Chemerin Forms: Their Generation and Activity
Abstract
:1. Introduction
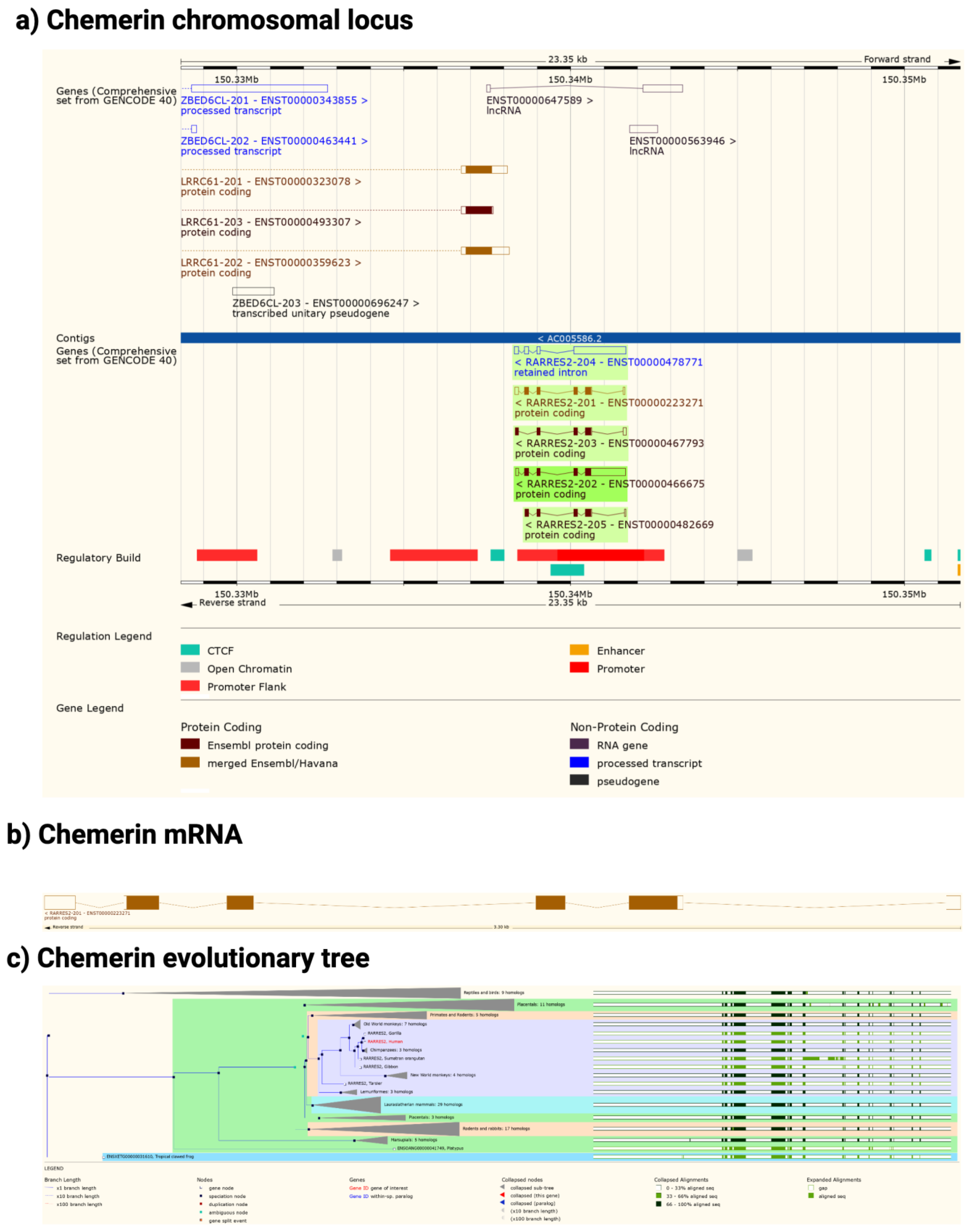
2. Chemerin System of Genes, Proteins, and Receptors
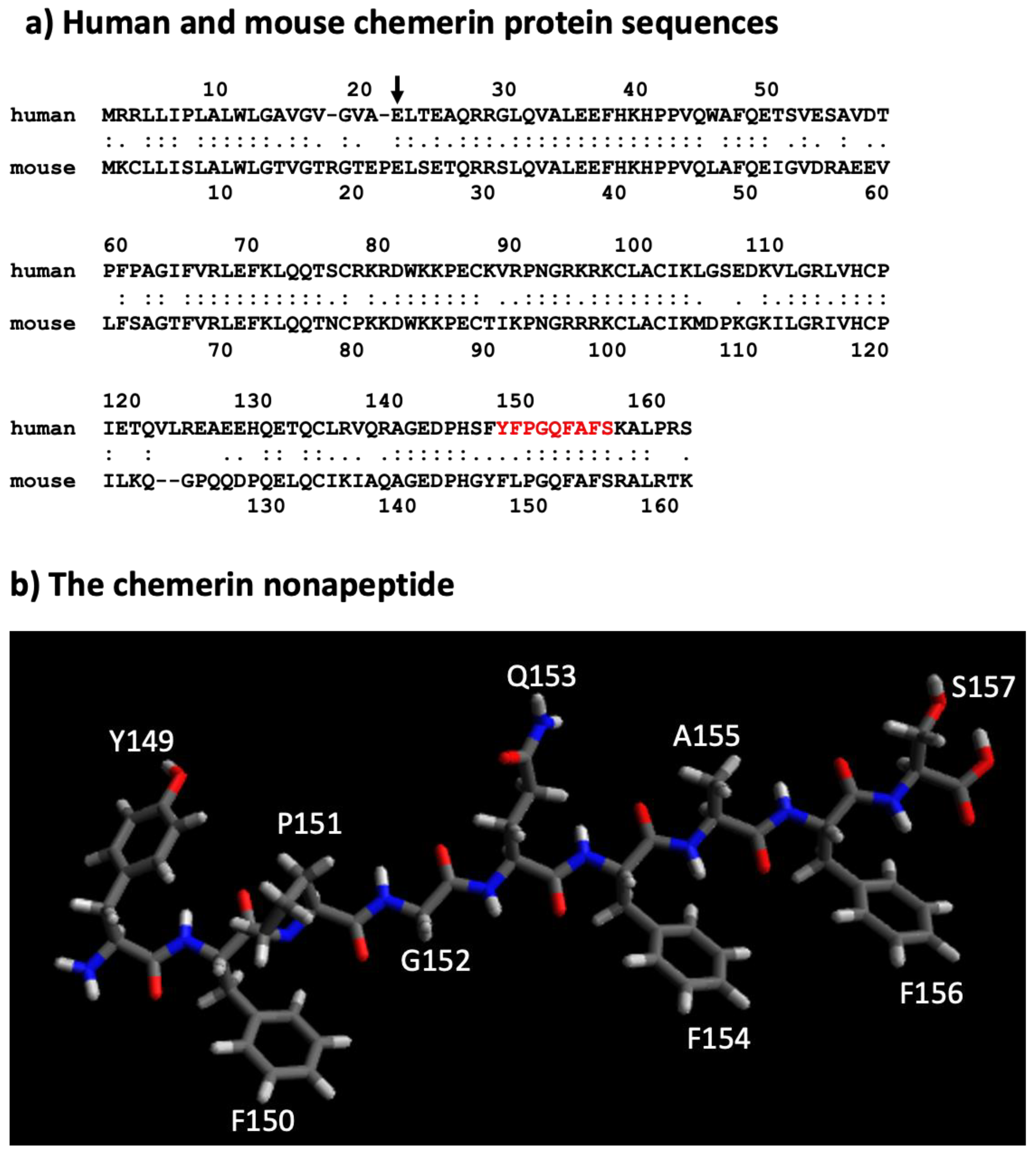
3. Chemerin System Evolution
4. Chemerin mRNA Regulation
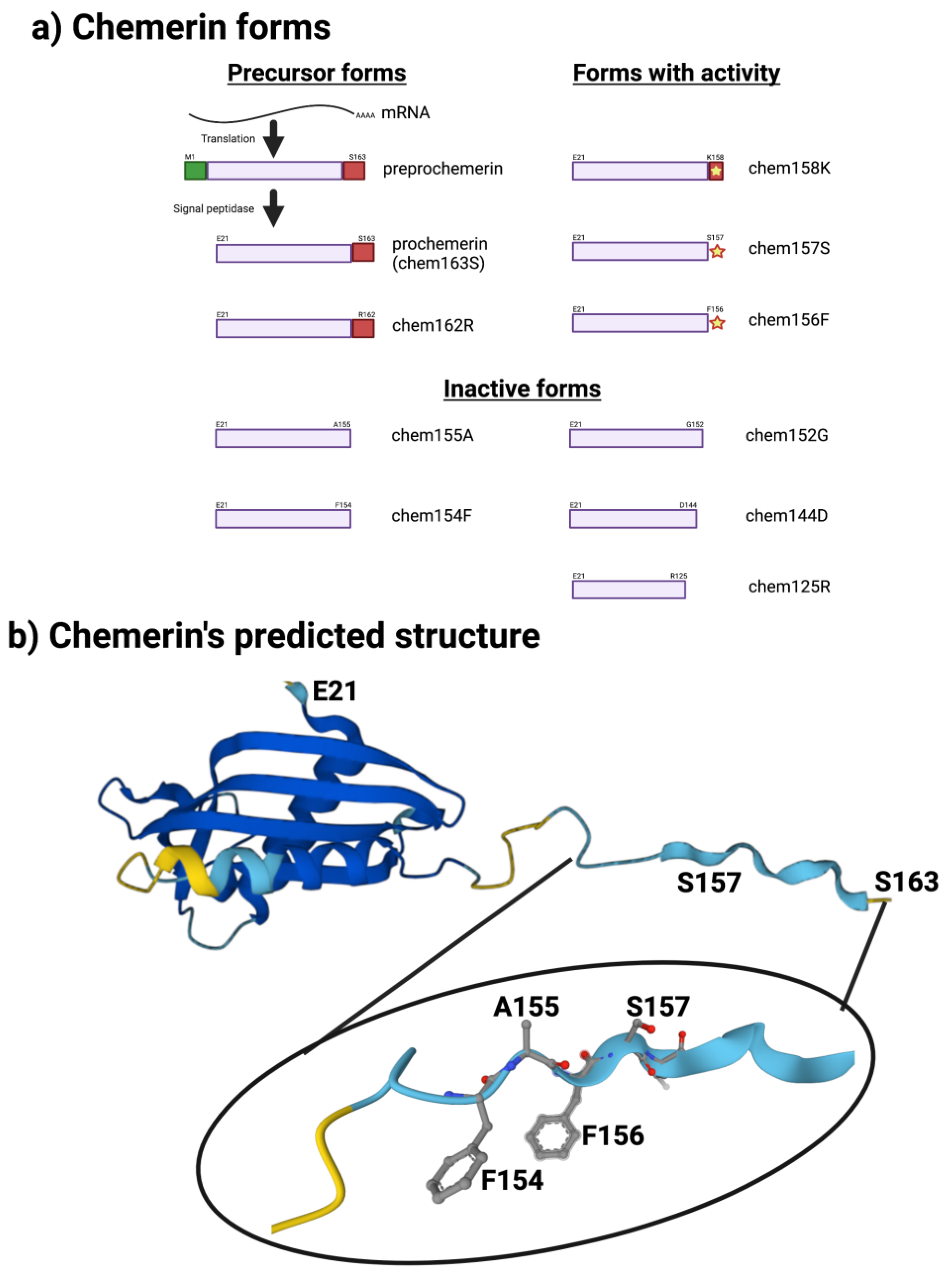
5. Different Forms of the Chemerin Protein
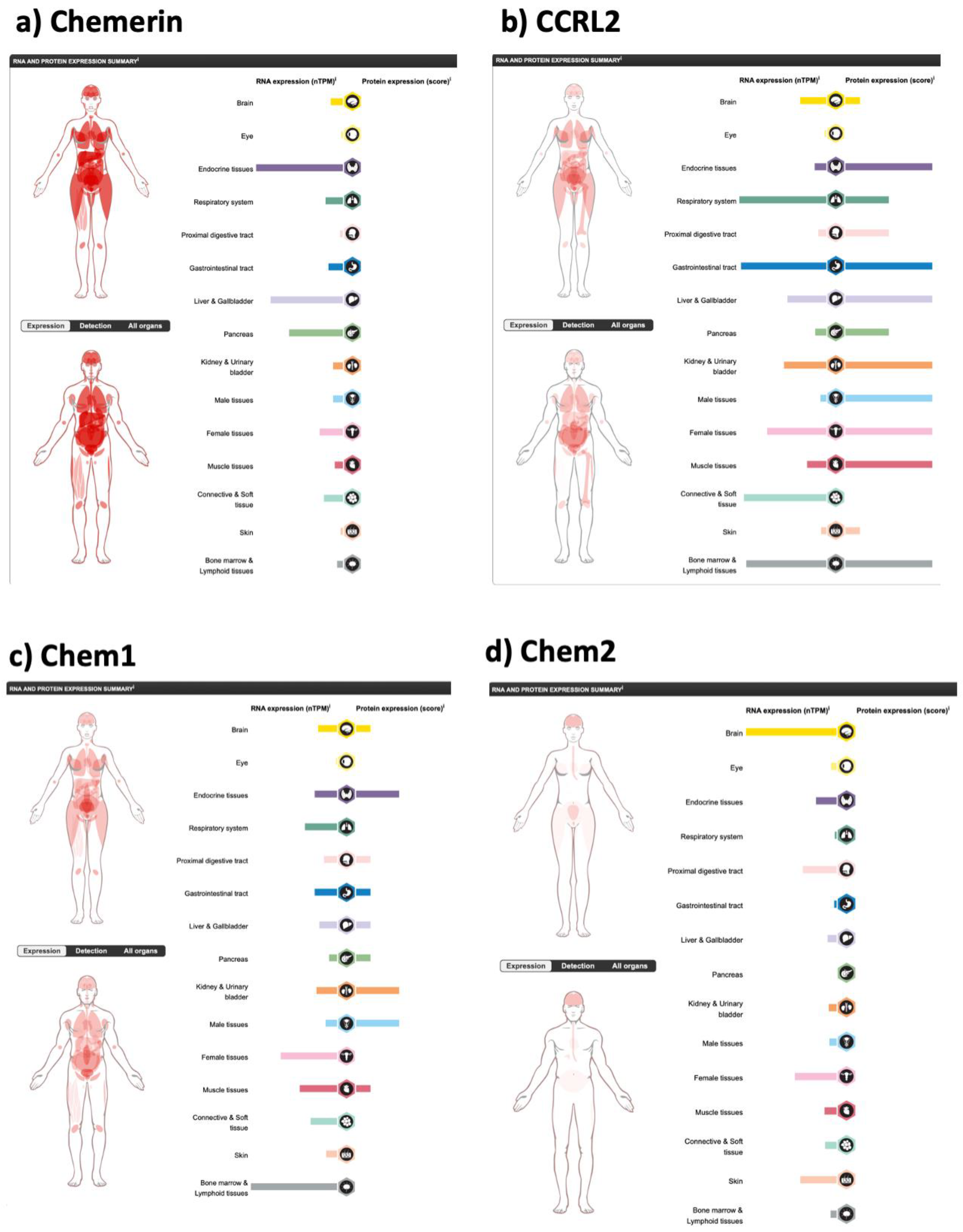
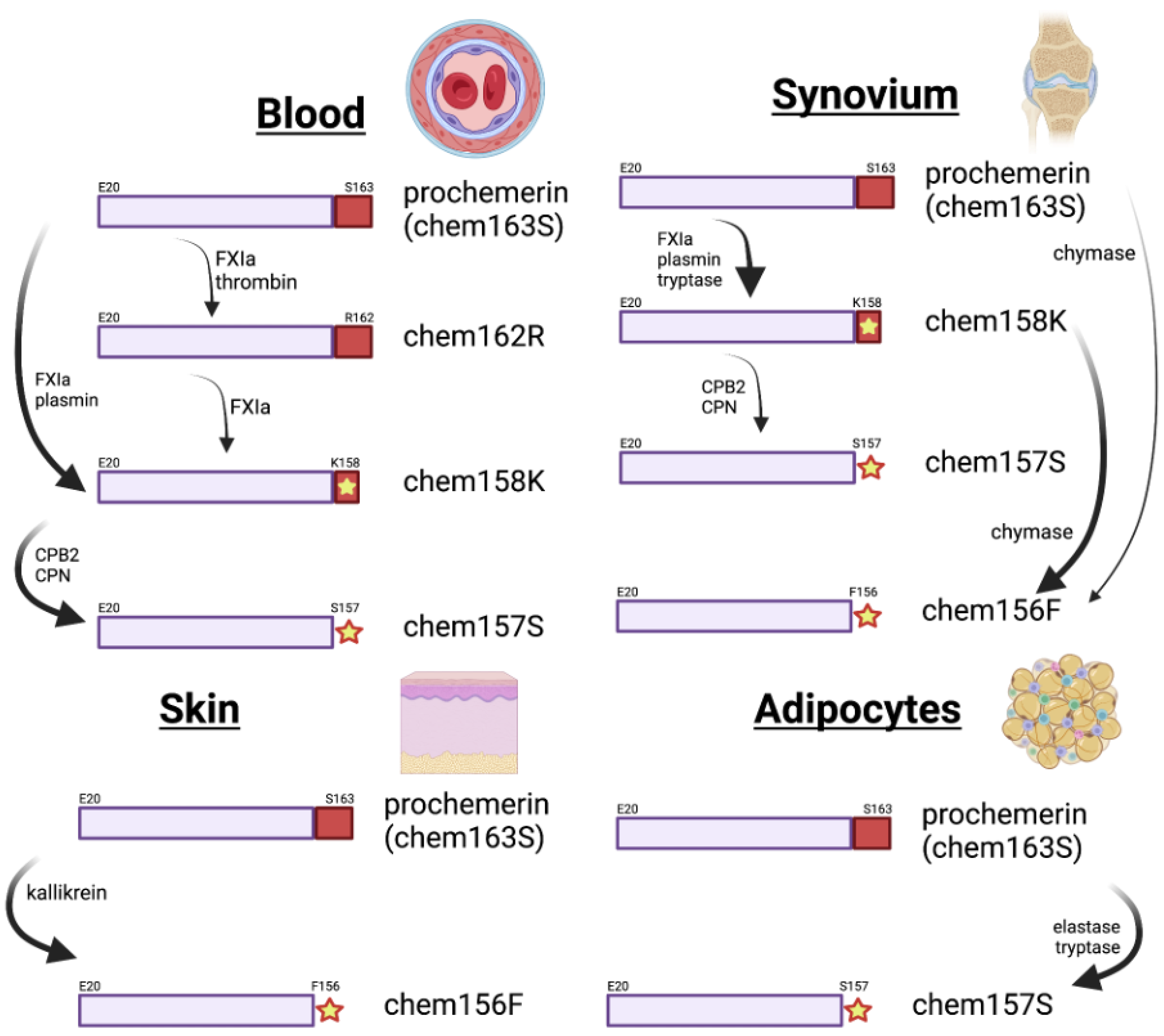
6. Chemerin Expression and Activity in Cells and Tissues
7. Proteolytic Enzymes in Chemerin Processing
8. Methods for Determining Levels of Chemerin Forms
9. Chemerin Forms and Disease
9.1. Cardiovascular Disease
9.2. Chemerin in Obesity and Diabetes
10. Discussion and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagpal, S.; Patel, S.; Jacobe, H.; DiSepio, D.; Ghosn, C.; Malhotra, M.; Teng, M.; Duvic, M.; Chandraratna, R.A. Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J. Investig. Dermatol. 1997, 109, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Wittamer, V.; Franssen, J.D.; Vulcano, M.; Mirjolet, J.F.; Le Poul, E.; Migeotte, I.; Brezillon, S.; Tyldesley, R.; Blanpain, C.; Detheux, M.; et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003, 198, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Meder, W.; Wendland, M.; Busmann, A.; Kutzleb, C.; Spodsberg, N.; John, H.; Richter, R.; Schleuder, D.; Meyer, M.; Forssmann, W.G. Characterization of human circulating TIG2 as a ligand for the orphan receptor ChemR23. FEBS Lett. 2003, 555, 495–499. [Google Scholar] [CrossRef]
- Zabel, B.A.; Silverio, A.M.; Butcher, E.C. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J. Immunol. 2005, 174, 244–251. [Google Scholar] [CrossRef]
- Allen, S.J.; Zabel, B.A.; Kirkpatrick, J.; Butcher, E.C.; Nietlispach, D.; Handel, T.M. NMR assignment of human chemerin, a novel chemoattractant. Biomol. NMR Assign. 2007, 1, 171–173. [Google Scholar] [CrossRef]
- Zaiou, M.; Nizet, V.; Gallo, R.L. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J. Investig. Dermatol. 2003, 120, 810–816. [Google Scholar] [CrossRef]
- Zabel, B.A.; Allen, S.J.; Kulig, P.; Allen, J.A.; Cichy, J.; Handel, T.M.; Butcher, E.C. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J. Biol. Chem. 2005, 280, 34661–34666. [Google Scholar] [CrossRef]
- Bondue, B.; Wittamer, V.; Parmentier, M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011, 22, 331–338. [Google Scholar] [CrossRef]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. CMKLR1 and GPR1 Mediate Chemerin Signaling Through the RhoA/ROCK Pathway. Mol. Cell. Endocrinol. 2015, 417, 36–51. [Google Scholar] [CrossRef]
- De Henau, O.; Degroot, G.N.; Imbault, V.; Robert, V.; De Poorter, C.; McHeik, S.; Gales, C.; Parmentier, M.; Springael, J.Y. Signaling Properties of Chemerin Receptors CMKLR1, GPR1 and CCRL2. PLoS ONE 2016, 11, e0164179. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Yang, P.; Read, C.; Kuc, R.E.; Yang, L.; Taylor, E.J.; Taylor, C.W.; Maguire, J.J.; Davenport, A.P. Chemerin Elicits Potent Constrictor Actions via Chemokine-Like Receptor 1 (CMKLR1), not G-Protein-Coupled Receptor 1 (GPR1), in Human and Rat Vasculature. J. Am. Heart Assoc. 2016, 5, e004421. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.T.; Luttrell, L.M. Diversity in arrestin function. Cell. Mol. Life Sci. 2009, 66, 2953–2973. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.J.; Davenport, A.P. International Union of Basic and Clinical Pharmacology CIII: Chemerin Receptors CMKLR1 (Chemerin1) and GPR1 (Chemerin2) Nomenclature, Pharmacology, and Function. Pharmacol. Rev. 2018, 70, 174–196. [Google Scholar] [CrossRef]
- Monnier, J.; Lewen, S.; O’Hara, E.; Huang, K.; Tu, H.; Butcher, E.C.; Zabel, B.A. Expression, regulation, and function of atypical chemerin receptor CCRL2 on endothelial cells. J. Immunol. 2012, 189, 956–967. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Deyama, S.; Shimoda, K.; Suzuki, H.; Ishikawa, Y.; Ishimura, K.; Fukuda, H.; Hitora-Imamura, N.; Ide, S.; Satoh, M.; Kaneda, K.; et al. Resolvin E1/E2 ameliorate lipopolysaccharide-induced depression-like behaviors via ChemR23. Psychopharmacology 2017, 235, 329–336. [Google Scholar] [CrossRef]
- Xu, H.; Chen, J.; Ge, J.; Xia, K.; Tao, S.; Su, Y.; Zhang, Q. Resolvin E1 Ameliorates Pulpitis by Suppressing Dental Pulp Fibroblast Activation in a Chemerin Receptor 23-dependent Manner. J. Endod. 2019, 45, 1126–1134. [Google Scholar] [CrossRef]
- Arita, M.; Ohira, T.; Sun, Y.P.; Elangovan, S.; Chiang, N.; Serhan, C.N. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 2007, 178, 3912–3917. [Google Scholar] [CrossRef]
- Peng, L.; Yu, Y.; Liu, J.; Li, S.; He, H.; Cheng, N.; Ye, R.D. The chemerin receptor CMKLR1 is a functional receptor for amyloid-beta peptide. J. Alzheimer’s Dis. 2015, 43, 227–242. [Google Scholar] [CrossRef]
- Kurowska, P.; Mlyczynska, E.; Dawid, M.; Sierpowski, M.; Estienne, A.; Dupont, J.; Rak, A. Adipokines change the balance of proliferation/apoptosis in the ovarian cells of human and domestic animals: A comparative review. Anim. Reprod. Sci. 2021, 228, 106737. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, K.; Brzoza, P.; Bak, M.; Majewski, P.; Skulimowska, I.; Bednarczyk, K.; Cichy, J.; Kwitniewski, M. The methylation status of the chemerin promoter region located from −252 to +258 bp regulates constitutive but not acute-phase cytokine-inducible chemerin expression levels. Sci. Rep. 2020, 10, 13702. [Google Scholar] [CrossRef] [PubMed]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes. Rev. 2013, 14, 245–262. [Google Scholar] [CrossRef]
- Ferland, D.J.; Seitz, B.; Darios, E.S.; Thompson, J.M.; Yeh, S.T.; Mullick, A.E.; Watts, S.W. Whole-Body but Not Hepatic Knockdown of Chemerin by Antisense Oligonucleotide Decreases Blood Pressure in Rats. J. Pharm. Exp. 2018, 365, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yamaguchi, Y.; Sharif, S.; Du, X.Y.; Song, J.J.; Lee, D.M.; Recht, L.D.; Robinson, W.H.; Morser, J.; Leung, L.L. Chemerin158K protein is the dominant chemerin isoform in synovial and cerebrospinal fluids but not in plasma. J. Biol. Chem. 2011, 286, 39520–39527. [Google Scholar] [CrossRef]
- Wittamer, V.; Gregoire, F.; Robberecht, P.; Vassart, G.; Communi, D.; Parmentier, M. The C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J. Biol. Chem. 2004, 279, 9956–9962. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Du, X.Y.; Zhao, L.; Morser, J.; Leung, L.L. Proteolytic cleavage of chemerin protein is necessary for activation to the active form, Chem157S, which functions as a signaling molecule in glioblastoma. J. Biol. Chem. 2011, 286, 39510–39519. [Google Scholar] [CrossRef]
- Degroot, G.N.; Lepage, V.; Parmentier, M.; Springael, J.Y. The Atypical Chemerin Receptor GPR1 Displays Different Modes of Interaction with beta-Arrestins in Humans and Mice with Important Consequences on Subcellular Localization and Trafficking. Cells 2022, 11, 1037. [Google Scholar] [CrossRef]
- Maggio, R.; Fasciani, I.; Carli, M.; Petragnano, F.; Marampon, F.; Rossi, M.; Scarselli, M. Integration and Spatial Organization of Signaling by G Protein-Coupled Receptor Homo-and Heterodimers. Biomolecules 2021, 11, 1828. [Google Scholar] [CrossRef]
- Sleno, R.; Hebert, T.E. The Dynamics of GPCR Oligomerization and Their Functional Consequences. Int. Rev. Cell Mol. Biol. 2018, 338, 141–171. [Google Scholar]
- Li, L.; Huang, C.; Zhang, X.; Wang, J.; Ma, P.; Liu, Y.; Xiao, T.; Zabel, B.A.; Zhang, J.V. Chemerin-derived peptide C-20 suppressed gonadal steroidogenesis. Am. J. Reprod. Immunol. 2014, 71, 265–277. [Google Scholar] [CrossRef]
- Fischer, T.F.; Schoeder, C.T.; Zellmann, T.; Stichel, J.; Meiler, J.; Beck-Sickinger, A.G. Cyclic Analogues of the Chemerin C-Terminus Mimic a Loop Conformation Essential for Activating the Chemokine-like Receptor 1. J. Med. Chem. 2021, 64, 3048–3058. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.F.; Czerniak, A.S.; Weiss, T.; Zellmann, T.; Zielke, L.; Els-Heindl, S.; Beck-Sickinger, A.G. Cyclic Derivatives of the Chemerin C-Terminus as Metabolically Stable Agonists at the Chemokine-like Receptor 1 for Cancer Treatment. Cancers 2021, 13, 3788. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, S.; Niederstadt, L.; Koziolek, E.J.; Gomez, J.D.C.; Prasad, S.; Wagener, A.; von Hacht, J.L.; Reinicke, S.; Exner, S.; Bandholtz, S.; et al. CMKLR1-targeting peptide tracers for PET/MR imaging of breast cancer. Theranostics 2019, 9, 6719–6733. [Google Scholar] [CrossRef] [PubMed]
- Friebus-Kardash, J.; Schulz, P.; Reinicke, S.; Karthaus, C.; Schefer, Q.; Bandholtz, S.; Grotzinger, C. A Chemerin Peptide Analog Stimulates Tumor Growth in Two Xenograft Mouse Models of Human Colorectal Carcinoma. Cancers 2021, 14, 125. [Google Scholar] [CrossRef]
- Zhao, L.; Yamaguchi, Y.; Ge, X.; Robinson, W.H.; Morser, J.; Leung, L.L.K. Chemerin 156F, generated by chymase cleavage of prochemerin, is elevated in joint fluids of arthritis patients. Arthritis Res. Ther. 2018, 20, 132. [Google Scholar] [CrossRef]
- Schultz, S.; Saalbach, A.; Heiker, J.T.; Meier, R.; Zellmann, T.; Simon, J.C.; Beck-Sickinger, A.G. Proteolytic activation of prochemerin by kallikrein 7 breaks an ionic linkage and results in C-terminal rearrangement. Biochem. J. 2013, 452, 271–280. [Google Scholar] [CrossRef]
- Zhao, L.; Yamaguchi, Y.; Shen, W.J.; Morser, J.; Leung, L.L.K. Dynamic and tissue-specific proteolytic processing of chemerin in obese mice. PLoS ONE 2018, 13, e0202780. [Google Scholar] [CrossRef]
- Chen, S.; Han, C.; Bian, S.; Chen, J.; Feng, X.; Li, G.; Wu, X. Chemerin-9 Attenuates Experimental Abdominal Aortic Aneurysm Formation in ApoE(−/−) Mice. J. Oncol. 2021, 2021, 6629204. [Google Scholar] [CrossRef]
- Sato, K.; Yoshizawa, H.; Seki, T.; Shirai, R.; Yamashita, T.; Okano, T.; Shibata, K.; Wakamatsu, M.J.; Mori, Y.; Morita, T.; et al. Chemerin-9, a potent agonist of chemerin receptor (ChemR23), prevents atherogenesis. Clin. Sci. 2019, 133, 1779–1796. [Google Scholar] [CrossRef]
- Du, X.Y.; Zabel, B.A.; Myles, T.; Allen, S.J.; Handel, T.M.; Lee, P.P.; Butcher, E.C.; Leung, L.L. Regulation of chemerin bioactivity by plasma carboxypeptidase N, carboxypeptidase B (activated thrombin-activable fibrinolysis inhibitor), and platelets. J. Biol. Chem. 2009, 284, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Yamaguchi, Y.; Zhao, L.; Bury, L.; Gresele, P.; Berube, C.; Leung, L.L.; Morser, J. Prochemerin cleavage by factor XIa links coagulation and inflammation. Blood 2018, 131, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Cash, J.L.; Hart, R.; Russ, A.; Dixon, J.P.; Colledge, W.H.; Doran, J.; Hendrick, A.G.; Carlton, M.B.; Greaves, D.R. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J. Exp. Med. 2008, 205, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Cash, J.L.; Bena, S.; Headland, S.E.; McArthur, S.; Brancaleone, V.; Perretti, M. Chemerin15 inhibits neutrophil-mediated vascular inflammation and myocardial ischemia-reperfusion injury through ChemR23. EMBO Rep. 2013, 14, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Ji, Q.; Wu, B.; Yu, K.; Zeng, Q.; Xin, S.; Liu, J.; Zhou, Y. Chemerin15-Ameliorated Cardiac Ischemia-Reperfusion Injury Is Associated with the Induction of Alternatively Activated Macrophages. Mediat. Inflamm. 2015, 2015, 563951. [Google Scholar] [CrossRef] [PubMed]
- Bondue, B.; De Henau, O.; Luangsay, S.; Devosse, T.; de Nadai, P.; Springael, J.Y.; Parmentier, M.; Vosters, O. The chemerin/ChemR23 system does not affect the pro-inflammatory response of mouse and human macrophages ex vivo. PLoS ONE 2012, 7, e40043. [Google Scholar] [CrossRef]
- Banas, M.; Zabieglo, K.; Kasetty, G.; Kapinska-Mrowiecka, M.; Borowczyk, J.; Drukala, J.; Murzyn, K.; Zabel, B.A.; Butcher, E.C.; Schroeder, J.M.; et al. Chemerin is an antimicrobial agent in human epidermis. PLoS ONE 2013, 8, e58709. [Google Scholar]
- Stables, J.; Green, A.; Marshall, F.; Fraser, N.; Knight, E.; Sautel, M.; Milligan, G.; Lee, M.; Rees, S. A bioluminescent assay for agonist activity at potentially any G-protein-coupled receptor. Anal. Biochem. 1997, 252, 115–126. [Google Scholar] [CrossRef]
- Samson, M.; Edinger, A.L.; Stordeur, P.; Rucker, J.; Verhasselt, V.; Sharron, M.; Govaerts, C.; Mollereau, C.; Vassart, G.; Doms, R.W.; et al. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur. J. Immunol. 1998, 28, 1689–1700. [Google Scholar] [CrossRef]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef]
- Watts, S.W.; Dorrance, A.M.; Penfold, M.E.; Rourke, J.L.; Sinal, C.J.; Seitz, B.; Sullivan, T.J.; Charvat, T.T.; Thompson, J.M.; Burnett, R.; et al. Chemerin connects fat to arterial contraction. Arter. Thromb. Vasc. Biol. 2013, 33, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Miyabe, Y.; Takayasu, A.; Fukuda, S.; Miyabe, C.; Ebisawa, M.; Yokoyama, W.; Watanabe, K.; Imai, T.; Muramoto, K.; et al. Chemerin activates fibroblast-like synoviocytes in patients with rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, R158. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, W.J.; Qiu, S.; Yang, P.; Dempsey, G.; Zhao, L.; Zhou, Q.; Hao, X.; Dong, D.; Stahl, A.; et al. Chemerin regulates formation and function of brown adipose tissue: Ablation results in increased insulin resistance with high fat challenge and aging. FASEB J. 2021, 35, e21687. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Han, L.; Yu, M.; Han, S.; Wang, M.; Huang, Y.; Guo, W.; Wei, Q.; Shang, W.; Min, W. Development of metabolic dysfunction in mice lacking chemerin. Mol. Cell. Endocrinol. 2021, 535, 111369. [Google Scholar] [CrossRef]
- Ferland, D.J.; Flood, E.D.; Garver, H.; Yeh, S.T.; Riney, S.; Mullick, A.E.; Fink, G.D.; Watts, S.W. Different blood pressure responses in hypertensive rats following chemerin mRNA inhibition in dietary high fat compared to dietary high salt conditions. Physiol. Genom. 2019, 51, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Banas, M.; Zegar, A.; Kwitniewski, M.; Zabieglo, K.; Marczynska, J.; Kapinska-Mrowiecka, M.; LaJevic, M.; Zabel, B.A.; Cichy, J. The expression and regulation of chemerin in the epidermis. PLoS ONE 2015, 10, e0117830. [Google Scholar] [CrossRef]
- Wang, Y.; Huo, J.; Zhang, D.; Hu, G.; Zhang, Y. Chemerin/ChemR23 axis triggers an inflammatory response in keratinocytes through ROS-sirt1-NF-kappaB signaling. J. Cell. Biochem. 2019, 120, 6459–6470. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Huang, C.; Wang, Y.F.; Ren, P.G.; Chen, L.; Xiao, T.X.; Wang, B.B.; Pan, Y.F.; Tsang, B.K.; Zabel, B.A.; et al. CMKLR1 deficiency maintains ovarian steroid production in mice treated chronically with dihydrotestosterone. Sci. Rep. 2016, 6, 21328. [Google Scholar] [CrossRef]
- Yang, Y.L.; Ren, L.R.; Sun, L.F.; Huang, C.; Xiao, T.X.; Wang, B.B.; Chen, J.; Zabel, B.A.; Ren, P.; Zhang, J.V. The role of GPR1 signaling in mice corpus luteum. J. Endocrinol. 2016, 230, 55–65. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Toulany, J.; Parlee, S.D.; Sinal, C.J.; Slayter, K.; McNeil, S.; Goralski, K.B. CMKLR1 activation ex vivo does not increase proportionally to serum total chemerin in obese humans. Endocr. Connect. 2016, 5, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Haberl, E.M.; Pohl, R.; Rein-Fischboeck, L.; Feder, S.; Eisinger, K.; Krautbauer, S.; Sinal, C.J.; Buechler, C. Ex vivo analysis of serum chemerin activity in murine models of obesity. Cytokine 2018, 104, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.; Shen, Z.; Li, X.; Jiang, W.; Peng, L.; Yuan, H.; Yang, K.; Wang, J. Effects of chemerin/CMKLR1 in obesity-induced hypertension and potential mechanism. Am. J. Transl. Res. 2017, 9, 3096–3104. [Google Scholar] [PubMed]
- Helfer, G.; Ross, A.W.; Thomson, L.M.; Mayer, C.D.; Stoney, P.N.; McCaffery, P.J.; Morgan, P.J. A neuroendocrine role for chemerin in hypothalamic remodelling and photoperiodic control of energy balance. Sci. Rep. 2016, 6, 26830. [Google Scholar] [CrossRef]
- Yun, H.; Dumbell, R.; Hanna, K.; Bowen, J.; McLean, S.L.; Kantamneni, S.; Pors, K.; Wu, Q.F.; Helfer, G. The Chemerin-CMKLR1 Axis is Functionally important for Central Regulation of Energy Homeostasis. Front. Physiol. 2022, 13, 897105. [Google Scholar] [CrossRef]
- Ernst, M.C.; Sinal, C.J. Chemerin: At the crossroads of inflammation and obesity. Trends Endocrinol. Metab. TEM 2010, 21, 660–667. [Google Scholar] [CrossRef]
- Roman, A.A.; Parlee, S.D.; Sinal, C.J. Chemerin: A potential endocrine link between obesity and type 2 diabetes. Endocrine 2012, 42, 243–251. [Google Scholar] [CrossRef]
- John, H.; Hierer, J.; Haas, O.; Forssmann, W.G. Quantification of angiotensin-converting-enzyme-mediated degradation of human chemerin 145-154 in plasma by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. Anal. Biochem. 2007, 362, 117–125. [Google Scholar] [CrossRef]
- Buechler, C.; Feder, S.; Haberl, E.M.; Aslanidis, C. Chemerin Isoforms and Activity in Obesity. Int. J. Mol. Sci. 2019, 20, 1128. [Google Scholar] [CrossRef]
- Doolittle, R.F. The structure and evolution of vertebrate fibrinogen: A comparison of the lamprey and mammalian proteins. Adv. Exp. Med. Biol. 1990, 281, 25–37. [Google Scholar]
- Doolittle, R.F. The structure and evolution of vertebrate fibrinogen. Ann. N. Y. Acad. Sci. 1983, 408, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Morser, J. Thrombomodulin links coagulation to inflammation and immunity. Curr. Drug Targets 2012, 13, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.L.K.; Morser, J. Carboxypeptidase B2 and carboxypeptidase N in the crosstalk between coagulation, thrombosis, inflammation, and innate immunity. J. Thromb. Haemost. 2018, 16, 1474–1486. [Google Scholar] [CrossRef]
- Bouma, B.N.; Mosnier, L.O.; Meijers, J.C.; Griffin, J.H. Factor XI dependent and independent activation of thrombin activatable fibrinolysis inhibitor (TAFI) in plasma associated with clot formation. Thromb. Haemost. 1999, 82, 1703–1708. [Google Scholar] [PubMed]
- Dranse, H.J.; Muruganandan, S.; Fawcett, J.P.; Sinal, C.J. Adipocyte-secreted chemerin is processed to a variety of isoforms and influences MMP3 and chemokine secretion through an NFkB-dependent mechanism. Mol. Cell. Endocrinol. 2016, 436, 114–129. [Google Scholar] [CrossRef]
- Parlee, S.D.; McNeil, J.O.; Muruganandan, S.; Sinal, C.J.; Goralski, K.B. Elastase and tryptase govern TNFalpha-mediated production of active chemerin by adipocytes. PLoS ONE 2012, 7, e51072. [Google Scholar] [CrossRef]
- Kulig, P.; Kantyka, T.; Zabel, B.A.; Banas, M.; Chyra, A.; Stefanska, A.; Tu, H.; Allen, S.J.; Handel, T.M.; Kozik, A.; et al. Regulation of chemerin chemoattractant and antibacterial activity by human cysteine cathepsins. J. Immunol. 2011, 187, 1403–1410. [Google Scholar] [CrossRef]
- Guillabert, A.; Wittamer, V.; Bondue, B.; Godot, V.; Imbault, V.; Parmentier, M.; Communi, D. Role of neutrophil proteinase 3 and mast cell chymase in chemerin proteolytic regulation. J. Leukoc. Biol. 2008, 84, 1530–1538. [Google Scholar] [CrossRef]
- Chang, S.S.; Eisenberg, D.; Zhao, L.; Adams, C.; Leib, R.; Morser, J.; Leung, L. Chemerin activation in human obesity. Obesity 2016, 24, 1522–1529. [Google Scholar] [CrossRef]
- Huang, H.; Tong, T.T.; Yau, L.F.; Wang, J.R.; Lai, M.H.; Zhang, C.R.; Wen, X.H.; Li, S.N.; Li, K.Y.; Liu, J.Q.; et al. Chemerin isoform analysis in human biofluids using an LC/MRM-MS-based targeted proteomics approach with stable isotope-labeled standard. Anal. Chim. Acta 2020, 1139, 79–87. [Google Scholar] [CrossRef]
- Fischer, T.F.; Beck-Sickinger, A.G. Chemerin—Exploring a versatile adipokine. Biol. Chem. 2022, 403, 625–642. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Cheng, Y.; Zhang, G.; Wang, B. Chemerin in inflammatory diseases. Clin. Chim. Acta 2021, 517, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Fan, J.; Zhu, J.; Ling, Y.; Mi, S.; Chen, H.; Fan, C.; Li, Y. Circulating chemerin level and risk of cancer: A systematic review and meta-analysis. Biomark. Med. 2020, 14, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Mattu, H.S.; Randeva, H.S. Chemerin in human cardiovascular disease. Vasc. Pharmacol. 2018, 110, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mussig, K.; Staiger, H.; Machicao, F.; Thamer, C.; Machann, J.; Schick, F.; Claussen, C.D.; Stefan, N.; Fritsche, A.; Haring, H.U. RARRES2, encoding the novel adipokine chemerin, is a genetic determinant of disproportionate regional body fat distribution: A comparative magnetic resonance imaging study. Metabolism 2009, 58, 519–524. [Google Scholar] [CrossRef]
- Hasanvand, Z.; Sadeghi, A.; Rezvanfar, M.R.; Goodarzi, M.T.; Rahmannezhad, G.; Mashayekhi, F.J. Association between chemerin rs17173608 and rs4721 gene polymorphisms and gestational diabetes mellitus in Iranian pregnant women. Gene 2018, 649, 87–92. [Google Scholar] [CrossRef]
- Tonjes, A.; Scholz, M.; Breitfeld, J.; Marzi, C.; Grallert, H.; Gross, A.; Ladenvall, C.; Schleinitz, D.; Krause, K.; Kirsten, H.; et al. Genome wide meta-analysis highlights the role of genetic variation in RARRES2 in the regulation of circulating serum chemerin. PLoS Genet. 2014, 10, e1004854. [Google Scholar] [CrossRef]
- Huang, H.W.; Liang, B.Y.; Li, Y.X. Association of Polymorphisms in STRA6 and RARRES2 Genes with Type 2 Diabetes in Southern Han Chinese. BioMed Res. Int. 2016, 2016, 6589793. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Moncayo, S.; Insenser, M.; Alvarez-Blasco, F.; Luque-Ramirez, M.; Escobar-Morreale, H.F. Metabolic Cytokines at Fasting and During Macronutrient Challenges: Influence of Obesity, Female Androgen Excess and Sex. Nutrients 2019, 11, 2566. [Google Scholar] [CrossRef]
- Parlee, S.D.; Ernst, M.C.; Muruganandan, S.; Sinal, C.J.; Goralski, K.B. Serum chemerin levels vary with time of day and are modified by obesity and tumor necrosis factor-{alpha}. Endocrinology 2010, 151, 2590–2602. [Google Scholar] [CrossRef]
- Zylla, S.; Pietzner, M.; Kuhn, J.P.; Volzke, H.; Dorr, M.; Nauck, M.; Friedrich, N. Serum chemerin is associated with inflammatory and metabolic parameters-results of a population-based study. Obesity 2017, 25, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.H.; Teng, M.S.; Hsu, L.A.; Er, L.K.; Wu, S.; Ko, Y.L. Circulating chemerin level is associated with metabolic, biochemical, and hematological parameters—A population-based study. Clin. Endocrinol. 2021, 94, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Inomata, S.; Okimura, Y.; Iguchi, G.; Fukuoka, H.; Miyake, K.; Koga, D.; Akamatsu, S.; Kasuga, M.; Takahashi, Y. Decreased serum chemerin levels in male Japanese patients with type 2 diabetes: Sex dimorphism. Endocr. J. 2013, 60, 37–44. [Google Scholar] [CrossRef]
- Ferland, D.J.; Mullick, A.E.; Watts, S.W. Chemerin as a driver of hypertension: A consideration. Am. J. Hypertens. 2020, 33, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Kunimoto, H.; Kazama, K.; Takai, M.; Oda, M.; Okada, M.; Yamawaki, H. Chemerin promotes the proliferation and migration of vascular smooth muscle and increases mouse blood pressure. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1017–H1028. [Google Scholar] [CrossRef]
- Eichelmann, F.; Schulze, M.B.; Wittenbecher, C.; Menzel, J.; Weikert, C.; di Giuseppe, R.; Biemann, R.; Isermann, B.; Fritsche, A.; Boeing, H.; et al. Chemerin as a Biomarker Linking Inflammation and Cardiovascular Diseases. J. Am. Coll. Cardiol. 2019, 73, 378–379. [Google Scholar] [CrossRef]
- Ismaiel, A.; Ashfaq, M.Z.; Leucuta, D.C.; Ismaiel, M.; Ensar Ismaiel, D.; Popa, S.L.; Dumitrascu, D.L. Chemerin Levels in Acute Coronary Syndrome: Systematic Review and Meta-Analysis. Lab. Med. 2022. [Google Scholar] [CrossRef]
- Zhou, X.; Tao, Y.; Chen, Y.; Xu, W.; Qian, Z.; Lu, X. Serum Chemerin as a Novel Prognostic Indicator in Chronic Heart Failure. J. Am. Heart Assoc. 2019, 8, e012091. [Google Scholar] [CrossRef]
- Takahashi, M.; Okimura, Y.; Iguchi, G.; Nishizawa, H.; Yamamoto, M.; Suda, K.; Kitazawa, R.; Fujimoto, W.; Takahashi, K.; Zolotaryov, F.N.; et al. Chemerin regulates beta-cell function in mice. Sci. Rep. 2011, 1, 123. [Google Scholar] [CrossRef]
- Reinehr, T.; Roth, C.L. Inflammation Markers in Type 2 Diabetes and the Metabolic Syndrome in the Pediatric Population. Curr. Diabetes Rep. 2018, 18, 131. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, H.; Ju, H.; Sun, M. Circulating chemerin levels and gestational diabetes mellitus: A systematic review and meta-analysis. Lipids Health Dis. 2018, 17, 169. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, L. Role of Chemerin/ChemR23 axis as an emerging therapeutic perspective on obesity-related vascular dysfunction. J. Transl. Med. 2022, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Askarpour, M.; Alizadeh, S.; Hadi, A.; Symonds, M.E.; Miraghajani, M.; Sheikhi, A.; Ghaedi, E. Effect of Bariatric Surgery on the Circulating Level of Adiponectin, Chemerin, Plasminogen Activator Inhibitor-1, Leptin, Resistin, and Visfatin: A Systematic Review and Meta-Analysis. Horm. Metab. Res. Horm. Stoffwechs. Horm. Metab. 2020, 52, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Jouan, Y.; Blasco, H.; Bongrani, A.; Couet, C.; Dupont, J.; Maillot, F. Preoperative Chemerin Level Is Predictive of Inflammatory Status 1 Year After Bariatric Surgery. Obes. Surg. 2020, 30, 3852–3861. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Auguet, T.; Guiu-Jurado, E.; Berlanga, A.; Orellana-Gavalda, J.M.; Hernandez, M.; Sabench, F.; Porras, J.A.; Llutart, J.; Martinez, S.; et al. Long-term changes in leptin, chemerin and ghrelin levels following different bariatric surgery procedures: Roux-en-Y gastric bypass and sleeve gastrectomy. Obes. Surg. 2013, 23, 1790–1798. [Google Scholar] [CrossRef]
- Parlee, S.D.; Wang, Y.; Poirier, P.; Lapointe, M.; Martin, J.; Bastien, M.; Cianflone, K.; Goralski, K.B. Biliopancreatic diversion with duodenal switch modifies plasma chemerin in early and late post-operative periods. Obesity 2015, 23, 1201–1208. [Google Scholar] [CrossRef]
- Sell, H.; Divoux, A.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Bedossa, P.; Tordjman, J.; Eckel, J.; Clement, K. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J. Clin. Endocrinol. Metab. 2010, 95, 2892–2896. [Google Scholar] [CrossRef]
- Helfer, G.; Wu, Q.F. Chemerin: A multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef]
- Ernst, M.C.; Issa, M.; Goralski, K.B.; Sinal, C.J. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology 2010, 151, 1998–2007. [Google Scholar] [CrossRef]
- Ferland, D.J.; Garver, H.; Contreras, G.A.; Fink, G.D.; Watts, S.W. Chemerin contributes to in vivo adipogenesis in a location-specific manner. PLoS ONE 2020, 15, e0229251. [Google Scholar] [CrossRef]
- Leniz, A.; Gonzalez, M.; Besne, I.; Carr-Ugarte, H.; Gomez-Garcia, I.; Portillo, M.P. Role of chemerin in the control of glucose homeostasis. Mol. Cell. Endocrinol. 2021, 541, 111504. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Leung, L.L.; Morser, J. Chemerin Forms: Their Generation and Activity. Biomedicines 2022, 10, 2018. https://doi.org/10.3390/biomedicines10082018
Zhao L, Leung LL, Morser J. Chemerin Forms: Their Generation and Activity. Biomedicines. 2022; 10(8):2018. https://doi.org/10.3390/biomedicines10082018
Chicago/Turabian StyleZhao, Lei, Lawrence L. Leung, and John Morser. 2022. "Chemerin Forms: Their Generation and Activity" Biomedicines 10, no. 8: 2018. https://doi.org/10.3390/biomedicines10082018
APA StyleZhao, L., Leung, L. L., & Morser, J. (2022). Chemerin Forms: Their Generation and Activity. Biomedicines, 10(8), 2018. https://doi.org/10.3390/biomedicines10082018





