Abstract
In multiple sclerosis (MS), fatigue is a frequent symptom that negatively affects quality of life. The pathogenesis of fatigue is multifactorial and inflammation may play a specific role. To explore the association between fatigue, central inflammation and disease course in MS in 106 relapsing-remitting (RR)-MS patients, clinical characteristics, including fatigue and mood, were explored at the time of diagnosis. NEDA (no evidence of disease activity)-3 status after one-year follow up was calculated. Cerebrospinal fluid (CSF) levels of a set of proinflammatory and anti-inflammatory molecules and peripheral blood markers of inflammation were also analyzed. MRI structural measures were explored in 35 patients. A significant negative correlation was found at diagnosis between fatigue measured with the Modified Fatigue Impact Scale (MFIS) and the CSF levels of interleukin (IL)-10. Conversely, no significant associations were found with peripheral markers of inflammation. Higher MFIS scores were associated with reduced probability to reach NEDA-3 status after 1-year follow up. Finally, T2 lesion load showed a positive correlation with MFIS scores and a negative correlation with CSF IL-10 levels at diagnosis. CSF inflammation, and particularly the reduced expression of the anti-inflammatory molecule IL-10, may exacerbate fatigue. Fatigue in MS may reflect subclinical CSF inflammation, predisposing to greater disease activity.
1. Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease of the central nervous system (CNS) characterized by inflammation, demyelination, and neurodegeneration. The inflammatory process in MS is initiated and sustained by infiltration of autoreactive T and B lymphocytes, the activation of microglia and astrocytes, and the release of proinflammatory cytokines [1].
Fatigue which negatively affects quality of life in patients with MS is observed in all disease phenotypes [2]. It is frequently present at the time of diagnosis, representing the onset symptom [3]. The pathogenesis of fatigue in MS is thought to be multifactorial [4]. Although it has been associated with the degree of disability and structural damage, alternative mechanisms have been implicated, including dysfunction of the hypothalamic–pituitary–adrenal axis, altered brain activation, and neurotransmitter release [4,5].
Although not completely elucidated, immunological and inflammatory mechanisms may play important roles in the pathogenesis of MS fatigue [4]. Notably, fatigue is very common in patients with autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis [6]. Moreover, increased serum levels of proinflammatory cytokines have been associated with fatigue in different autoimmune conditions [7,8,9] and have been demonstrated in chronic fatigue syndrome [7]. Previous studies have reported some relationship between fatigue and peripheral inflammatory biomarkers. In particular, in MS patients with fatigue, higher production capacity, increased mRNA expression, and serum levels of specific inflammatory molecules such as IL-6 and TNF have been reported [8,10,11]. However, the association between fatigue in MS and peripheral inflammatory molecules is still unclear; in fact, some investigations have not reported significant associations, and most studies have been conducted on small samples [12,13,14,15,16]. Assessment of intrathecal cytokine levels may help to identify markers of fatigue directly related to MS-specific mechanisms [17]. In addition, cerebrospinal fluid (CSF) cytokines represent a reliable marker of prospective disease activity in MS [18]. It is worthy of note that associations have been reported between fatigue and worse MS course [19,20], suggesting that higher levels of fatigue could characterize a subgroup of patients prone to develop greater disease activity. Therefore, studying the association between fatigue and CSF inflammation could be extremely useful to identify reliable biomarkers to predict disease course and tailor treatment from the time of diagnosis.
In this paper, we explored in a group of relapsing-remitting (RR)-MS patients whether fatigue assessed at diagnosis is associated with specific clinical features, and especially with a particular cytokine profile in the CSF. Additionally, we examined whether fatigue at the time of diagnosis could be a factor that predicts no evidence of disease activity (NEDA)-3, after a one-year follow-up.
2. Materials and Methods
2.1. RR-MS Patients
We retrospectively analyzed the data of 106 consecutive MS patients enrolled between 2018 and 2020. Patients were admitted to the neurology unit of Neuromed hospital (Pozzilli, Italy) and later diagnosed as suffering from RR-MS, based on clinical, laboratory and MRI parameters according to McDonald’s diagnostic criteria [21]. The study, performed in accordance with the Declaration of Helsinki, was approved by the Ethics Committee of Neuromed hospital (CE numbers 06/17 and 11/17). All patients gave written informed consent to participate in the study and to give their demographic and clinical data and biological material for scientific research. Inclusion criteria were the established RR-MS diagnosis and the ability to provide written informed consent to the study. Patients with different autoimmune and inflammatory disease and with clinically relevant medical or surgical conditions which might affect fatigue were excluded. No patient had a major psychiatric diagnosis. At the diagnosis, demographic and clinical characteristics were recorded, including disease duration, clinical disability evaluated with the Expanded Disability Status Scale (EDSS) [22], and body mass index (BMI) calculated as weight (kg)/height (m2). Fatigue, depression, and anxiety were also evaluated at the time of diagnosis in all patients. Fatigue was measured with the Modified Fatigue Impact Scale (MFIS), a 21-item questionnaire measuring the physical, cognitive, and psychosocial impact of fatigue (maximum score of 84) [23]. In line with a previous study, we used a cut-off of 38 for MFIS scores [24], and clinically significant fatigue was evidenced. The Beck Depression Inventory-Second Edition (BDI-II) was used to assess depressive symptoms [25]. Anxiety was measured with the State-Trait Anxiety Inventory (STAI) form Y (STAI-Y) [26].
Patients were followed up for one year. NEDA status was calculated for each patient at the end of the follow up period. NEDA was defined as the absence of radiological and clinical disease activity and the absence of disability progression (NEDA-3) [27].
Patients had not previously received either corticosteroids or immunoactive therapies. Disease modifying therapies (DMT) were initiated after diagnosis as follows: I line (interferon beta-1a 22 mcg = one patient; peginterferon beta-1a = one patient; teriflunomide = three patients; glatiramer acetate = 12 patients; dimethyl fumarate = 57 patients), II line (ocrelizumab = four patients; cladribine = five patients; fingolimod = eight patients; natalizumab = 10 patients). DMTs were selected according to practical guidelines [28] by neurologists blinded to MFIS values and CSF cytokines levels.
2.2. MRI Examination
Brain and spine MRI was performed at diagnosis, six months and one year after diagnosis. Additional MRI scans were performed if clinical relapses occurred. MRI included dual-echo proton density sequences, FLAIR, T1-weighted spin-echo (SE), T2-weighted fast SE, and contrast-enhanced T1-weighted SE after intravenous gadolinium (Gd) infusion (0.2 mL/kg), performed on 1.5- or 3.0-Tesla scanners. Radiological disease activity was defined as the presence of a gadolinium-enhancing (Gd+) lesion on MRI scans performed at diagnosis.
In 35 patients, cortical thickness and T2 lesion load were analyzed at diagnosis. All the images were acquired using the 3T MR scanner (GE Signa HDxt, GE Healthcare, Milwaukee, WI, USA). We used a 3D Spoiled Gradient Recalled (SPGR) T1-weighted sequence (178 contiguous sagittal slices, voxel size 1 × 1 × 1 mm3, TR 7 ms, TE 2.856 ms, inversion time 450 ms) and a 3D FLAIR sequence (208 contiguous sagittal 1.6 mm slices, voxel size, 0.8 × 0.8 × 0.8 mm3, TR 6000 ms, TE 139.45 ms; inversion time 1827 ms). We first segmented white matter lesions from FLAIR and T1 images by using the lesion growth algorithm as implemented in version 2.0.15 of the lesion segmentation tool (www.statistical-modelling.de/lst.html (accessed on 14 May 2022)) for SPM12 (https://www.fl.ion.ucl.ac.uk/spm (accessed on 14 May 2022)). Furthermore, we used the computational anatomy toolbox (CAT12, version 916, https://dbm.neuro.uni-jena.de/cat/ (accessed on 14 May 2022)) as implemented in SPM12 to extract individual cortical thickness values from lesion-filled MR images. Finally, T2 lesion load was computed from 3D T1 and 3d FLAIR images by using a well-established pipeline.
2.3. CSF Collection and Analysis
In all patients, CSF was collected at diagnosis by lumbar puncture. Lactate levels and oligoclonal bands (OCB) were assessed. After withdrawal, CSF was centrifuged and stored at −80 °C. Proinflammatory and anti-inflammatory molecules were analyzed using a Bio-Plex multiplex cytokine assay (Bio-Rad Laboratories, Hercules, CA, USA). All samples were analyzed in triplicate. Concentrations were expressed as picograms/milliliter. The CSF molecules examined included interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-17, IL-1receptor antagonist (ra), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon (IFN)γ, tumor necrosis factor (TNF), monocyte chemoattractant protein 1 (MCP-1)/CCL2, macrophage inflammatory protein (MIP)-1α/CCL3, and MIP-1 β/CCL4.
2.4. Blood Collection and Analysis of Peripheral Inflammatory Biomarkers
Blood samples were collected at diagnosis. Erythrocyte sedimentation rate (ESR) and fibrinogen levels were measured. In addition, total white blood cells (WBC), neutrophils, lymphocytes and platelets counts were assessed. The neutrophil-lymphocytes ratio (NRL) was calculated as the quotient between the neutrophils and lymphocytes [29].
2.5. Statistical Analysis
A Kolmogorov–Smirnov test was used to assess the normality distribution of variables. Data were shown as median (interquartile range, IQR). Categorical variables were presented as number (N) and frequency (%). A p value ≤ 0.05 was considered statistically significant. Spearman’s nonparametric correlation was used to explore associations between not normally distributed variables. A Benjamini-Hockberg (B-H) test was applied to control the false discovery rate and the Type I errors (false positives) when analyzing correlations between MFIS and CSF inflammatory molecules. A box plot was used to depict statistically significant differences between groups.
Logistic regression analysis was undertaken after one year (yes/no) with NEDA-3 as the dependent variable. All variables with significance levels ≥0.05 by univariate analysis were included in the multivariate models (method: enter).
Logistic regression analysis was used to explore the association between fatigue, as measured by MFIS, and reaching NEDA-3 after one year, after adjustment by multiple potential confounding factors.
All analyses were performed using IBM SPSS Statistics for Windows (IBM Corp., Armonk, NY, USA).
Missing data: disease duration in 3/106 patients (2.83%); radiological activity in 5/106 patients (4.71%); OCB in 2/106 patients (1.89%); CSF lactate in 2/106 patients (1.89%); BMI in 4/106 patients (3.77%); BDI-II, STAI-Y state/trait in 6/106 patients (5.66%); ESR in 1/106 patients (0.94%); fibrinogen in 2/106 patients (1.89%); WBC, lymphocytes, neutrophils, and NLR in 3/106 patients (2.83%).
3. Results
3.1. Fatigue and Clinical Characteristics at the Time of MS Diagnosis
The clinical characteristics of MS patients are shown in Table 1.

Table 1.
Clinical characteristics at MS diagnosis.
The MFIS total score showed strong positive correlations with the three subscales: MFIS-physical, MFIS-cognitive, and MFIS-psychosocial (all Spearman’s r ≥ 0.80, all p < 0.0001).
Using a cut-off of 38 for psychosocial scores, clinically significant fatigue was evidenced in 23/106 individuals (21.7%) of the MS cohort.
We explored the correlations between fatigue and other clinical characteristics at the time of diagnosis. A strong correlation was found between MFIS and mood scales. In particular, positive correlations emerged with BDI-II (Spearman’s r = 0.539, p < 0.0001), STAI-Y state (Spearman’s r = 0.347, p < 0.001) and STAI-Y trait (Spearman’s r = 0.491, p < 0.0001). No significant correlations were found between MFIS and the other clinical characteristics explored (data not presented).
The subgroups qualified for I and II line DMT differed neither in the baseline MFIS total score (p = 0.182), nor in any of its domains (MFIS physical p = 0.252; MFIS cognitive p = 0.135; MFIS psychosocial p = 0.796).
3.2. Fatigue Is Associated with Reduced IL-10 CSF Expression
We explored the correlations between MFIS and the CSF levels of a set of proinflammatory and anti-inflammatory molecules at the time of MS diagnosis.
A significant association was found between MFIS and the CSF levels of IL-10 (Spearman’s r = −0.309, p = 0.001, and B-H adjusted p = 0.02, N = 106) (Table 2, Figure 1). The median CSF IL-10 concentration was 1.59 pg/mL (IQR 0.71–2.66). No significant correlations were found between MFIS and the other CSF molecules analyzed (Table 2).

Table 2.
Correlations between MFIS and CSF inflammatory molecules at MS diagnosis.
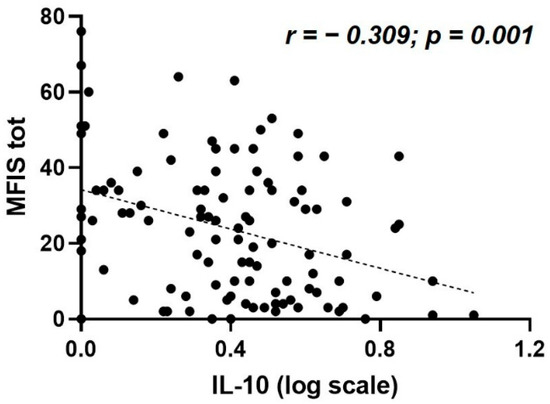
Figure 1.
Correlation between MFIS total score and CSF IL-10 levels at MS diagnosis. Abbreviations: CSF, cerebrospinal fluid; IL, interleukin; MFIS tot, Modified Fatigue Impact Scale total score; MS, multiple sclerosis.
3.3. Fatigue Is not Associated with Peripheral Inflammation
We explored the association between MFIS and peripheral inflammation in MS patients. A set of peripheral blood inflammatory biomarkers has been evaluated at the time of MS diagnosis including ESR (median [IQR] = 10 [5.5–20]), fibrinogen (median [IQR] = 311 [265–352]), WBC (median [IQR] = 6.9 [5.7–8.4]), lymphocytes (median [IQR] = 1.9 [1.5–2.3]), neutrophils (median [IQR] = 4.3 [3.3–5.5]), and NLR (median [IQR] = 2.37 [1.77–2.95]).
No significant correlations were found between MFIS scores and the peripheral inflammatory biomarkers analyzed: ESR (Spearman’s r = 0.024, p = 0.809, N = 105), fibrinogen (Spearman’s r = −0.19, p = 0.849, N = 104), WBC (Spearman’s r = 0.04, p = 0.685, N = 103), lymphocytes (Spearman’s r = −0.039, p = 0.693, N = 103), neutrophils (Spearman’s r = ‒0.001, p = 0.992, N = 103), and NLR (Spearman’s r = 0.004, p = 0.971, N = 103).
3.4. Baseline Fatigue Predicts NEDA-3 after One Year Follow-Up
We investigated predictive values of all variables listed in the Table 3 to reach NEDA-3 after one year in our RR-MS cohort. None of these variables was demonstrated to be statistically significant predictors, except the MFIS total score and its psychosocial domain. NEDA-3 was achieved in 61/101 (60%) of the patients independently on the type of DMTs (50/74 for the I line and 11/27 for the II line).

Table 3.
Logistic regression analysis of the association of the different MFIS domains and total score, with reaching NEDA-3 in RR-MS patients, after one-year follow-up. Bold values denote statistical significance.
Results of the logistic regression analyses of the association of the MFIS total score and different domains with reaching NEDA-3 in RR-MS patients, after a one-year follow-up, are presented in Table 3. We analyzed predictive factors for reaching NEDA-3 using this variable as a dependent factor in the models. In the logistic regression analyses (dependent variable: NEDA-3, yes/no), it has been demonstrated that only MFIS psychosocial domain and total MFIS score were independent predictors of NEDA-3, after adjustment by several demographic and clinical variables at baseline: sex, age, disease duration, EDSS, radiological disease activity, total number of relapses, BDI-II, STAI-Y state, STAI-Y trait, BMI, treatment with DMTs and concentration of IL-10 in the CSF. A lower score of MFIS psychosocial domain and MFIS total score predicted higher probability of reaching NEDA-3 (OR = 0.67, 95% CI 0.49–0.90, p = 0.009; OR = 0.96; 95% CI 0.92–0.99; p = 0.039), respectively. This means that patients whose baseline score of MFIS in the psychosocial domain will increase for one point after one year, and will have a 33% lower chance of reaching NEDA-3. Additionally, those whose baseline total MFIS score will increase for one point after one year will have a 4% lower chance of reaching NEDA-3.
3.5. Correlations between Structural MRI Measures, Fatigue, and CSF IL-10 Levels at the Time of MS Diagnosis
Structural MRI measures were available for 35 MS patients (Supplementary Figure S1): cortical thickness (median [IQR] = 2.76 [2.65–2.83]), and T2 lesion load (median [IQR] = 0.31 [0.09–0.90]).
A significant correlation was found between MFIS-total and T2 lesion load (Spearman’s r = 0.420 p = 0.012, N = 35); conversely, no significant correlation emerged between MFIS-tot and cortical thickness (Spearman’s r = 0.306, p = 0.074, N = 35) (Figure 2A).
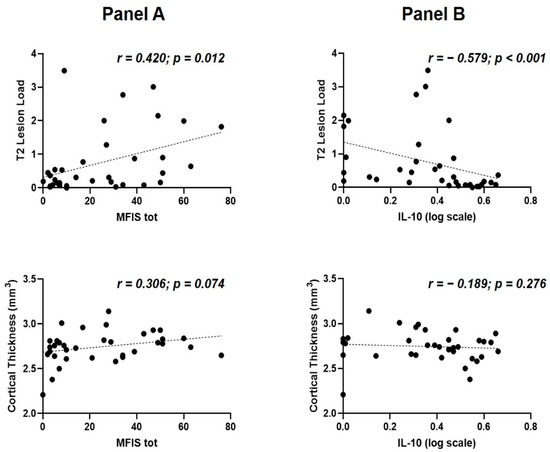
Figure 2.
Fatigue, IL-10 and structural MRI measures at the time of MS diagnosis. (A) correlations between fatigue, T2 lesion load (upper) and cortical thickness (lower); (B) correlations between IL-10 concentrations, T2 lesion load (upper) and cortical thickness (lower). Abbreviations: IL, interleukin; MFIS tot, Modified Fatigue Impact Scale total score; MS, multiple sclerosis.
A significant correlation was also found between IL-10 and T2 lesion load (Spearman’s r = −0.579, p = 0.000269, N = 35). No significant association was found between the CSF levels of IL-10 and cortical thickness (Spearman’s r = −0.189, p = 0.276, N = 35) (Figure 2B).
4. Discussion
Numerous findings support the finding that activation of the immune system plays a role in the pathogenesis of fatigue; however, studies exploring the association between inflammation and fatigue in MS have yielded conflicting results [17,30]. Some association has been reported between fatigue and serum levels of the proinflammatory molecule IL-6 [12], serum levels of TNF mRNA, [10] and increased TNF and IFN𝛾 [8]. As inflammation in MS is primarily confined to the CNS compartment, inflammatory biomarkers in the CSF may provide a reliable and specific marker of fatigue in MS. After analyzing a large set of proinflammatory and anti-inflammatory CSF cytokines, we found a negative correlation between fatigue and CSF levels of the anti-inflammatory molecule IL-10 at the diagnosis. Although no association between MS fatigue and routine CSF parameters (cell count, lactate, albumin and intrathecal immunoglobulin synthesis) have been reported [30], a previous study reported a positive correlation between fatigue and levels of the proinflammatory molecule IL-6 in the CSF of patients with MS [31]. In line with our findings, an exacerbated inflammatory milieu in the CSF, resulting from the increased expression of proinflammatory cytokines or reduced levels of regulatory molecules, may characterize MS patients with fatigue. Notably, a previous study examining CSF cytokine profiles in a group of 18 patients with chronic fatigue syndrome found that IL-10 levels were significantly reduced [32]. Similarly, studies in animal models of MS (i.e., experimental autoimmune encephalomyelitis, EAE), have shown that IL-10 gene therapy has a beneficial effect on both motor and non-motor symptoms, including fatigue, anxiety and neuropathic pain [33].
IL-10 is a major anti-inflammatory cytokine and plays an important regulatory role on the inflammatory response by inhibiting the production of several proinflammatory molecules [34]. The reduced expression of IL-10 in MS patients compared to controls has been reported, particularly during disease exacerbations [35,36]. Conversely, higher levels of IL-10 have been reported in stable disease phases [37], and have been associated with reduced brain damage and disability in MS patients [38].
An altered CSF inflammatory milieu in RR-MS patients with higher levels of fatigue may be associated with increased brain damage at diagnosis and promote a worse disease course. To clarify this hypothesis, we analyzed structural MRI data available in a subgroup of 35 RR-MS patients. The negative correlation demonstrated between IL-10 CSF concentration and T2 lesion load, although limited by the low number of patients analyzed, could be consistent with the anti-inflammatory role of this molecule and might suggest that reduced CSF expression of IL-10 could be associated with increased disease activity before establishing the diagnosis of RR-MS. In addition, we found a positive correlation between MFIS and T2 lesion load in patients with RR-MS at diagnosis. An association between fatigue and white matter lesion load has been previously reported in MS [39,40]; however, other studies failed to find significant correlations [41,42]. Although differences in patients’ characteristics or MRI methodology may partly explain such discrepancies [43], it has been proposed that other factors may play a role, including lesion location, alterations in normal-appearing white matter [44], or global and regional atrophy [39,40,44]. It is conceivable that the reduced expression of anti-inflammatory molecules and the ensuing brain damage may determine higher levels of fatigue at diagnosis.
When analyzing the impact of fatigue on disease activity, we found that lower MFIS psychosocial domain and MFIS total predicted higher probability of reaching NEDA-3 (p = 0.009 and p = 0.039, respectively). In particular, patients whose baseline total MFIS score will increase for one point after one year, and will have a 4% lower chance of reaching NEDA-3. These findings were independent of mood alterations, which was consistent with previous studies [19,20] which showed that fatigue at diagnosis predicted disability progression in RR-MS and was associated with a higher risk of conversion in patients with CIS. The association between MFIS psychosocial subscale and NEDA-3 should be carefully interpreted, as results of the MFIS psychosocial subscale could be affected by possible confounding factors [23,45]. However, our results are in line with the hypothesis that in patients with RR-MS, higher levels of fatigue at diagnosis might reflect a proinflammatory state of the CSF which, in turn, is associated with higher brain damage and greater prospective disease activity.
Fatigue in MS is a complex phenomenon, and understanding the pathogenesis of this severely disabling symptom is of paramount importance to design effective therapeutic strategies to improve patients’ quality of life. In our RR-MS cohort, the prevalence of fatigue at the time of diagnosis was 21.7%. Previous studies using a similar MFIS cut-off value for fatigue have found higher proportions [24]. To explain this discrepancy, it should be noted that age at diagnosis, disease duration and disability were lower in our study; moreover, only patients with RR-MS were included.
Although it is likely that EDSS score might influence the probability of achieving NEDA-3 status, in our study, which included patients at diagnosis with mild disability, NEDA-3 after one year of follow-up was not predicted by EDSS score. The observation that other predictors of disease activity analyzed were also not associated with NEDA-3, highlights the need to study longer follow-ups in a larger number of patients even with more severe or progressive MS. The relatively short follow up duration and the lack of a healthy controls group represent major limitations of the present study. Further studies exploring fatigue fluctuations over time are important to assess the relationship between perceived fatigue and ongoing disease activity. Moreover, identifying specific CSF cytokine clusters may help to better explain the possible synergistic effect of the different molecules. Although no significant correlations emerged between fatigue and the peripheral inflammatory markers explored (ESR, fibrinogen, WBC, neutrophils, lymphocytes, NLR), the lack of peripheral cytokine levels in our study, which could have allowed simultaneous assessment of serum and CSF cytokine profiles, cannot help disentangle the differential contribution of peripheral and central inflammatory mechanisms in MS-related fatigue. Finally, studies with structural MRI measures involving a larger population are crucial to better define the influence of both IL-10 CSF levels and fatigue at diagnosis on disease activity and progression.
In conclusion, our findings could potentially implicate that self-reported fatigue at MS onset may reflect the specific manifestation of a subclinical neuroinflammatory state, predisposing a worse disease outcome.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomedicines10092058/s1, Figure S1: Structural MRI image samples.
Author Contributions
L.G., F.B., T.P., J.D., D.C., M.S.B. contributed to the study design and literature search; F.B., G.G., E.D., F.A., A.B. (Antonio Bruno), A.B. (Angela Borrelli), contributed to the patient recruitment; L.G., L.P., G.G., M.S.B., E.D., F.A., A.B. (Antonio Bruno), A.B. (Angela Borrelli), contributed to data collection; L.G., L.P., E.I., T.P., J.D., M.S.B. writing the manuscript; T.P. performed the data analysis; L.G., L.P., E.I., T.P., J.D., G.M., D.C., M.S.B. contributed to data interpretation; M.S., R.F., A.F., G.M., D.F., V.V. contributed to the sample collection and experimental implementation; L.G., L.P., E.I., T.P., J.D., D.C., M.S.B. contributed to data interpretation. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by: Ministero della Salute (Ministry of Health, Italy): Diego Centonze and Georgia Mandolesi RF-2018-12366144; Ministero della Salute (Ministry of Health, Italy): Fabio Buttari GR-2018-12366154; Ministero della Salute (Ministry of Health, Italy): Progetto Ricerca Corrente to IRCCS Neuromed; Ministero della Salute (Ministry of Health, Italy): Progetto Ricerca Corrente to IRCCS San Raffaele; Georgia Mandolesi; Fondazione Italiana Sclerosi Multipla (FISM): Diego Centonze cod. 2019/S/1 and financed or co-financed with the ‘5 per mille’ public funding; Fondazione Italiana Sclerosi Multipla (FISM): Mario Stampanoni Bassi cod. 2020/R-Multi/018 and financed or co-financed with the ‘5 per mille’ public funding; Private donation in memory of Chiara Sardi to Diego Centonze; Project ‘Nuovi Biomarker Diagnostici e Terapeutici delle Malattie Neurodegenerative’—ADOPT co-funded by FOE 2020—funding from CNR to Diego Centonze.
Institutional Review Board Statement
The study, performed in accordance with the Declaration of Helsinki, was approved by the Ethics Committee of Neuromed hospital (CE numbers 06/17 and 11/17).
Informed Consent Statement
Written informed consent was obtained from all participants prior to study participation.
Data Availability Statement
The data that support the fundings of this study are available from the corresponding author, upon reasonable request.
Conflicts of Interest
The authors declared the following potential conflict of interest with respect to the research, authorship, and/or publication of this article: TP has been an advisor or speaker for Sanofi-Genzyme, Medis, Hemofarm, Merck, Teva, and Roche. J.D. has been an advisor or speaker for Bayer HealthCare, Sanofi-Genzyme, Medis, Merck, Teva, Novartis, Hemofarm, Biogen and Roche. F.B. acted as Advisory Board members of Teva and Roche and received honoraria for speaking or consultation fees from Merck Serono, Teva, Bio-gen Idec, Sanofi, and Novartis and non-financial support from Merck Serono, Teva, Biogen Idec, and Sanofi. R.F. received honoraria for serving on scientific advisory boards or as a speaker from Biogen, Novartis, Roche, and Merck and funding for research from Merck. DC is an Advisory Board member of Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novar-tis, Roche, Sanofi-Genzyme, and Teva and received honoraria for speaking or consultation fees from Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva. He is also the principal investigator in clinical trials for Bayer Schering, Biogen, Merck Serono, Mitsubishi, Novartis, Roche, Sanofi-Genzyme, and Teva. His preclinical and clinical research was supported by grants from Bayer Schering, Biogen Idec, Celgene, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. L.G., L.P., E.I., G.G., E.D., F.A., A.B. (Antonio Bruno), A.B. (Angela Borrelli), M.S., A.F., G.M., D.F., V.V., M.S.B.: nothing to report.
Abbreviations
BDI-II: Beck Depression Inventory-Second Edition; B-H: Benjamini-Hockberg; BMI: body mass index; CNS: central nervous system; CSF: cerebrospinal fluid; DMT: Disease modifying therapies; EDSS: Expanded Disability Status Scale; ESR: Erythrocyte sedimentation rate; G-CSF: granulocyte colony-stimulating factor; Gd+: gadolinium-enhancing; GM-CSF: granulocyte-macrophage colony-stimulating factor; IFN: interferon; IL: interleukin; IL1-ra: IL-1receptor antagonist; IQR: interquartile range; MCP-1: monocyte chemoattractant protein 1; MFIS: Modified Fatigue Impact Scale; MIP: macrophage inflammatory protein; MS: multiple sclerosis; N: number; NEDA: no evidence of disease activity; NRL: Neutrophil-lymphocytes ratio; OCB: oligoclonal bands; PRO: patient-reported outcomes; RR-MS: relapsing-remitting multiple sclerosis; STAI-Y: State-Trait Anxiety Inventory-form Y; TNF: tumor necrosis factor; WBC: total white blood cells; %: frequency.
References
- Lassmann, H.; Bradl, M. Multiple sclerosis: Experimental models and reality. Acta Neuropathol. 2017, 133, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Herring, T.E.; Alschuler, K.N.; Knowles, L.M.; Phillips, K.M.; Morean, W.M.; Turner, A.P.; Ehde, D.M. Differences in correlates of fatigue between relapsing and progressive forms of multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 54, 103109. [Google Scholar] [CrossRef] [PubMed]
- Patrick, E.; Christodoulou, C.; Krupp, L.; New York State MS Consortium. Longitudinal correlates of fatigue in multiple sclerosis. Mult. Scler. 2009, 15, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Manjaly, Z.M.; Harrison, N.A.; Critchley, H.D.; Do, C.T.; Stefanics, G.; Wenderoth, N.; Lutterotti, A.; Müller, A.; Stephan, K.E. Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 642–651. [Google Scholar] [CrossRef]
- Dantzer, R.; Heijnen, C.J.; Kavelaars, A.; Laye, S.; Capuron, L. The neuroimmune basis of fatigue. Trends Neurosci. 2014, 37, 39–46. [Google Scholar] [CrossRef]
- Ramsey-Goldman, R.; Rothrock, N. Fatigue in systemic lupus erythematosus and rheumatoid arthritis. PM R 2010, 2, 384–392. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M. Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab. Brain Dis. 2014, 29, 19–36. [Google Scholar] [CrossRef]
- Heesen, C.; Nawrath, L.; Reich, C.; Bauer, N.; Schulz, K.H.; Gold, S.M. Fatigue in multiple sclerosis: An example of cytokine mediated sickness behaviour? J. Neurol. Neurosurg. Psychiatry 2006, 77, 34–39. [Google Scholar] [CrossRef]
- Lee, Y.C.; Frits, M.L.; Iannaccone, C.K.; Weinblatt, M.E.; Shadick, N.A.; Williams, D.A.; Cui, J. Subgrouping of patients with rheumatoid arthritis based on pain, fatigue, inflammation, and psychosocial factors. Arthritis Rheumatol. 2014, 66, 2006–2014. [Google Scholar] [CrossRef]
- Flachenecker, P.; Bihler, I.; Weber, F.; Gottschalk, M.; Toyka, K.V.; Rieckmann, P. Cytokine mRNA expression in patients with multiple sclerosis and fatigue. Mult. Scler. 2004, 10, 165–169. [Google Scholar] [CrossRef]
- Alvarenga-Filho, H.; Salles, M.; Hygino, J.; Ferreira, T.B.; Sacramento, P.M.; Monteiro, C.; Vasconcelos, C.C.; Alvarenga, R.M.; Bento, C.A. Fatigue favors in vitro Th1 and Th17-like cell expansion and reduces corticoid sensitivity in MS patients. J. Neuroimmunol. 2017, 303, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh, A.; Van de Geer-Peeters, W.; De Groot, V.; Teunissen, C.E.; Beckerman, H.; TREFAMS-ACE Study Group. Fatigue in patients with multiple sclerosis: Is it related to pro- and anti-inflammatory cytokines? Dis. Markers 2015, 2015, 758314. [Google Scholar] [CrossRef] [PubMed]
- Akcali, A.; Zengin, F.; Aksoy, S.N.; Zengin, O. Fatigue in Multiple Sclerosis: Is it related to cytokines and hypothalamic-pituitary-adrenal axis? Mult. Scler. Relat. Disord. 2017, 15, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Iriarte, J.; Subirá, M.L.; Castro, P. Modalities of fatigue in multiple sclerosis: Correlation with clinical and biological factors. Mult. Scler. 2000, 6, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Thompson, A.J.; Miller, D.H.; Thompson, E.J. Fatigue is not associated with raised inflammatory markers in multiple sclerosis. Neurology 2001, 57, 676–681. [Google Scholar] [CrossRef]
- Yaldizli, O.; Kumar, M.; Vago, S.; Kreuzfelder, E.; Limmroth, V.; Putzki, N. Fatigue is not associated with impaired function of regulatory T cells in untreated patients with multiple sclerosis. Eur. Neurol. 2009, 62, 321–326. [Google Scholar] [CrossRef]
- Patejdl, R.; Penner, I.K.; Noack, T.K.; Zettl, U. Multiple sclerosis and fatigue: A review on the contribution of inflammation and immune-mediated neurodegeneration. Autoimmun. Rev. 2016, 15, 210–220. [Google Scholar] [CrossRef]
- Stampanoni Bassi, M.; Iezzi, E.; Landi, D.; Monteleone, F.; Gilio, L.; Simonelli, I.; Musella, A.; Mandolesi, G.; De Vito, F.; Furlan, R.; et al. Delayed treatment of MS is associated with high CSF levels of IL-6 and IL-8 and worse future disease course. J. Neurol. 2018, 265, 2540–2547. [Google Scholar] [CrossRef]
- Runia, T.F.; Jafari, N.; Siepman, D.A.; Hintzen, R.Q. Fatigue at time of CIS is an independent predictor of a subsequent diagnosis of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 543–546. [Google Scholar] [CrossRef]
- Cavallari, M.; Palotai, M.; Glanz, B.I.; Egorova, S.; Prieto, J.C.; Healy, B.C.; Chitnis, T.; Guttmann, C.R. Fatigue predicts disease worsening in relapsing-remitting multiple sclerosis patients. Mult. Scler. 2016, 22, 1841–1849. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Kos, D.; Kerckhofs, E.; Carrea, I.; Verza, R.; Ramos, M.; Jansa, J. Evaluation of the Modified Fatigue Impact Scale in four different European countries. Mult. Scler. 2005, 11, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Flachenecker, P.; Kümpfel, T.; Kallmann, B.; Gottschalk, M.; Grauer, O.; Rieckmann, P.; Trenkwalder, C.; Toyka, K.V. Fatigue in multiple sclerosis: A comparison of different rating scales and correlation to clinical parameters. Mult. Scler. 2002, 8, 523–526. [Google Scholar] [CrossRef]
- Sica, C.; Ghisi, M. The Italian versions of the Beck Anxiety Inventory and the Beck Depression Inventory-II: Psychometric properties and discriminant power. In Leading-Edge Psychological Tests and Testing Research; Lange, M.A., Ed.; Nova Science Publishers: New York, NY, USA, 2007; pp. 27–50. [Google Scholar]
- Pedrabissi, L.; Santinello, M. Manuale Dell’adattamento Italiano dello STAI di Spielberger, Forma Y; Organizzazioni Speciali: Firenze, Italy, 1989. [Google Scholar]
- Havrdova, E.; Galetta, S.; Hutchinson, M.; Stefoski, D.; Bates, D.; Polman, C.H.; O’Connor, P.W.; Giovannoni, G.; Phillips, J.T.; Lublin, F.D.; et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: A retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 2009, 8, 254–260. [Google Scholar] [CrossRef]
- Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2019, 92, 112. [CrossRef]
- Hasselbalch, I.C.; Søndergaard, H.B.; Koch-Henriksen, N.; Olsson, A.; Ullum, H.; Sellebjerg, F.; Oturai, A.B. The neutrophil-to-lymphocyte ratio is associated with multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2018, 4, 2055217318813183. [Google Scholar] [CrossRef]
- Biberacher, V.; Schmidt, P.; Selter, R.C.; Pernpeinter, V.; Kowarik, M.C.; Knier, B.; Buck, D.; Hoshi, M.M.; Korn, T.; Berthele, A.; et al. Fatigue in multiple sclerosis: Associations with clinical, MRI and CSF parameters. Mult. Scler. 2018, 24, 1115–1125. [Google Scholar] [CrossRef]
- Brenner, P.; Granqvist, M.; Königsson, J.; Al Nimer, F.; Piehl, F.; Jokinen, J. Depression and fatigue in multiple sclerosis: Relation to exposure to violence and cerebrospinal fluid immunomarkers. Psychoneuroendocrinology 2018, 89, 53–58. [Google Scholar] [CrossRef]
- Peterson, D.; Brenu, E.W.; Gottschalk, G.; Ramos, S.; Nguyen, T.; Staines, D.; Marshall-Gradisnik, S. Cytokines in the cerebrospinal fluids of patients with chronic fatigue syndrome/myalgic encephalomyelitis. Mediat. Inflamm. 2015, 2015, 929720. [Google Scholar] [CrossRef]
- Grace, P.M.; Loram, L.C.; Christianson, J.P.; Strand, K.A.; Flyer-Adams, J.G.; Penzkover, K.R.; Forsayeth, J.R.; van Dam, A.M.; Mahoney, M.J.; Maier, S.F. Behavioral assessment of neuropathic pain, fatigue, and anxiety in experimental autoimmune encephalomyelitis (EAE) and attenuation by interleukin-10 gene therapy. Brain Behav. Immun. 2017, 59, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kwilasz, A.J.; Grace, P.M.; Serbedzija, P.; Maier, S.F.; Watkins, L.R. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology 2015, 96, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Van Boxel-Dezaire, A.H.; Hoff, S.C.; van Oosten, B.W.; Verweij, C.L.; Dräger, A.M.; Adèr, H.J.; van Houwelingen, J.C.; Barkhof, F.; Polman, C.H.; Nagelkerken, L. Decreased interleukin-10 and increased interleukin-12p40 mRNA are associated with disease activity and characterize different disease stages in multiple sclerosis. Ann. Neurol. 1999, 45, 695–703. [Google Scholar] [CrossRef]
- Inogés, S.; Merino, J.; Bandrés, E.; De Castro, P.; Subirá, M.L.; Sánchez-Ibarrola, A. Cytokine flow cytometry differentiates the clinical status of multiple sclerosis (MS) patients. Clin. Exp. Immunol. 1999, 115, 521–525. [Google Scholar] [CrossRef]
- Waubant, E.; Gee, L.; Bacchetti, P.; Sloan, R.; Cotleur, A.; Rudick, R.; Goodkin, D. Relationship between serum levels of IL-10, MRI activity and interferon beta-1a therapy in patients with relapsing remitting MS. J. Neuroimmunol. 2001, 112, 139–145. [Google Scholar] [CrossRef]
- Petereit, H.F.; Pukrop, R.; Fazekas, F.; Bamborschke, S.U.; Röpele, S.; Kölmel, H.W.; Merkelbach, S.; Japp, G.; Jongen, P.J.; Hartung, H.P.; et al. Low interleukin-10 production is associated with higher disability and MRI lesion load in secondary progressive multiple sclerosis. J. Neurol. Sci. 2003, 206, 209–214. [Google Scholar] [CrossRef]
- Tedeschi, G.; Dinacci, D.; Lavorgna, L.; Prinster, A.; Savettieri, G.; Quattrone, A.; Livrea, P.; Messina, C.; Reggio, A.; Servillo, G.; et al. Correlation between fatigue and brain atrophy and lesion load in multiple sclerosis patients independent of disability. J. Neurol. Sci. 2007, 263, 15–19. [Google Scholar] [CrossRef]
- Sepulcre, J.; Masdeu, J.C.; Goñi, J.; Arrondo, G.; Vélez de Mendizábal, N.; Bejarano, B.; Villoslada, P. Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult. Scler. 2009, 15, 337–344. [Google Scholar] [CrossRef]
- Bakshi, R.; Miletich, R.S.; Henschel, K.; Shaikh, Z.A.; Janardhan, V.; Wasay, M.; Stengel, L.M.; Ekes, R.; Kinkel, P.R. Fatigue in multiple sclerosis: Cross-sectional correlation with brain MRI findings in 71 patients. Neurology 1999, 53, 1151–1153. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Müller-Lenke, N.; Naegelin, Y.; Kalt, G.; Bendfeldt, K.; Kuster, P.; Stoecklin, M.; Gass, A.; Sprenger, T.; Radue, E.W.; et al. Contribution of cortical and white matter lesions to cognitive impairment in multiple sclerosis. Mult. Scler. 2013, 19, 1290–1296. [Google Scholar] [CrossRef]
- Popescu, V.; Schoonheim, M.M.; Versteeg, A.; Chaturvedi, N.; Jonker, M.; Xavier de Menezes, R.; Gallindo Garre, F.; Uitdehaag, B.M.; Barkhof, F.; Vrenken, H. Grey Matter Atrophy in Multiple Sclerosis: Clinical Interpretation Depends on Choice of Analysis Method. PLoS ONE 2016, 11, e0143942. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.A.; Parisi, L.; Pagani, E.; Copetti, M.; Rodegher, M.; Colombo, B.; Comi, G.; Falini, A.; Filippi, M. Regional but not global brain damage contributes to fatigue in multiple sclerosis. Radiology 2014, 273, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.D. Psychometric properties of the modified fatigue impact scale. Int. J. MS Care 2013, 15, 15–20. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).