Role of microRNAs in B-Cell Compartment: Development, Proliferation and Hematological Diseases
Abstract
1. Introduction
2. microRNAs: Biogenesis, Activity and Function
3. B-Cells: Populations, Location and Functions
4. B-2 Cell Development
5. B-1 Cell Development
6. microRNAs in the Early B-Cell Development
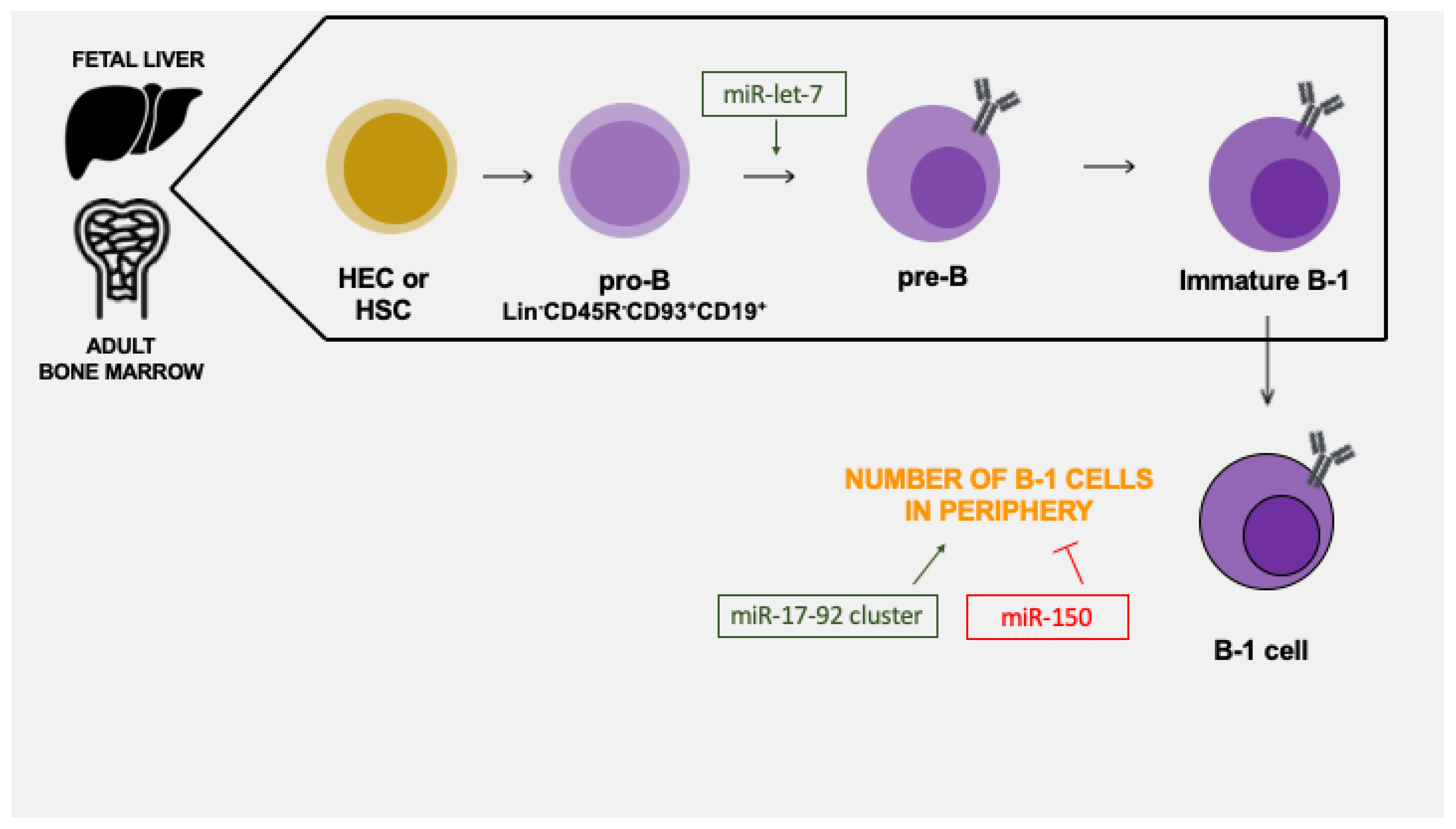
7. microRNAs in the Early B-1 Cell Development
8. miRNAs Regulate B-1 Cell Numbers in the Periphery
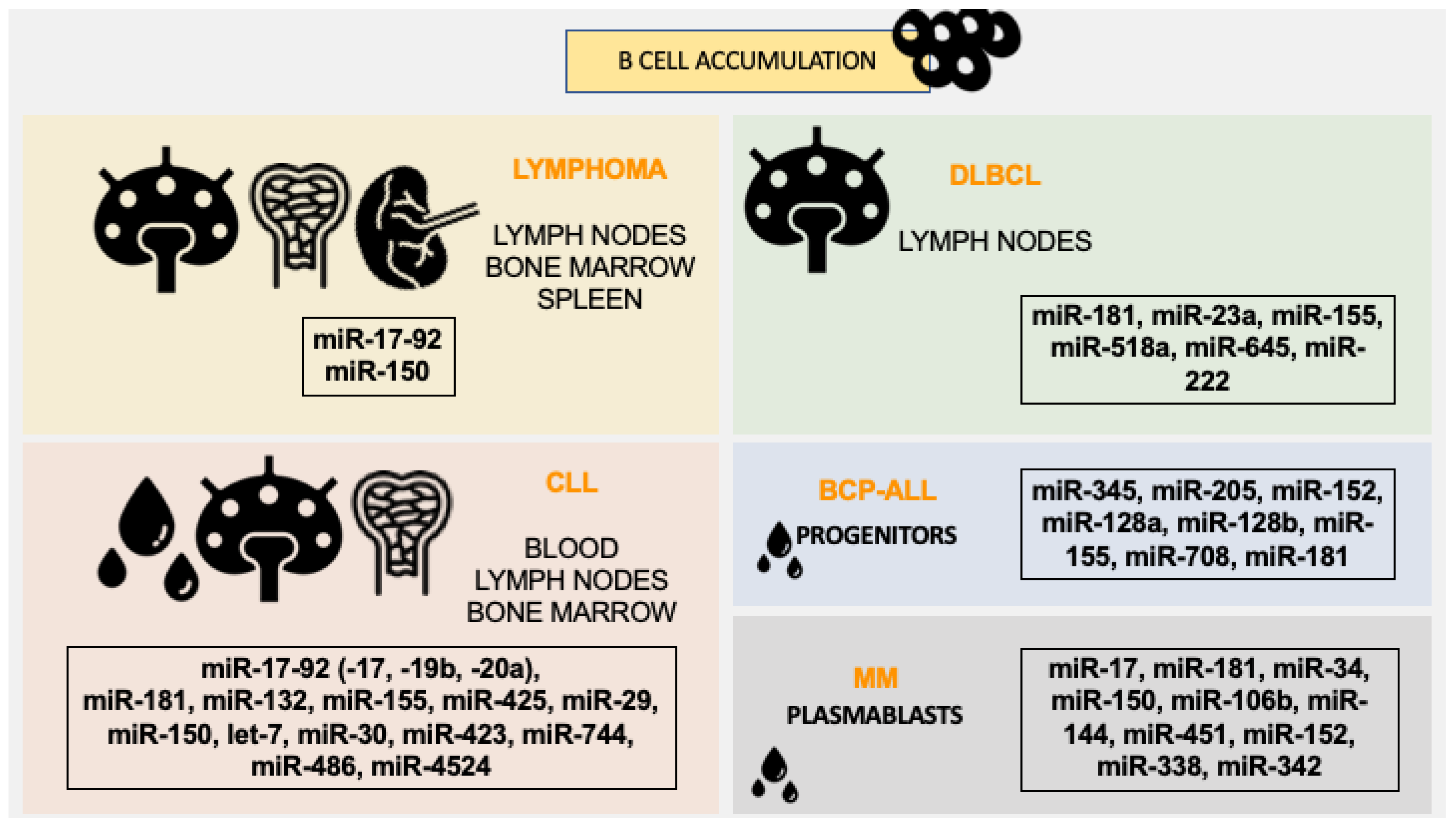
9. miRNAs Involvement in Regulating Periphery B-Cells
10. miRNAs Derived from Exosomes
11. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Selbach, M.; Schwanhäusser, B.; Thierfelder, N.; Fang, Z.; Khanin, R.; Rajewsky, N. Widespread changes in protein synthesis induced by microRNAs. Nature 2008, 455, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Yu, D.; Weiss, M.J. MicroRNAs in erythropoiesis. Curr. Opin. Hematol. 2010, 17, 155–162. [Google Scholar] [CrossRef]
- Koralov, S.B.; Muljo, S.A.; Galler, G.R.; Krek, A.; Chakraborty, T.; Kanellopoulou, C.; Jensen, K.; Cobb, B.S.; Merkenschlager, M.; Rajewsky, N. Dicer Ablation Affects Antibody Diversity and Cell Survival in the B Lymphocyte Lineage. Cell 2008, 132, 860–874. [Google Scholar] [CrossRef]
- Monticelli, S.; Ansel, K.M.; Xiao, C.; Socci, N.D.; Krichevsky, A.M.; Thai, T.-H.; Rajewsky, N.; Marks, D.S.; Sander, C.; Rajewsky, K.; et al. MicroRNA profiling of the murine hematopoietic system. Genome Biol. 2005, 6, R71. [Google Scholar] [CrossRef]
- Baskerville, S.; Bartel, D.P. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA 2005, 11, 241–247. [Google Scholar] [CrossRef]
- Kuchen, S.; Resch, W.; Yamane, A.; Kuo, N.; Li, Z.; Chakraborty, T.; Wei, L.; Laurence, A.; Yasuda, T.; Peng, S.; et al. Regulation of MicroRNA expression and abundance during lymphopoiesis. Immunity 2010, 32, 828–839. [Google Scholar] [CrossRef]
- Mehta, A.; Baltimore, D. MicroRNAs as regulatory elements in immune system logic. Nat. Rev. Immunol. 2016, 16, 279–294. [Google Scholar] [CrossRef]
- Winter, J.; Jung, S.; Keller, S.; Gregory, R.I.; Diederichs, S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 2009, 11, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hagedorn, C.H.; Cullen, B.R. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 2004, 10, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Rådmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003, 425, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Qin, Y.; Macara, I.G.; Cullen, B.R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003, 17, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Kok, K.H.; Ng, M.-H.J.; Ching, Y.-P.; Jin, D.-Y. Human TRBP and PACT Directly Interact with Each Other and Associate with Dicer to Facilitate the Production of Small Interfering RNA. J. Biol. Chem. 2007, 282, 17649–17657. [Google Scholar] [CrossRef]
- Bhayani, M.K.; Calin, G.A.; Lai, S.Y. Functional relevance of miRNA* sequences in human disease. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2012, 731, 14–19. [Google Scholar] [CrossRef]
- Meister, G.; Landthaler, M.; Patkaniowska, A.; Dorsett, Y.; Teng, G.; Tuschl, T. Human Argonaute2 Mediates RNA Cleavage Targeted by miRNAs and siRNAs. Mol. Cell 2004, 15, 185–197. [Google Scholar] [CrossRef]
- Jakymiw, A.; Lian, S.; Eystathioy, T.; Li, S.; Satoh, M.; Hamel, J.C.; Fritzler, M.J.; Chan, E. Disruption of GW bodies impairs mammalian RNA interference. Nat. Cell Biol. 2005, 7, 1267–1274. [Google Scholar] [CrossRef]
- Liu, J.; Rivas, F.V.; Wohlschlegel, J.; Yates, J.R.; Parker, R.; Hannon, G.J. A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 2005, 7, 1261–1266. [Google Scholar] [CrossRef]
- Meister, G.; Landthaler, M.; Peters, L.; Chen, P.Y.; Urlaub, H.; Lührmann, R.; Tuschl, T. Identification of Novel Argonaute-Associated Proteins. Curr. Biol. 2005, 15, 2149–2155. [Google Scholar] [CrossRef]
- Sibley, C.; Seow, Y.; Saayman, S.; Dijkstra, K.; El Andaloussi, S.; Weinberg, M.; Wood, M.J.A. The biogenesis and characterization of mammalian microRNAs of mirtron origin. Nucleic Acids Res. 2011, 40, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; You, X.; Chen, T.; Mackowiak, S.D.; Friedländer, M.R.; Weigt, M.; Du, H.; Gogol-Döring, A.; Chang, Z.; Dieterich, C.; et al. Global profiling of miRNAs and the hairpin precursors: Insights into miRNA processing and novel miRNA discovery. Nucleic Acids Res. 2013, 41, 3619–3634. [Google Scholar] [CrossRef] [PubMed]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Baumgarth, N. B-Cell Immunophenotyping. Methods Cell Biol. 2004, 75, 643–662. [Google Scholar]
- Smith, F.L.; Baumgarth, N. B-1 cell responses to infections. Curr. Opin. Immunol. 2019, 57, 23–31. [Google Scholar] [CrossRef]
- Hayakawa, K.; Hardy, R.R.; Parks, D.R.; Herzenberg, L.A. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J. Exp. Med. 1983, 157, 202–218. Available online: https://www.ncbi.nlm.nih.gov/pubmed/6600267 (accessed on 28 July 2022). [CrossRef]
- Herzenberg, L.A.; Stall, A.M.; Lalor, P.A.; Sidman, C.; Moore, W.A.; Parks, D.R.; Herzenberg, L.A. The Ly-1 B cell lineage. Immunol. Rev. 1986, 93, 81–102. Available online: https://www.ncbi.nlm.nih.gov/pubmed/3096879 (accessed on 28 July 2022). [CrossRef]
- Tung, J.W.; Mrazek, M.D.; Yang, Y.; Herzenberg, L.A.; Herzenberg, L.A.; Herzenberg, L.A. Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse. Proc. Natl. Acad. Sci. USA 2006, 103, 6293–6298. [Google Scholar] [CrossRef]
- Griffin, D.O.; Holodick, N.E.; Rothstein, T.L. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+CD27+CD43+CD70-. J. Exp. Med. 2011, 208, 67–80. [Google Scholar] [CrossRef]
- Wang, C.Y.; Good, R.A.; Ammirati, P.; Dymbort, G.; Evans, R.L. Identification of a p69,71 complex expressed on human T cells sharing determinants with B-type chronic lymphatic leukemic cells. J. Exp. Med. 1980, 151, 1539–1544. [Google Scholar] [CrossRef]
- Lanier, L.L.; Warner, N.L.; Ledbetter, J.A.; Herzenberg, L.A. Expression of Lyt-1 antigen on certain murine B cell lymphomas. J. Exp. Med. 1981, 153, 998–1003. [Google Scholar] [CrossRef]
- Griffin, D.; Rothstein, T.L. A small CD11b+ human B1 cell subpopulation stimulates T cells and is expanded in lupus. J. Exp. Med. 2011, 208, 2591–2598. [Google Scholar] [CrossRef] [PubMed]
- Holodick, N.E.; Rodríguez-Zhurbenko, N.; Hernández, A.M. Defining Natural Antibodies. Front. Immunol. 2017, 8, 872. [Google Scholar] [CrossRef]
- Li, Y.-S.; Zhou, Y.; Tang, L.; Shinton, S.A.; Hayakawa, K.; Hardy, R.R. A developmental switch between fetal and adult B lymphopoiesis. Ann. N. Y. Acad. Sci. 2015, 1362, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T. Microenvironmental niches in the bone marrow required for B-cell development. Nat. Rev. Immunol. 2006, 6, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Bain, G.; Murre, C. The role of E-proteins in B- and T-lymphocyte development. Semin. Immunol. 1998, 10, 143–153. [Google Scholar] [CrossRef]
- Georgopoulos, K. The making of a lymphocyte: The choice among disparate cell fates and the IKAROS enigma. Genes Dev. 2017, 31, 439–450. [Google Scholar] [CrossRef]
- Singh, H.; Pongubala, J.M.R.; Medina, K.L. Gene Regulatory Networks that Orchestrate the Development of B Lymphocyte Precursors. Mech. Lymph. Act. Immune Regul. XI 2007, 596, 57–62. [Google Scholar] [CrossRef]
- Mercer, E.M.; Lin, Y.C.; Benner, C.; Jhunjhunwala, S.; Dutkowski, J.; Flores, M.; Sigvardsson, M.; Ideker, T.; Glass, C.K.; Murre, C. Multilineage Priming of Enhancer Repertoires Precedes Commitment to the B and Myeloid Cell Lineages in Hematopoietic Progenitors. Immunity 2011, 35, 413–425. [Google Scholar] [CrossRef]
- Medvedovic, J.; Ebert, A.; Tagoh, H.; Busslinger, M. Pax5: A Master Regulator of B Cell Development and Leukemogenesis. Adv. Immunol. 2011, 111, 179–206. [Google Scholar]
- Zandi, S.; Mansson, R.; Tsapogas, P.; Zetterblad, J.; Bryder, D.; Sigvardsson, M. EBF1 Is Essential for B-Lineage Priming and Establishment of a Transcription Factor Network in Common Lymphoid Progenitors. J. Immunol. 2008, 181, 3364–3372. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Y. B Cells in Immunity and Tolerance; Springer: Singapore, 2020; Volume 1254, ISBN 978-981-15-3531-4. [Google Scholar] [CrossRef]
- Pieper, K.; Grimbacher, B.; Eibel, H. B-cell biology and development. J. Allergy Clin. Immunol. 2013, 131, 959–971. [Google Scholar] [CrossRef]
- Pillai, S.; Cariappa, A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. Immunol. 2009, 9, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, A.; Cols, M.; Puga, I. Marginal zone B cells: Virtues of innate-like antibody-producing lymphocytes. Nat. Rev. Immunol. 2013, 13, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Okada, T.; Cyster, J.G. Germinal-Center Organization and Cellular Dynamics. Immunity 2007, 27, 190–202. [Google Scholar] [CrossRef]
- Kantor, A.B.; Herzenberg, L.A. Origin of murine B cell lineages. Annu. Rev. Immunol. 1993, 11, 501–538. Available online: https://www.ncbi.nlm.nih.gov/pubmed/8476571 (accessed on 28 July 2022). [CrossRef]
- Montecino-Rodriguez, E.; Dorshkind, K. B-1 B Cell Development in the Fetus and Adult. Immunity 2012, 36, 13–21. [Google Scholar] [CrossRef]
- Hayakawa, K.; Hardy, R.R.; Herzenberg, L.A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J. Exp. Med. 1985, 161, 1554–1568. [Google Scholar] [CrossRef]
- Herzenberg, L.A. Toward a layered immune system. Cell 1989, 59, 953–954. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Montecino-Rodriguez, E.; Ferkowicz, M.J.; Porayette, P.; Shelley, W.C.; Conway, S.J.; Dorshkind, K.; Yoder, M.C. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc. Natl. Acad. Sci. USA 2011, 108, 1468–1473. [Google Scholar] [CrossRef]
- Ghosn, E.E.B.; Waters, J.; Phillips, M.; Yamamoto, R.; Long, B.R.; Yang, Y.; Gerstein, R.; Stoddart, C.A.; Nakauchi, H.; Herzenberg, L.A. Fetal Hematopoietic Stem Cell Transplantation Fails to Fully Regenerate the B-Lymphocyte Compartment. Stem Cell Rep. 2015, 6, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Shelley, W.C.; Seo, W.; Vemula, S.; Lin, Y.; Liu, Y.; Kapur, R.; Taniuchi, I.; Yoshimoto, M. Functional B-1 progenitor cells are present in the hematopoietic stem cell-deficient embryo and depend on Cbfβ for their development. Proc. Natl. Acad. Sci. USA 2014, 111, 12151–12156. [Google Scholar] [CrossRef]
- Rodriguez, E.M.; Fice, M.; Casero, D.; Berent-Maoz, B.; Barber, C.L.; Dorshkind, K. Distinct Genetic Networks Orchestrate the Emergence of Specific Waves of Fetal and Adult B-1 and B-2 Development. Immunity 2016, 45, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Godin, I.E.; Garcia-Porrero, J.A.; Coutinho, A.; Dieterlen-Lièvre, F.; Marcos, M.A.R. Fran Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature 1993, 364, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.R.; Hayakawa, K. Perspectives on fetal derived CD5+ B1 B cells. Eur. J. Immunol. 2015, 45, 2978–2984. [Google Scholar] [CrossRef]
- Pelayo, R.; Welner, R.S.; Nagai, Y.; Kincade, P.W. Life before the pre-B cell receptor checkpoint: Specification and commitment of primitive lymphoid progenitors in adult bone marrow. Semin. Immunol. 2006, 18, 2–11. [Google Scholar] [CrossRef]
- Beaudin, A.E.; Boyer, S.W.; Perez-Cunningham, J.; Hernandez, G.E.; Derderian, S.C.; Jujjavarapu, C.; Aaserude, E.; MacKenzie, T.; Forsberg, E.C. A Transient Developmental Hematopoietic Stem Cell Gives Rise to Innate-like B and T Cells. Cell Stem Cell 2016, 19, 768–783. [Google Scholar] [CrossRef]
- Li, Y.-S.; Wasserman, R.; Hayakawa, K.; Hardy, R.R. Identification of the Earliest B Lineage Stage in Mouse Bone Marrow. Immunity 1996, 5, 527–535. [Google Scholar] [CrossRef]
- Rodriguez, E.M.; Leathers, H.; Dorshkind, K. Identification of a B-1 B cell–specified progenitor. Nat. Immunol. 2006, 7, 293–301. [Google Scholar] [CrossRef]
- Popi, A.F. B-1 phagocytes: The myeloid face of B-1 cells. Ann. N. Y. Acad. Sci. 2015, 1362, 86–97. [Google Scholar] [CrossRef]
- Hayakawa, K.; Asano, M.; Shinton, S.A.; Gui, M.; Allman, D.; Stewart, C.L.; Silver, J.; Hardy, R.R. Positive Selection of Natural Autoreactive B Cells. Science 1999, 285, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Melchers, F.; ten Boekel, E.; Seidl, T.; Kong, X.C.; Yamagami, T.; Onishi, K.; Shimizu, T.; Rolink, A.G.; Andersson, J. Repertoire selection by pre-B-cell receptors and B-cell receptors, and genetic control of B-cell development from immature to mature B cells. Immunol. Rev. 2000, 175, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.; Young, A.G.; Winslow, M.M.; Lintault, L.; Meissner, A.; Erkeland, S.J.; Newman, J.; Bronson, R.T.; Crowley, D.; Stone, J.R.; et al. Targeted Deletion Reveals Essential and Overlapping Functions of the miR-17~92 Family of miRNA Clusters. Cell 2008, 132, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Gonzalez-Martin, A.; Cooper, A.B.; Oda, H.; Jin, H.Y.; Shepherd, J.; He, L.; Zhu, J.; Nemazee, D.; Xiao, C. Regulation of B-cell development and tolerance by different members of the miR-17~1/492 family microRNAs. Nat. Commun. 2016, 7, 12207. [Google Scholar] [CrossRef]
- Tanzer, A.; Stadler, P.F. Molecular evolution of a microRNA cluster. J. Mol. Biol. 2004, 339, 327–335. [Google Scholar] [CrossRef]
- Fuziwara, C.S.; Kimura, E.T. Insights into Regulation of the miR-17-92 Cluster of miRNAs in Cancer. Front. Med. 2015, 2, 64. [Google Scholar] [CrossRef]
- Ghisi, M.; Corradin, A.; Basso, K.; Frasson, C.; Serafin, V.; Mukherjee, S.; Mussolin, L.; Ruggero, K.; Bonanno, L.; Guffanti, A.; et al. Modulation of microRNA expression in human T-cell development: Targeting of NOTCH3 by miR-150. Blood 2011, 117, 7053–7062. [Google Scholar] [CrossRef]
- Del Gaizo, M.; Sergio, I.; Lazzari, S.; Cialfi, S.; Pelullo, M.; Screpanti, I.; Felli, M.P. MicroRNAs as Modulators of the Immune Response in T-Cell Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2022, 23, 829. [Google Scholar] [CrossRef]
- Xiao, C.; Srinivasan, L.; Calado, D.; Patterson, H.C.; Zhang, B.; Wang, J.; Henderson, J.M.; Kutok, J.L.; Rajewsky, K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat. Immunol. 2008, 9, 405–414. [Google Scholar] [CrossRef]
- He, L.; Thomson, J.M.; Hemann, M.T.; Hernando-Monge, E.; Mu, D.; Goodson, S.; Powers, S.; Cordon-Cardo, C.; Lowe, S.W.; Hannon, G.J.; et al. A microRNA polycistron as a potential human oncogene. Nature 2005, 435, 828–833. [Google Scholar] [CrossRef]
- Alencar, A.J.; Malumbres, R.; Kozloski, G.A.; Advani, R.; Talreja, N.; Chinichian, S.; Briones, J.; Natkunam, Y.; Sehn, L.H.; Gascoyne, R.D.; et al. MicroRNAs Are Independent Predictors of Outcome in Diffuse Large B-Cell Lymphoma Patients Treated with R-CHOP. Clin. Cancer Res. 2011, 17, 4125–4135. [Google Scholar] [CrossRef] [PubMed]
- Willimott, S.; Wagner, S. Stromal cells and CD40 ligand (CD154) alter the miRNome and induce miRNA clusters including, miR-125b/miR-99a/let-7c and miR-17-92 in chronic lymphocytic leukaemia. Leukemia 2011, 26, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Moussay, E.; Wang, K.; Cho, J.-H.; van Moer, K.; Pierson, S.; Paggetti, J.; Nazarov, P.V.; Palissot, V.; Hood, L.E.; Berchem, G.; et al. MicroRNA as biomarkers and regulators in B-cell chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2011, 108, 6573–6578. [Google Scholar] [CrossRef]
- Kong, Y.; Hu, L.; Lu, K.; Wang, Y.; Xie, Y.; Gao, L.; Yang, G.; Xie, B.; He, W.; Chen, G.; et al. Ferroportin downregulation promotes cell proliferation by modulating the Nrf2–miR-17-5p axis in multiple myeloma. Cell Death Dis. 2019, 10, 624. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, J.; Zhang, H.; Liu, H.; Yao, H.; Wang, X.; Zhang, W.; Zhao, Y.; Yang, N. KLF10 inhibits cell growth by regulating PTTG1 in multiple myeloma under the regulation of microRNA-106b-5p. Int. J. Biol. Sci. 2020, 16, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Z.; Li, L.; Lodish, H.F.; Bartel, D.P. MicroRNAs Modulate Hematopoietic Lineage Differentiation. Science 2004, 303, 83–86. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Williams, A.; Goff, L.A.; Staron, M.; Licona-Limón, P.; Kaech, S.M.; Nakayama, M.; Rinn, J.L.; Flavell, R.A. The MicroRNA miR-181 Is a Critical Cellular Metabolic Rheostat Essential for NKT Cell Ontogenesis and Lymphocyte Development and Homeostasis. Immunity 2013, 38, 984–997. [Google Scholar] [CrossRef]
- Kozloski, G.A.; Jiang, X.; Bhatt, S.; Ruiz, J.; Vega, F.; Shaknovich, R.; Melnick, A.; Lossos, I.S. MiR-181a negatively regulates NF-κB signaling and affects activated B-cell-like diffuse large B-cell lymphoma pathogenesis. Blood J. Am. Soc. Hematol. 2016, 127, 2856–2866. [Google Scholar] [CrossRef]
- Zhu, D.; Fang, C.; He, W.; Wu, C.; Li, X.; Wu, J. MicroRNA-181a Inhibits Activated B-Cell-Like Diffuse Large B-Cell Lymphoma Progression by Repressing. CARD11 J. Oncol. 2019, 2019, 9832956. [Google Scholar] [CrossRef]
- Yan, X.; Gao, M.; Zhang, P.; Ouyang, G.; Mu, Q.; Xu, K. MiR-181a functions as an oncogene by regulating CCND1 in multiple myeloma. Oncol. Lett. 2020, 20, 758–764. [Google Scholar] [CrossRef]
- Pop-Bica, C.; Pintea, S.; Cojocneanu-Petric, R.; Del Sal, G.; Piazza, S.; Wu, Z.-H.; Alencar, A.J.; Lossos, I.S.; Berindan-Neagoe, I.; Calin, G.A. MiR-181 family-specific behavior in different cancers: A meta-analysis view. Cancer Metastasis Rev. 2018, 37, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Kou, Z.; Liu, H.; Wang, Y.-C.; Huang, Q.; Wang, Z.-S.; Gu, Z.-L.N.E.; Lang, T.; Nie, Y.-L.; An, L.; Li, A.Z.; et al. Expression Level and Target Gene Prediction of miR-181b in Patients with Chronic Lymphocytic Leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2020, 28, 808–814. [Google Scholar] [PubMed]
- Rao, D.S.; O’Connell, R.M.; Chaudhuri, A.A.; Garcia-Flores, Y.; Geiger, T.L.; Baltimore, D. MicroRNA-34a Perturbs B Lymphocyte Development by Repressing the Forkhead Box Transcription Factor Foxp1. Immunity 2010, 33, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ren, W.; Chen, K. MiR-34a Promotes Apoptosis and Inhibits Autophagy by Targeting HMGB1 in Acute Myeloid Leukemia Cells. Cell. Physiol. Biochem. 2017, 41, 1981–1992. [Google Scholar] [CrossRef]
- Di Martino, M.T.; Leone, E.; Amodio, N.; Foresta, U.; Lionetti, M.; Pitari, M.R.; Cantafio, M.E.G.; Gullà, A.; Conforti, F.; Morelli, E.; et al. Synthetic miR-34a mimics as a novel therapeutic agent for multiple myeloma: In vitro and in vivo evidence. Clin. Cancer Res. 2012, 18, 6260–6270. [Google Scholar] [CrossRef]
- Xiao, X.; Gu, Y.; Wang, G.; Chen, S. c-Myc, RMRP, and miR-34a-5p form a positive-feedback loop to regulate cell proliferation and apoptosis in multiple myeloma. Int. J. Biol. Macromol. 2018, 122, 526–537. [Google Scholar] [CrossRef]
- Xiao, C.; Calado, D.P.; Galler, G.; Thai, T.H.; Patterson, H.C.; Wang, J.; Rajewsky, N.; Bender, T.P.; Rajewsky, K. MiR-150 Controls B Cell Differentiation by Targeting the Transcription Factor c-Myb. Cell 2007, 131, 146–159. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, S.; Mayr, C.; Bartel, D.P.; Lodish, H.F. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc. Natl. Acad. Sci. USA 2007, 104, 7080–7085. [Google Scholar] [CrossRef]
- Musilova, K.; Devan, J.; Cerna, K.; Seda, V.; Pavlasova, G.; Sharma, S.; Oppelt, J.; Pytlik, R.; Prochazka, V.; Prouzova, Z.; et al. miR-150 downregulation contributes to the high-grade transformation of follicular lymphoma by upregulating FOXP1 levels. Blood 2018, 132, 2389–2400. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, H.; Yang, J.; Ma, Y.; Liu, Z.; Li, C.; Wang, T.; Yan, Z.; Du, N. MiR-150-5p regulates melanoma proliferation, invasion and metastasis via SIX1-mediated Warburg Effect. Biochem. Biophys. Res. Commun. 2019, 515, 85–91. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, C.; Cao, Y.; Liu, E. miR-150 Suppresses Tumor Growth in Melanoma Through Downregulation of MYB. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2019, 27, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.Y.; Owens, K.S.; Rogers, J.H.; Mullenix, J.; Velu, C.S.; Grimes, H.L.; Dahl, R. MIR-23A microRNA cluster inhibits B-cell development. Exp. Hematol. 2010, 38, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Kurkewich, J.L.; Bikorimana, E.; Nguyen, T.; Klopfenstein, N.; Zhang, H.; Hallas, W.M.; Stayback, G.; McDowell, M.A.; Dahl, R. The mirn23a microRNA cluster antagonizes B cell development. J. Leukoc. Biol. 2016, 100, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xu, T. Expression and clinical significance of miR-23a and MTSS1 in diffuse large B-cell lymphoma. Oncol. Lett. 2018, 16, 371–377. [Google Scholar] [CrossRef]
- Che, F.; Wan, C.; Dai, J.; Chen, J. Increased expression of miR-27 predicts poor prognosis and promotes tumorigenesis in human multiple myeloma. Biosci. Rep. 2019, 39, BSR20182502. [Google Scholar] [CrossRef]
- Mehta, A.; Mann, M.; Zhao, J.L.; Marinov, G.K.; Majumdar, D.; Garcia-Flores, Y.; Du, X.; Erikci, E.; Chowdhury, K.; Baltimore, D. The microRNA-212/132 cluster regulates B cell development by targeting Sox4. J. Exp. Med. 2015, 212, 1679–1692. [Google Scholar] [CrossRef]
- Calin, G.A.; Liu, C.-G.; Sevignani, C.; Ferracin, M.; Felli, N.; Dumitru, C.D.; Shimizu, M.; Cimmino, A.; Zupo, S.; Dono, M.; et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc. Natl. Acad. Sci. USA 2004, 101, 11755–11760. [Google Scholar] [CrossRef]
- Benhamou, D.; Labi, V.; Novak, R.; Dai, I.; Shafir-Alon, S.; Weiss, A.; Gaujoux, R.; Arnold, R.; Shen-Orr, S.S.; Rajewsky, K.; et al. A c-Myc/miR17-92/Pten Axis Controls PI3K-Mediated Positive and Negative Selection in B Cell Development and Reconstitutes CD19 Deficiency. Cell Rep. 2016, 16, 419–431. [Google Scholar] [CrossRef]
- Xu, S.; Ou, X.; Huo, J.; Lim, K.; Huang, Y.; Chee, S.; Lam, K.-P. Mir-17–92 regulates bone marrow homing of plasma cells and production of immunoglobulin G2c. Nat. Commun. 2015, 6, 6764. [Google Scholar] [CrossRef]
- King, J.K.; Ung, N.M.; Paing, M.H.; Contreras, J.R.; Alberti, M.O.; Fernando, T.R.; Zhang, K.; Pellegrini, M.; Rao, D.S. Regulation of Marginal Zone B-Cell Differentiation by MicroRNA-146a. Front. Immunol. 2017, 7, 670. [Google Scholar] [CrossRef][Green Version]
- Teng, G.; Hakimpour, P.; Landgraf, P.; Rice, A.; Tuschl, T.; Casellas, R.; Papavasiliou, F.N. MicroRNA-155 Is a Negative Regulator of Activation-Induced Cytidine Deaminase. Immunity 2008, 28, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Thai, T.-H.; Calado, D.P.; Casola, S.; Ansel, K.M.; Xiao, C.; Xue, Y.; Murphy, A.; Frendewey, D.; Valenzuela, D.; Kutok, J.L.; et al. Regulation of the Germinal Center Response by MicroRNA-155. Science 2007, 316, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, E.; Białopiotrowicz, E.; Szydłowski, M.; Prochorec-Sobieszek, M.; Juszczyński, P.; Szumera-Ciećkiewicz, A. DEPTOR is a microRNA-155 target regulating migration and cytokine production in diffuse large B-cell lymphoma cells. Exp. Hematol. 2020, 88, 56–67.e2. [Google Scholar] [CrossRef]
- Wang, F.; Xu, W.; Gu, W.-Y.; Li, J.-Y. Expression and Prognostic Value of MiR-155 in Patients with Chronic Lymphocytic Leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2020, 28, 34–39. [Google Scholar] [PubMed]
- Kaur, G.; Ruhela, V.; Rani, L.; Gupta, A.; Sriram, K.; Gogia, A.; Sharma, A.; Kumar, L.; Gupta, R. RNA-Seq profiling of deregulated miRs in CLL and their impact on clinical outcome. Blood Cancer J. 2020, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Jones, D. Setbacks shadow microRNA therapies in the clinic. Nat. Biotechnol. 2018, 36, 909–910. [Google Scholar] [CrossRef] [PubMed]
- Gururajan, M.; Haga, C.L.; Das, S.; Leu, C.-M.; Hodson, D.J.; Josson, S.; Turner, M.; Cooper, M.D. MicroRNA 125b inhibition of B cell differentiation in germinal centers. Int. Immunol. 2010, 22, 583–592. [Google Scholar] [CrossRef]
- Zhang, J.; Jima, D.D.; Jacobs, C.; Fischer, R.; Gottwein, E.; Huang, G.; Lugar, P.L.; Lagoo, A.S.; Rizzieri, D.A.; Friedman, D.R.; et al. Patterns of microRNA expression characterize stages of human B-cell differentiation. Blood 2009, 113, 4586–4594. [Google Scholar] [CrossRef]
- Jiang, Y.; Ding, J.; Li, J.; Chen, G. Effects of microRNA-125b on multiple myeloma cell growth in vitro and in vivo. Oncol. Rep. 2018, 40, 2864–2875. [Google Scholar] [CrossRef]
- Kramer, N.; Wang, W.-L.; Reyes, E.Y.; Kumar, B.; Chen, C.-C.; Ramakrishna, C.; Cantin, E.M.; Vonderfecht, S.L.; Taganov, K.D.; Chau, N.; et al. Altered lymphopoiesis and immunodeficiency in miR-142 null mice. Blood 2015, 125, 3720–3730. [Google Scholar] [CrossRef]
- Zelenetz, A.D.; Gordon, L.I.; Abramson, J.S.; Advani, R.H.; Bartlett, N.L.; Caimi, P.F.; Chang, J.E.; Chavez, J.C.; Christian, B.; Fayad, L.E.; et al. NCCN Guidelines Insights: B-Cell Lymphomas, Version 3.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Dai, Y.; Jiang, T.; Zhou, Y. miR-532-3p Inhibits Proliferation and Promotes Apoptosis of Lymphoma Cells by Targeting β-Catenin. J. Cancer 2020, 11, 4762–4770. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, F.; Ma, P.; Gan, S.; Li, X.; Chen, L.; Sun, L.; Sun, H.; Jiang, Z.; Guo, F. LncRNA CRNDE Promotes the Progression of B-cell Precursor Acute Lymphoblastic Leukemia by Targeting the miR-345-5p/CREB Axi. Mol. Cells 2020, 43, 718–727. [Google Scholar] [PubMed]
- Song, Y.; Guo, N.-H.; Zheng, J.-F. LncRNA-MALAT1 regulates proliferation and apoptosis of acute lymphoblastic leukemia cells via miR-205-PTK7 pathway. Pathol. Int. 2020, 70, 724–732. [Google Scholar] [CrossRef]
- Huang, M.; Zheng, J.; Ren, Y.; Zhu, J.; Kou, L.; Nie, J. LINC00221 suppresses the malignancy of children acute lymphoblastic leukemia. Biosci. Rep. 2020, 40, BSR20194070. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Camino, A.; Garcia-Obregon, S.; Lopez-Lopez, E.; Astigarraga, I.; Garcia-Orad, A. miRNA deregulation in childhood acute lymphoblastic leukemia: A systematic review. Epigenomics 2020, 12, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, F.; Fu, H.; Shen, J. Epigenetic regulation of miR-518a-5p-CCR6 feedback loop promotes both proliferation and invasion in diffuse large B cell lymphoma. Epigenetics 2020, 16, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shen, J.; Su, N.; Wang, Q.; Zhang, M.; Liu, C. MiR-645 regulates the proliferation and apoptosis of diffuse large B-cell lymphoma by targeting DACH1. Hum. Cell 2020, 33, 1091–1098. [Google Scholar] [CrossRef]
- Fu, J.; Lou, X.; Wan, S.; Zhao, X.; Chen, Z.; Zhu, M.; Guo, L.; Wu, D.; Wang, S. microRNA-196a-3p inhibits cell proliferation and promotes cell apoptosis by targeting ADP ribosylation factor 4 in diffuse large B-cell lymphoma. Oncol. Rep. 2020, 45, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, H.; Ji, M. Overexpression of miR-222-3p Promotes the Proliferation and Inhibits the Apoptosis of Diffuse Large B-Cell Lymphoma Cells via Suppressing PPP2R2A. Technol. Cancer Res. Treat. 2019, 18, 1533033819892256. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Xie, X. MicroRNA-425 inhibits proliferation of chronic lymphocytic leukaemia cells through regulation of the Bruton’s tyrosine kinase/phospholipase Cγ2 signalling pathway. Exp. Ther. Med. 2020, 20, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Casabonne, D.; Benavente, Y.; Seifert, J.; Costas, L.; Armesto, M.; Arestin, M.; Besson, C.; Hosnijeh, F.S.; Duell, E.J.; Weiderpass, E.; et al. Serum levels of hsa-miR-16-5p, hsa-miR-29a-3p, hsa-miR-150-5p, hsa-miR-155-5p and hsa-miR-223-3p and subsequent risk of chronic lymphocytic leukemia in the EPIC study. Int. J. Cancer 2020, 147, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Rao, E.; Jiang, C.; Ji, M.; Huang, X.; Iqbal, J.; Lenz, G.; Wright, G.; Staudt, L.M.; Zhao, Y.; McKeithan, T.; et al. The miRNA-17~92 cluster mediates chemoresistance and enhances tumor growth in mantle cell lymphoma via PI3K/AKT pathway activation. Leukemia 2011, 26, 1064–1072. [Google Scholar] [CrossRef]
- Concepcion, C.P.; Bonetti, C.; Ventura, A. The MicroRNA-17-92 Family of MicroRNA Clusters in Development and Disease. Cancer J. 2012, 18, 262–267. [Google Scholar] [CrossRef]
- Lin, L.; Huang, Y.; Zhuang, W.; Lin, P.; Ma, X. miR-100 inhibits cell proliferation in mantle cell lymphoma by targeting mTOR. Exp. Hematol. Oncol. 2020, 9, 25. [Google Scholar] [CrossRef]
- Husby, S.; Geißler, C.; Grønbæk, K. MicroRNAs in mantle cell lymphoma. Leuk. Lymphoma 2013, 54, 1867–1875. [Google Scholar] [CrossRef]
- Wolowiec, D.; Berger, F.; Ffrench, P.; Bryon, P.A. CDK1 and Cyclin A Expression is Linked to Cell Proliferation and Associated with Prognosis in Non-Hodgkin’s Lymphomas. Leuk. Lymphoma 1999, 35, 147–157. [Google Scholar] [CrossRef]
- Marzec, M.; Kasprzycka, M.; Lai, R.; Gladden, A.B.; Wlodarski, P.; Tomczak, E.; Nowell, P.; DePrimo, S.E.; Sadis, S.; Eck, S.; et al. Mantle cell lymphoma cells express predominantly cyclin D1a isoform and are highly sensitive to selective inhibition of CDK4 kinase activity. Blood 2006, 108, 1744–1750. [Google Scholar] [CrossRef]
- Grossel, M.J.; Hinds, P.W. From Cell Cycle to Differentiation: An Expanding Role for Cdk6. Cell Cycle 2005, 5, 266–270. [Google Scholar] [CrossRef]
- Tian, F.; Wang, H.; Ma, H.; Zhong, Y.; Liao, A. miR-144-3p inhibits the proliferation, migration and angiogenesis of multiple myeloma cells by targeting myocyte enhancer factor 2A. Int. J. Mol. Med. 2020, 46, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xie, Z.; Lin, J.; Liu, P. MiR-144-3p inhibits cell proliferation and induces apoptosis in multiple myeloma by targeting c-Met. Am. J. Transl. Res. 2017, 9, 2437–2446. [Google Scholar] [PubMed]
- Wang, L.; Xu, M.; Xing, J.; Huang, Z.-H.; Liu, G.-Q. MiR-144 Inhibits the Malignant Biologic Behaviors of Multiple Myeloma Cells by Regulating Wnt4/β-Catenin Signaling Pathway. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2020, 28, 1256–1260. [Google Scholar]
- Lu, J.; Men, L.J.; Li, T.T.; Sun, L.; Wu, X.F. miR-451 inhibits malignant progression of multiple myeloma RPMI-8226 cells by targeting c-Myc. Zhonghua Zhong Liu Za Zhi [Chin. J. Oncol.] 2020, 42, 560–564. [Google Scholar] [PubMed]
- Wang, Y.; Lin, Q.; Song, C.; Ma, R.; Li, X. Circ_0007841 promotes the progression of multiple myeloma through targeting miR-338-3p/BRD4 signaling cascade. Cancer Cell Int. 2020, 20, 383. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-F.; Guo, N.-H.; Zi, F.-M.; Cheng, J. Long Noncoding RNA H19 Promotes Tumorigenesis of Multiple Myeloma by Activating BRD4 Signaling by Targeting MicroRNA 152-3p. Mol. Cell. Biol. 2020, 40, e00382-19. [Google Scholar] [CrossRef]
- Yang, X.; Huang, H.; Wang, X.; Liu, H.; Liu, H.; Lin, Z. Knockdown of lncRNA SNHG16 suppresses multiple myeloma cell proliferation by sponging miR-342-3p. Cancer Cell Int. 2020, 20, 38. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, H.; Fang, C.; Li, C.; Xhyliu, F.; Dysert, H.; Bodo, J.; Habermehl, G.; Russell, B.E.; Li, W.; et al. Targeting of CD38 by the Tumor Suppressor miR-26a Serves as a Novel Potential Therapeutic Agent in Multiple Myeloma. Cancer Res. 2020, 80, 2031–2044. [Google Scholar] [CrossRef]
- Zi, Y.; Zhang, Y.; Wu, Y.; Zhang, L.; Yang, R.; Huang, Y. Downregulation of microRNA-25-3p inhibits the proliferation and promotes the apoptosis of multiple myeloma cells via targeting the PTEN/PI3K/AKT signaling pathway. Int. J. Mol. Med. 2020, 47, 8. [Google Scholar] [CrossRef]
- Amodio, N.; Leotta, M.; Bellizzi, D.; Di Martino, M.T.; D’Aquila, P.; Lionetti, M.; Fabiani, F.; Leone, E.; Gullà, A.M.; Passarino, G.; et al. DNA-demethylating and anti-tumor activity of synthetic miR-29b mimics in multiple myeloma. Oncotarget 2012, 3, 1246. [Google Scholar] [CrossRef]
- Amodio, N.; Stamato, M.A.; Gullà, A.M.; Morelli, E.; Romeo, E.; Raimondi, L.; Pitari, M.R.; Ferrandino, I.; Misso, G.; Caraglia, M.; et al. Therapeutic Targeting of miR-29b/HDAC4 Epigenetic Loop in Multiple Myeloma. Mol. Cancer Ther. 2016, 15, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Lin, J.; Zhu, D.; Wang, X.; Brooks, D.; Chen, M.; Chu, Z.-B.; Takada, K.; Ciccarelli, B.; Admin, S.; et al. MiR-30-5p functions as a tumor suppressor and novel therapeutic tool by targeting the oncogenic Wnt/b-Catenin/BCL9 pathway. Cancer Res. 2014, 74, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; He, X.; Li, M.; Shi, F.; Wu, D.; Pan, M.; Guo, M.; Zhang, R.; Luo, S.; Gu, N.; et al. MiRNA-34a overexpression inhibits multiple myeloma cancer stem cell growth in mice by suppressing TGIF2. Am. J. Transl. Res. 2016, 8, 5433–5443. [Google Scholar]
- Liu, M.; Liu, H.; Zhou, J.; Yu, Z. miR-140-5p inhibits the proliferation of multiple myeloma cells by targeting VEGFA. Mol. Med. Rep. 2021, 23, 53. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, X.; Wu, T.; Yang, S. miR-489 suppresses multiple myeloma cells growth through inhibition of LDHA-mediated aerobic glycolysis. Genes Genom. 2019, 42, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X. Focus on exosomes—From pathogenic mechanisms to the potential clinical application value in lymphoma. J. Cell. Biochem. 2019, 120, 19220–19228. [Google Scholar] [CrossRef]
- Longjohn, M.N.; Hudson, J.-A.B.; Smith, N.C.; Rise, M.L.; Moorehead, P.C.; Christian, S.L. Deciphering the messages carried by extracellular vesicles in hematological malignancies. Blood Rev. 2020, 46, 100734. [Google Scholar] [CrossRef]
- Sinha, D.; Roy, S.; Saha, P.; Chatterjee, N.; Bishayee, A. Trends in Research on Exosomes in Cancer Progression and Anticancer Therapy. Cancers 2021, 13, 326. [Google Scholar] [CrossRef]
- Moloudizargari, M.; Hekmatirad, S.; Mofarahe, Z.S.; Asghari, M.H. Exosomal microRNA panels as biomarkers for hematological malignancies. Curr. Probl. Cancer 2021, 45, 100726. [Google Scholar] [CrossRef]
- Boyiadzis, M.; Whiteside, T.L. The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia 2017, 31, 1259–1268. [Google Scholar] [CrossRef]
- Yeh, Y.-Y.; Ozer, H.G.; Lehman, A.M.; Maddocks, K.; Yu, L.; Johnson, A.J.; Byrd, J.C. Characterization of CLL exosomes reveals a distinct microRNA signature and enhanced secretion by activation of BCR signaling. Blood 2015, 125, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; D’Arena, G.; Simeon, V.; Trino, S.; Laurenzana, I.; Caivano, A.; La Rocca, F.; Villani, O.; Mansueto, G.; Deaglio, S.; et al. Characterization and prognostic relevance of circulating microvesicles in chronic lymphocytic leukemia. Leuk. Lymphoma 2017, 58, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Caivano, A.; La Rocca, F.; Simeon, V.; Girasole, M.; Dinarelli, S.; Laurenzana, I.; De Stradis, A.; De Luca, L.; Trino, S.; Traficante, A.; et al. MicroRNA-155 in serum-derived extracellular vesicles as a potential biomarker for hematologic malignancies—A short report. Cell. Oncol. 2016, 40, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Zare, N.; Javanmard, S.H.; Mehrzad, V.; Eskandari, N.; Kefayat, A. Evaluation of exosomal miR-155, let-7g and let-7i levels as a potential noninvasive biomarker among refractory/relapsed patients, responsive patients and patients receiving R-CHOP. Leuk. Lymphoma 2019, 60, 1877–1889. [Google Scholar] [CrossRef]
- Manier, S.; Liu, C.-J.; Avet-Loiseau, H.; Park, J.; Shi, J.; Campigotto, F.; Salem, K.Z.; Huynh, D.; Glavey, S.V.; Rivotto, B.; et al. Prognostic role of circulating exosomal miRNAs in multiple myeloma. Blood 2017, 129, 2429–2436. [Google Scholar] [CrossRef]
- van Eijndhoven, M.A.J.; Zijlstra, J.M.; Groenewegen, N.J.; Drees, E.E.E.; van Niele, S.; Baglio, S.R.; Koppers-Lalic, D.; van der Voorn, H.; Libregts, S.F.W.M.; Wauben, M.H.M.; et al. Plasma vesicle miRNAs for therapy response monitoring in Hodgkin lymphoma patients. JCI Insight 2016, 1, e89631. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, Y.; Hu, S.; Cai, Y.; Yang, J.; Ren, S.; Zhao, Y.; Lu, T.; Zhou, X.; Wang, X. Circulating Exosomal MiR-107 Restrains Tumorigenesis in Diffuse Large B-Cell Lymphoma by Targeting 14-3-3η. Front. Cell Dev. Biol. 2021, 9, 667800. [Google Scholar] [CrossRef]
- Peixoto da Silva, S.; Caires, H.R.; Bergantim, R.; Guimarães, J.E.; Vasconcelos, M.H. miRNAs mediated drug resistance in hematological malignancies. Semin. Cancer Biol. 2022, 83, 283–302. [Google Scholar] [CrossRef]
- Han, Z.; Rosen, S.T.; Querfeld, C. Targeting microRNA in hematologic malignancies. Curr. Opin. Oncol. 2020, 32, 535–544. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, O.F.; Popi, A.F. Role of microRNAs in B-Cell Compartment: Development, Proliferation and Hematological Diseases. Biomedicines 2022, 10, 2004. https://doi.org/10.3390/biomedicines10082004
Souza OF, Popi AF. Role of microRNAs in B-Cell Compartment: Development, Proliferation and Hematological Diseases. Biomedicines. 2022; 10(8):2004. https://doi.org/10.3390/biomedicines10082004
Chicago/Turabian StyleSouza, Olívia Fonseca, and Ana Flavia Popi. 2022. "Role of microRNAs in B-Cell Compartment: Development, Proliferation and Hematological Diseases" Biomedicines 10, no. 8: 2004. https://doi.org/10.3390/biomedicines10082004
APA StyleSouza, O. F., & Popi, A. F. (2022). Role of microRNAs in B-Cell Compartment: Development, Proliferation and Hematological Diseases. Biomedicines, 10(8), 2004. https://doi.org/10.3390/biomedicines10082004





