Inflammatory Blood Parameters as Biomarkers for Response to Immune Checkpoint Inhibition in Metastatic Melanoma Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patient Cohort
2.2. Patient Characteristics
2.3. Blood Parameters
2.4. Clinical Outcome Short-Term Response to Immune Checkpoint Inhibition
2.5. Clinical Outcome Long-Term Response to Immune Checkpoint Inhibition
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
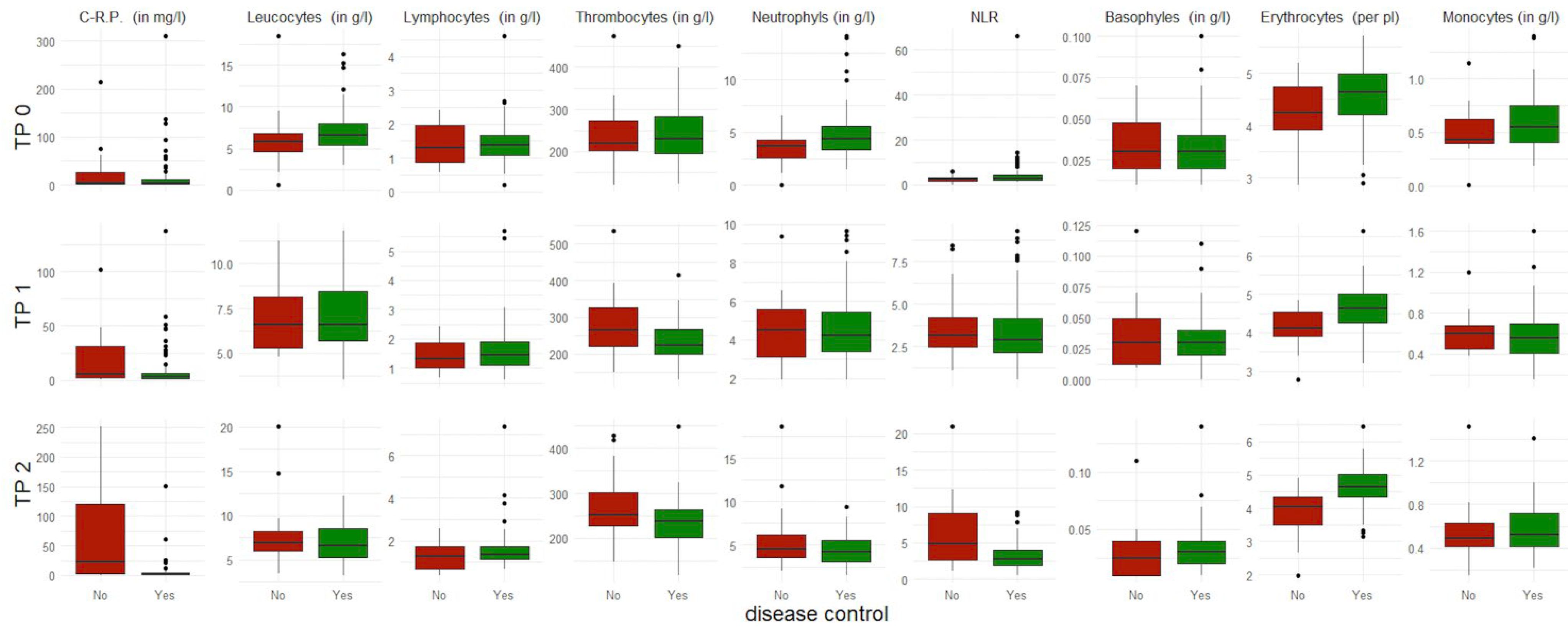
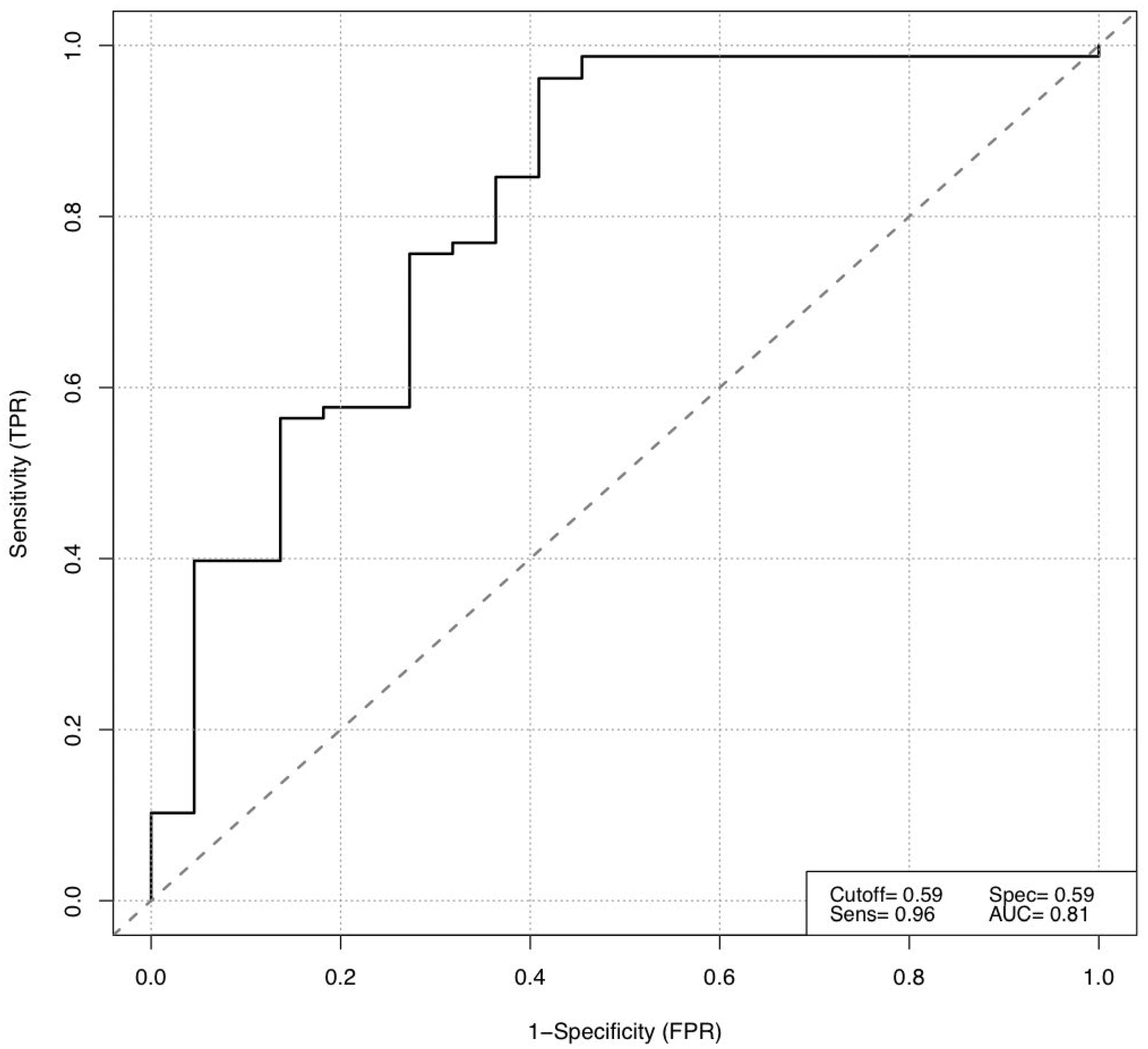
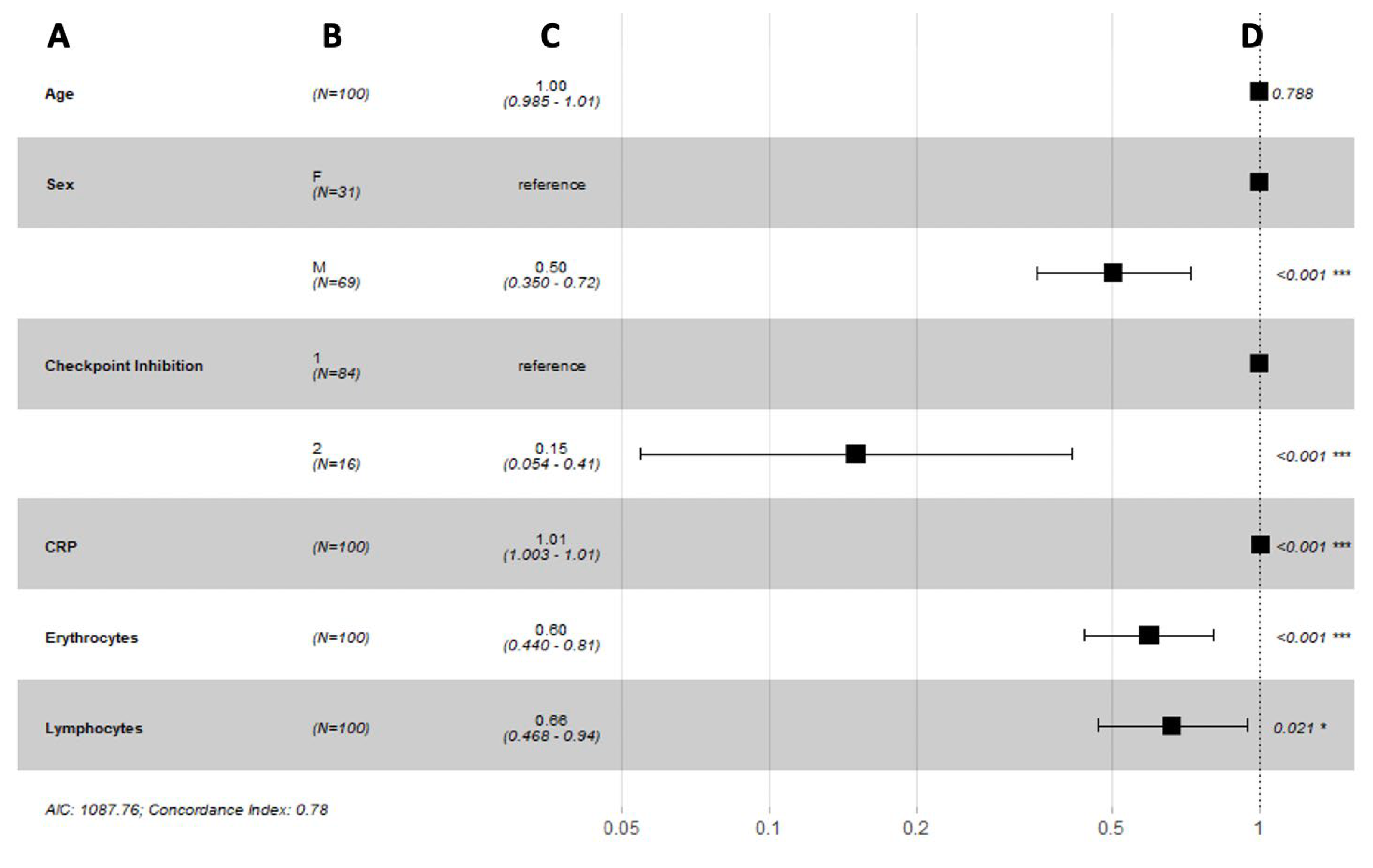
3.2. Short-Term Response to Immune Checkpoint Inhibition
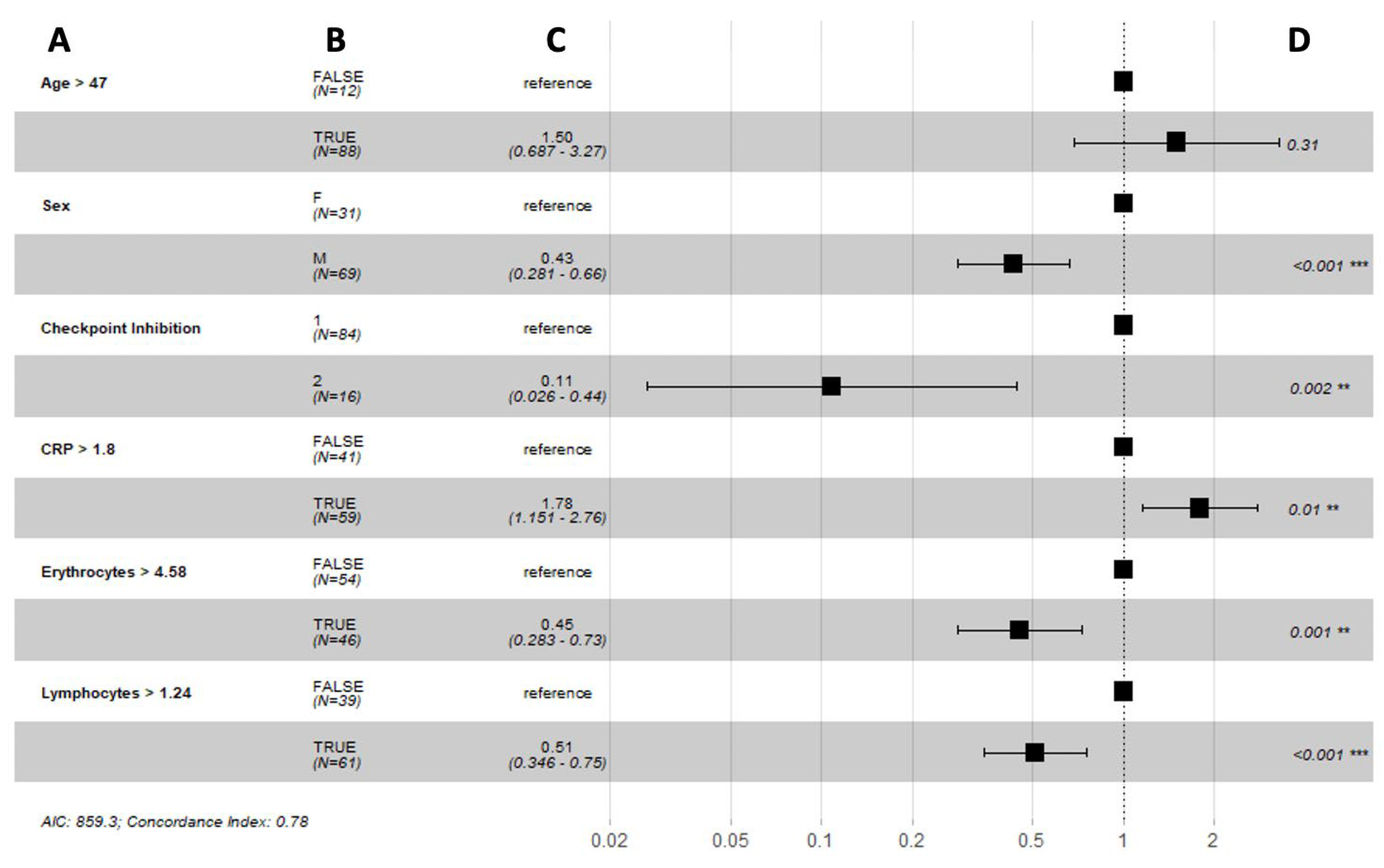
3.3. Long-Term Response to Immune Checkpoint Inhibition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTLA-4 | Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) |
| PD-1 | Programmed cell death protein-1 (PD-1) |
| PD-L1 | Programmed cell death ligand-1 |
| ICI | Immune checkpoint inhibitor |
| LDH | Lactate dehydrogenase |
| TMB | Tumor mutation burden |
| GCP | Good Clinical Practice |
| AJCC | American Joint Committee on Cancer |
| FDG-PET/CT | 18F-2-fluor-2-desoxy-D-glucose positron emission tomography/computed tomography |
| TP | Time point |
| NLR | Neutrophils-to-lymphocytes ratio |
| TOF | Time-of-flight |
| BMI | Body mass index |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| CR | Complete response |
| PR | Partial response |
| PD | Progressive disease |
| SD | Stable disease |
| OS | Overall survival |
| PFS | Progression-free survival |
| ROC | Receiver operating characteristic |
| IQR | Interquartile range |
| AIC | Akaike information criterion |
| CI | Confidence interval |
References
- Balatoni, T.; Ladányi, A.; Fröhlich, G.; Czirbesz, K.; Kovács, P.; Pánczél, G.; Bence, E.; Plótár, V.; Liszkay, G. Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Pathol. Oncol. Res. POR 2020, 26, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Forschner, A.; Battke, F.; Hadaschik, D.; Schulze, M.; Weißgraeber, S.; Han, C.-T.; Kopp, M.; Frick, M.; Klumpp, B.; Tietze, N.; et al. Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma–results of a prospective biomarker study. J. Immunother. Cancer 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Martens, A.; Wistuba-Hamprecht, K.; Foppen, M.G.; Yuan, J.; Postow, M.A.; Wong, P.; Romano, E.; Khammari, A.; Dreno, B.; Capone, M.; et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 2908–2918. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, H.; Takeuchi, S.; Miyagaki, T.; Kadono, T. Increase of lymphocytes and eosinophils, and decrease of neutrophils at an early stage of anti-PD-1 antibody treatment is a favorable sign for advanced malignant melanoma. Drug Discov. Ther. 2020, 14, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.W.; Boyle, S.M.; Pyke, R.M.; McDaniel, L.D.; Levy, E.; Navarro, F.C.; Mellacheruvu, D.; Zhang, S.V.; Tan, M.; Santiago, R.; et al. Prediction of Immunotherapy Response in Melanoma through Combined Modeling of Neoantigen Burden and Immune-Related Resistance Mechanisms. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 4265–4276. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, C.; Kirchberger, M.C.; Goldinger, S.M.; Weide, B.; Konrad, A.; Erdmann, M.; Schadendorf, D.; Croner, R.S.; Krähenbühl, L.; Kähler, K.C.; et al. Predictive value of PD-L1 based on mRNA level in the treatment of stage IV melanoma with ipilimumab. J. Cancer Res. Clin. Oncol. 2017, 143, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Klempner, S.J.; Fabrizio, D.; Bane, S.; Reinhart, M.; Peoples, T.; Ali, S.M.; Sokol, E.S.; Frampton, G.; Schrock, A.B.; Anhorn, R.; et al. Tumor Mutational Burden as a Predictive Biomarker for Response to Immune Checkpoint Inhibitors: A Review of Current Evidence. Oncologist 2020, 25, e147–e159. [Google Scholar] [CrossRef] [PubMed]
- McGrail, D.; Pilié, P.; Rashid, N.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Ardito, R.; Merelli, B.; Lonati, V.; Cabiddu, M.; Seghezzi, S.; Barni, S.; Ghidini, A. Prognostic and predictive role of elevated lactate dehydrogenase in patients with melanoma treated with immunotherapy and BRAF inhibitors: A systematic review and meta-analysis. Melanoma Res. 2019, 29, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Cabiddu, M.; Coinu, A.; Borgonovo, K.; Ghilardi, M.; Lonati, V.; Barni, S. Prognostic role of lactate dehydrogenase in solid tumors: A systematic review and meta-analysis of 76 studies. Acta Oncol. 2015, 54, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Santos-Briz, A.; Cañueto, J.; del Carmen, S.; Barrios, B.; Yuste, M.; Bellido, L.; Ludeña, M.D.; Román, C. Value of PD-L1, PD-1, and CTLA-4 Expression in the Clinical Practice as Predictors of Response to Nivolumab and Ipilimumab in Monotherapy in Patients with Advanced Stage Melanoma. Am. J. Dermatopathol. 2021, 43, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, J.; Du, C.; Wu, Y.; Xia, D.; Lv, W.; Hu, J. The Predictive Value of Tumor Mutation Burden on Efficacy of Immune Checkpoint Inhibitors in Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 1161. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Li, L.; Chen, X.; Chen, N.; Song, W.; Zhang, Y.; Lv, Z.; Han, F.; Zhao, Y.; Li, W.; et al. Correlation of Peripheral Blood Parameters and Immune-Related Adverse Events with the Efficacy of Immune Checkpoint Inhibitors. J. Oncol. 2021, 2021, 9935076. [Google Scholar] [CrossRef] [PubMed]
- Capone, M.; Giannarelli, D.; Mallardo, D.; Madonna, G.; Festino, L.; Grimaldi, A.M.; Vanella, V.; Simeone, E.; Paone, M.; Palmieri, G.; et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer 2018, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Marra, A.; Morganti, S.; Zagami, P.; Viale, G.; Esposito, A.; Curigliano, G. Pretreatment Blood Parameters Predict Efficacy from Immunotherapy Agents in Early Phase Clinical Trials. Oncologist 2020, 25, e1732–e1742. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.T.; Miner, T.J.; Vezeridis, M.P. Is the neutrophil-to-lymphocyte ratio a useful prognostic indicator in melanoma patients? Melanoma Manag. 2020, 7, MMT47. [Google Scholar] [CrossRef] [PubMed]
- Sacdalan, D.B.; Lucero, J.A.; Sacdalan, D.L. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: A review and meta-analysis. OncoTargets Ther. 2018, 11, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Viñal, D.; Gutierrez-Sainz, L.; Martinez, D.; Garcia-Cuesta, J.A.; Pedregosa, J.; Villamayor, J.; Ostios, L.; Sanchez-Cabrero, D.; Higuera, O.; Pinto, A.; et al. Prognostic value of neutrophil-to-lymphocyte ratio in advanced cancer patients receiving immunotherapy. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2021, 23, 1185–1192. [Google Scholar] [CrossRef] [PubMed]




| Median age at treatment start in years (IQR) | 68 (53–74) |
| Sex | N = 100 |
| Male | 69 |
| Female | 31 |
| AJCC-stage at treatment start | N = 100 |
| III | 5 |
| IV | 95 |
| Histopathology of primary tumor, n (%) | N = 100 |
| Superficial spreading | 31 |
| Nodular | 27 |
| Lentigo maligna | 6 |
| Acral lentiginous | 8 |
| Sinonasal | 3 |
| Mucosal | 3 |
| Amelanotic | 1 |
| Ocular | 3 |
| Unknown | 18 |
| Site of primary tumor, n (%) | N = 100 |
| Head and Neck | 40 |
| Upper extremity | 22 |
| Body trunk | 14 |
| Lower extremity | 1 |
| Eye | 2 |
| Vagina | 1 |
| Sinonasal | 11 |
| No primary tumor | 9 |
| Site of melanoma metastases at treatment start, n (%) | N = 670 |
| Soft tissue | 359 |
| Lung | 142 |
| Liver/Spleen | 125 |
| Bone | 42 |
| Heart | 2 |
| Number of patients with one metastasis and n metastases at treatment start | N = 100 |
| 1 | 21 |
| 2 | 10 |
| 3 | 11 |
| 4 | 6 |
| 5 | 4 |
| 6–10 | 30 |
| 11–15 | 13 |
| >15 | 5 |
| Prior treatment, n (%) | N = 100 |
| Native | 24 |
| Pretreated | 76 |
| Ipilimumab | 46 |
| Nivolumab | 13 |
| Ipilimumab + Nivolumab | 4 |
| BRAF Inhibitor | 3 |
| MEK Inhibitor | 4 |
| Chemotherapy | 4 |
| Intratumoral | 2 |
| Checkpoint inhibition, n (%) | N = 100 |
| Single | 84 |
| Double | 16 |
| Characteristics | Minimum | 1st Quantile | Median | Mean | 3rd Quantile | Maximum |
|---|---|---|---|---|---|---|
| TP 0 | ||||||
| Basophiles (in g/L) | 0.01 | 0.02 | 0.03 | 0.03 | 0.04 | 0.10 |
| C-reactive protein (in mg/L) | 0.30 | 1.20 | 3.90 | 17.46 | 11.75 | 310.00 |
| Erythrocytes (per pL) | 2.91 | 4.22 | 4.64 | 4.57 | 4.98 | 5.71 |
| Leucocytes (in g/L) | 3.02 | 5.35 | 6.54 | 7.07 | 7.99 | 16.28 |
| Lymphocytes (in g/L) | 0.21 | 1.08 | 1.35 | 1.45 | 1.67 | 4.60 |
| Monocytes (in g/L) | 0.19 | 0.40 | 0.54 | 0.59 | 0.74 | 1.40 |
| Neutrophils (in g/L) | 1.44 | 3.32 | 4.36 | 4.81 | 5.59 | 14.10 |
| Thrombocytes (in g/L) | 122.00 | 195.25 | 230.50 | 239.82 | 283.75 | 449.00 |
| NLR | 1.04 | 2.16 | 2.95 | 4.54 | 4.41 | 66.19 |
| TP 1 | ||||||
| Basophiles (in g/L) | 0.00 | 0.02 | 0.03 | 0.03 | 0.04 | 0.11 |
| C-reactive protein (in mg/L) | 0.30 | 1.00 | 2.95 | 9.63 | 6.45 | 137.00 |
| Erythrocytes (per pL) | 3.21 | 4.25 | 4.63 | 4.63 | 5.00 | 6.64 |
| Leucocytes (in g/L) | 3.53 | 5.72 | 6.62 | 6.98 | 8.45 | 11.79 |
| Lymphocytes (in g/L) | 0.60 | 1.11 | 1.44 | 1.60 | 1.90 | 5.68 |
| Monocytes (in g/L) | 0.15 | 0.40 | 0.55 | 0.57 | 0.69 | 1.60 |
| Neutrophils (in g/L) | 1.93 | 3.40 | 4.21 | 4.58 | 5.43 | 9.64 |
| Thrombocytes (in g/L) | 130.00 | 199.25 | 225.00 | 233.41 | 269.00 | 416.00 |
| NLR | 0.54 | 2.16 | 2.89 | 3.49 | 4.14 | 9.28 |
| TP 2 | ||||||
| Basophiles (in g/L) | 0.01 | 0.02 | 0.03 | 0.03 | 0.04 | 0.14 |
| C-reactive protein (in mg/L) | 0.30 | 1.10 | 2.40 | 6.71 | 4.70 | 151.00 |
| Erythrocytes (per pL) | 3.16 | 4.35 | 4.63 | 4.64 | 5.01 | 6.46 |
| Leucocytes (in g/L) | 3.24 | 5.29 | 6.56 | 6.79 | 8.55 | 12. 17 |
| Lymphocytes (in g/L) | 0.65 | 1.12 | 1.35 | 1.56 | 1.74 | 7.38 |
| Monocytes (in g/L) | 0.21 | 0.41 | 0.53 | 0.56 | 0.72 | 1.41 |
| Neutrophils (in g/L) | 1.55 | 3.06 | 4.25 | 4.39 | 5.54 | 9.44 |
| Thrombocytes (in g/L) | 118.00 | 201.50 | 238.50 | 233.65 | 264.00 | 449.00 |
| NLR | 0.584 | 1.93 | 2.75 | 3.37 | 4.07 | 9.25 |
| Characteristics | Minimum | 1st Quantile | Median | Mean | 3rd Quantile | Maximum |
|---|---|---|---|---|---|---|
| TP 0 | ||||||
| Basophiles (in g/L) | 0.01 | 0.02 | 0.03 | 0.03 | 0.05 | 0.07 |
| C-reactive protein (in mg/L) | 0.40 | 1.35 | 3.45 | 24.83 | 27.25 | 2 14.00 |
| Erythrocytes (per pL) | 2.87 | 3.92 | 4.25 | 4.23 | 4.75 | 5.19 |
| Leucocytes (in g/L) | 0.65 | 4.60 | 5.87 | 6.26 | 6.78 | 18.47 |
| Lymphocytes (in g/L) | 0.58 | 0.88 | l.31 | l.39 | l.96 | 2.43 |
| Monocytes (in g/L) | 0.01 | 0.40 | 0.43 | 0.51 | 0.62 | 1.15 |
| Neutrophils (in g/L) | 0.01 | 2.60 | 3.69 | 3.52 | 4.31 | 6.59 |
| Thrombocytes (in g/L) | 121.00 | 202.50 | 219.00 | 247.04 | 273.25 | 473.00 |
| NLR | 0.02 | 1.73 | 2.54 | 2.71 | 3.27 | 5.89 |
| TP l | ||||||
| Basophiles (in g/L) | 0.01 | 0.01 | 0.03 | 0.04 | 0.05 | 0.12 |
| C-reactive protein (in mg/L) | 0.40 | 2.32 | 5.95 | 17.63 | 31.00 | 102.00 |
| Erythrocytes (per pL) | 2.77 | 3.90 | 4.12 | 4.11 | 4.54 | 4.85 |
| Leucocytes (in g/L) | 4.78 | 5.27 | 6.59 | 6.89 | 8.15 | l l.24 |
| Lymphocytes (in g/L) | 0.66 | 1.01 | 1.30 | l.42 | l.88 | 2.43 |
| Monocytes (in g/L) | 0.38 | 0.45 | 0.60 | 0.60 | 0.67 | 1.20 |
| Neutrophils (in g/L) | 1.92 | 3.11 | 4.50 | 4.55 | 5.57 | 9.39 |
| Thrombocytes (in g/L) | 150.00 | 221.25 | 266.00 | 279.27 | 325.75 | 534.00 |
| NLR | 1.11 | 2.49 | 3.17 | 3.63 | 4.21 | 8.45 |
| TP 2 | ||||||
| Basophiles (in g/L) | 0.01 | 0.01 | 0.02 | 0.03 | 0.04 | 0.11 |
| C-reactive protein (in mg/L) | 0.30 | 3.35 | 23.20 | 64.40 | 120.50 | 252.00 |
| Erythrocytes (per pL) | l.99 | 3.50 | 4.03 | 3.91 | 4.35 | 4.90 |
| Leucocytes (in g/L) | 3.45 | 5.94 | 6.85 | 7.63 | 8.25 | 20.11 |
| Lymphocytes(in g/L) | 0.38 | 0.64 | 1.25 | 1.24 | 1.74 | 2.58 |
| Monocytes (in g/L) | 0.15 | 0.41 | 0.48 | 0.55 | 0.63 | 1.52 |
| Neutrophy ls (in g/L) | 2.01 | 3.54 | 4.56 | 5.60 | 6.16 | 18.66 |
| Thrombocytes (in g/L) | 147.00 | 227.00 | 251.00 | 270.04 | 302.50 | 429.00 |
| NLR | 1.12 | 2.62 | 4.85 | 6.04 | 9.03 | 20.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudura, K.; Nussbaumer, L.; Foerster, R.; Basler, L. Inflammatory Blood Parameters as Biomarkers for Response to Immune Checkpoint Inhibition in Metastatic Melanoma Patients. Biomedicines 2022, 10, 2135. https://doi.org/10.3390/biomedicines10092135
Kudura K, Nussbaumer L, Foerster R, Basler L. Inflammatory Blood Parameters as Biomarkers for Response to Immune Checkpoint Inhibition in Metastatic Melanoma Patients. Biomedicines. 2022; 10(9):2135. https://doi.org/10.3390/biomedicines10092135
Chicago/Turabian StyleKudura, Ken, Lukas Nussbaumer, Robert Foerster, and Lucas Basler. 2022. "Inflammatory Blood Parameters as Biomarkers for Response to Immune Checkpoint Inhibition in Metastatic Melanoma Patients" Biomedicines 10, no. 9: 2135. https://doi.org/10.3390/biomedicines10092135
APA StyleKudura, K., Nussbaumer, L., Foerster, R., & Basler, L. (2022). Inflammatory Blood Parameters as Biomarkers for Response to Immune Checkpoint Inhibition in Metastatic Melanoma Patients. Biomedicines, 10(9), 2135. https://doi.org/10.3390/biomedicines10092135





