Role of Neuropilin 1 in COVID-19 Patients with Acute Ischemic Stroke
Abstract
:1. Introduction
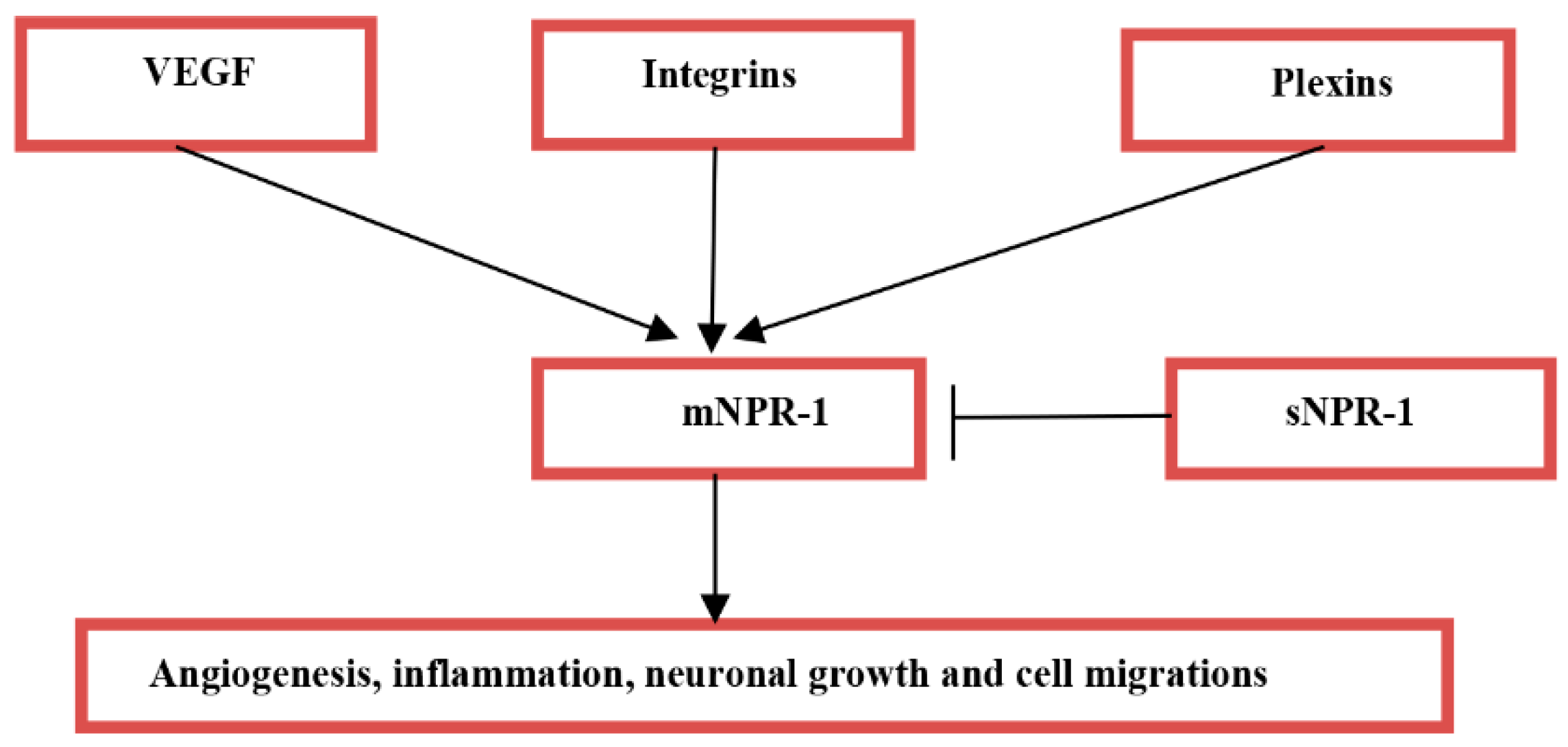
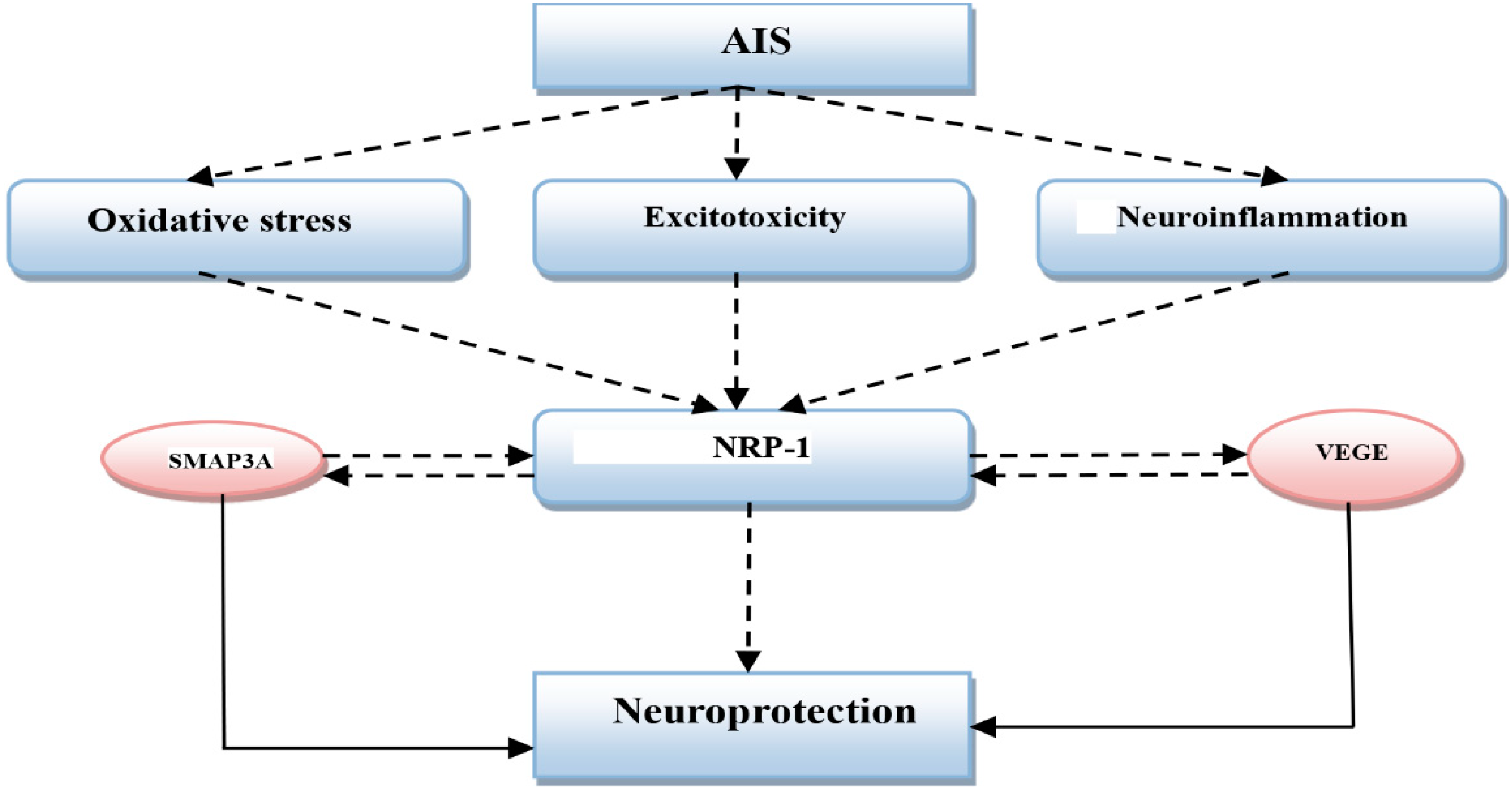
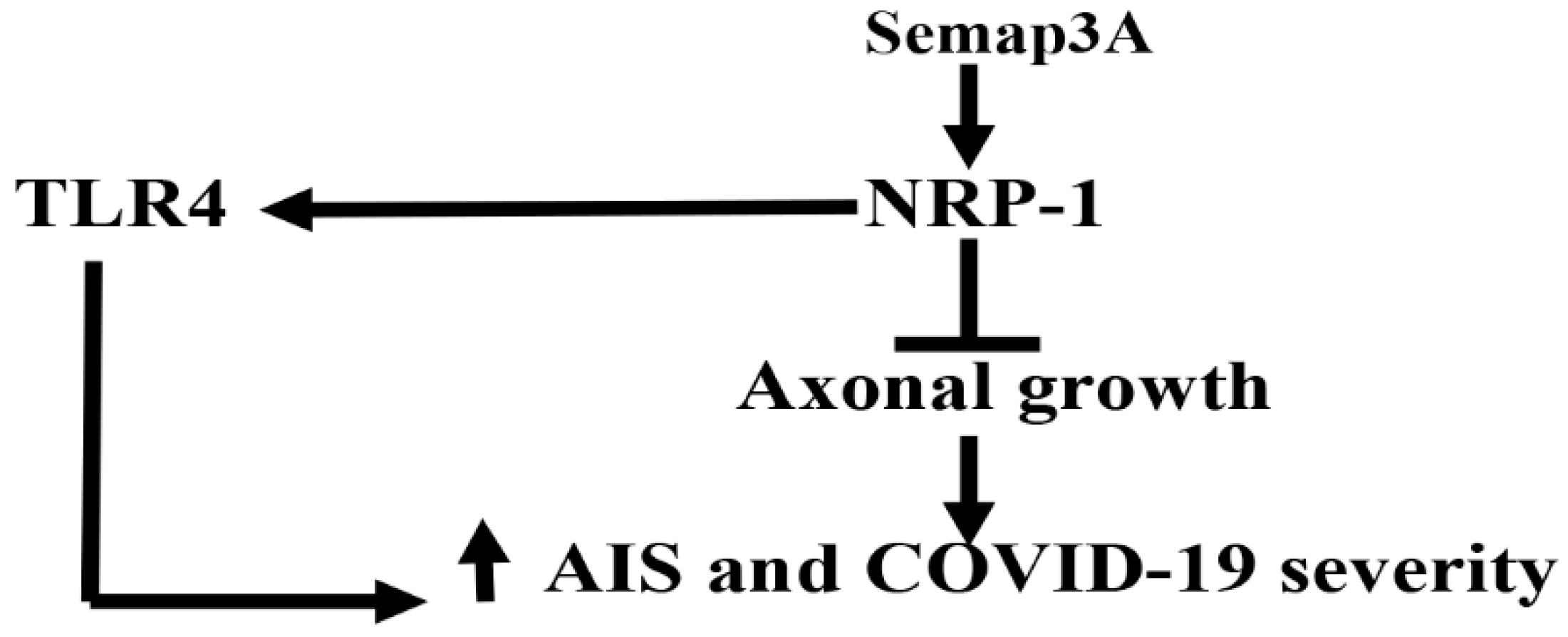
2. Acute Ischemic Stroke and Neuropilin-1
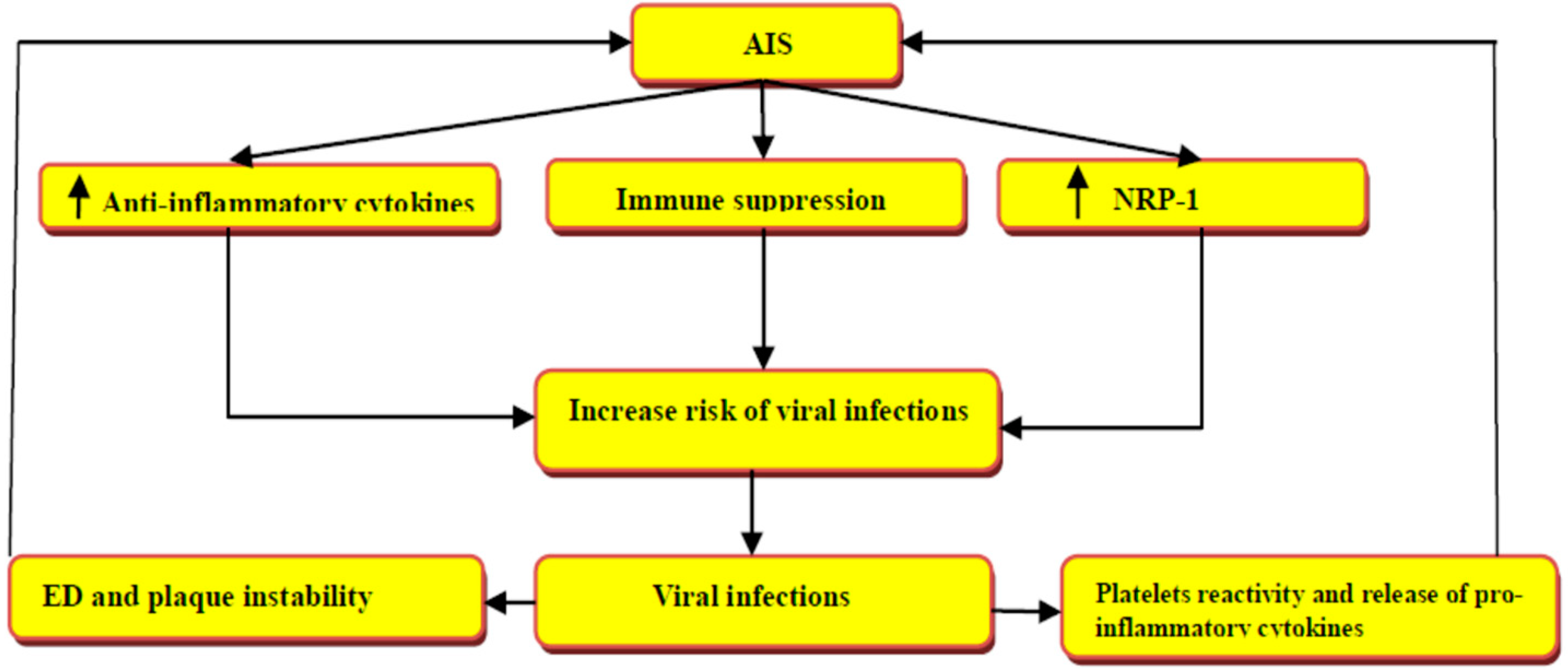
3. Acute Ischemic Stroke in Viral Infections
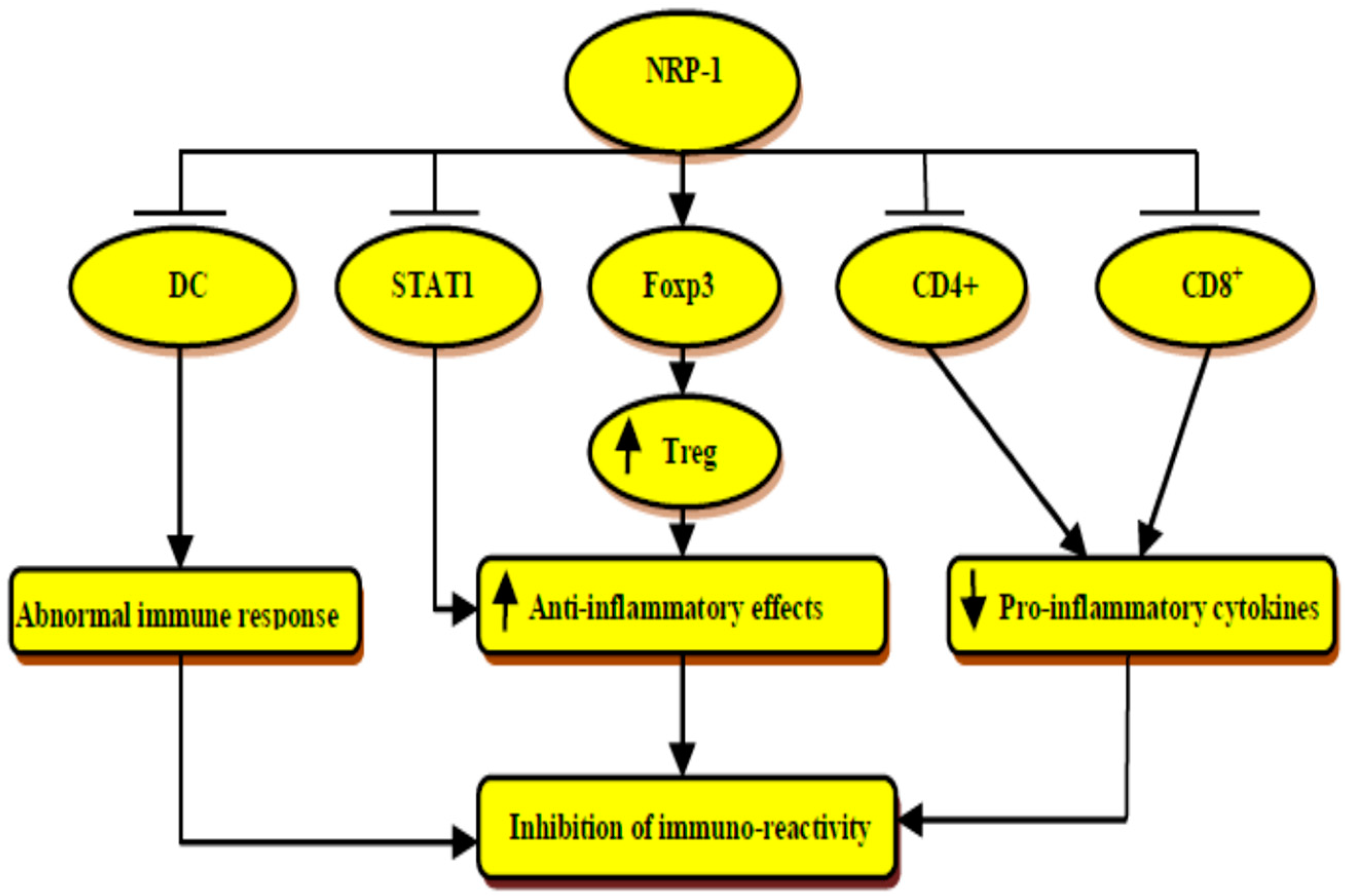
4. Immunological Role of Neuropilin-1
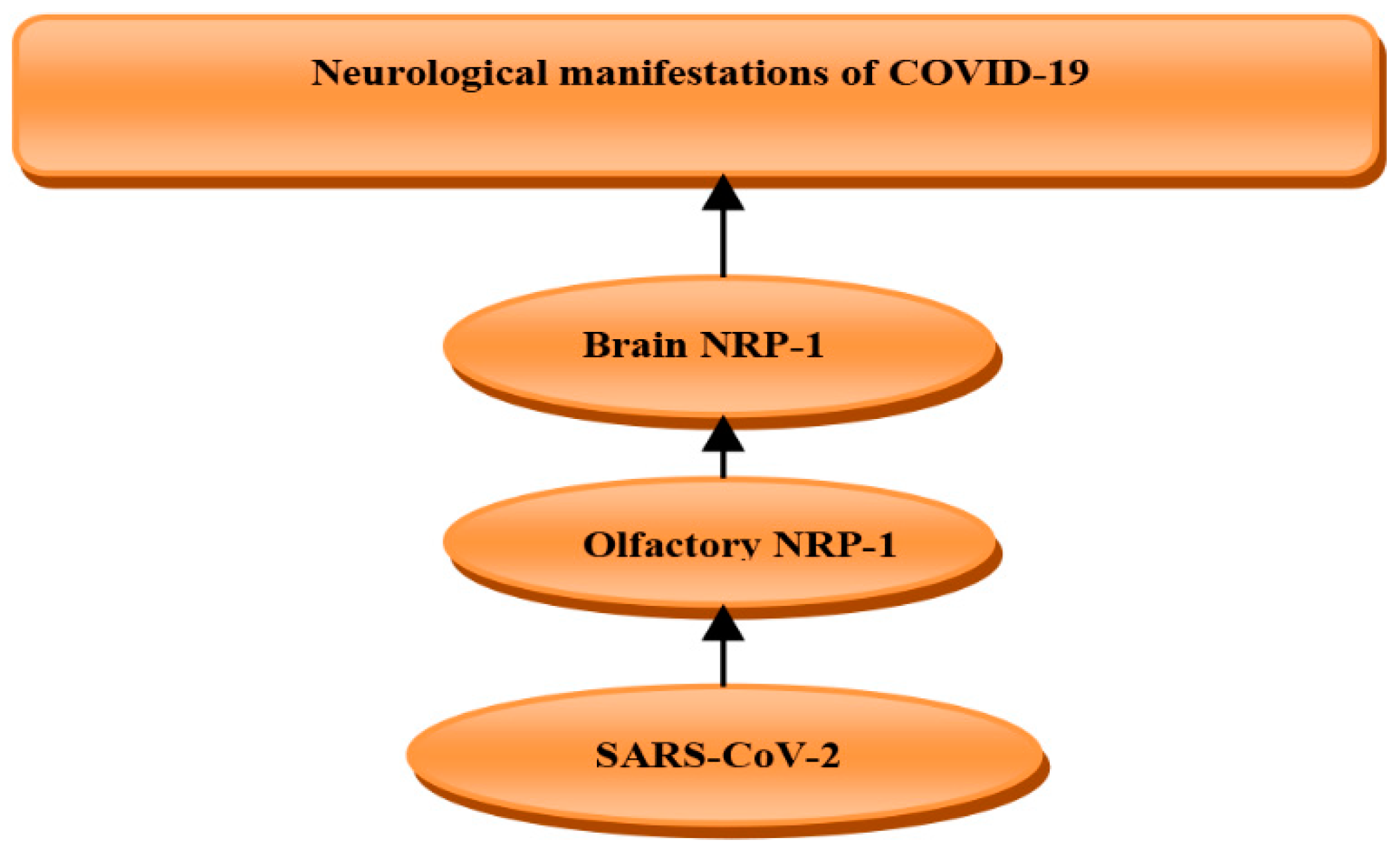
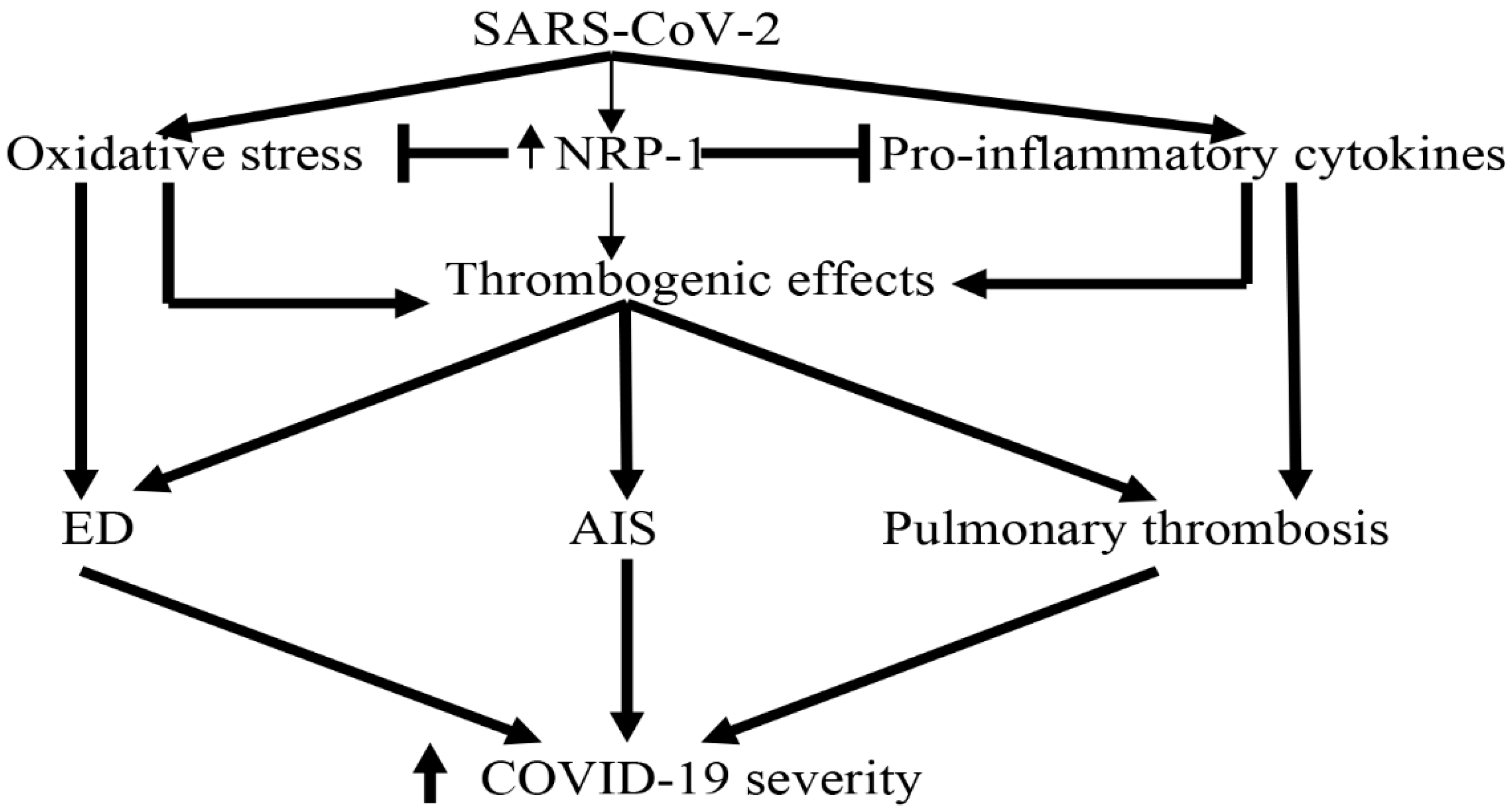
5. Role of Neuropilin-1 in COVID-19
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alzahrani, K.J.; Cruz-Martins, N.; Batiha, G.E.-S. The potential role of neopterin in COVID-19: A new perspective. Mol. Cell. Biochem. 2021, 476, 4161–4166. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Gari, A.; Elshony, N.; Shaheen, H.M.; Abubakar, M.B.; Adeyemi, S.B.; Al-Kuraishy, H.M. Hypertension and its management in COVID-19 patients: The assorted view. Int. J. Cardiol. Cardiovasc. Risk Prev. 2021, 11, 200121. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Qusti, S.; Alshammari, E.M.; Atanu, F.O.; Batiha, G.E.-S. Arginine vasopressin and pathophysiology of COVID-19: An innovative perspective. Biomed. Pharmacother. 2021, 143, 112193. [Google Scholar] [CrossRef]
- Lemuel, A.M.; Usman, I.M.; Kasozi, K.I.; Alghamdi, S.; Aigbogun, E.O.; Archibong, V.; Ssebuufu, R.; Kabanyoro, A.; Ifie, J.E.; Swase, D.T. COVID-19-Related Mental Health Burdens: Impact of Educational Level and Relationship Status Among Low-Income Earners of Western Uganda. Front. Public Health. 2021, 9, 739270. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alkazmi, L.; Alexiou, A.; Batiha, G.E.-S. Levamisole therapy in COVID-19. Viral Immunol. 2021, 34, 722–725. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Qusty, N.; Cruz-Martins, N.; Batiha, G.E.-S. Sequential doxycycline and colchicine combination therapy in COVID-19: The salutary effects. Pulm. Pharmacol. Ther. 2021, 67, 102008. [Google Scholar] [CrossRef]
- Lugnier, C.; Al-Kuraishy, H.M.; Rousseau, E. PDE4 inhibition as a therapeutic strategy for improvement of pulmonary dysfunctions in COVID-19 and cigarette smoking. Biochem. Pharmacol. 2021, 185, 114431. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alblihed, M.; Cruz-Martins, N.; Batiha, G.E.-S. COVID-19 and risk of acute ischemic stroke and acute lung injury in patients with type ii diabetes mellitus: The anti-inflammatory role of metformin. Front. Med. 2021, 110, 644295. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Faidah, H.; Al-Maiahy, T.J.; Cruz-Martins, N.; Batiha, G.E.-S. The looming effects of estrogen in COVID-19: A rocky rollout. Front. Nutr. 2021, 8, 649128. [Google Scholar] [CrossRef]
- Guglielmetti, G.; Quaglia, M.; Sainaghi, P.P.; Castello, L.M.; Vaschetto, R.; Pirisi, M.; Corte, F.D.; Avanzi, G.C.; Stratta, P.; Cantaluppi, V. “War to the knife” against thromboinflammation to protect endothelial function of COVID-19 patients. Crit. Care 2020, 24, 365. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Mostafa-Hedeab, G.; Kasozi, K.I.; Zirintunda, G.; Aslam, A.; Allahyani, M.; Welburn, S.C.; Batiha, G.E.-S. Effects of β-Blockers on the Sympathetic and Cytokines Storms in COVID-19. Front. Immunol. 2021, 12, 749291. [Google Scholar] [CrossRef] [PubMed]
- Getu, S.; Tiruneh, T.; Andualem, H.; Hailemichael, W.; Kiros, T.; Belay, D.M.; Kiros, M. Coagulopathy in SARS-CoV-2 infected patients: Implication for the management of COVID-19. J. Blood Med. 2021, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Qusti, S.; Alshammari, E.M.; Gyebi, G.A.; Batiha, G.E.-S. COVID-19-induced dysautonomia: A menace of sympathetic storm. ASN Neuro 2021, 13, 17590914211057635. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alkazmi, L.; Habotta, O.A.; Batiha, G.E.-S. High-mobility group box 1 (HMGB1) in COVID-19: Extrapolation of dangerous liaisons. Inflammopharmacology 2022, 30, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Al-Hussaniy, H.A.; Al-Harcan, N.A.H.; Alexiou, A.; Batiha, G.E.-S. Neutrophil Extracellular Traps (NETs) and COVID-19: A new frontiers for therapeutic modality. Int. Immunopharmacol. 2022, 104, 108516. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Saha, S.C.; Sunita, K.; Majumder, M.; Ghorai, M.; Mane, A.B.; Prasanth, D.A.; Kumar, P.; Pandey, D.K.; Al-Tawaha, A.R. Promising botanical-derived monoamine oxidase (MAO) inhibitors: Pharmacological aspects and structure-activity studies. S. Afr. J. Bot. 2022, 146, 127–145. [Google Scholar] [CrossRef]
- Kruse, J.M.; Magomedov, A.; Kurreck, A.; Münch, F.H.; Koerner, R.; Kamhieh-Milz, J.; Kahl, A.; Gotthardt, I.; Piper, S.K.; Eckardt, K.-U. Thromboembolic complications in critically ill COVID-19 patients are associated with impaired fibrinolysis. Crit. Care 2020, 24, 676. [Google Scholar] [CrossRef] [PubMed]
- Diab, H.; Ahmed, A.; Batiha, G.; Alkazmi, L.; El-Zamkan, M. Molecular surveillance of lumpy skin disease outbreak, 2019 in sohag, egypt: Enzootic potential, phylogenetic assessment and implications on cattle herds health. J. Anim. Health Prod 2021, 9, 406–416. [Google Scholar]
- Hu, R.; Han, C.; Pei, S.; Yin, M.; Chen, X. Procalcitonin levels in COVID-19 patients. Int. J. Antimicrob. Agents 2020, 56, 106051. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Onohuean, H.; El-Saber Batiha, G. COVID-19 and erythrocrine function: The roller coaster and danger. Int. J. Immunopathol. Pharmacol. 2022, 36, 03946320221103151. [Google Scholar] [CrossRef]
- Onohuean, H.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Qusti, S.; Alshammari, E.M.; Batiha, G.E.-S. COVID-19 and development of heart failure: Mystery and truth. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Alkhayyat, S.S.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; El-Bouseary, M.M.; AboKamer, A.M.; Batiha, G.E.-S.; Simal-Gandara, J. Fenofibrate for COVID-19 and related complications as an approach to improve treatment outcomes: The missed key for Holy Grail. Inflamm. Res. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.; Al-Gareeb, A.; Naji, M. Brain natriuretic peptide in patients with acute ischemic stroke: Role of statins. Biomed. Biotechnol. Res. J. 2020, 4, 239–245. [Google Scholar] [CrossRef]
- Zalpoor, H.; Akbari, A.; Samei, A.; Forghaniesfidvajani, R.; Kamali, M.; Afzalnia, A.; Manshouri, S.; Heidari, F.; Pornour, M.; Khoshmirsafa, M. The roles of Eph receptors, neuropilin-1, P2X7, and CD147 in COVID-19-associated neurodegenerative diseases: Inflammasome and JaK inhibitors as potential promising therapies. Cell. Mol. Biol. Lett. 2022, 27, 10. [Google Scholar] [CrossRef]
- Niland, S.; Eble, J.A. Neuropilin: Handyman and power broker in the tumor microenvironment. In Tumor Microenvironment. Advances in Experimental Medicine and Biology, 1st ed.; Birbrair, A., Ed.; Springer: Cham, Switzerland, 2020; Volume 1223, pp. 31–67. [Google Scholar]
- Nedaei, K.; Hesaraki, M.; Mazloomzadeh, S.; Totonchi, M.; Biglari, A.R. Lentiviral mediated expression of soluble Neuropilin 1 inhibits Semaphorin 3A-mediated Collapse Activity in vitro. Basic Clin. Neurosci. 2021, 12, 223. [Google Scholar] [CrossRef]
- Gil, V.; Del Río, J.A. Functions of plexins/neuropilins and their ligands during hippocampal development and neurodegeneration. Cells 2019, 8, 206. [Google Scholar] [CrossRef] [Green Version]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Naji, M.T.; Al-Mamorry, F. Role of vinpocetine in ischemic stroke and poststroke outcomes: A critical review. Brain Circ. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Al-kuraishy, H.M.; Al-Gareeb, A.I. Vinpocetine and ischemic stroke. In Ischemic Stroke; IntechOpen: London, UK, 2020; p. 27. [Google Scholar]
- Della-Morte, D.; Guadagni, F.; Palmirotta, R.; Testa, G.; Caso, V.; Paciaroni, M.; Abete, P.; Rengo, F.; Ferroni, P.; Sacco, R.L. Genetics of ischemic stroke, stroke-related risk factors, stroke precursors and treatments. Pharmacogenomics 2012, 13, 595–613. [Google Scholar] [CrossRef]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, M.; Yang, S.; Chen, X.; Wu, J.; Wen, M.; Yan, K.; Bi, X. Enriched environment improves post-stroke cognitive impairment and inhibits neuroinflammation and oxidative stress by activating Nrf2-ARE pathway. Int. J. Neurosci. 2021, 131, 641–649. [Google Scholar] [CrossRef]
- Dabrowska, S.; Andrzejewska, A.; Kozlowska, H.; Strzemecki, D.; Janowski, M.; Lukomska, B. Neuroinflammation evoked by brain injury in a rat model of lacunar infarct. Exp. Neurol. 2021, 336, 113531. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Huang, Q.; Hu, Z.; Tang, X. Potential neuroprotective treatment of stroke: Targeting excitotoxicity, oxidative stress, and inflammation. Front. Neurosci. 2019, 13, 1036. [Google Scholar] [CrossRef] [PubMed]
- Pawluk, H.; Woźniak, A.; Grześk, G.; Kołodziejska, R.; Kozakiewicz, M.; Kopkowska, E.; Grzechowiak, E.; Kozera, G. The role of selected pro-inflammatory cytokines in pathogenesis of ischemic stroke. Clin. Interv. Aging 2020, 15, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staton, C.; Kumar, I.; Reed, M.; Brown, N. Neuropilins in physiological and pathological angiogenesis. J. Pathol. 2007, 212, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Zhang, L.; Jiang, Q.; Zhang, R.; Davies, K.; Powers, C.; van Bruggen, N.; Chopp, M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Investig. 2000, 106, 829–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.G.; Tsang, W.; Zhang, L.; Powers, C.; Chopp, M. Up-regulation of neuropilin-1 in neovasculature after focal cerebral ischemia in the adult rat. J. Cereb. Blood Flow Metab. 2001, 21, 541–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foxton, R.H.; Finkelstein, A.; Shima, D.T.; Ng, Y.-S. Direct VEGF-A Mediated Neuroprotection: Mechanistic Studies in RGCs. Invest. Ophthalmol. Vis. Sci. 2011, 52, 5438. [Google Scholar]
- Ergul, A.; Abdelsaid, M.; Fouda, A.Y.; Fagan, S.C. Cerebral neovascularization in diabetes: Implications for stroke recovery and beyond. J. Cereb. Blood Flow Metab. 2014, 34, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Jin, K.L.; Mao, X.O.; Greenberg, D.A. Vascular endothelial growth factor: Direct neuroprotective effect in in vitro ischemia. Proc. Natl. Acad. Sci. USA 2000, 97, 10242–10247. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, T.; Kitsukawa, T.; Bekku, Y.; Matsuda, Y.; Sanbo, M.; Yagi, T.; Fujisawa, H. A requirement for neuropilin-1 in embryonic vessel formation. Development 1999, 126, 4895–4902. [Google Scholar] [CrossRef]
- Jiang, S.X.; Sheldrick, M.; Desbois, A.; Slinn, J.; Hou, S.T. Neuropilin-1 is a direct target of the transcription factor E2F1 during cerebral ischemia-induced neuronal death in vivo. Mol. Cell. Biol. 2007, 27, 1696–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, V.; Fields, L.; Evans, I.M.; Yamaji, M.; Pellet-Many, C.; Jones, T.; Mahmoud, M.; Zachary, I. VEGF (vascular endothelial growth factor) induces NRP1 (neuropilin-1) cleavage via ADAMs (a disintegrin and metalloproteinase) 9 and 10 to generate novel carboxy-terminal NRP1 fragments that regulate angiogenic signaling. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1845–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Zechariah, A.; Qu, Y.; Hermann, D.M. Effects of vascular endothelial growth factor in ischemic stroke. J. Neurosci. Res. 2012, 90, 1873–1882. [Google Scholar] [CrossRef]
- Slevin, M.; Krupinski, J.; Slowik, A.; Kumar, P.; Szczudlik, A.; Gaffney, J. Serial measurement of vascular endothelial growth factor and transforming growth factor-β1 in serum of patients with acute ischemic stroke. Stroke 2000, 31, 1863–1870. [Google Scholar] [CrossRef]
- Ionita, C.C.; Siddiqui, A.H.; Levy, E.I.; Hopkins, L.N.; Snyder, K.V.; Gibbons, K.J. Acute ischemic stroke and infections. J. Stroke Cerebrovasc. Dis. 2011, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Manousakis, G.; Jensen, M.B.; Chacon, M.R.; Sattin, J.A.; Levine, R.L. The interface between stroke and infectious disease: Infectious diseases leading to stroke and infections complicating stroke. Curr. Neurol. Neurosci. Rep. 2009, 9, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, R.; Bliden, K.; Tantry, U.; Gurbel, P. Viral respiratory tract infections increase platelet reactivity and activation: An explanation for the higher rates of myocardial infarction and stroke during viral illness. J. Thromb. Haemost. 2005, 3, 2108. [Google Scholar] [CrossRef]
- Chow, F.C.; Regan, S.; Zanni, M.V.; Looby, S.E.; Bushnell, C.D.; Meigs, J.B.; Grinspoon, S.K.; Feske, S.K.; Triant, V.A. Elevated ischemic stroke risk among women living with HIV infection. AIDS 2018, 32, 59. [Google Scholar] [CrossRef]
- Grandhi, G.R.; Mszar, R.; Vahidy, F.; Valero-Elizondo, J.; Blankstein, R.; Blaha, M.J.; Virani, S.S.; Andrieni, J.D.; Omer, S.B.; Nasir, K. Sociodemographic disparities in influenza vaccination among adults with atherosclerotic cardiovascular disease in the United States. JAMA Cardiol. 2021, 6, 87–91. [Google Scholar] [CrossRef]
- Seyoum, M.; Enawgaw, B.; Melku, M. Human blood platelets and viruses: Defense mechanism and role in the removal of viral pathogens. Thromb. J. 2018, 16, 16. [Google Scholar] [CrossRef]
- Huo, Y.; Schober, A.; Forlow, S.B.; Smith, D.F.; Hyman, M.C.; Jung, S.; Littman, D.R.; Weber, C.; Ley, K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat. Med. 2003, 9, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Mammarella, A.; Martini, F.; Paoletti, V.; Cardarello, C.M.; Labbadia, G.; Donnarumma, L.; De Matteis, A.; Gazzaniga, P.P.; Musca, A. Increased soluble P-selectin levels in hepatitis C virus-related chronic hepatitis: Correlation with viral load. J. Investig. Med. 2001, 49, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, E.C.; Hottz, E.D.; Amancio, R.T.; Carneiro, A.B.; Palhinha, L.; Coelho, L.E.; Grinsztejn, B.; Zimmerman, G.A.; Rondina, M.T.; Weyrich, A.S. Persistent platelet activation and apoptosis in virologically suppressed HIV-infected individuals. Sci. Rep. 2018, 8, 14999. [Google Scholar] [CrossRef] [PubMed]
- Ghez, D.; Lepelletier, Y.; Lambert, S.; Fourneau, J.-M.; Blot, V.; Janvier, S.; Arnulf, B.; van Endert, P.M.; Heveker, N.; Pique, C. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J. Virol. 2006, 80, 6844–6854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.-B.; Zhang, H.; Zhang, J.-P.; Li, Y.; Zhao, B.; Feng, G.-K.; Du, Y.; Xiong, D.; Zhong, Q.; Liu, W.-L. Neuropilin 1 is an entry factor that promotes EBV infection of nasopharyngeal epithelial cells. Nat. Commun. 2015, 6, 6240. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-C.; Huang, P.-N.; Hung, H.-C.; Tseng, S.-N.; Chang, C.-C.; Tsai, Y.-R.; Wang, Y.-M.; Shih, S.-R.; Hsu, J.T.-A. Effect of a neuropilin-1-derived virus receptor trap on enterovirus A71 infection in vitro. Antimicrob. Agents Chemother. 2021, 65, e00695-20. [Google Scholar] [CrossRef]
- Lane, R.K.; Guo, H.; Fisher, A.D.; Diep, J.; Lai, Z.; Chen, Y.; Upton, J.W.; Carette, J.; Mocarski, E.S.; Kaiser, W.J. Necroptosis-based CRISPR knockout screen reveals Neuropilin-1 as a critical host factor for early stages of murine cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 2020, 117, 20109–20116. [Google Scholar] [CrossRef]
- Delgoffe, G.M.; Woo, S.-R.; Turnis, M.E.; Gravano, D.M.; Guy, C.; Overacre, A.E.; Bettini, M.L.; Vogel, P.; Finkelstein, D.; Bonnevier, J. Stability and function of regulatory T cells is maintained by a neuropilin-1–semaphorin-4a axis. Nature 2013, 501, 252–256. [Google Scholar] [CrossRef]
- Bruder, D.; Probst-Kepper, M.; Westendorf, A.M.; Geffers, R.; Beissert, S.; Loser, K.; von Boehmer, H.; Buer, J.; Hansen, W. Frontline: Neuropilin-1: A surface marker of regulatory T cells. Eur. J. Immunol. 2004, 34, 623–630. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Sun, Y.; Carroll, C.R.; Usherwood, E.J. Neuropilin-1 regulates the secondary CD8 T cell response to virus infection. Msphere 2019, 4, e00221-19. [Google Scholar] [CrossRef] [Green Version]
- Solomon, B.D.; Mueller, C.; Chae, W.-J.; Alabanza, L.M.; Bynoe, M.S. Neuropilin-1 attenuates autoreactivity in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2011, 108, 2040–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tordjman, R.; Lepelletier, Y.; Lemarchandel, V.; Cambot, M.; Gaulard, P.; Hermine, O.; Roméo, P.-H. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat. Immunol. 2002, 3, 477–482. [Google Scholar] [CrossRef]
- Leclerc, M.; Voilin, E.; Gros, G.; Corgnac, S.; de Montpréville, V.; Validire, P.; Bismuth, G.; Mami-Chouaib, F. Regulation of antitumour CD8 T-cell immunity and checkpoint blockade immunotherapy by Neuropilin-1. Nat. Commun. 2019, 10, 3345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Somasundaram, A.; Manne, S.; Gocher, A.M.; Szymczak-Workman, A.L.; Vignali, K.M.; Scott, E.N.; Normolle, D.P.; John Wherry, E.; Lipson, E.J. Neuropilin-1 is a T cell memory checkpoint limiting long-term antitumor immunity. Nat. Immunol. 2020, 21, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Sinha, D.; Mukherjee, S.; Biswas, R.; Biswas, T. LPS stimulates and Hsp70 down-regulates TLR4 to orchestrate differential cytokine response of culture-differentiated innate memory CD8+ T cells. Cytokine 2015, 73, 44–52. [Google Scholar] [CrossRef]
- Gao, Y.-L.; Wang, C.-X.; Wang, Z.-Y.; Li, W.-J.; Liu, Y.-C.; Shou, S.-T.; Chai, Y.-F. Targeting neuropilin-1 suppresses the stability of CD4+ CD25+ regulatory T cells via the NF-κB signaling pathway in sepsis. Infect. Immun. 2021, 89, e00399-20. [Google Scholar] [CrossRef]
- Abdel-aziz, H.; Ahmed, S.M.; Mohammed, M.Z.; Abdel Nour, H.M. Efficacy of cerebrolysin on dentate gyrus of hippocampus after experimentally induced acute ischemic stroke in adult albino rats (Histological, immunohistochemical and biochemical study). Egypt. J. Histol. 2019, 42, 229–244. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cao, Y.; Mangalam, A.K.; Guo, Y.; LaFrance-Corey, R.G.; Gamez, J.D.; Atanga, P.A.; Clarkson, B.D.; Zhang, Y.; Wang, E. Neuropilin-1 modulates interferon-γ-stimulated signaling in brain microvascular endothelial cells. J. Cell Sci. 2016, 129, 3911–3921. [Google Scholar] [CrossRef] [Green Version]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflammation 2019, 16, 142. [Google Scholar] [CrossRef] [Green Version]
- Lalor, S.J.; Segal, B.M. T h1-mediated experimental autoimmune encephalomyelitis is CXCR 3 independent. Eur. J. Immunol. 2013, 43, 2866–2874. [Google Scholar] [CrossRef]
- Argaw, A.T.; Asp, L.; Zhang, J.; Navrazhina, K.; Pham, T.; Mariani, J.N.; Mahase, S.; Dutta, D.J.; Seto, J.; Kramer, E.G. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Investig. 2012, 122, 2454–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.H.-P.; Arra, M.; Mbalaviele, G.; Swarnkar, G.; Abu-Amer, Y. Inflammatory responses reprogram Tregs through impairment of neuropilin-1. Sci. Rep. 2019, 9, 10429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudowska-Sawczuk, M.; Mroczko, B. The role of neuropilin-1 (NRP-1) in SARS-CoV-2 infection. J. Clin. Med. 2021, 10, 2772. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.-E.; Williamson, M.K.; Antón-Plágaro, C.; Shoemark, D.K.; Simón-Gracia, L.; Bauer, M.; Hollandi, R. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Moin, A.S.M.; Sathyapalan, T.; Atkin, S.L.; Butler, A.E. The relationship of soluble neuropilin-1 to severe COVID-19 risk factors in polycystic ovary syndrome. Metab. Open 2021, 9, 100079. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Alnomasy, S.F. Virus-receptor interactions of SARS-CoV-2 spikereceptor-binding domain and human neuropilin-1 b1 domain. Saudi J. Biol. Sci. 2021, 28, 3926–3928. [Google Scholar] [CrossRef]
- Kyrou, I.; Randeva, H.S.; Spandidos, D.A.; Karteris, E. Not only ACE2—the quest for additional host cell mediators of SARS-CoV-2 infection: Neuropilin-1 (NRP1) as a novel SARS-CoV-2 host cell entry mediator implicated in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 21. [Google Scholar] [CrossRef]
- Davies, J.; Randeva, H.S.; Chatha, K.; Hall, M.; Spandidos, D.A.; Karteris, E.; Kyrou, I. Neuropilin-1 as a new potential SARS-CoV-2 infection mediator implicated in the neurologic features and central nervous system involvement of COVID-19. Mol. Med. Report. 2020, 22, 4221–4226. [Google Scholar] [CrossRef]
- Heinonen, S.; Helve, O.; Andersson, S.; Janér, C.; Süvari, L.; Kaskinen, A. Nasal expression of SARS-CoV-2 entry receptors in newborns. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 95–97. [Google Scholar] [CrossRef]
- Romano, M.; Fanelli, G.; Albany, C.J.; Giganti, G.; Lombardi, G. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front. Immunol. 2019, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- Rouas, R.; Merimi, M.; Najar, M.; El Zein, N.; Fayyad-Kazan, M.; Berehab, M.; Agha, D.; Bron, D.; Burny, A.; Rachidi, W. Human CD8+ CD25+ CD127 low regulatory T cells: MicroRNA signature and impact on TGF-β and IL-10 expression. J. Cell. Physiol. 2019, 234, 17459–17472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, D.L.; Sjaastad, L.E.; Farrar, M.A. Regulatory T cell development in the thymus. J. Immunol. Res. 2019, 203, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Abdullah, S.M.; Cruz-Martins, N.; Batiha, G.E.-S. Case report: Hyperbilirubinemia in gilbert syndrome attenuates COVID-19-induced metabolic disturbances. Front. Cardiovasc. Med. 2021, 8, 642181. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, N.; Seif, F.; Ostadrahimi, A.; Pouraghaei, M.; Ghaffari, S. Targeting cytokine storm to manage patients with COVID-19: A mini-review. Arch. Med. Res. 2020, 51, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafiz, A.S.; Fouad, M.A.; Sayed-Ahmed, M.M.; Kamel, M.M.; Ali, A.; Fouda, M.; Khalil, M.A.; Abdel-Moneim, A.S.; Kamal, L.M. Upregulation of FOXP3 is associated with severity of hypoxia and poor outcomes in COVID-19 patients. Virology 2021, 563, 74–81. [Google Scholar] [CrossRef]
- Mohebbi, S.R.; Baghaei, K.; Rostami-Nejad, M.; Mojarad, E.N.; Mirjalali, H.; Yadegar, A.; Asri, N.; Abdoulahi, S.; Aghdaei, H.A. Significant changes of CD4, FOXP3, CD25, and IL6 expression level in Iranian COVID-19 patients. Gastroenterol. Hepatol. Bed Bench 2020, 13, 388. [Google Scholar]
- Sarris, M.; Andersen, K.G.; Randow, F.; Mayr, L.; Betz, A.G. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity 2008, 28, 402–413. [Google Scholar] [CrossRef] [Green Version]
- Mone, P.; Gambardella, J.; Wang, X.; Jankauskas, S.S.; Matarese, A.; Santulli, G. miR-24 targets SARS-CoV-2 co-factor Neuropilin-1 in human brain microvascular endothelial cells: Insights for COVID-19 neurological manifestations. Res. Sq. 2021. (preprint). [Google Scholar]
- Gambardella, J.; Coppola, A.; Izzo, R.; Fiorentino, G.; Trimarco, B.; Santulli, G. Role of endothelial miR-24 in COVID-19 cerebrovascular events. Crit. Care 2021, 25, 306. [Google Scholar] [CrossRef]
- Abdelhafez, M.; El-Gamasy, M.; Mehrez, M.; Fakhreldin, A. A disintegrin and metalloproteinase with thrombospondin type 1 repeats 13 in children with end-stage renal disease on regular hemodialysis. J. Integr. Nephrol. Androl. 2017, 4, 93–100. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J. Identification of miRNA-21 and miRNA-24 in plasma as potential early stage markers of acute cerebral infarction. Mol. Med. Report. 2014, 10, 971–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moutal, A.; Martin, L.F.; Boinon, L.; Gomez, K.; Ran, D.; Zhou, Y.; Stratton, H.J.; Cai, S.; Luo, S.; Gonzalez, K.B. SARS-CoV-2 spike protein co-opts VEGF-A/neuropilin-1 receptor signaling to induce analgesia. Pain 2021, 162, 243. [Google Scholar] [CrossRef]
- Parker, M.W.; Xu, P.; Li, X.; Vander Kooi, C.W. Structural basis for selective vascular endothelial growth factor-A (VEGF-A) binding to neuropilin-1. J. Biol. Chem. 2012, 287, 11082–11089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ascanio, L.; Pandolfini, M.; Cingolani, C.; Latini, G.; Gradoni, P.; Capalbo, M.; Frausini, G.; Maranzano, M.; Brenner, M.J.; Di Stadio, A. Olfactory dysfunction in COVID-19 patients: Prevalence and prognosis for recovering sense of smell. Otolaryngol. Head Neck Surg. 2021, 164, 82–86. [Google Scholar] [CrossRef]
- Yin, X.-X.; Zheng, X.-R.; Peng, W.; Wu, M.-L.; Mao, X.-Y. Vascular endothelial growth factor (VEGF) as a vital target for brain inflammation during the COVID-19 outbreak. ACS Chem. Neurosci. 2020, 11, 1704–1705. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Y.; Chen, S.; Xuran, L.; Dong, J.; Chen, W.; Tao, S.; Yang, W.; Zhang, Y. The role of vascular endothelial growth factor in ischemic stroke. Pharmazie 2021, 76, 127–131. [Google Scholar] [PubMed]
- Chua, C.C.; Hamdy, R.C.; Chua, B.H. Upregulation of vascular endothelial growth factor by angiotensin II in rat heart endothelial cells. Biochim. Biophys. Acta 1998, 1401, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Ishibashi, M.; Hiasa, K.-I.; Tan, C.; Takeshita, A.; Egashira, K. Essential role of vascular endothelial growth factor in angiotensin II–induced vascular inflammation and remodeling. Hypertension 2004, 44, 264–270. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Hussien, N.R.; Al-Naimi, M.S.; Al-Buhadily, A.K.; Al-Gareeb, A.I.; Lungnier, C. Renin–Angiotensin system and fibrinolytic pathway in COVID-19: One-way skepticism. Biomed. Biotechnol. Res. J. 2020, 4, 33. [Google Scholar]
- Chen, I.-C.; Lin, J.-Y.; Liu, Y.-C.; Chai, C.-Y.; Yeh, J.-L.; Hsu, J.-H.; Wu, B.-N.; Dai, Z.-K. Angiotensin-converting enzyme 2 activator ameliorates severe pulmonary hypertension in a rat model of left pneumonectomy combined with VEGF inhibition. Front. Med. 2021, 8, 619133. [Google Scholar] [CrossRef] [PubMed]
- Hira, K.; Ueno, Y.; Tanaka, R.; Miyamoto, N.; Yamashiro, K.; Inaba, T.; Urabe, T.; Okano, H.; Hattori, N. Astrocyte-derived exosomes treated with a semaphorin 3A inhibitor enhance stroke recovery via prostaglandin D2 synthase. Stroke 2018, 49, 2483–2494. [Google Scholar] [CrossRef]
- Pekcec, A.; Yigitkanli, K.; Jung, J.E.; Pallast, S.; Xing, C.; Antipenko, A.; Minchenko, M.; Nikolov, D.B.; Holman, T.R.; Lo, E.H. Following experimental stroke, the recovering brain is vulnerable to lipoxygenase-dependent semaphorin signaling. FASEB J. 2013, 27, 437–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, D.X.; Hardeland, R. Potential utility of melatonin in deadly infectious diseases related to the overreaction of innate immune response and destructive inflammation: Focus on COVID-19. Melatonin Res. 2020, 3, 120–143. [Google Scholar] [CrossRef]
- Caravita, S.; Senni, M.; Parati, G. Reply to:‘The hyperdynamic circulatory profile of patients with COVID-19-related acute vascular distress syndrome. Letter regarding the article Haemodynamic characteristics of COVID-19 patients with acute respiratory distress syndrome requiring mechanical ventilation. An invasive assessment using right heart catheterization’. Eur. J. Heart Fail. 2021, 23, 493–494. [Google Scholar] [PubMed]
- Bechet, D.; Tirand, L.; Faivre, B.; Plénat, F.; Bonnet, C.; Bastogne, T.; Frochot, C.; Guillemin, F.; Barberi-Heyob, M. Neuropilin-1 targeting photosensitization-induced early stages of thrombosis via tissue factor release. Pharm. Res. 2010, 27, 468–479. [Google Scholar] [CrossRef]
- AlSheef, M.; Zaidi, A.R.Z.; Abonab, M.; Obaid, M.; Bawazir, K.; Zaidi, S.Z.A.; Kullab, G.J.; Alawfi, S.; Enani, M. Dual catastrophe of COVID-19: Massive pulmonary embolism and stroke in a previously healthy young patient: A case report. J. Infect. Public Health 2021, 14, 647–650. [Google Scholar] [CrossRef]
- Mostafa-Hedeab, G.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Welson, N.N.; Batiha, G.E.-S.; Conte-Junior, C.A. Selinexor and COVID-19: The Neglected Warden. Front. Pharmacol. 2022, 13, 884228. [Google Scholar] [CrossRef]
- Issitt, T.; Bosseboeuf, E.; De Winter, N.; Dufton, N.; Gestri, G.; Senatore, V.; Chikh, A.; Randi, A.M.; Raimondi, C. Neuropilin-1 controls endothelial homeostasis by regulating mitochondrial function and iron-dependent oxidative stress. Iscience 2019, 11, 205–223. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Zhu, L. The role of semaphorins in metabolic disorders. Int. J. Mol. Sci. 2020, 21, 5641. [Google Scholar] [CrossRef]
- Usman, F.; Shah, H.S.; Zaib, S.; Manee, S.; Mudassir, J.; Khan, A.; Batiha, G.E.-S.; Abualnaja, K.M.; Alhashmialameer, D.; Khan, I. Fabrication and biological assessment of antidiabetic α-Mangostin loaded nanosponges: In vitro, in vivo, and in silico studies. Molecules 2021, 26, 6633. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, N. The Regulation of Osteopontin and Soluble Neuropilin-1 in HIV-Associated Preeclampsia. Master’s Dissertation, University of KwaZulu-Natal, Durban, South Africa, 2020. [Google Scholar]
- El-Arabey, A.A.; Abdalla, M. Neuropilin-1 may be responsible for retinal findings in patients with COVID-19. Hum. Cell 2021, 34, 1280–1281. [Google Scholar]
- Sodhi, A.; Ma, T.; Menon, D.; Deshpande, M.; Jee, K.; Dinabandhu, A.; Vancel, J.; Lu, D.; Montaner, S. Angiopoietin-like 4 binds neuropilins and cooperates with VEGF to induce diabetic macular edema. J. Clin. Investig. 2019, 129, 4593–4608. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Goh, Y.Y.; Chin, H.F.A.; Kersten, S.; Tan, N.S. Angiopoietin-like 4: A decade of research. Biosci. Rep. 2012, 32, 211–219. [Google Scholar] [CrossRef]
- Lei, X.; Shi, F.; Basu, D.; Huq, A.; Routhier, S.; Day, R.; Jin, W. Proteolytic processing of angiopoietin-like protein 4 by proprotein convertases modulates its inhibitory effects on lipoprotein lipase activity. J. Biol. Chem. 2011, 286, 15747–15756. [Google Scholar] [CrossRef] [Green Version]
- Dewey, F.E.; Gusarova, V.; O’Dushlaine, C.; Gottesman, O.; Trejos, J.; Hunt, C.; Van Hout, C.V.; Habegger, L.; Buckler, D.; Lai, K.-M.V. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N. Engl. J. Med. 2016, 374, 1123–1133. [Google Scholar] [CrossRef]
- Kadomatsu, T.; Tabata, M.; Oike, Y. Angiopoietin-like proteins: Emerging targets for treatment of obesity and related metabolic diseases. FEBS J. 2011, 278, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.L.; Kash, J.C.; Beres, S.B.; Sheng, Z.M.; Musser, J.M.; Taubenberger, J.K. High-throughput RNA sequencing of a formalin-fixed, paraffin-embedded autopsy lung tissue sample from the 1918 influenza pandemic. J. Pathol. 2013, 229, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Foo, B.J.W.; Kwok, K.W.; Sakamoto, N.; Mukae, H.; Izumikawa, K.; Mandard, S.; Quenot, J.-P.; Lagrost, L.; Teh, W.K. Antibody treatment against angiopoietin-like 4 reduces pulmonary edema and injury in secondary pneumococcal pneumonia. MBio 2019, 10, e02469-18. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yu, J.; Mu, R.; Dong, S. Clinical effect of electroacupuncture on lung injury patients caused by severe acute pancreatitis. Evid. Based Complement. Alternat. Med. 2017, 2017, 3162851. [Google Scholar]
- Aarsetøy, R.; Ueland, T.; Aukrust, P.; Michelsen, A.E.; de la Fuente, R.L.; Pönitz, V.; Brügger-Andersen, T.; Grundt, H.; Staines, H.; Nilsen, D.W. Angiopoietin-2 and angiopoietin-like 4 protein provide prognostic information in patients with suspected acute coronary syndrome. J. Intern. Med. 2021, 290, 894–909. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Guo, Z.-N.; Yang, Y.; Xu, J.; Burchell, S.R.; Tang, J.; Zhang, J.; Xu, J.; Zhang, J.H. Angiopoietin-like 4: A double-edged sword in atherosclerosis and ischemic stroke? Exp. Neurol. 2015, 272, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, X.; Chu, X.; Yu, X.; Zhao, Y. Protective effects of angiopoietin-like 4 on the blood-brain barrier in acute ischemic stroke treated with thrombolysis in mice. Neurosci. Lett. 2017, 645, 113–120. [Google Scholar] [CrossRef]
- Alay, H.; Laloglu, E. The role of angiopoietin-2 and surfactant protein-D levels in SARS-CoV-2-related lung injury: A prospective, observational, cohort study. J. Med. Virol. 2021, 93, 6008–6015. [Google Scholar] [CrossRef] [PubMed]
- Smadja, D.M.; Guerin, C.L.; Chocron, R.; Yatim, N.; Boussier, J.; Gendron, N.; Khider, L.; Hadjadj, J.; Goudot, G.; Debuc, B. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis 2020, 23, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Connors, J.M.; Levy, J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. 2020, 69, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Y.; Xie, X.; Wu, H.; Su, X.N.; Qi, J.; Xin, W.; Gao, L.; Zhang, Y.; Shah, V.H. Neuropilin-1 aggravates liver cirrhosis by promoting angiogenesis via VEGFR2-dependent PI3K/Akt pathway in hepatic sinusoidal endothelial cells. eBioMedicine 2019, 43, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Pan, X.; Ma, A. Effect of leucine-rich α2-glycoprotein-1 on ischemic stroke by regulating transforming growth factor β signaling pathway. Int. J. Cerebrovasc. Dis. 2021, 12, 53–57. [Google Scholar]
- Ghali, M.G.Z.; Srinivasan, V.M.; Johnson, J.; Kan, P.; Britz, G. Therapeutically targeting platelet-derived growth factor-mediated signaling underlying the pathogenesis of subarachnoid hemorrhage-related vasospasm. J. Stroke Cerebrovasc. Dis. 2018, 27, 2289–2295. [Google Scholar] [CrossRef]
- Meshaal, A.K.; Hetta, H.F.; Yahia, R.; Abualnaja, K.M.; Mansour, A.T.; Al-Kadmy, I.M.; Alghamdi, S.; Dablool, A.S.; Emran, T.B.; Sedky, H. In Vitro Antimicrobial Activity of Medicinal Plant Extracts against Some Bacterial Pathogens Isolated from Raw and Processed Meat. Life 2021, 11, 1178. [Google Scholar] [CrossRef]
- Chen, W. A potential treatment of COVID-19 with TGF-β blockade. Int. J. Biol. Sci. 2020, 16, 1954. [Google Scholar] [CrossRef] [PubMed]
- Teibo, J.O.; Ayinde, K.S.; Olaoba, O.T.; Adelusi, T.I.; Teibo, T.K.A.; Bamikunle, M.V.; Jimoh, Y.A.; Alghamdi, S.; Abdulaziz, O.; Rauf, A. Functional foods’ bioactive components and their chemoprevention mechanism in cervical, breast, and liver cancers: A systematic review. Funct. Food Health Dis. 2021, 11, 559–585. [Google Scholar] [CrossRef]
- Hondermarck, H.; Bartlett, N.W.; Nurcombe, V. The role of growth factor receptors in viral infections: An opportunity for drug repurposing against emerging viral diseases such as COVID-19? FASEB BioAdvances 2020, 2, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Bano, D.; Prehn, J.H. Apoptosis-inducing factor (AIF) in physiology and disease: The tale of a repented natural born killer. eBioMedicine 2018, 30, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Hayat, K.; Khan, A.; Bibi, F.; Murad, W.; Fu, Y.; Batiha, G.E.-S.; Alqarni, M.; Khan, A.; Al-Harrasi, A. Effect of Cadmium and Copper Exposure on Growth, Physio-Chemicals and Medicinal Properties of Cajanus cajan L.(Pigeon Pea). Metabolites 2021, 11, 769. [Google Scholar] [CrossRef]
- Xing, J.; Lu, J. HIF-1α activation attenuates IL-6 and TNF-α pathways in hippocampus of rats following transient global ischemia. Cell. Physiol. Biochem. 2016, 39, 511–520. [Google Scholar] [CrossRef]
- Serebrovska, Z.O.; Chong, E.Y.; Serebrovska, T.V.; Tumanovska, L.V.; Xi, L. Hypoxia, HIF-1α, and COVID-19: From pathogenic factors to potential therapeutic targets. Acta Pharmacol. Sin. 2020, 41, 1539–1546. [Google Scholar] [CrossRef]
- Wang, C.; Niu, F.; Ren, N.; Wang, X.; Zhong, H.; Zhu, J.; Li, B. Hyperbaric oxygen improves cerebral ischemia/reperfusion injury in rats probably via inhibition of autophagy triggered by the downregulation of hypoxia-inducing factor-1 alpha. BioMed Res. Int. 2021, 2021, 6615685. [Google Scholar] [CrossRef]
- Jiang, Q.; Geng, X.; Warren, J.; Cosky, E.E.P.; Kaura, S.; Stone, C.; Li, F.; Ding, Y. Hypoxia inducible factor-1α (HIF-1α) mediates NLRP3 inflammasome-dependent-pyroptotic and apoptotic cell death following ischemic stroke. Neuroscience 2020, 448, 126–139. [Google Scholar] [CrossRef]
- Eguiburu-Jaime, J.L.; Delmiro, A.; Lalueza, A.; Valenzuela, P.L.; Aguado, J.M.; Lumbreras, C.; Arenas, J.; Martín, M.A.; Lucia, A.; López-Jiménez, E.A. Soluble fms-like tyrosine kinase-1: A potential early predictor of respiratory failure in COVID-19 patients. Clin. Chem. Lab. Med. 2021, 59, e289–e292. [Google Scholar] [CrossRef]
- Hamrick, S.E.G.; McQuillen, P.S.; Jiang, X.; Mu, D.; Madan, A.; Ferriero, D.M. A role for hypoxia-inducible factor-1α in desferoxamine neuroprotection. Neurosci. Lett. 2005, 379, 96–100. [Google Scholar] [CrossRef]
- Davis, C.K.; Jain, S.A.; Bae, O.-N.; Majid, A.; Rajanikant, G. Hypoxia mimetic agents for ischemic stroke. Front. Cell Dev. Biol. 2019, 6, 175. [Google Scholar] [CrossRef] [PubMed]
- Bok, S.; Kim, Y.-E.; Woo, Y.; Kim, S.; Kang, S.-J.; Lee, Y.; Park, S.K.; Weissman, I.L.; Ahn, G.-O. Hypoxia-inducible factor-1α regulates microglial functions affecting neuronal survival in the acute phase of ischemic stroke in mice. Oncotarget 2017, 8, 111508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, R.M.; Bajaj, M.S.; Kale, V.P. Vasculogenic mimicry of HT1080 tumour cells in vivo: Critical role of HIF-1α-neuropilin-1 axis. PLoS ONE 2012, 7, e50153. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Thomali, A.W.; Al-kuraishy, H.M.; Al-Gareeb, A.I.; K. Al-buhadiliy, A.; De Waard, M.; Sabatier, J.-M.; Khan Khalil, A.A.; Saad, H.M.; Batiha, G.E.-S. Role of Neuropilin 1 in COVID-19 Patients with Acute Ischemic Stroke. Biomedicines 2022, 10, 2032. https://doi.org/10.3390/biomedicines10082032
Al-Thomali AW, Al-kuraishy HM, Al-Gareeb AI, K. Al-buhadiliy A, De Waard M, Sabatier J-M, Khan Khalil AA, Saad HM, Batiha GE-S. Role of Neuropilin 1 in COVID-19 Patients with Acute Ischemic Stroke. Biomedicines. 2022; 10(8):2032. https://doi.org/10.3390/biomedicines10082032
Chicago/Turabian StyleAl-Thomali, Asma W., Hayder M. Al-kuraishy, Ali I. Al-Gareeb, Ali K. Al-buhadiliy, Michel De Waard, Jean-Marc Sabatier, Atif Ali Khan Khalil, Hebatallah M. Saad, and Gaber El-Saber Batiha. 2022. "Role of Neuropilin 1 in COVID-19 Patients with Acute Ischemic Stroke" Biomedicines 10, no. 8: 2032. https://doi.org/10.3390/biomedicines10082032
APA StyleAl-Thomali, A. W., Al-kuraishy, H. M., Al-Gareeb, A. I., K. Al-buhadiliy, A., De Waard, M., Sabatier, J.-M., Khan Khalil, A. A., Saad, H. M., & Batiha, G. E.-S. (2022). Role of Neuropilin 1 in COVID-19 Patients with Acute Ischemic Stroke. Biomedicines, 10(8), 2032. https://doi.org/10.3390/biomedicines10082032







