. Nat. Med. 2018, 24, 203–212. [Google Scholar] [CrossRef]
- Tang, J.; Zhou, H.; Hou, X.; Wang, L.; Li, Y.; Pang, Y.; Chen, C.; Jiang, G.; Liu, Y. Enhanced anti-tumor efficacy of temozolomide-loaded carboxylated poly(amido-amine) combined with photothermal/photodynamic therapy for melanoma treatment. Cancer Lett. 2018, 423, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Bazylińska, U.; Kulbacka, J.; Schmidt, J.; Talmon, Y.; Murgia, S. Polymer-free cubosomes for simultaneous bioimaging and photodynamic action of photosensitizers in melanoma skin cancer cells. J. Colloid Interface Sci. 2018, 522, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Clemente, N.; Miletto, I.; Gianotti, E.; Invernizzi, M.; Marchese, L.; Dianzani, U.; Renò, F. Verteporfin-loaded mesoporous silica nanoparticles inhibit mouse melanoma proliferation in vitro and in vivo. J. Photochem. Photobiol. B Biol. 2019, 197, 111533. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Lim, S.J.; Lee, M.K. Chitosan-coated liposomes to stabilize and enhance transdermal delivery of indocyanine green for photodynamic therapy of melanoma. Carbohydr. Polym. 2019, 224, 115143. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xue, Y.; Tian, J.; Liu, Z.; Zhuang, A.; Gu, P.; Zhou, H.; Zhang, W.; Fan, X. Fluorinated-functionalized hyaluronic acid nanoparticles for enhanced photodynamic therapy of ocular choroidal melanoma by ameliorating hypoxia. Carbohydr. Polym. 2020, 237, 116119. [Google Scholar] [CrossRef]
- Li, D.; Ren, J.; Li, J.; Zhang, Y.; Lou, Y.; Zhu, J.; Liu, P.; Chen, Y.; Yu, Z.; Zhao, L.; et al. Ferroptosis-apoptosis combined anti-melanoma immunotherapy with a NIR-responsive upconverting mSiO2 photodynamic platform. Chem. Eng. J. 2021, 419, 129557. [Google Scholar] [CrossRef]
- Ghazaeian, M.; Khorsandi, K.; Hosseinzadeh, R.; Naderi, A.; Abrahamse, H. Curcumin–silica nanocomplex preparation, hemoglobin and DNA interaction and photocytotoxicity against melanoma cancer cells. J. Biomol. Struct. Dyn. 2021, 39, 6606–6616. [Google Scholar] [CrossRef]
- Omura, G.; Honma, Y.; Matsumoto, Y.; Shinozaki, T.; Itoyama, M.; Eguchi, K.; Sakai, T.; Yokoyama, K.; Watanabe, T.; Ohara, A.; et al. Transnasal photoimmunotherapy with cetuximab sarotalocan sodium: Outcomes on the local recurrence of nasopharyngeal squamous cell carcinoma. Auris Nasus Larynx 2022, in press. [Google Scholar] [CrossRef]
- DeWitt, J.M.; Sandrasegaran, K.; O’Neil, B.; House, M.G.; Zyromski, N.J.; Sehdev, A.; Perkins, S.M.; Flynn, J.; McCranor, L.; Shahda, S. Phase 1 study of EUS-guided photodynamic therapy for locally advanced pancreatic cancer. Gastrointest. Endosc. 2019, 89, 390–398. [Google Scholar] [CrossRef]
- Hanada, Y.; Pereira, S.P.; Pogue, B.; Maytin, E.V.; Hasan, T.; Linn, B.; Mangels-Dick, T.; Wang, K.K. EUS-guided verteporfin photodynamic therapy for pancreatic cancer. Gastrointest. Endosc. 2021, 94, 179–186. [Google Scholar] [CrossRef]
- Doustvandi, M.A.; Mohammadnejad, F.; Mansoori, B.; Tajalli, H.; Mohammadi, A.; Mokhtarzadeh, A.; Baghbani, E.; Khaze, V.; Hajiasgharzadeh, K.; Moghaddam, M.M.; et al. Photodynamic therapy using zinc phthalocyanine with low dose of diode laser combined with doxorubicin is a synergistic combination therapy for human SK-MEL-3 melanoma cells. Photodiagnosis Photodyn. Ther. 2019, 28, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Cherukula, K.; Bang, Y.J.; Vijayan, V.; Moon, M.J.; Thiruppathi, J.; Puth, S.; Jeong, Y.Y.; Park, I.-K.; Lee, S.E.; et al. Combination of Photodynamic Therapy and a Flagellin-Adjuvanted Cancer Vaccine Potentiated the Anti-PD-1-Mediated Melanoma Suppression. Cells 2020, 9, 2432. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, K.; Hosseinzadeh, R.; Chamani, E. Molecular interaction and cellular studies on combination photodynamic therapy with rutoside for melanoma A375 cancer cells: An in vitro study. Cancer Cell Int. 2020, 20, 525. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef]
- Xie, Q.; Li, Z.; Liu, Y.; Zhang, D.; Su, M.; Niitsu, H.; Lu, Y.; Coffey, R.J.; Bai, M. Translocator protein-targeted photodynamic therapy for direct and abscopal immunogenic cell death in colorectal cancer. Acta Biomater. 2021, 134, 716–729. [Google Scholar] [CrossRef]
- Lou, J.; Aragaki, M.; Bernards, N.; Kinoshita, T.; Mo, J.; Motooka, Y.; Ishiwata, T.; Gregor, A.; Chee, T.; Chen, Z.; et al. Repeated porphyrin lipoprotein-based photodynamic therapy controls distant disease in mouse mesothelioma via the abscopal effect. Nanophotonics 2021, 10, 3279–3294. [Google Scholar] [CrossRef]
- Ghosh, P.; Hanada, Y.; Linn, B.; Mangels-Dick, T.; Roy, B.; Wang, K. Abscopal Effect of Intratumoral Photodynamic Therapy Is Associated with Increased Tumor Directed T Cells. Am. J. Gastroenterol. 2021, 116, S676–S677. [Google Scholar] [CrossRef]

| Photosensitiser | Cancer | Country/Region of Approval | Year of First Approval |
|---|---|---|---|
| Photofrin | Bladder cancer, lung cancer, advanced obstructive oesophageal cancer, early-stage non-small-cell lung cancer, cervical cancer | Canada, Japan, USA, Europe | 1993 |
| Foscan | Advanced head and neck squamous cell carcinoma | Europe | 2001 |
| Talaporfin sodium/Laserphyrin | Early-stage lung cancer | Japan | 2004 |
| 5-ALA Ameluz/Levulan Metvix/Metvixia | Basal cell carcinoma, optical imaging in high-grade gliomas and bladder cancer | USA, Europe, New Zealand | 2007 |
| Redaporfin | Biliary tract cancer | Europe, USA | 2015 |
| SGX301 (synthetic hypericin) | Early stage cutaneous T-cell lymphoma | USA | 2021 |
| NIR-PIT (IR700) with Erbitux anti-EGFR mAb | Recurrent head and neck cancer | Japan | 2021 |
| Study (Authors, Year) | Photosensitiser | Absorption Wavelength (nm) | Antibody | Nanoparticle |
|---|---|---|---|---|
| Tang et al., 2018 [165] | Indocyanine green (ICG) | 808 | None | Carboxylated poly (amido-amine) |
| Bazylińska et al., 2018 [166] | Chlorin e6 (Ce6) | 600–630 | None | Cubosomes |
| Bazylińska et al., 2018 [166] | Meso-tetraphenylporphine-Mn (III) chloride (TPP-Mn (III)cl) | 530–570 | None | Cubosomes |
| Clemente et al., 2019 [167] | Verteporfin (Ver) | 690 | None | Mesoporous silica nanoparticles (MSNs) |
| Lee et al., 2019 [168] | ICG | 785 | None | Chitosan-coated liposomes |
| Li et al., 2020 [169] | Pyropheophorbide a (Ppa) | 670 | None | Amphiphilic micelles |
| Li et al., 2020 [170] | Ce6 | 980 | None | Mesoporous coated upconverting nanoparticles |
| Naidoo et al., 2019 [103] | Zinc phthalocyanine tetra-sulphonic acid | 673 | Melanoma inhibitory activity antigen | Gold |
| Ghazaeian et al., 2021 [171] | Curcumin | 465 | None | Silica |
| Biteghe et al., 2020 [14] | Hypericin with doxorubicin | 561 | None | None |
| Zhang et al., 2019 [30] | 5-ALA with 5-FU and 5-MA pre-treatment | 633 ± 10 | None | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malindi, Z.; Barth, S.; Abrahamse, H. The Potential of Antibody Technology and Silver Nanoparticles for Enhancing Photodynamic Therapy for Melanoma. Biomedicines 2022, 10, 2158. https://doi.org/10.3390/biomedicines10092158
Malindi Z, Barth S, Abrahamse H. The Potential of Antibody Technology and Silver Nanoparticles for Enhancing Photodynamic Therapy for Melanoma. Biomedicines. 2022; 10(9):2158. https://doi.org/10.3390/biomedicines10092158
Chicago/Turabian StyleMalindi, Zaria, Stefan Barth, and Heidi Abrahamse. 2022. "The Potential of Antibody Technology and Silver Nanoparticles for Enhancing Photodynamic Therapy for Melanoma" Biomedicines 10, no. 9: 2158. https://doi.org/10.3390/biomedicines10092158
APA StyleMalindi, Z., Barth, S., & Abrahamse, H. (2022). The Potential of Antibody Technology and Silver Nanoparticles for Enhancing Photodynamic Therapy for Melanoma. Biomedicines, 10(9), 2158. https://doi.org/10.3390/biomedicines10092158








