Abstract
Non-alcoholic fatty liver (NAFL) is the most common chronic liver disease. Activation of mitogen-activated kinases (MAPK) cascade, which leads to c-Jun N-terminal kinase (JNK) activation occurs in the liver in response to the nutritional and metabolic stress. The aberrant activation of MAPKs, especially c-Jun-N-terminal kinases (JNKs), leads to unwanted genetic and epi-genetic modifications in addition to the metabolic stress adaptation in hepatocytes. A mechanism of sustained P-JNK activation was identified in acute and chronic liver diseases, suggesting an important role of aberrant JNK activation in NASH. Therefore, modulation of JNK activation, rather than targeting JNK protein levels, is a plausible therapeutic application for the treatment of chronic liver disease.
Keywords:
JNK; SAB; JNK activation loop; ROS; MAP kinase cascade; drug therapy; non-alcoholic steatohepatitis 1. Introduction
Non-alcoholic fatty liver (NAFL) is a disorder where excess fat accumulates in the liver (steatosis) due to non-alcoholic or non-viral causes. Non-alcoholic steatohepatitis (NASH) is hepatic steatosis associated with hepatocellular injury, innate immune cell-mediated inflammation, and progressive fibrosis in the liver. A total of 30–40% of adults in the United States have NAFL, defined as hepatic fat >5% of total liver weight, and 3–12% have NASH. Twenty percent of people with NAFL will develop NASH. NASH can progress to irreversible liver diseases such as cirrhosis and hepatocellular carcinoma (HCC). Up to 25% of adults with NASH may have cirrhosis [1,2,3,4,5,6]. While liver transplant is the ultimate solution for cirrhosis or HCC, new drugs are emerging that target molecules and pathways such as stress kinases, de novo lipogenesis, lipid oxidation and transport, lipotoxicity, inflammation and fibrogenesis to prevent or treat steatosis and steatohepatitis in liver [4,7,8,9]. Hypertension and cardiovascular diseases (CVD) due to increased risk of arteriosclerosis, hyperglycemia due to hepatic gluconeogenesis, and hyperinsulinemia due to insulin resistance cause the metabolic syndrome in NAFL disease [1,3]. Therefore, an effective therapeutic management is required for NAFL disease to prevent progression to NASH and its resulting complications. While the mechanism of developing NAFL/NASH is not fully understood, the hepatic metabolic stress response via JNK activation has been identified as a common pathway in numerous models of liver injury [10,11,12,13,14,15]. The relationship of JNK activation pathway to oxidative stress, lipotoxic stress and cell death, inflammation, and cytokines, fibrogenesis, de novo lipogenesis, lipolysis, lipid oxidation and transport are being studied to determine the mechanistic significant of MAPKs in the development of NASH (Figure 1) [6,16,17,18,19,20]. In addition, the development of NAFL/NASH is influenced by many common diseases whose primary etiology is not in liver, such as type II diabetes, hyper-insulinemia, obesity, changes in sex hormone regulation, adipokines and adipose tissue lipolysis, dysfunctional muscle metabolism, bile acid dyshomeostasis, gut dysbiosis, and brain lesions, and those are beyond the scope of this review [21,22,23,24,25,26,27,28,29]. This review discusses the hepatic stress kinase activation in NASH and plausible pharmaceutical targets.
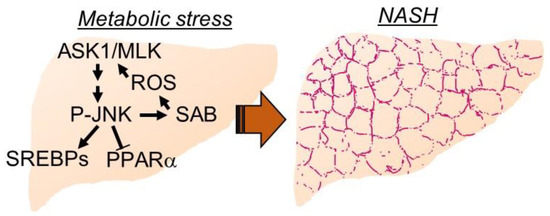
Figure 1.
Key mediators of hepatic metabolic stress. Carbohydrate and free fatty acid overload to hepatocytes activate stress kinase cascade to upregulate de novo lipogenesis genes to adapt metabolic stress. Dial-up feedforward activation of stress kinase cascade through P-JNK-SAB interaction attenuates β-oxidation and lipid oxidation genes. Damage signals, receptors, and extracellular vesicles from hepatocytes recruit inflammation, and activate hepatic stellate cells and fibrogenesis.
2. Hepatic MAPK Family in Metabolic Stress and the Mechanism of Sustained Activation
The liver is composed of 60% parenchymal cells, i.e., hepatocytes, and 30% to 35% non-parenchymal cells, i.e., Kupffer cells (KCs), hepatic stellate cells (HSCs) and liver sinusoidal endothelial cells (LSECs). Hepatocytes are the work horse of the liver and carry out a vast array of metabolic, regulatory, and toxicological functions. Hepatocytes express MAPKs which transduce extracellular and intracellular signals to regulate cell proliferation, differentiation, apoptosis, and metabolism [30]. Metabolic stress induced MAPK cascade activation in hepatocytes includes an upstream MAPK kinase kinases (MAP3K) such as ASK1, MLKs, TAK1, MAPK kinases (MAP2K) such as MKK4, MKK7, and terminal stress kinases such as JNK, and p38 [31,32,33,34]. Diets, specifically hyper-nutrition type diets such as high fat, high carbohydrate, activate hepatic JNK longer than standard fat and carbohydrate diet [35]. It is important to mention that activated JNK (P-JNK) level decreases between meals, and fasting overnight alleviates basal low level of P-JNK. Hyper-nutrition type diets activate JNK by metabolites, endoplasmic reticulum (ER) stress, and mitochondrial stress via the modification of acetylation/deacetylation status, transcriptional activation/inhibition, energy metabolism and lipid oxidation, and oxidative stress [36,37,38].
JNK and p38 stress kinase are self-regulated via dual-specificity phosphatase (DUSP) group, which is the vital mechanism to dampen and terminate kinase activation via direct inactivation or inhibiting upstream kinase activation [39,40]. Individual DUSP has different tissue expression, subcellular localization, and has differing preferences for the kinases in MAPK cascade such as DUSP22 in ASK1-MKK7-JNK signaling pathway, DUSP5 on ERK signaling pathway [41,42,43]. DUSP9, 10, 12, 14 and 26 in liver have been found to prevent the hepatic steatosis [44,45,46,47,48,49,50]. In addition, the scaffold function of DUSP has been noted and extended reviews on DUSPs are available for further reading [41].
The sustained activation of JNK via the self-amplified feed-forward activation loop has been discovered recently [51] and found to play a key role in several disease models such as drug induced acute liver injury and cell death, cytokine induced hepatic apoptosis, lipotoxicity, ER stress induced mitochondrial dysfunction and cell death, ischemia-reperfusion injury in heart, cardiotoxicity, neuronal activity in brain, neurotoxicity, and ovarian cancer treatment [52,53,54,55,56,57,58,59]. Initial activated P-JNKs translocate to mitochondria where P-JNKs phosphorylate mitochondrial outer membrane protein SH3 homology associated BTK binding protein, SH3BP5 (SAB). P-JNK binding to SAB initiates intramitochondrial release of tyrosine phosphatase SH2 phosphatase 1 (Shp1) from SAB leading to dephosphorylation of activated-Src at tyrosine 419, which occurs on and requires the platform, docking protein 4 (DOK4), located on the mitochondrial inner membrane. P-Src is required to maintain electron transport [51]. Decreased P-Src inhibits mitochondrial respiration and enhances reactive oxygen species (ROS) production especially when mitochondrial aerobic metabolism via tricarboxylic acid cycle (TCA cycle) is upregulated by Ca+2 flux from mitochondria-associated membrane (MAM), ER and cytoplasm (Figure 1 and Figure 2) [54]. ROS facilitates ASK1 N-terminal dimerization and activation by oxidation and removal of thioredoxin, which binds and inhibits ASK1 [60]. Activated ASK1 activates MKK4/7 which in turn activates JNKs to amplify and increase the level of P-JNK [30,61,62]. Interestingly, P-MKK4 is associated with P-JNK on mitochondria [63]. Importantly, SAB is a pivotal molecule determining the level of P-JNK to cellular stress and damage [51,64]. Depletion or inhibition of ASK1, MKK4 or SAB prevents sustained P-JNK activation and prevents JNK mediated cellular stress but does not prevent initial JNK activation from the stress inducers such as reactive adducts in toxic drug metabolites, metabolic reprogram, IRE1α and PERK activation in unfolded protein or ER stress, membrane associated Src activated in lipid induced modification of plasma membrane plasticity and fluidity [52,65,66,67,68]. Therefore, the feed-forward activation to sustain JNK activation, the JNK-SAB-ROS activation loop, is a key player in the mechanism of cellular stress and damage [30,51].
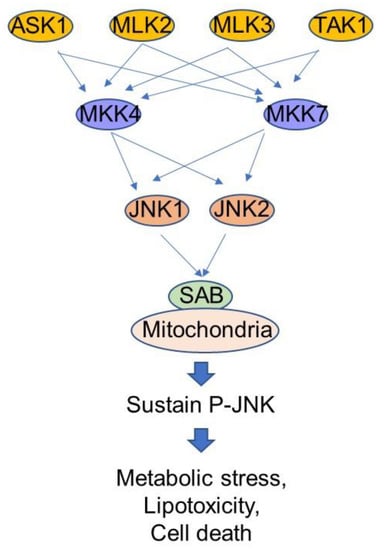
Figure 2.
Stress response kinase cascade. MAP3K such as ASK1, MLK2/3, TAK1 are upstream kinases which are activated by reactive oxygen species, membrane lipid composition and changes. MKK4 and MKK7 are abundant MAP2K in liver. JNK1 and JNK2 are MAP kinases with functional redundancy in liver. SAB is a mitochondrial outer membrane protein and directly interacts with P-JNK, but not interact with p38 in vivo. Suppression of SAB expression or inhibition of P-JNK-SAB interaction is a plausible therapeutic target of hepatic metabolic stress in NASH.
The JNK-interacting protein (JIP) family plays an integral role in MAPK cascade activation by creating proximity of upstream kinases and terminal kinases [69,70]. JIP1 is a key platform in MLKs mediated MKK7 and JNKs activation [71,72]. However, JIP1 also mediates ASK1-MEK-JNK signaling [73] and more than one JIP could be involved in P-JNK inactivation and activation loop [74,75]. We believe MLKs activation is an important signal for initial JNK activation at least in the acute liver injury models because initial JNK activation occur in the liver of acetaminophen treated ASK1 KO mice [76]. Role and function of JIP2, 3 and 4 are yet to be examined. Stress kinases target various intracellular molecules such as transcriptional and non-transcriptional targets via kinase interacting motif (KIM) and substrates are predicted to be phosphorylated serine/threonine-proline (SP) sites [30,61,62]. As an example, JNK targeted GCLC are ubiquitinated and degraded leading to decreased GSH level and increased oxidative stress as occur in the acute acetaminophen hepatotoxicity [64,77]. In NASH, P-JNK targeting of transcriptional factors, such as SREBFs to increase lipogenesis and cholesterol synthesis, NCoR1 to repress PPARα and fatty acid oxidation, leads to cumulative lipotoxic stress [78,79,80]. Therefore, P-JNK-SAB ROS activation loop is a key mediator in sustained activation of JNK.
3. Hepatic MAPK Mediates Progression of NAFL/NASH
3.1. Hepatocytes: Hepatic Steatosis, Oxidative Stress, and Autophagy
Hepatic fat is mostly triglyceride formed by esterification of free fatty acid from the diet, adipose tissue lipolysis and de novo lipogenesis. Fat accumulation in hepatocytes causes lipotoxicity and apoptosis [6,16,53] and ballooning degeneration of hepatocytes which are associated with oxidative stress in steatosis/steatohepatitis [78]. In the course of disease progression, high calorie diet increases mitochondrial function and metabolism accompanied with stress kinase activation [78,81]. Interaction of activated P-JNK with mitochondrial protein SAB impairs mitochondrial respiration and produces ROS leading to amplify feed-forward activation of JNK, known as the JNK-SAB-ROS activation loop [30,51,82]. Decreased hepatic SAB (SH3BP5) expression by Sab knockdown or knockout decreases ROS and decreases the stress kinase JNK activation in established experimental models of steatosis and steatohepatitis [78]. Notably, increased expression of SAB in steatotic liver plays a pivotal role in the higher activation of stress kinases and progression to steatohepatitis [64,78]. In line with this observation, depletion of hepatic JNK1/2 prevents diet induced lipogenesis and steatosis [79]. In addition, WW domain containing transcription regulator 1 (Wwtr1/TAZ) induced hepatic NADPH oxidase 2 (NOX2/Cybb) expression mediates the oxidative DNA damage in diet induced NASH and HCC [7]. NOX2 is a superoxide generating enzyme, which delivers activated oxygen into phagocytic vacuole in inflammatory cells such as granulocytic neutrophil in NASH [83]. The mechanism may be associated with microbiome dysregulation in the gut and translocation to liver [84]. Nevertheless, the inter-regulation of MAPK family and TAZ expression and activity in the progression of NASH needs to be explored.
The critical role of JNK activation in the progression of NASH is supported by studies of MAP3Ks such as ASK1 and MLKs, and DUSPs. For instance, ASK1−/− mice have reduced hepatic steatosis. CASP8 and a FADD-like apoptosis regulator (CFLAR) disrupts the N-terminus-mediated dimerization of ASK1 and favors ASK1 degradation and prevents JNK activation and hepatic steatosis [85]. CFLAR peptide is proposed as a potential therapeutic agent. In addition, upregulation of TRAF1 promotes hepatic steatosis through enhanced activation of ASK1-mediated JNK/P38 activation [86]. However, contradictory data is reported in hepatocyte specific ASK1 deleted mice which is cross-bred with albumin-Cre mice or in mice treated with ASK1 inhibitor. Deletion of hepatic ASK1 decreases P-JNK/P-38, impairs autophagosome formation in the liver and increase the liver triglyceride and fibrosis [87]. Hepatocyte specific ASK1 expression in mice prevents hepatic steatosis [87]. It should be noted that the content of diet, age and background strain of mice, housing environment such as temperature and microbiome affect the animal model of NASH [88], and direct or indirect effect of ASK1 and CFLAR on NASH is required to reconcile. MLK2 and 3 are redundant and abundant in the liver. MLK−/− mice prevents high fat diet (HFD) induced hepatic steatosis and triglyceride accumulation. The mechanism is explained by reduced JNK/p38 activation in HFD fed MLK−/− mice, and in mice treated with MLK3 inhibitor URMC-099 [89]. However, further studies are required to examine the possible activation of ASK1 in HFD fed MLK KO mice, and vice versa to identify the specific or overlapping function in progression to NASH. Nevertheless, JNK activation is an important mechanism in development of hepatic steatosis.
The liver expresses several dual-specificity phosphatases (DUSPs). DUSP9 and 12 have the substrate preference for JNK and p38 [41]. Moreover, the expression of DUSPs protects against steatohepatitis through suppression of ASK1 activation [44,45]. The suppression of ASK1 activation could be via inhibition of JNK-SAB-ROS activation in DUSP9 and 12 over-expressed HFD fed mice. In line with these results, DUSP10(MKP5)−/− mice progress to severe steatosis with aging or HFD [46]. DUSP14 and 26 prevent JNK/p38 activation in hepatic steatosis and inflammation via inhibition of TAK1 [47,48]. In addition, DUSP14 protects against hepatic ischemia-reperfusion injury via suppressing TAK1 and subsequent NF-κB signaling and JNK activation [49]. The results of HFD fed DUSP1−/− mice are difficult to reconcile. Hepatic DUSP1−/− mice are protected from hepatic steatosis but have elevated JNK/p38 phosphorylation [90].
The dual role of JNK1/2 in HFD induced steatosis and steatohepatitis has been elucidated hepatic specific JNK1/2 depletion studies [79] and settled the earlier studies using gene specific KO mice [91]. Increased JNK activation with the progression of hepatic steatosis reduces peroxisome β-oxidation by suppressing peroxisome proliferator-activated receptor α (PPARα) activity, in part through the upregulation of nuclear receptor corepressor 1 (NCoR1) [79]. Moreover, the hepatic metabolic stress accompanied by increased JNK activation and increased flux of acetyl-CoA from mitochondria to cytosol favors acetylation of transcription factors and histones leading to de novo lipogenesis via transcriptional factors SREBFs and the activation of downstream lipogenesis genes [92]. In addition, the carbohydrate sensor ChREBP is upregulated following ingestion of a carbohydrate-enriched meal, leading to the increased expression of genes regulating lipogenesis and fatty acid esterification that promote liver steatosis [93]. Direct association of JNK activity and ChREBP expression and activation is unknown. P38 isoforms (α, β, γ/δ) expression and activity are difficult to reconcile in several studies of NASH. A recent study using liver-specific p38α KO mice suggested that hepatic p38α protects mice from steatohepatitis in a diet feeding model [94]. However, macrophage p38α promotes progression of steatohepatitis [95]. It is important to stress that pharmaceutical inhibition or knockdown of p38 causes liver injury [96,97] and isoform specific and conditional studies may be required to examine the hypothesis.
Autophagy, induced by extra- and intra-cellular stress, is the important part of liver homeostasis by removing damaged organelles (mitophagy, ER-phagy, pexo-phagy), lipid droplets (lipophagy) and protein aggregates, and recycling nutrients to preserve the cellular energy, integrity, and survival [98,99]. The mammalian target of rapamycin (mTOR) is the one major inhibitor of autophagy [100]. Growth factors and insulin repress autophagy via PI-3K-AKT-mTOR activation pathway [101,102]. During starvation, specifically glucose or amino acid deprivation, autophagy pathway in liver is upregulated by interfering mTOR activation via activation of cAMP-AMPK-TSC1/2 inhibitory pathway [101,102]. In diet-induced NASH, autophagy, especially lipophagy, occurs [103,104,105,106]. Increased lipophagy in NASH may reflect decreased mTOR activity due to P-JNK inhibition of insulin receptor signaling in NASH. However, the direct involvement of the P-JNK and P-JNK activation loop in a lipophagy mechanism is unknown. Nevertheless, pharmaceutical enhancement of autophagy by rapamycin or carbamazepine which inhibits mTOR reduced hepatic steatosis in NASH model [107], and severe hepatic steatosis occurs in mice with hepatic deletion of Atg5 [104]. Viral expression of Atg7 increases autophagy, reduces ER stress, and improves insulin sensitivity [108]. More importantly, ER stress in NASH may upregulate autophagy pathway because tunicamycin induces autophagy and IRE1α-JNK pathway is involved [109,110]. Though, JNK1 promotes autophagy through phosphorylation of BCL2, which releases BECN1 from BCL2 to contribute in autophagosome [111], JNK1 can phosphorylate RPTOR at Ser863 which is necessary to assemble MTOTC1 complex to block autophagy [112]. Direct evidence that JNK mediates these effects is needed to fully prove in NASH model.
Sex differences also exist in NAFLD [113]. The prevalence and severity of NAFLD are higher in men than in women during the reproductive age (age ≤ 50–60 years); however, women after menopause (age ≥ 50–60 years) have higher rates of NAFLD [113]. The hepatic ERα-p53-miR34a signaling axis decreases SAB protein level in women of reproductive age [64]. This is explained in hormone mediated suppression of SAB. SAB expression is reduced in adult females, compared to male and postmenopausal females [64]. Increased hepatic SAB expression increases JNK activation in steatosis and steatohepatitis [78]. Additional factors such as sex difference in metabolic homeostasis contributed by skeletal muscle, adipose tissue, and thyroid hormones [114,115,116] are beyond the scope of current review. Clinical and epidemiological studies have shown that postmenopausal women receiving hormone replacement therapy had a lower prevalence of NAFLD compared to postmenopausal women not receiving replacement therapy [113]. While these findings suggest that estrogen is protective, further studies are needed to define the hepatic molecular mechanisms of sex difference in NAFLD. Sex, age, reproductive status, and synthetic hormone usage are needed to include in future clinical investigation and gene association studies of NAFLD [113].
A meta-analysis of recent studies has also shown that the PNPLA3, also known as adiponutrin, rs738409 [G] allele is associated with an increased risk of diet related hepatocellular carcinoma [117]. The PNPLA3 Ile148Met variant is resistant to degradation and disrupts ATGL lipolysis activity [118]. In addition, levels of PNPLA3 and CGI-58 in lipid droplets determine the lipolysis activity of ATGL. A recent study demonstrated that cJUN inhibits RORα-mediated PNPLA3 expression [119], leading to downregulation of the ATGL activity. cJUN is a JNK activated transcriptional factor [62]. Thus, the relationship between hepatic stress kinase activity and hepatic lipase expression and activity needs to be further explored. An allele in PNPLA3 (rs738409[G], encoding Ile148Met) is associated with increased liver fat, hepatic inflammation, and fibrosis [120].
P-JNK activates transcriptional co-repressor NCoR1 and suppresses PPARα activation in NASH, affecting decreased expression of genes involved in fatty acid transport and oxidative degradation in mitochondria and peroxisomes [79]. Consistent with the hepatocyte-specific JNK deletion model, Elafibranor (GFT505, Genfit), a PPARα/β agonist, Saroglitazar, a PPARα/γ agonist, and lanifibranor, a pan-PPAR agonist normalize serum lipid profiles, insulin resistance and improve NASH [121]. PPAR agonists, one of the most advanced classes of anti-NASH molecules, are in phase II or III clinical studies and may need to improve the efficacy and safety [122]. In addition, long-term studies in rodents showed an association of PPARα agonists with hepatic carcinogenesis and the result could be species-specific effect [123,124]. In line with this observation, hepatic JNK KO increases the cholangiocyte proliferation, and intrahepatic cholangiocarcinoma [125]. JNK KO activates the transcription factor PPARα and its target genes related to hepatic cholesterol and bile acid synthesis resulting in cholestasis. Therefore, a pharmaceutical target that directly interferes and dampens the sustained P-JNK activation is required to overcome the effect of complete deletion of JNK.
3.2. Non-Hepatocytes: Immune Cells, Sinusoidal Endothelial Cells, Hepatic Stellate Cells
NASH is the hepatic steatosis with liver cell inflammation and innate-immune system activation involving Kupffer cells (KC) and production of pro-inflammatory cytokines in the liver. Extracellular vesicles, fatty acids, cholesterol released from steatotic hepatocytes and lipopolysaccharides (LPS) activate KCs and hepatic stellate cells (HSCs) via damage-associated molecular pattern receptors, known as Toll-like receptor 4 (TLR4) and TNF-related apoptosis-inducing ligand death receptor (TRAIL-R2) [126]. Mice without TLR4 in KCs are protected against steatosis and NAFLD progression [126]. KCs activated by the hepatocyte-derived mitochondrial DNA produce pro-inflammatory chemokines such as TNF-α, monocyte chemotactic protein-1 (MCP-1), transforming growth factor-β (TGF-β), and tissue inhibitors of metalloproteinase, leading to fibrosis of the liver [127]. Moreover, chemokines and chemokine receptors involved in leukocyte recruitment such as CXCL8/CXCR1; CXCL1 & 3/CXCR2; CCL3-5/CCR5 and the chemokines CXCL9-11 and CCL2 (MCP1) are upregulated in steatohepatitis [128]. Importantly, bacteria toxins, cytokine and chemokines activate JNK and JNK activation is required for synthesis of proinflammatory cytokines [129]. Blood lymphocytes, monocytes and macrophages, and tissue residence immune cells express JNK, SAB and MAP kinase cascades ubiquitously [130]. Therefore, JNK-SAB-ROS activation loop may play a role in sustained activation of P-JNK, and immune cell function and migration are regulated by P-JNK activity. Deletion of SAB or inhibition of JNK kinase activity by SP600125 prevented TNF induced sustained P-JNK activation and cell death [52]. Consistently, conditional mononuclear cell JNK KO mice are prevented from diet-induced NASH [131]. However, a recent study on the differential role of recruited monocytes in steatohepatitis has been discovered [132] and role of MAP kinase signaling in subpopulation of macrophage needs to be revisited.
In addition, CD62E (E-Selectin) and CD44 which recruit leukocytes into inflammation sites are upregulated in NASH patients [128]. P-selectin produced from platelets and endothelial cells by TNF, IL1, or LPS upregulate E-selectin expression in liver sinusoidal endothelial cell (LSEC) [133]. Similar to hepatocytes, LSECs express MAP kinase cascade and SAB, and lipotoxic stress also occurs in LSECs contributing to NOX1 expression and ROS generation [134]. The gut microbiota also seems to contribute to liver endothelial dysfunction. Restoration of a healthy microbiota via fecal transplantation normalizes portal hypertension by improving intrahepatic vascular resistance and endothelial dysfunction in rats [135]. Gut microbiome dysregulation (dysbiosis) is one of the important factors in the progression of NAFLD to advanced fibrosis and cirrhosis [84]. A low fiber, high fat, and high carbohydrate diet changes gut microbiome which in turn changes gut and systemic dietary metabolites such as acetate and cytokines. Gut bacteria and their metabolites translocate to the liver through a disrupted gut barrier and induce hepatic inflammatory reaction [84]. Further diet induced NASH studies are required to explore the effect of microbiome on JNK activation loop in LSEC.
JNK activation in HSCs in response to TGF-β and platelet-derived growth factor (PDGF) activates Smad2/3 leading to α-smooth muscle actin (αSMA) expression, migration of resident HSCs and myofibroblasts, and fibrosis in NASH [17]. Notably, follistatin like 1 (Fstl1), glycoprotein, is secreted from HSCs/myofibroblast induced by TGF-β1. Fstl1 binds to TGF-β1 and negatively regulates TGF-β1 signaling in lung development. Interestingly, Fstl1 neutralizing antibody attenuates CCL4 induced liver fibrosis [136]. Further studies are required to reconcile results from developmental and disease models. Importantly, we found that JNK, SAB and MAP kinase cascade express in HSC, but how JNK activation is involved in HSC function and liver fibrosis is yet to be examined.
4. Perspective of the Sustained JNK Activation Loop in Therapeutic Development
Chronic hepatic inflammation causes fibrosis leading to liver cirrhosis. One-carbon metabolism, mitochondrial stress, ER stress, DNA damage, inflammation, and obesity are involved in carcinogenesis in NAFLD. Patients who develop cirrhosis related to NASH are at risk for hepatocellular carcinoma and/or end-stage liver failure and liver transplantation. Currently there is no drug approved by US Food and Drug Administration (FDA) for the treatment of NASH. Lifestyle alterations remain the only treatment. Steatosis, inflammation, ballooning, and fibrosis were improved in those achieving >5% weight loss, with even greater improvement in patients achieving >10% weight loss. Several molecules are in clinical trials (www.clinicaltrials.gov, accessed on 18 July 2022). However, the study that aims to reduce level of P-JNK activation via targeting sustained-JNK-activation-loop is very sparse and mentioned here, and prospective targets are discussed.
Oxidative stress and antioxidant supplement: Deletion of Nrf2 results in rapid progression of NASH [137]. In addition, NADPH oxidases (NOXs), which links NAFL progression to NASH and HCC, are membrane-bound enzymatic complexes generating ROS and are abundant in liver associated with inflammation and immune responses [138]. Evidence supports that in early steatosis phase of diet induced NASH oxidized protein adducts increase in liver [78], suggesting that ROS produced in both hepatocytes and non-hepatocytes contribute the progression of NASH. Nevertheless, antioxidants, such as vitamin E prevent NASH [139]. The effectiveness of Vitamin E supplementation is currently under study in clinical trials of NAFLD. Further studies may be indicated because of possible association of prostate cancer and insulin resistance with the long-term usage of Vitamin E [140].
JNK and JNK inhibitors: JNK inhibitors inhibit the kinase activity of JNK isoforms in both hepatocytes and non-hepatocytes. SP600125, the most well tested JNK inhibitor, inhibits JNK kinase activity via inhibition of self-activation or MKK4 by competitive reversible inhibition at ATP binding site, but not at a JNK substrate binding site [141]. New and more specific JNK inhibitors, JNK-IN-8 and JNK-IN-10, are irreversible inhibitors [142]. Chemical inhibitors may potentially target a wide variety of JNK substrates in hepatocytes, KCs, HSCs, sinusoidal endothelial cells, and immune cells in liver. This non-selective inhibition precludes the development of JNK inhibitors as drugs for clinical use. The antisense oligo nucleotide targeting to JNK (JNK-ASO) to reduce JNKs expression is a selective and potential therapeutic agent and can efficiently target to hepatocytes [143]. However, hepatic JNK1/2 embryonic KO studies demonstrated that hepatocyte proliferation at 48 h is reduced in an experimental model of liver regeneration [144], though overall regeneration after 72 h is not different. Importantly, prolonged hepatic JNK deficiency increased cholangiocyte proliferation and intrahepatic cholangiocarcinoma [125].
JNK-SAB interaction and targeting SAB: SAB is a mitochondrial outer membrane protein. The interaction of P-JNK and SAB inhibits the mitochondrial respiration [51]. SAB expression is increased in human and murine NAFL and NASH [78]. Increased level of SAB amplifies and sustains JNK activation via the JNK-SAB-ROS activation loop. Blocking JNK-SAB interaction by a SAB peptide selectively prevents the sustained amplification of P-JNK and cell death in various animal models [51,52,53,54,55,56,57,58,59,78]. SAB-ASO treatment, which decrease SAB expression and JNK activation, effectively prevents NASH progression and reverses NASH score [78]. Notably, complete depletion of SAB prevents sustained P-JNK activation but does not interfere the initial activation of JNK and stress response signaling required for cellular response and survival such as JNK/ATF2 dependent early phase induction of DUSP1 and 10, and late phase transcriptional activation of DUSP4 and 16 [30].
JNK-JIP1 interaction and inhibitor: JNK-interacting protein-1 (JIP1) is a scaffolding protein where upstream MAPKs activate JNK. BI-78D3 is able to compete with the D-domain of JIP1 for JNK binding and thus inhibits JNK activation. BI-78D3 effectively prevent CCL4 induced acute liver injury [145], yet the effect of BI-78D3 in NASH models need to be examined.
ASK1 and ASK1 inhibitor: Apoptosis signaling-regulating kinase 1 (ASK1) activity directly involves P-JNK-SAB-ROS activation loop through redox sensitive thioredoxin [146,147]. ASK1 is a MAP3 kinase which activates downstream terminal kinases both JNK and p38. JNK activity on mitochondria reversely corresponds to mitochondrial respiration [51], but function of p38 on mitochondria is not known. ASK1 inhibitor, Selonsertib (GS-444217) ameliorates NASH and improved fibrosis in preclinical studies and in a short-term clinical trial [148] but was not effective in late-phase clinical trials [149]. Selonsertib, which blocks ASK1 activation but not MLKs, may not be enough to revert the progression of NASH in human disease. Notably, hepatic JNK1 and JNK2 are cross-activated by the upstream MAP3Ks, ASK1, MLK2/3 and TAK1.
MLKs and MLK inhibitor: The stress kinase mixed lineage kinase is also a MAP3K activating MKK4/7 and then JNK/p38. MLK3-JNK mediates the hepatic extracellular vesicle release [150,151]. Genetic or pharmacological inhibition of MLK3 results in reduction of the potent C-X-C motif chemokine ligand 10 (CXCL10) in extracellular vesicles (EV) derived from LPC-treated hepatocytes. NASH-inducing diet fed MLK3−/− mice have reduced CXCL10 levels in their plasma EVs and, hepatoprotection against injury and inflammation. Furthermore, pharmacological MLK3 inhibitor, URMC099, reduces circulating CXCL10 and attenuates murine NASH [151]. It is important to stress that circulating EVs derived from hepatocytes, immune cells and platelets can be a biomarker for NAFL resolution in response to weight loss surgery [152,153]. Preclinical studies of URMC099 on Alzheimer’s disease, Parkinson’s disease and NASH model are encouraging [154,155]. Efficacy in human clinical trials has not yet been examined.
5. Conclusions
The understanding the mechanism of kinase activation and signal regulation will generate therapeutic target molecules to develop a safe and effective treatment. However, limitations and caveats do exist because of similarity of the kinase activation and interaction with its substrates through a pattern of amino acid sequence, motif, creating unwanted off target side effects. Finding the targetable proximity molecules in the P-JNK activation loop, Table 1 such as CFLAR in ASK1 activation, could be an alternative strategy. Advances in our current understanding of molecular mechanisms using improved animal models and small molecule screening could support the new pharmaceutical development especially target specific peptides or antisense oligos, which are the promising technologies for the future prevention and treatment of NASH.

Table 1.
Regulators of JNK activation in liver diseases. MAPKs, JNK and p38, are activated by upstream MAP kinase cascade which activity is dampened by interacting proteins or proteasomal degradation, or enhanced by membrane associated signal activation or reactive oxygen species (ROS). DUSP family phosphatases inhibition of MAP kinase cascade is critical in preventing susceptibility of hepatocyte toxicity and steatosis. Sustained activation of JNK via SAB-ROS-MAP3K overcomes the cellular protective mechanism and causes hepatocyte toxicity and lipogenesis.
Author Contributions
R.W.M.M. and S.W. wrote the original draft preparation. R.W.M.M., F.W.M.A., B.L., A.A. and S.W. contributed writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The study did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Diehl, A.M.; Day, C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2017, 377, 2063–2072. [Google Scholar] [CrossRef]
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef]
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.C.; van Rooyen, D.; Gan, L.; Chitturi, S. NASH is an Inflammatory Disorder: Pathogenic, Prognostic and Therapeutic Implications. Gut Liver 2012, 6, 149–171. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.H.; Kohli, R.; Gores, G.J. Mechanisms of lipotoxicity in NAFLD and clinical implications. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 131–140. [Google Scholar] [CrossRef]
- Albhaisi, S.A.M.; Sanyal, A.J. New drugs for NASH. Liver Int. 2021, 41, 112–118. [Google Scholar] [CrossRef]
- Parlati, L.; Régnier, M.; Guillou, H.; Postic, C. New targets for NAFLD. JHEP Rep. 2021, 3, 100346. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Noureddin, M.; Alkhouri, N.; Sanyal, A.J. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 373–392. [Google Scholar] [CrossRef]
- Czaja, M.J. JNK regulation of hepatic manifestations of the metabolic syndrome. Trends Endocrinol. Metab. 2010, 21, 707–713. [Google Scholar] [CrossRef]
- Hirata, Y.; Inoue, A.; Suzuki, S.; Takahashi, M.; Matsui, R.; Kono, N.; Noguchi, T.; Matsuzawa, A. trans-Fatty acids facilitate DNA damage-induced apoptosis through the mitochondrial JNK-Sab-ROS positive feedback loop. Sci. Rep. 2020, 10, 2743. [Google Scholar] [CrossRef] [PubMed]
- Kluwe, J.; Pradere, J.P.; Gwak, G.Y.; Mencin, A.; De Minicis, S.; Osterreicher, C.H.; Colmenero, J.; Bataller, R.; Schwabe, R.F. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology 2010, 138, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, J.; Huang, P.; Yang, L.; Liu, Y.; Li, Y.; Wang, J.; Song, H.; Zheng, P. Scoparone Improves Nonalcoholic Steatohepatitis Through Alleviating JNK/Sab Signaling Pathway-Mediated Mitochondrial Dysfunction. Front. Pharmacol. 2022, 13, 863756. [Google Scholar] [CrossRef]
- Wei, Y.; Pagliassotti, M.J. Hepatospecific effects of fructose on c-jun NH2-terminal kinase: Implications for hepatic insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E926–E933. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Brenner, D.A.; Karin, M. A liver full of JNK: Signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology 2012, 143, 307–320. [Google Scholar] [CrossRef]
- Fuchs, M.; Sanyal, A.J. Lipotoxicity in NASH. J. Hepatol. 2012, 56, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Baglieri, J.; Kisseleva, T.; Brenner, D.A. Mechanisms of liver fibrosis and its role in liver cancer. Exp. Biol. Med. 2020, 245, 96–108. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, J.; Lu, X.; Thewke, D.P.; Mason, R.J. KGF induces lipogenic genes through a PI3K and JNK/SREBP-1 pathway in H292 cells. J. Lipid Res. 2005, 46, 2624–2635. [Google Scholar] [CrossRef]
- Rozo, A.V.; Vijayvargia, R.; Weiss, H.R.; Ruan, H. Silencing Jnk1 and Jnk2 accelerates basal lipolysis and promotes fatty acid re-esterification in mouse adipocytes. Diabetologia 2008, 51, 1493–1504. [Google Scholar] [CrossRef][Green Version]
- Haberzettl, P.; Hill, B.G. Oxidized lipids activate autophagy in a JNK-dependent manner by stimulating the endoplasmic reticulum stress response. Redox Biol. 2013, 1, 56–64. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed]
- Eshraghian, A.; Hamidian Jahromi, A. Non-alcoholic fatty liver disease and thyroid dysfunction: A systematic review. World J. Gastroenterol. 2014, 20, 8102–8109. [Google Scholar] [CrossRef] [PubMed]
- Arata, M.; Nakajima, J.; Nishimata, S.; Nagata, T.; Kawashima, H. Nonalcoholic steatohepatitis and insulin resistance in children. World J. Diabetes 2014, 5, 917–923. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.G.; Kim, S.M.; Caussy, C.; Fu, T.; Guo, J.; Bassirian, S.; Singh, S.; Madamba, E.V.; Bettencourt, R.; Richards, L.; et al. A Universal Gut-Microbiome-Derived Signature Predicts Cirrhosis. Cell Metab. 2020, 32, 878–888. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Targeting bile acids and lipotoxicity for NASH treatment. Hepatol. Commun. 2017, 1, 1002–1004. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Shibolet, O.; Elinav, E. The role of the microbiome in NAFLD and NASH. EMBO Mol. Med. 2019, 11, e9302. [Google Scholar] [CrossRef]
- Lombardi, R.; Fargion, S.; Fracanzani, A.L. Brain involvement in non-alcoholic fatty liver disease (NAFLD): A systematic review. Dig. Liver Dis. 2019, 51, 1214–1222. [Google Scholar] [CrossRef]
- Win, S.; Than, T.A.; Zhang, J.; Oo, C.; Min, R.W.M.; Kaplowitz, N. New insights into the role and mechanism of c-Jun- N-Terminal kinase signaling in the pathobiology of liver diseases. Hepatology 2018, 67, 2013–2024. [Google Scholar] [CrossRef]
- Schuster-Gaul, S.; Geisler, L.J.; McGeough, M.D.; Johnson, C.D.; Zagorska, A.; Li, L.; Wree, A.; Barry, V.; Mikaelian, I.; Jih, L.J.; et al. ASK1 inhibition reduces cell death and hepatic fibrosis in an Nlrp3 mutant liver injury model. JCI Insight 2020, 5, e123294. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.X.; Török, N.J. MLK3 as a regulator of disease progression in Non-alcoholic steatohepatitis. Liver Int. 2014, 34, 1131–1132. [Google Scholar] [CrossRef][Green Version]
- Seki, E. TAK1-dependent autophagy: A suppressor of fatty liver disease and hepatic oncogenesis. Mol. Cell. Oncol. 2014, 1, e968507. [Google Scholar] [CrossRef] [PubMed]
- Cicuéndez, B.; Ruiz-Garrido, I.; Mora, A.; Sabio, G. Stress kinases in the development of liver steatosis and hepatocellular carcinoma. Mol. Metab. 2021, 50, 101190. [Google Scholar] [CrossRef] [PubMed]
- Vernia, S.; Cavanagh-Kyros, J.; Barrett, T.; Tournier, C.; Davis, R.J. Fibroblast Growth Factor 21 Mediates Glycemic Regulation by Hepatic JNK. Cell Rep. 2016, 14, 2273–2280. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, H.; Bu, Q.; Wei, S.; Li, L.; Zhou, J.; Zhou, S.; Su, W.; Liu, M.; Liu, Z.; et al. Role of XBP1 in regulating the progression of non-alcoholic steatohepatitis. J. Hepatol. 2022, 77, 312–325. [Google Scholar] [CrossRef]
- Guo, L.; Guo, Y.Y.; Li, B.Y.; Peng, W.Q.; Chang, X.X.; Gao, X.; Tang, Q.Q. Enhanced acetylation of ATP-citrate lyase promotes the progression of nonalcoholic fatty liver disease. J. Biol. Chem. 2019, 294, 11805–11816. [Google Scholar] [CrossRef]
- Li, Y.F.; Xie, Z.F.; Song, Q.; Li, J.Y. Mitochondria homeostasis: Biology and involvement in hepatic steatosis to NASH. Acta Pharmacol. Sin. 2022, 43, 1141–1155. [Google Scholar] [CrossRef]
- Ha, J.; Kang, E.; Seo, J.; Cho, S. Phosphorylation Dynamics of JNK Signaling: Effects of Dual-Specificity Phosphatases (DUSPs) on the JNK Pathway. Int. J. Mol. Sci. 2019, 20, 6157. [Google Scholar] [CrossRef]
- Staples, C.J.; Owens, D.M.; Maier, J.V.; Cato, A.C.; Keyse, S.M. Cross-talk between the p38α and JNK MAPK pathways mediated by MAP kinase phosphatase-1 determines cellular sensitivity to UV radiation. J. Biol. Chem. 2010, 285, 25928–25940. [Google Scholar] [CrossRef]
- Jeffrey, K.L.; Camps, M.; Rommel, C.; Mackay, C.R. Targeting dual-specificity phosphatases: Manipulating MAP kinase signalling and immune responses. Nat. Rev. Drug Discov. 2007, 6, 391–403. [Google Scholar] [CrossRef]
- Ju, A.; Cho, Y.C.; Kim, B.R.; Park, S.G.; Kim, J.H.; Kim, K.; Lee, J.; Park, B.C.; Cho, S. Scaffold Role of DUSP22 in ASK1-MKK7-JNK Signaling Pathway. PLoS ONE 2016, 11, e0164259. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Cho, Y.C.; Ju, A.; Lee, S.; Park, B.C.; Park, S.G.; Kim, J.H.; Kim, K.; Cho, S. Dual-specificity phosphatase 5 acts as an anti-inflammatory regulator by inhibiting the ERK and NF-κB signaling pathways. Sci. Rep. 2017, 7, 17348. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Xiang, M.; Liao, H.; Liu, J.; Luo, H.; Wang, Y.; Huang, L.; Chen, M.; Xia, J. Dual-Specificity Phosphatase 9 Protects Against Nonalcoholic Fatty Liver Disease in Mice Through ASK1 Suppression. Hepatology 2019, 69, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, L.M.; Zhang, J.L.; Sabri, A.; Wang, S.J.; Qin, G.J.; Guo, C.Q.; Wen, H.T.; Du, B.B.; Zhang, D.H.; et al. Dual Specificity Phosphatase 12 Regulates Hepatic Lipid Metabolism Through Inhibition of the Lipogenesis and Apoptosis Signal-Regulating Kinase 1 Pathways. Hepatology 2019, 70, 1099–1118. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Low, H.B.; Png, C.W.; Torta, F.; Kumar, J.K.; Lim, H.Y.; Zhou, Y.; Yang, H.; Angeli, V.; Shabbir, A.; et al. Protective Function of Mitogen-Activated Protein Kinase Phosphatase 5 in Aging- and Diet-Induced Hepatic Steatosis and Steatohepatitis. Hepatol. Commun. 2019, 3, 748–762. [Google Scholar] [CrossRef]
- Ye, P.; Liu, J.; Xu, W.; Liu, D.; Ding, X.; Le, S.; Zhang, H.; Chen, S.; Chen, M.; Xia, J. Dual-Specificity Phosphatase 26 Protects Against Nonalcoholic Fatty Liver Disease in Mice Through Transforming Growth Factor Beta-Activated Kinase 1 Suppression. Hepatology 2019, 69, 1946–1964. [Google Scholar] [CrossRef]
- Wang, S.; Yan, Z.Z.; Yang, X.; An, S.; Zhang, K.; Qi, Y.; Zheng, J.; Ji, Y.X.; Wang, P.X.; Fang, C.; et al. Hepatocyte DUSP14 maintains metabolic homeostasis and suppresses inflammation in the liver. Hepatology 2018, 67, 1320–1338. [Google Scholar] [CrossRef]
- Wang, X.; Mao, W.; Fang, C.; Tian, S.; Zhu, X.; Yang, L.; Huang, Z.; Li, H. Dusp14 protects against hepatic ischaemia-reperfusion injury via Tak1 suppression. J. Hepatol. 2018, 68, 118–129. [Google Scholar] [CrossRef]
- Ng, K.Y.; Chan, L.H.; Chai, S.; Tong, M.; Guan, X.Y.; Lee, N.P.; Yuan, Y.; Xie, D.; Lee, T.K.; Dusetti, N.J.; et al. TP53INP1 Downregulation Activates a p73-Dependent DUSP10/ERK Signaling Pathway to Promote Metastasis of Hepatocellular Carcinoma. Cancer Res. 2017, 77, 4602–4612. [Google Scholar] [CrossRef]
- Win, S.; Than, T.A.; Min, R.W.M.; Aghajan, M.; Kaplowitz, N. c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial Src. Hepatology 2016, 63, 1987–2003. [Google Scholar] [CrossRef]
- Win, S.; Than, T.A.; Han, D.; Petrovic, L.M.; Kaplowitz, N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J. Biol. Chem. 2011, 286, 35071–35078. [Google Scholar] [CrossRef] [PubMed]
- Win, S.; Than, T.A.; Le, B.H.; García-Ruiz, C.; Fernandez-Checa, J.C.; Kaplowitz, N. SAB (Sh3bp5) dependence of JNK mediated inhibition of mitochondrial respiration in palmitic acid induced hepatocyte lipotoxicity. J. Hepatol. 2015, 62, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Win, S.; Than, T.A.; Fernandez-Checa, J.C.; Kaplowitz, N. JNK interaction with Sab mediates ER stress induced inhibition of mitochondrial respiration and cell death. Cell Death Dis. 2014, 5, e989. [Google Scholar] [CrossRef]
- Chambers, J.W.; Pachori, A.; Howard, S.; Iqbal, S.; LoGrasso, P.V. Inhibition of JNK mitochondrial localization and signaling is protective against ischemia/reperfusion injury in rats. J. Biol. Chem. 2013, 288, 4000–4011. [Google Scholar] [CrossRef]
- Chambers, T.P.; Santiesteban, L.; Gomez, D.; Chambers, J.W. Sab mediates mitochondrial dysfunction involved in imatinib mesylate-induced cardiotoxicity. Toxicology 2017, 382, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Sodero, A.O.; Rodriguez-Silva, M.; Salio, C.; Sassoè-Pognetto, M.; Chambers, J.W. Sab is differentially expressed in the brain and affects neuronal activity. Brain Res. 2017, 1670, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.W.; Howard, S.; LoGrasso, P.V. Blocking c-Jun N-terminal kinase (JNK) translocation to the mitochondria prevents 6-hydroxydopamine-induced toxicity in vitro and in vivo. J. Biol. Chem. 2013, 288, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Paudel, I.; Hernandez, S.M.; Portalatin, G.M.; Chambers, T.P.; Chambers, J.W. Sab concentrations indicate chemotherapeutic susceptibility in ovarian cancer cell lines. Biochem. J. 2018, 475, 3471–3492. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, P.X.; Zhao, L.P.; Zhang, X.; Ji, Y.X.; Zhang, X.J.; Fang, C.; Lu, Y.X.; Yang, X.; Gao, M.M.; et al. The deubiquitinating enzyme TNFAIP3 mediates inactivation of hepatic ASK1 and ameliorates nonalcoholic steatohepatitis. Nat. Med. 2018, 24, 84–94. [Google Scholar] [CrossRef]
- Win, S.; Than, T.A.; Kaplowitz, N. The Regulation of JNK Signaling Pathways in Cell Death through the Interplay with Mitochondrial SAB and Upstream Post-Translational Effects. Int. J. Mol. Sci. 2018, 19, 3657. [Google Scholar] [CrossRef] [PubMed]
- Zeke, A.; Misheva, M.; Reményi, A.; Bogoyevitch, M.A. JNK Signaling: Regulation and Functions Based on Complex Protein-Protein Partnerships. Microbiol. Mol. Biol. Rev. 2016, 80, 793–835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Min, R.W.M.; Le, K.; Zhou, S.; Aghajan, M.; Than, T.A.; Win, S.; Kaplowitz, N. The role of MAP2 kinases and p38 kinase in acute murine liver injury models. Cell Death Dis. 2017, 29, e2903. [Google Scholar] [CrossRef] [PubMed]
- Win, S.; Min, R.W.M.; Chen, C.Q.; Zhang, J.; Chen, Y.; Li, M.; Suzuki, A.; Abdelmalek, M.F.; Wang, Y.; Aghajan, M.; et al. Expression of mitochondrial membrane-linked SAB determines severity of sex-dependent acute liver injury. J. Clin. Investig. 2019, 129, 5278–5293. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Choy, P.M.; Bubici, C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene 2019, 38, 2223–2240. [Google Scholar] [CrossRef] [PubMed]
- Urano, F.; Wang, X.; Bertolotti, A.; Zhang, Y.; Chung, P.; Harding, H.P.; Ron, D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000, 287, 664–666. [Google Scholar] [CrossRef]
- Liang, S.H.; Zhang, W.; McGrath, B.C.; Zhang, P.; Cavener, D.R. PERK (eIF2α kinase) is required to activate the stress-activated MAPKs and induce the expression of immediate-early genes upon disruption of ER calcium homoeostasis. Biochem. J. 2006, 393, 201–209. [Google Scholar] [CrossRef]
- Holzer, R.G.; Park, E.J.; Li, N.; Tran, H.; Chen, M.; Choi, C.; Solinas, G.; Karin, M. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell 2011, 147, 173–184. [Google Scholar] [CrossRef]
- Whitmarsh, A.J.; Cavanagh, J.; Tournier, C.; Yasuda, J.; Davis, R.J. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science 1998, 281, 1671–1674. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Nihalani, D.; Meyer, D.; Pajni, S.; Holzman, L.B. Mixed lineage kinase-dependent JNK activation is governed by interactions of scaffold protein JIP with MAPK module components. EMBO J. 2001, 20, 3447–3458. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Hasegawa, K.; Masaki, M.; Moriguchi, T.; Nishida, E.; Kozutsumi, Y.; Oka, S.; Kawasaki, T. Mixed lineage kinase LZK forms a functional signaling complex with JIP-1, a scaffold protein of the c-Jun NH(2)-terminal kinase pathway. J. Biochem. 2001, 130, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Lee, Y.J. Dissociation of Akt1 from its negative regulator JIP1 is mediated through the ASK1-MEK-JNK signal transduction pathway during metabolic oxidative stress: A negative feedback loop. J. Cell Biol. 2005, 170, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, E.A.; Perkins, G.R.; Collins, M.K.; Whitmarsh, A.J. The JNK-interacting protein-1 scaffold protein targets MAPK phosphatase-7 to dephosphorylate JNK. J. Biol. Chem. 2003, 278, 10731–10736. [Google Scholar] [CrossRef]
- Kelkar, N.; Standen, C.L.; Davis, R.J. Role of the JIP4 scaffold protein in the regulation of mitogen-activated protein kinase signaling pathways. Mol. Cell. Biol. 2005, 25, 2733–2743. [Google Scholar] [CrossRef]
- Nakagawa, H.; Maeda, S.; Hikiba, Y.; Ohmae, T.; Shibata, W.; Yanai, A.; Sakamoto, K.; Ogura, K.; Noguchi, T.; Karin, M.; et al. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology 2008, 135, 1311–1321. [Google Scholar] [CrossRef]
- Shinohara, M.; Ybanez, M.D.; Win, S.; Than, T.A.; Jain, S.; Gaarde, W.A.; Han, D.; Kaplowitz, N. Silencing glycogen synthase kinase-3β inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J. Biol. Chem. 2010, 285, 8244–8255. [Google Scholar] [CrossRef]
- Win, S.; Min, R.W.M.; Zhang, J.; Kanel, G.; Wanken, B.; Chen, Y.; Li, M.; Wang, Y.; Suzuki, A.; Aung, F.W.M.; et al. Hepatic Mitochondrial SAB Deletion or Knockdown Alleviates Diet-Induced Metabolic Syndrome, Steatohepatitis, and Hepatic Fibrosis. Hepatology 2021, 74, 3127–3145. [Google Scholar] [CrossRef]
- Vernia, S.; Cavanagh-Kyros, J.; Garcia-Haro, L.; Sabio, G.; Barrett, T.; Jung, D.Y.; Kim, J.K.; Xu, J.; Shulha, H.P.; Garber, M.; et al. The PPARα-FGF21 hormone axis contributes to metabolic regulation by the hepatic JNK signaling pathway. Cell Metab. 2014, 20, 512–525. [Google Scholar] [CrossRef]
- Nikolic, I.; Leiva, M.; Sabio, G. The role of stress kinases in metabolic disease. Nat. Rev. Endocrinol. 2020, 16, 697–716. [Google Scholar] [CrossRef]
- Stein, L.R.; Imai, S. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol. Metab. 2012, 23, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.W.; LoGrasso, P.V. Mitochondrial c-Jun N-terminal kinase (JNK) signaling initiates physiological changes resulting in amplification of reactive oxygen species generation. J. Biol. Chem. 2011, 286, 16052–16062. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeldin, S.; Shi, H.; Zhu, C.; Saito, Y.; Corey, K.E.; Osganian, S.A.; Remotti, H.E.; Verna, E.C.; Pajvani, U.B.; et al. TAZ-induced Cybb contributes to liver tumor formation in non-alcoholic steatohepatitis. J. Hepatol. 2022, 76, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Brandl, K.; Schnabl, B. Intestinal microbiota and nonalcoholic steatohepatitis. Curr. Opin. Gastroenterol. 2017, 33, 128–133. [Google Scholar] [CrossRef]
- Wang, P.X.; Ji, Y.X.; Zhang, X.J.; Zhao, L.P.; Yan, Z.Z.; Zhang, P.; Shen, L.J.; Yang, X.; Fang, J.; Tian, S.; et al. Targeting CASP8 and FADD-like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nat. Med. 2017, 23, 439–449. [Google Scholar] [CrossRef]
- Xiang, M.; Wang, P.X.; Wang, A.B.; Zhang, X.J.; Zhang, Y.; Zhang, P.; Mei, F.H.; Chen, M.H.; Li, H. Targeting hepatic TRAF1-ASK1 signaling to improve inflammation, insulin resistance, and hepatic steatosis. J. Hepatol. 2016, 64, 1365–1377. [Google Scholar] [CrossRef]
- Challa, T.D.; Wueest, S.; Lucchini, F.C.; Dedual, M.; Modica, S.; Borsigova, M.; Wolfrum, C.; Blüher, M.; Konrad, D. Liver ASK1 protects from non-alcoholic fatty liver disease and fibrosis. EMBO Mol. Med. 2019, 11, e10124. [Google Scholar] [CrossRef]
- Giles, D.A.; Moreno-Fernandez, M.E.; Stankiewicz, T.E.; Graspeuntner, S.; Cappelletti, M.; Wu, D.; Mukherjee, R.; Chan, C.C.; Lawson, M.J.; Klarquist, J.; et al. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat. Med. 2017, 23, 829–838. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Hirsova, P.; Tomita, K.; Bronk, S.F.; Werneburg, N.W.; Harrison, S.A.; Goodfellow, V.S.; Malhi, H.; Gores, G.J. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology 2016, 63, 731–744. [Google Scholar] [CrossRef]
- Lawan, A.; Zhang, L.; Gatzke, F.; Min, K.; Jurczak, M.J.; Al-Mutairi, M.; Richter, P.; Camporez, J.P.; Couvillon, A.; Pesta, D.; et al. Hepatic mitogen-activated protein kinase phosphatase 1 selectively regulates glucose metabolism and energy homeostasis. Mol. Cell. Biol. 2015, 35, 26–40. [Google Scholar] [CrossRef]
- Schattenberg, J.M.; Singh, R.; Wang, Y.; Lefkowitch, J.H.; Rigoli, R.M.; Scherer, P.E.; Czaja, M.J. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology 2006, 43, 163–172. [Google Scholar] [CrossRef]
- Kotzka, J.; Knebel, B.; Haas, J.; Kremer, L.; Jacob, S.; Hartwig, S.; Nitzgen, U.; Muller-Wieland, D. Preventing phosphorylation of sterol regulatory element-binding protein 1a by MAP-kinases protects mice from fatty liver and visceral obesity. PLoS ONE 2012, 7, e32609. [Google Scholar] [CrossRef] [PubMed]
- Benhamed, F.; Denechaud, P.D.; Lemoine, M.; Robichon, C.; Moldes, M.; Bertrand-Michel, J.; Ratziu, V.; Serfaty, L.; Housset, C.; Capeau, J.; et al. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J. Clin. Investig. 2012, 122, 2176–2194. [Google Scholar] [CrossRef]
- Hwang, S.; Wang, X.; Rodrigues, R.M.; Ma, J.; He, Y.; Seo, W.; Park, S.H.; Kim, S.J.; Feng, D.; Gao, B. Protective and Detrimental Roles of p38α Mitogen-Activated Protein Kinase in Different Stages of Nonalcoholic Fatty Liver Disease. Hepatology 2020, 72, 873–891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, L.; Wu, J.; Xu, H.; Leung, W.Y.; Fu, K.; Wu, J.; Liu, K.; Man, K.; Yang, X.; et al. Macrophage p38α promotes nutritional steatohepatitis through M1 polarization. J. Hepatol. 2019, 71, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Tormos, A.M.; Arduini, A.; Talens-Visconti, R.; del Barco Barrantes, I.; Nebreda, A.R.; Sastre, J. Liver-specific p38α deficiency causes reduced cell growth and cytokinesis failure during chronic biliary cirrhosis in mice. Hepatology 2013, 57, 1950–1961. [Google Scholar] [CrossRef]
- Xu, J.J.; Hendriks, B.S.; Zhao, J.; de Graaf, D. Multiple effects of acetaminophen and p38 inhibitors: Towards pathway toxicology. FEBS Lett. 2008, 582, 1276–1282. [Google Scholar] [CrossRef]
- Czaja, M.J. Function of Autophagy in Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1304–1313. [Google Scholar] [CrossRef]
- Yan, S.; Huda, N.; Khambu, B.; Yin, X.M. Relevance of autophagy to fatty liver diseases and potential therapeutic applications. Amino Acids 2017, 49, 1965–1979. [Google Scholar] [CrossRef]
- Yin, X.M.; Ding, W.X.; Gao, W. Autophagy in the liver. Hepatology 2008, 47, 1773–1785. [Google Scholar] [CrossRef]
- Zhang, C.S.; Hardie, D.G.; Lin, S.C. Glucose Starvation Blocks Translation at Multiple Levels. Cell Metab. 2020, 31, 217–218. [Google Scholar] [CrossRef] [PubMed]
- Leprivier, G.; Rotblat, B. How does mTOR sense glucose starvation? AMPK is the usual suspect. Cell Death Discov. 2020, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Carotti, S.; Aquilano, K.; Zalfa, F.; Ruggiero, S.; Valentini, F.; Zingariello, M.; Francesconi, M.; Perrone, G.; Alletto, F.; Antonelli-Incalzi, R.; et al. Lipophagy Impairment Is Associated with Disease Progression in NAFLD. Front. Physiol. 2020, 11, 850. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Li, H.Y.; Peng, Z.G. Targeting lipophagy as a potential therapeutic strategy for nonalcoholic fatty liver disease. Biochem. Pharmacol. 2022, 197, 114933. [Google Scholar] [CrossRef] [PubMed]
- Uehara, K.; Sostre-Colón, J.; Gavin, M.; Santoleri, D.; Leonard, K.A.; Jacobs, R.L.; Titchenell, P.M. Activation of Liver mTORC1 Protects Against NASH via Dual Regulation of VLDL-TAG Secretion and De Novo Lipogenesis. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1625–1647. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Zhang, H.; Li, M.; Xiong, X.; Chen, X.; Chen, X.; Dong, X.C.; Yin, X.M. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J. Hepatol. 2013, 58, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, P.; Fu, S.; Calay, E.S.; Hotamisligil, G.S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010, 11, 467–478. [Google Scholar] [CrossRef]
- Ding, W.X.; Ni, H.M.; Gao, W.; Hou, Y.F.; Melan, M.A.; Chen, X.; Stolz, D.B.; Shao, Z.M.; Yin, X.M. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J. Biol. Chem. 2007, 282, 4702–4710. [Google Scholar] [CrossRef]
- Ogata, M.; Hino, S.; Saito, A.; Morikawa, K.; Kondo, S.; Kanemoto, S.; Murakami, T.; Taniguchi, M.; Tanii, I.; Yoshinaga, K.; et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 2006, 26, 9220–9231. [Google Scholar] [CrossRef]
- Wei, Y.; Pattingre, S.; Sinha, S.; Bassik, M.; Levine, B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 2008, 30, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.; Choi, S.; Jeong, H.; Jang, J.H.; Lee, Y.; Jeon, H.; Lee, M.N.; Noh, J.; Cho, K.; Yoo, J.S.; et al. Osmotic stress regulates mammalian target of rapamycin (mTOR) complex 1 via c-Jun N-terminal Kinase (JNK)-mediated Raptor protein phosphorylation. J. Biol. Chem. 2012, 287, 18398–18407. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Ribas, V.; Drew, B.G.; Zhou, Z.; Phun, J.; Kalajian, N.Y.; Soleymani, T.; Daraei, P.; Widjaja, K.; Wanagat, J.; de Aguiar Vallim, T.Q.; et al. Skeletal muscle action of estrogen receptor α is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci. Transl. Med. 2016, 8, 334ra54. [Google Scholar] [CrossRef] [PubMed]
- Ayonrinde, O.T.; Olynyk, J.K.; Beilin, L.J.; Mori, T.A.; Pennell, C.E.; de Klerk, N.; Oddy, W.H.; Shipman, P.; Adams, L.A. Gender-specific differences in adipose distribution and adipocytokines influence adolescent nonalcoholic fatty liver disease. Hepatology 2011, 53, 800–809. [Google Scholar] [CrossRef]
- Lee, Y.K.; Shin, D.Y.; Shin, H.; Lee, E.J. Sex-specific genetic influence on thyroid-stimulating hormone and free thyroxine levels, and interactions between measurements: KNHANES 2013–2015. PLoS ONE 2018, 13, e0207446. [Google Scholar] [CrossRef]
- Valenti, L.; Dongiovanni, P.; Ginanni Corradini, S.; Burza, M.A.; Romeo, S. PNPLA3 I148M variant and hepatocellular carcinoma: A common genetic variant for a rare disease. Dig. Liver Dis. 2013, 45, 619–624. [Google Scholar] [CrossRef]
- Smagris, E.; BasuRay, S.; Li, J.; Huang, Y.; Lai, K.M.; Gromada, J.; Cohen, J.C.; Hobbs, H.H. Pnpla3I148M knockin mice accumulate PNPLA3 on lipid droplets and develop hepatic steatosis. Hepatology 2015, 61, 108–118. [Google Scholar] [CrossRef]
- Han, Y.H.; Kim, H.J.; Lee, M.O. RORα regulates hepatic lipolysis by inducing transcriptional expression of PNPLA3 in mice. Mol. Cell. Endocrinol. 2021, 522, 111122. [Google Scholar] [CrossRef]
- Li, J.Z.; Huang, Y.; Karaman, R.; Ivanova, P.T.; Brown, H.A.; Roddy, T.; Castro-Perez, J.; Cohen, J.C.; Hobbs, H.H. Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis. J. Clin. Investig. 2012, 122, 4130–4144. [Google Scholar] [CrossRef]
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Boeckmans, J.; Natale, A.; Rombaut, M.; Buyl, K.; Rogiers, V.; De Kock, J.; Vanhaecke, T.; Rodrigues, R.M. Anti-NASH Drug Development Hitches a Lift on PPAR Agonism. Cells 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Hays, T.; Rusyn, I.; Burns, A.M.; Kennett, M.J.; Ward, J.M.; Gonzalez, F.J.; Peters, J.M. Role of peroxisome proliferator-activated receptor-alpha (PPARalpha) in bezafibrate-induced hepatocarcinogenesis and cholestasis. Carcinogenesis 2005, 26, 219–227. [Google Scholar] [CrossRef]
- Foreman, J.E.; Koga, T.; Kosyk, O.; Kang, B.H.; Zhu, X.; Cohen, S.M.; Billy, L.J.; Sharma, A.K.; Amin, S.; Gonzalez, F.J.; et al. Diminished Hepatocarcinogenesis by a Potent, High-Affinity Human PPARα Agonist in PPARA-Humanized Mice. Toxicol. Sci. 2021, 183, 70–80. [Google Scholar] [CrossRef]
- Manieri, E.; Folgueira, C.; Rodríguez, M.E.; Leiva-Vega, L.; Esteban-Lafuente, L.; Chen, C.; Cubero, F.J.; Barrett, T.; Cavanagh-Kyros, J.; Seruggia, D.; et al. JNK-mediated disruption of bile acid homeostasis promotes intrahepatic cholangiocarcinoma. Proc. Natl. Acad. Sci. USA 2020, 117, 16492–16499. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.A.; Adegboyega, P.; van Rooijen, N.; Tagalicud, A.; Allman, M.; Wallace, M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J. Hepatol. 2007, 47, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, I.; Santoro, N.; Chen, Y.; Hoque, R.; Ouyang, X.; Caprio, S.; Shlomchik, M.J.; Coffman, R.L.; Candia, A.; Mehal, W.Z. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J. Clin. Investig. 2016, 126, 859–864. [Google Scholar] [CrossRef]
- Bertola, A.; Bonnafous, S.; Anty, R.; Patouraux, S.; Saint-Paul, M.C.; Iannelli, A.; Gugenheim, J.; Barr, J.; Mato, J.M.; Le Marchand-Brustel, Y.; et al. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PLoS ONE 2010, 5, e13577. [Google Scholar] [CrossRef]
- Baud, V.; Liu, Z.G.; Bennett, B.; Suzuki, N.; Xia, Y.; Karin, M. Signaling by proinflammatory cytokines: Oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 1999, 13, 1297–1308. [Google Scholar] [CrossRef]
- Kobayashi, K.; Yamaguchi, M.; Miyazaki, K.; Imai, H.; Yokoe, K.; Ono, R.; Nosaka, T.; Katayama, N. Expressions of SH3BP5, LMO3, and SNAP25 in diffuse large B-cell lymphoma cells and their association with clinical features. Cancer Med. 2016, 5, 1802–1809. [Google Scholar] [CrossRef]
- Han, M.S.; Barrett, T.; Brehm, M.A.; Davis, R.J. Inflammation Mediated by JNK in Myeloid Cells Promotes the Development of Hepatitis and Hepatocellular Carcinoma. Cell Rep. 2016, 15, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ma, C. The hepatic macrophage pool in NASH. Cell. Mol. Immunol. 2021, 18, 2059–2060. [Google Scholar] [CrossRef] [PubMed]
- Lorenzon, P.; Vecile, E.; Nardon, E.; Ferrero, E.; Harlan, J.M.; Tedesco, F.; Dobrina, A. Endothelial cell E- and P-selectin and vascular cell adhesion molecule-1 function as signaling receptors. J. Cell Biol. 1998, 142, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Zhang, J.; Zhang, X.; Liu, J.; Jiang, J.X.; Yamaguchi, K.; Taruno, A.; Katsuyama, M.; Iwata, K.; Ibi, M.; et al. The NOX1 isoform of NADPH oxidase is involved in dysfunction of liver sinusoids in nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2018, 115, 412–420. [Google Scholar] [CrossRef]
- García-Lezana, T.; Raurell, I.; Bravo, M.; Torres-Arauz, M.; Salcedo, M.T.; Santiago, A.; Schoenenberger, A.; Manichanh, C.; Genescà, J.; Martell, M.; et al. Restoration of a healthy intestinal microbiota normalizes portal hypertension in a rat model of nonalcoholic steatohepatitis. Hepatology 2018, 67, 1485–1498. [Google Scholar] [CrossRef]
- Xu, X.Y.; Du, Y.; Liu, X.; Ren, Y.; Dong, Y.; Xu, H.Y.; Shi, J.S.; Jiang, D.; Xu, X.; Li, L.; et al. Targeting Follistatin like 1 ameliorates liver fibrosis induced by carbon tetrachloride through TGF-β1-miR29a in mice. Cell Commun. Signal. 2020, 18, 151. [Google Scholar] [CrossRef]
- Okada, K.; Warabi, E.; Sugimoto, H.; Horie, M.; Gotoh, N.; Tokushige, K.; Hashimoto, E.; Utsunomiya, H.; Takahashi, H.; Ishii, T.; et al. Deletion of Nrf2 leads to rapid progression of steatohepatitis in mice fed atherogenic plus high-fat diet. J. Gastroenterol. 2013, 48, 620–632. [Google Scholar] [CrossRef]
- Gabbia, D.; Cannella, L.; De Martin, S. The Role of Oxidative Stress in NAFLD-NASH-HCC Transition-Focus on NADPH Oxidases. Biomedicines 2021, 9, 687. [Google Scholar] [CrossRef]
- Perumpail, B.J.; Li, A.A.; John, N.; Sallam, S.; Shah, N.D.; Kwong, W.; Cholankeril, G.; Kim, D.; Ahmed, A. The Role of Vitamin E in the Treatment of NAFLD. Diseases 2018, 6, 86. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- Bennett, B.L.; Sasaki, D.T.; Murray, B.W.; O’Leary, E.C.; Sakata, S.T.; Xu, W.; Leisten, J.C.; Motiwala, A.; Pierce, S.; Satoh, Y.; et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 2001, 98, 13681–13686. [Google Scholar] [CrossRef]
- Zhang, T.; Inesta-Vaquera, F.; Niepel, M.; Zhang, J.; Ficarro, S.B.; Machleidt, T.; Xie, T.; Marto, J.A.; Kim, N.; Sim, T.; et al. Discovery of potent and selective covalent inhibitors of JNK. Chem. Biol. 2012, 19, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, N.; Shinohara, M.; Saberi, B.; Gaarde, W.A.; Han, D.; Kaplowitz, N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008, 283, 13565–13577. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Garlick, D.S.; Greiner, D.L.; Davis, R.J. The role of JNK in the development of hepatocellular carcinoma. Genes Dev. 2011, 25, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Yu, L.R.; Abdelmegeed, M.A.; Gao, Y.; Banerjee, A.; Song, B.J. Critical role of c-jun N-terminal protein kinase in promoting mitochondrial dysfunction and acute liver injury. Redox Biol. 2015, 6, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Fujino, G.; Noguchi, T.; Matsuzawa, A.; Yamauchi, S.; Saitoh, M.; Takeda, K.; Ichijo, H. Thioredoxin and TRAF family proteins regulate reactive oxygen species-dependent activation of ASK1 through reciprocal modulation of the N-terminal homophilic interaction of ASK1. Mol. Cell Biol. 2007, 27, 8152–8163. [Google Scholar] [CrossRef]
- Liu, Y.; Min, W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ. Res. 2002, 90, 1259–1266. [Google Scholar] [CrossRef]
- Loomba, R.; Lawitz, E.; Mantry, P.S.; Jayakumar, S.; Caldwell, S.H.; Arnold, H.; Diehl, A.M.; Djedjos, C.S.; Han, L.; Myers, R.P.; et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology 2018, 67, 549–559. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Gores, G.J.; Hirsova, P.; Kirby, M.; Miles, L.; Jaeschke, A.; Kohli, R. Mixed lineage kinase 3 deficient mice are protected against the high fat high carbohydrate diet-induced steatohepatitis. Liver Int. 2014, 34, 427–437. [Google Scholar] [CrossRef]
- Tomita, K.; Kabashima, A.; Freeman, B.L.; Bronk, S.F.; Hirsova, P.; Ibrahim, S.H. Mixed Lineage Kinase 3 Mediates the Induction of CXCL10 by a STAT1-Dependent Mechanism During Hepatocyte Lipotoxicity. J. Cell. Biochem. 2017, 118, 3249–3259. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Mauer, A.S.; Lucien, F.; Raiter, A.; Bandla, H.; Mounajjed, T.; Yin, Z.; Glaser, K.J.; Yin, M.; et al. Characterization of Cellular Sources and Circulating Levels of Extracellular Vesicles in a Dietary Murine Model of Nonalcoholic Steatohepatitis. Hepatol. Commun. 2019, 3, 1235–1249. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Amrollahi, P.; Parthasarathy, G.; Mauer, A.S.; Sehrawat, T.S.; Vanderboom, P.; Nair, K.S.; Nakao, K.; Allen, A.M.; Hu, T.Y.; et al. Circulating extracellular vesicles are a biomarker for NAFLD resolution and response to weight loss surgery. Nanomedicine 2021, 36, 102430. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, T.; Machhi, J.; Lu, Y.; Dyavarshetty, B.; Nemati, M.; Zhang, G.; Mosley, R.L.; Gelbard, H.A.; Gendelman, H.E. URMC-099 facilitates amyloid-β clearance in a murine model of Alzheimer’s disease. J. Neuroinflamm. 2018, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Marker, D.F.; Tremblay, M.È.; Puccini, J.M.; Barbieri, J.; Gantz Marker, M.A.; Loweth, C.J.; Muly, E.C.; Lu, S.M.; Goodfellow, V.S.; Dewhurst, S.; et al. The new small-molecule mixed-lineage kinase 3 inhibitor URMC-099 is neuroprotective and anti-inflammatory in models of human immunodeficiency virus-associated neurocognitive disorders. J. Neurosci. 2013, 33, 9998–10010. [Google Scholar] [CrossRef]
- Wang, P.X.; Zhang, X.J.; Luo, P.; Jiang, X.; Zhang, P.; Guo, J.; Zhao, G.N.; Zhu, X.; Zhang, Y.; Yang, S.; et al. Hepatocyte TRAF3 promotes liver steatosis and systemic insulin resistance through targeting TAK1-dependent signaling. Nat. Commun. 2016, 17, 10592. [Google Scholar] [CrossRef]
- Yang, L.; Inokuchi, S.; Roh, Y.S.; Song, J.; Loomba, R.; Park, E.J.; Seki, E. Transforming growth factor-β signaling in hepatocytes promotes hepatic fibrosis and carcinogenesis in mice with hepatocyte-specific deletion of TAK1. Gastroenterology 2013, 144, 1042–1054. [Google Scholar] [CrossRef]
- Schnabl, B.; Bradham, C.A.; Bennett, B.L.; Manning, A.M.; Stefanovic, B.; Brenner, D.A. TAK1/JNK and p38 have opposite effects on rat hepatic stellate cells. Hepatology 2001, 34, 953–963. [Google Scholar] [CrossRef]
- Inokuchi, S.; Aoyama, T.; Miura, K.; Osterreicher, C.H.; Kodama, Y.; Miyai, K.; Akira, S.; Brenner, D.A.; Seki, E. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 844–849. [Google Scholar] [CrossRef]
- Sun, Y.; Li, T.Y.; Song, L.; Zhang, C.; Li, J.; Lin, Z.Z.; Lin, S.C.; Lin, S.Y. Liver-specific deficiency of unc-51 like kinase 1 and 2 protects mice from acetaminophen-induced liver injury. Hepatology 2018, 67, 2397–2413. [Google Scholar] [CrossRef]
- Torres, S.; Baulies, A.; Insausti-Urkia, N.; Alarcón-Vila, C.; Fucho, R.; Solsona-Vilarrasa, E.; Núñez, S.; Robles, D.; Ribas, V.; Wakefield, L.; et al. Endoplasmic Reticulum Stress-Induced Upregulation of STARD1 Promotes Acetaminophen-Induced Acute Liver Failure. Gastroenterology 2019, 157, 552–568. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).