Differential Effects of D9 Tetrahydrocannabinol (THC)- and Cannabidiol (CBD)-Based Cannabinoid Treatments on Macrophage Immune Function In Vitro and on Gastrointestinal Inflammation in a Murine Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cannabis Extracts and Cannabinoids
2.2. Mice
2.3. Peritoneal Macrophages
2.4. Nitric Oxide (NO•) Determination
2.5. Flow Cytometry
2.6. RNA Extraction and Real-Time PCR Analysis
2.7. Induction of Colitis in Mice
2.8. Histopathology and Immunohistochemistry
2.9. Proinflammatory Chemokine and Cytokine Analysis
2.10. Statistical Analysis
3. Results
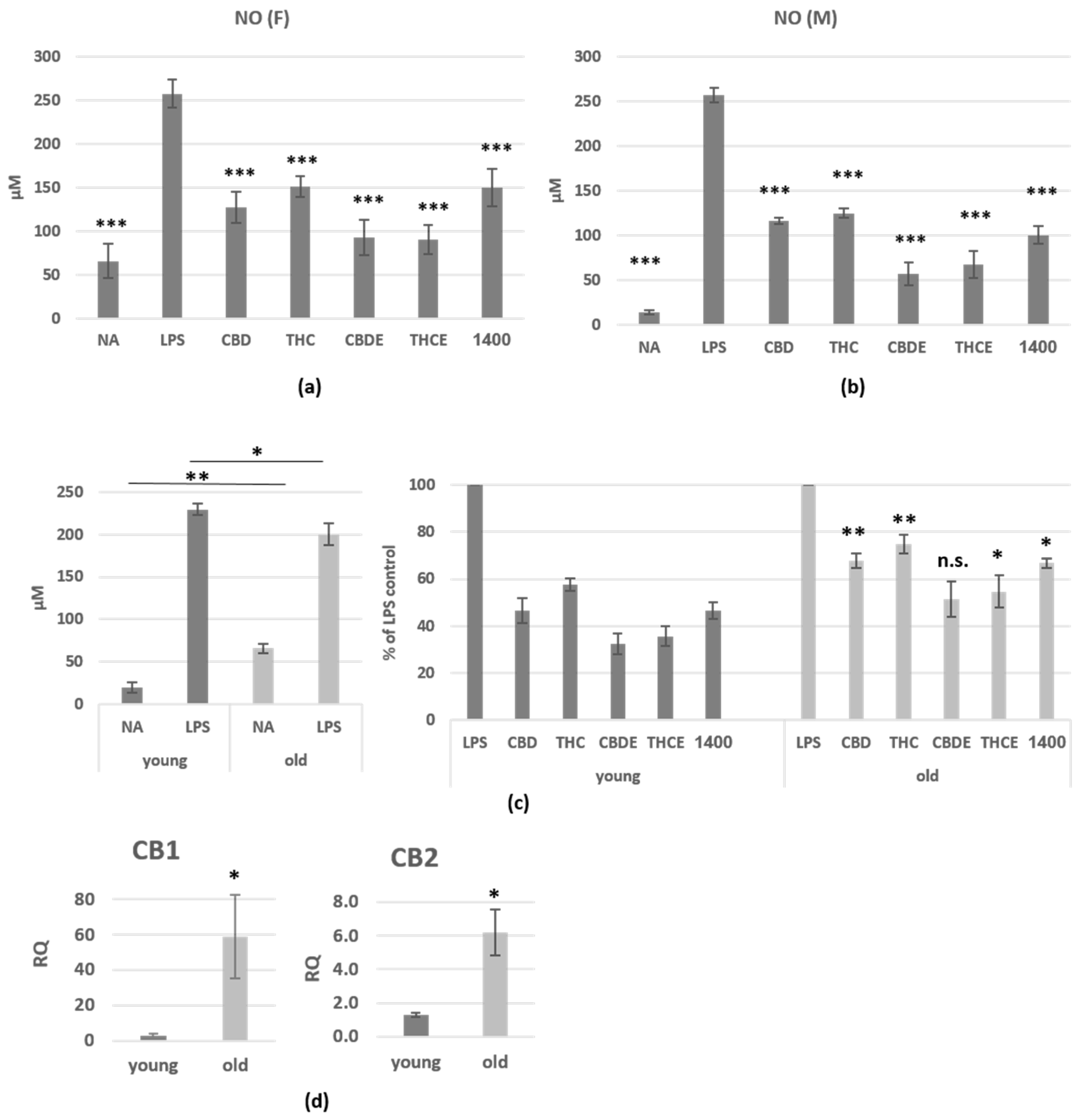
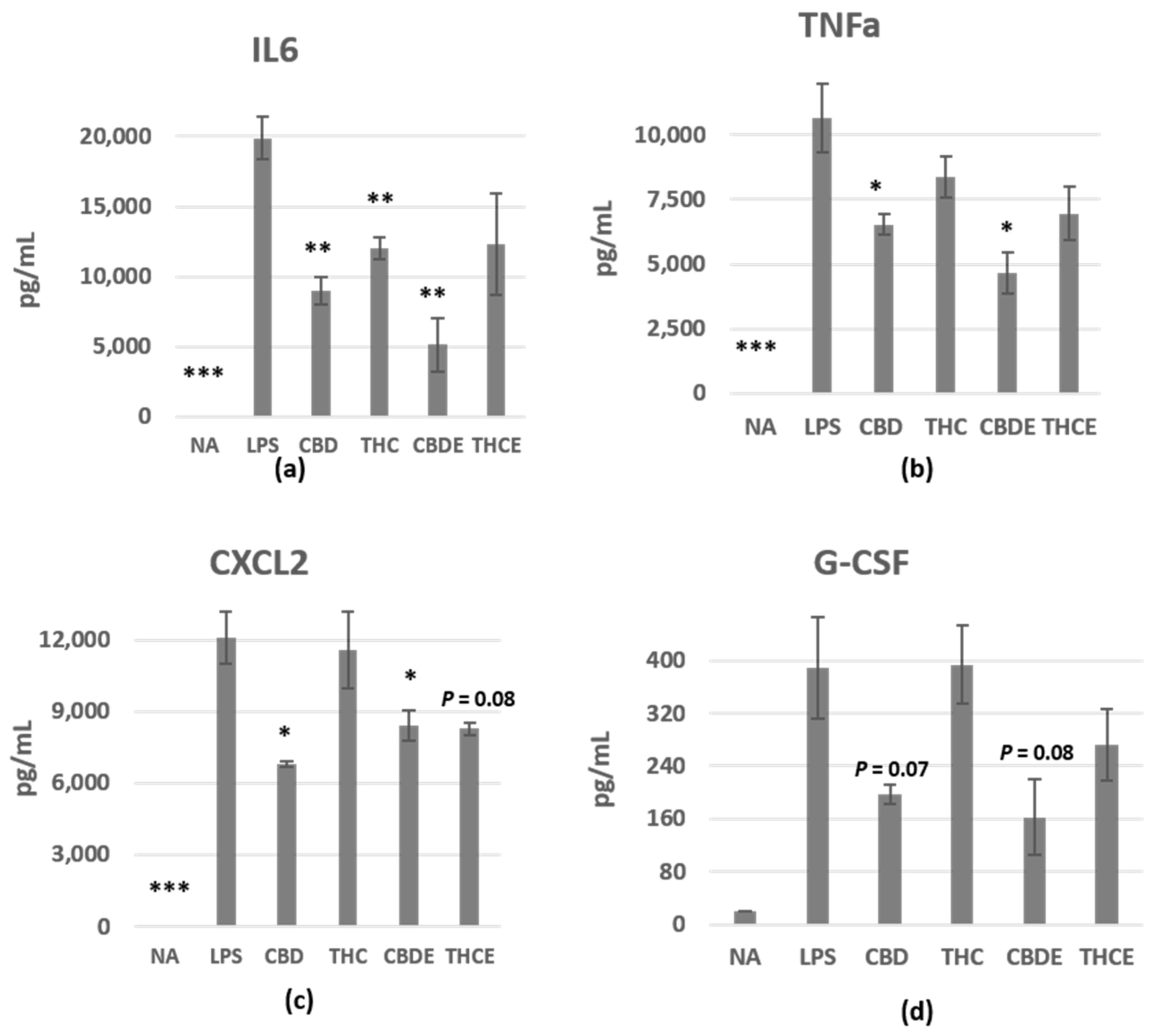
3.1. Cannabinoid Treatments Reduce Nitric Oxide and Cytokine Production of LPS-Activated Peritoneal Macrophages
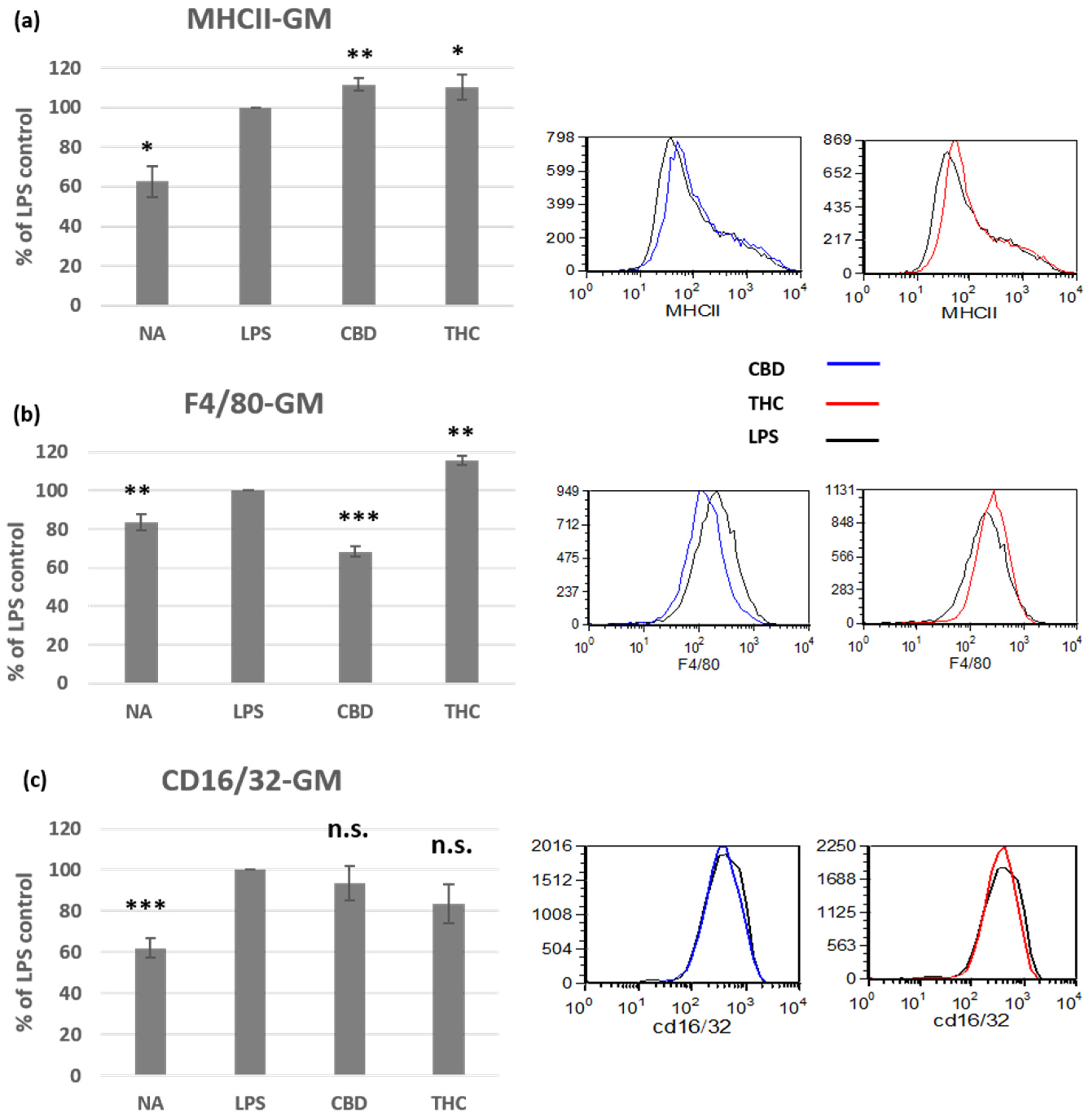
3.2. Cannabinoid Treatments Affect the Phenotype of Activated Peritoneal Macrophages
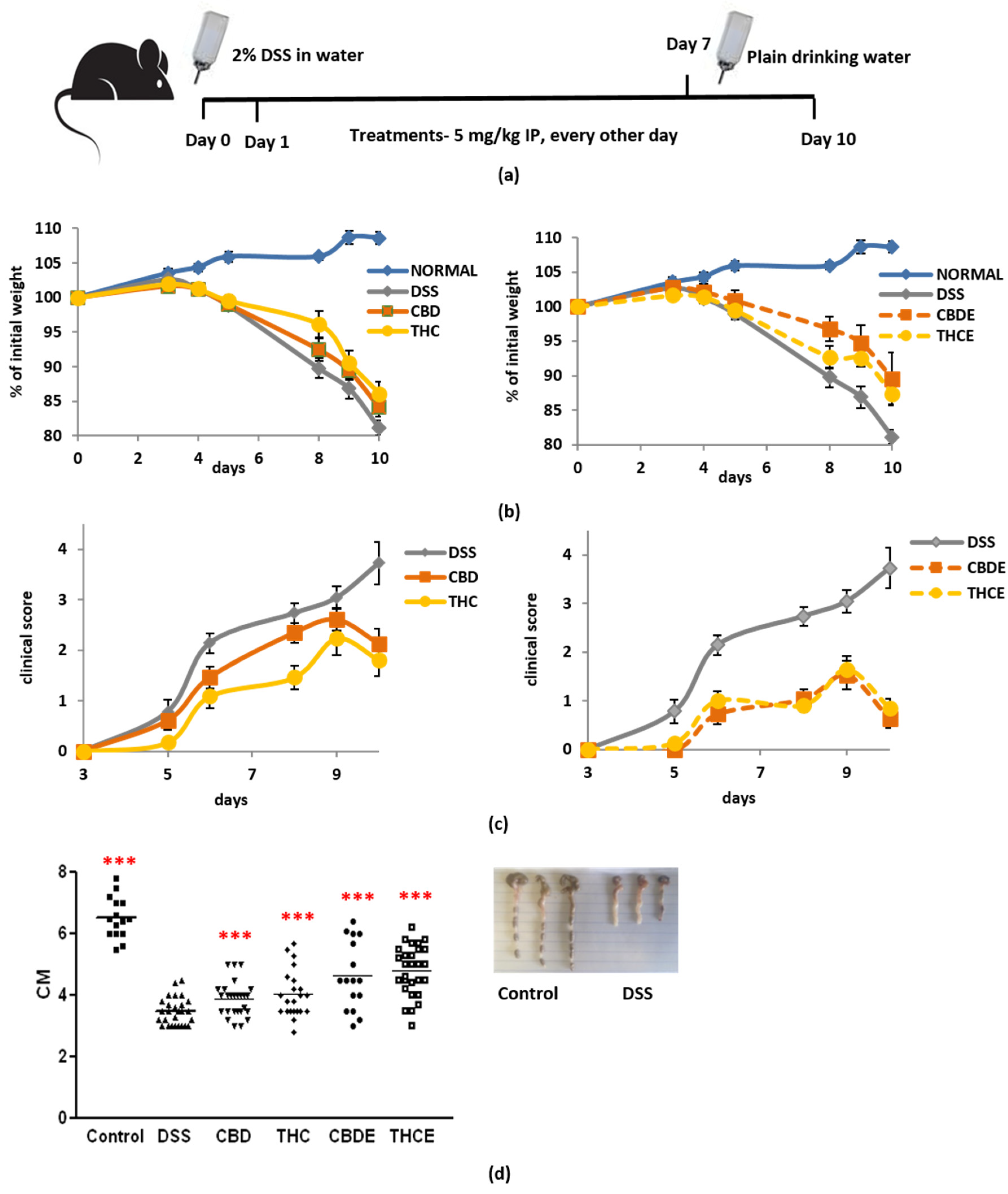
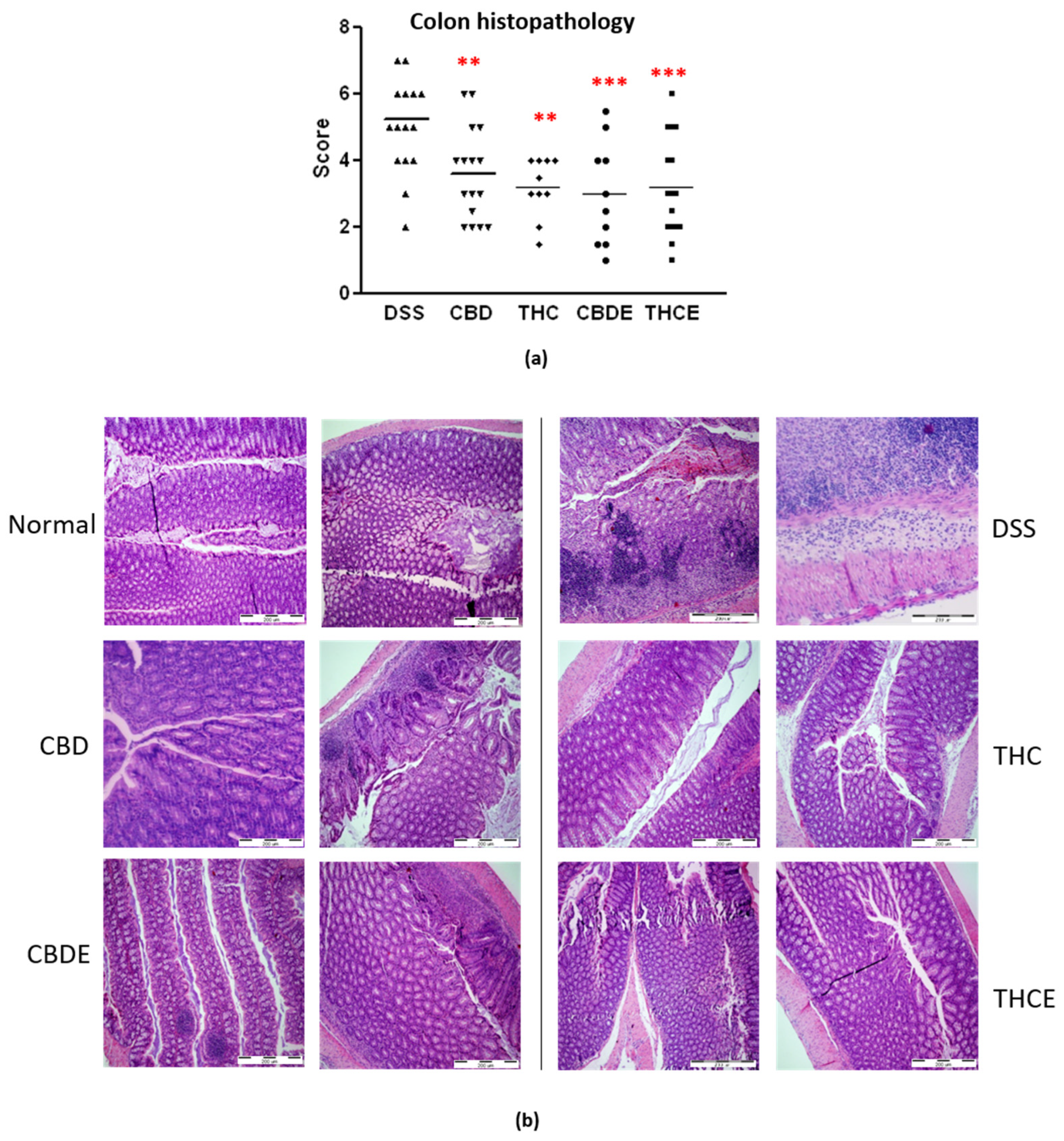
3.3. Cannabis Extracts Have Improved Effect in Murine Colitis DSS Model Mice as Compared with Pure Cannabinoids
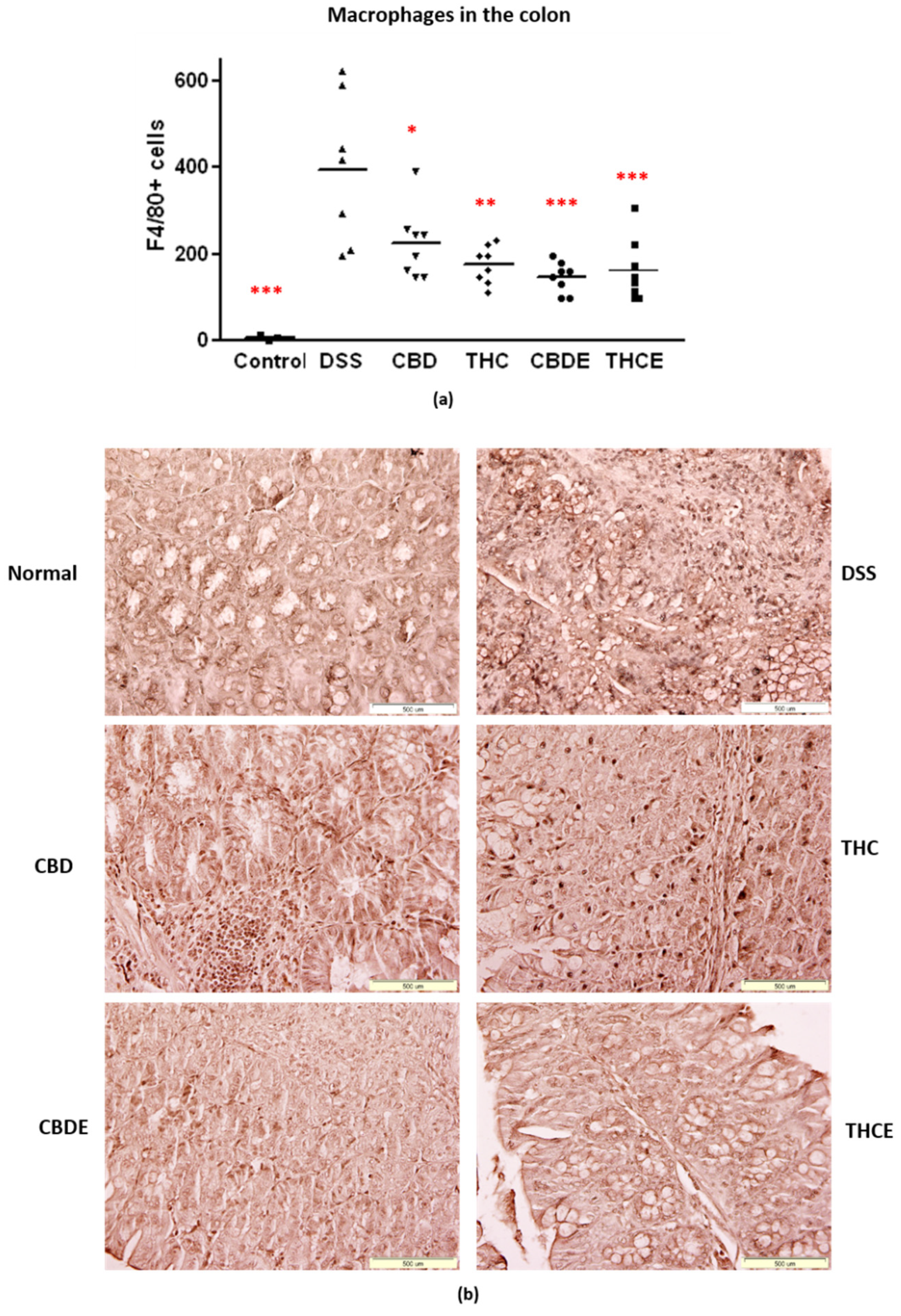
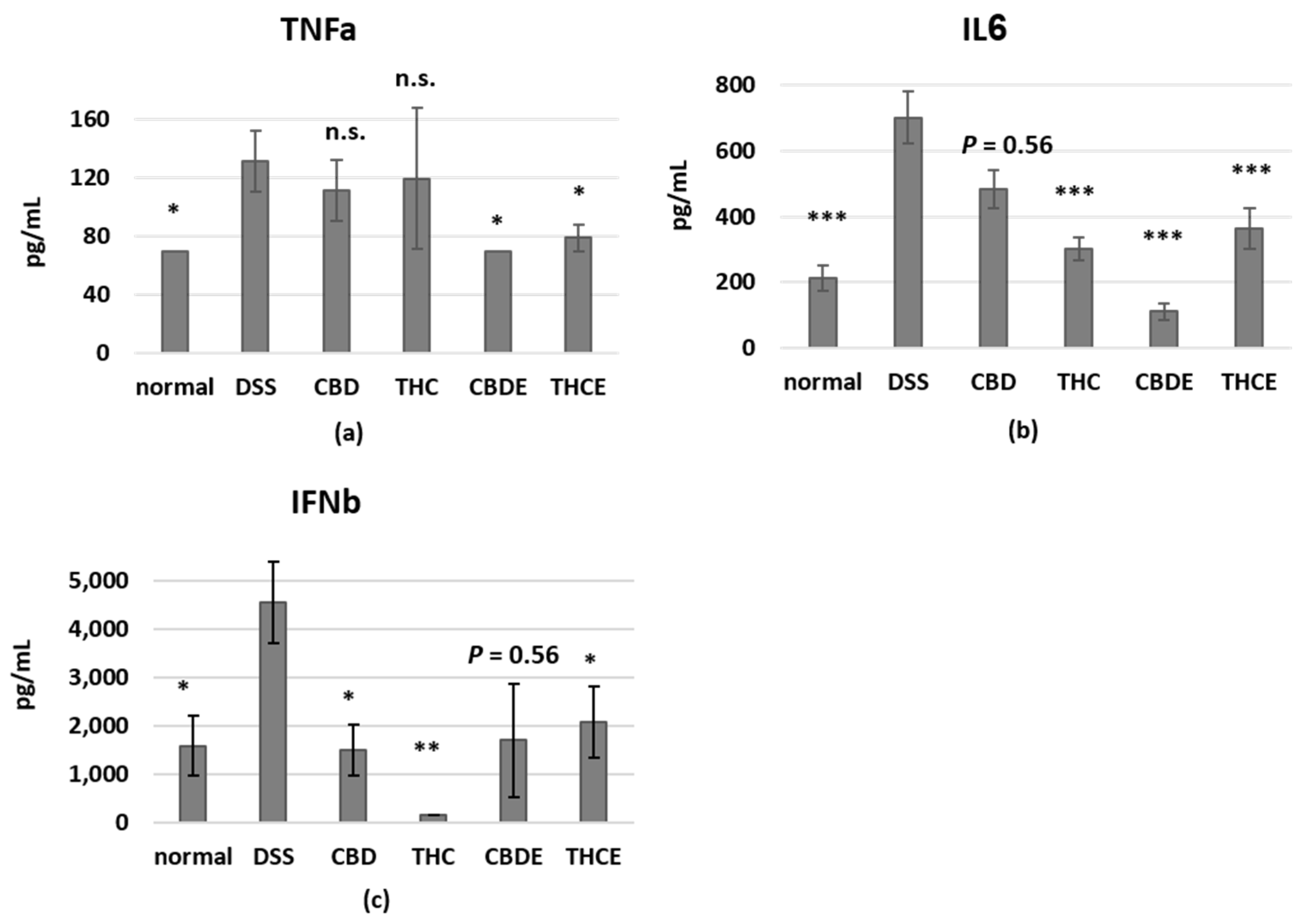
3.4. Cannabinoid Treatments Reduce Intestinal Macrophage Infiltration and the Levels of Inflammatory Cytokines in the Plasma of DSS Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [Green Version]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity-the Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef]
- Tomar, S.; Zumbrun, E.E.; Nagarkatti, M.; Nagarkatti, P.S. Protective role of cannabinoid receptor 2 activation in galactosamine/lipopolysaccharide-induced acute liver failure through regulation of macrophage polarization and microRNAs. J. Pharmacol. Exp. Ther. 2015, 353, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.P.; Yuan, Q.H.; Zhang, B.; Yang, L.; He, Q.W.; Chen, K.; Liu, Q.S.; Li, Z.; Zhan, J. Cannabinoid receptor 2 activation alleviates septic lung injury by promoting autophagy via inhibition of inflammatory mediator release. Cell. Signal. 2020, 69, 109556. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, Y.; Huang, X.; Yuan, A.; Shao, Q.; Pu, J.; He, B. Selective activation of CB2 receptor improves efferocytosis in cultured macrophages. Life Sci. 2016, 161, 10–18. [Google Scholar] [CrossRef]
- Denaes, T.; Lodder, J.; Chobert, M.N.; Ruiz, I.; Pawlotsky, J.M.; Lotersztajn, S.; Teixeira-Clerc, F. The Cannabinoid Receptor 2 Protects Against Alcoholic Liver Disease Via a Macrophage Autophagy-Dependent Pathway. Sci. Rep. 2016, 6, 28806. [Google Scholar] [CrossRef] [Green Version]
- Duncan, M.; Galic, M.A.; Wang, A.; Chambers, A.P.; McCafferty, D.M.; McKay, D.M.; Sharkey, K.A.; Pittman, Q.J. Cannabinoid 1 receptors are critical for the innate immune response to TLR4 stimulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R224–R231. [Google Scholar] [CrossRef] [Green Version]
- Mai, P.; Yang, L.; Tian, L.; Wang, L.; Jia, S.; Zhang, Y.; Liu, X.; Yang, L.; Li, L. Endocannabinoid System Contributes to Liver Injury and Inflammation by Activation of Bone Marrow-Derived Monocytes/Macrophages in a CB1-Dependent Manner. J. Immunol. 2015, 195, 3390–3401. [Google Scholar] [CrossRef] [Green Version]
- Mai, P.; Tian, L.; Yang, L.; Wang, L.; Yang, L.; Li, L. Cannabinoid receptor 1 but not 2 mediates macrophage phagocytosis by G(alpha)i/o /RhoA/ROCK signaling pathway. J. Cell. Physiol. 2015, 230, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.; Mehrpouya-Bahrami, P.; Nagarkatti, P.S.; Nagarkatti, M. Cannabinoid Receptor 1 Blockade Attenuates Obesity and Adipose Tissue Type 1 Inflammation Through miR-30e-5p Regulation of Delta-Like-4 in Macrophages and Consequently Downregulation of Th1 Cells. Front. Immunol. 2019, 10, 1049. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Rakotoarivelo, V.; Di Marzo, V.; Flamand, N. Expression and Functions of the CB2 Receptor in Human Leukocytes. Front Pharmacol. 2022, 13, 826400. [Google Scholar] [CrossRef] [PubMed]
- Khuja, I.; Yekhtin, Z.; Or, R.; Almogi-Hazan, O. Cannabinoids Reduce Inflammation but Inhibit Lymphocyte Recovery in Murine Models of Bone Marrow Transplantation. Int. J. Mol. Sci. 2019, 20, 668. [Google Scholar] [CrossRef] [Green Version]
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 14280. [Google Scholar] [CrossRef] [Green Version]
- Shapira, A.; Berman, P.; Futoran, K.; Guberman, O.; Meiri, D. Tandem Mass Spectrometric Quantification of 93 Terpenoids in Cannabis Using Static Headspace Injections. Anal. Chem. 2019, 91, 11425–11432. [Google Scholar] [CrossRef] [Green Version]
- Ezekowitz, R.A.; Gordon, S. Surface properties of activated macrophages: Sensitized lymphocytes, specific antigen and lymphokines reduce expression of antigen F4/80 and FC and mannose/fucosyl receptors, but induce Ia. Adv. Exp. Med. Biol. 1982, 155, 401–407. [Google Scholar] [CrossRef]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Chiurchiu, V.; Lanuti, M.; Catanzaro, G.; Fezza, F.; Rapino, C.; Maccarrone, M. Detailed characterization of the endocannabinoid system in human macrophages and foam cells, and anti-inflammatory role of type-2 cannabinoid receptor. Atherosclerosis 2014, 233, 55–63. [Google Scholar] [CrossRef]
- Galiegue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carriere, D.; Carayon, P.; Bouaboula, M.; Shire, D.; Le Fur, G.; Casellas, P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.F.; Newton, C.; Widen, R.; Friedman, H.; Klein, T.W. Differential expression of cannabinoid CB(2) receptor mRNA in mouse immune cell subpopulations and following B cell stimulation. Eur. J. Pharmacol. 2001, 423, 235–241. [Google Scholar] [CrossRef]
- Staiano, R.I.; Loffredo, S.; Borriello, F.; Iannotti, F.A.; Piscitelli, F.; Orlando, P.; Secondo, A.; Granata, F.; Lepore, M.T.; Fiorelli, A.; et al. Human lung-resident macrophages express CB1 and CB2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. J. Leukoc. Biol. 2016, 99, 531–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugamura, K.; Sugiyama, S.; Nozaki, T.; Matsuzawa, Y.; Izumiya, Y.; Miyata, K.; Nakayama, M.; Kaikita, K.; Obata, T.; Takeya, M.; et al. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation 2009, 119, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajan, T.S.; Scionti, D.; Diomede, F.; Grassi, G.; Pollastro, F.; Piattelli, A.; Cocco, L.; Bramanti, P.; Mazzon, E.; Trubiani, O. Gingival Stromal Cells as an In Vitro Model: Cannabidiol Modulates Genes Linked With Amyotrophic Lateral Sclerosis. J. Cell. Biochem. 2017, 118, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Yeisley, D.J.; Arabiyat, A.S.; Hahn, M.S. Cannabidiol-Driven Alterations to Inflammatory Protein Landscape of Lipopolysaccharide-Activated Macrophages In Vitro May Be Mediated by Autophagy and Oxidative Stress. Cannabis Cannabinoid Res. 2021, 6, 253–263. [Google Scholar] [CrossRef]
- Fitzpatrick, J.M.; Minogue, E.; Curham, L.; Tyrrell, H.; Gavigan, P.; Hind, W.; Downer, E.J. MyD88-dependent and -independent signalling via TLR3 and TLR4 are differentially modulated by Delta(9)-tetrahydrocannabinol and cannabidiol in human macrophages. J. Neuroimmunol. 2020, 343, 577217. [Google Scholar] [CrossRef]
- Muthumalage, T.; Rahman, I. Cannabidiol differentially regulates basal and LPS-induced inflammatory responses in macrophages, lung epithelial cells, and fibroblasts. Toxicol. Appl. Pharmacol. 2019, 382, 114713. [Google Scholar] [CrossRef]
- Dopkins, N.; Miranda, K.; Wilson, K.; Holloman, B.; Nagarkatti, P.; Nagarkatti, M. Effects of Orally Administered Cannabidiol on Neuroinflammation and Intestinal Inflammation in the Attenuation of Experimental Autoimmune Encephalomyelitis. J. Neuroimmune Pharmacol. 2021, 1–18. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Lipina, C.; Hundal, H.S. The endocannabinoid system: ‘NO’ longer anonymous in the control of nitrergic signalling? J. Mol. Cell Biol. 2017, 9, 91–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, B.; Pagano, E.; Orlando, P.; Capasso, R.; Cascio, M.G.; Pertwee, R.; Marzo, V.D.; Izzo, A.A.; Borrelli, F. Pure Delta(9)-tetrahydrocannabivarin and a Cannabis sativa extract with high content in Delta(9)-tetrahydrocannabivarin inhibit nitrite production in murine peritoneal macrophages. Pharmacol. Res. 2016, 113, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Di Benedetto, S.; Pawelec, G. The Immune System and Its Dysregulation with Aging. Subcell. Biochem. 2019, 91, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Pascual, A.C.; Gaveglio, V.L.; Giusto, N.M.; Pasquare, S.J. Aging modifies the enzymatic activities involved in 2-arachidonoylglycerol metabolism. Biofactors 2013, 39, 209–220. [Google Scholar] [CrossRef]
- Paradisi, A.; Oddi, S.; Maccarrone, M. The endocannabinoid system in ageing: A new target for drug development. Curr. Drug Targets 2006, 7, 1539–1552. [Google Scholar] [CrossRef] [Green Version]
- Mosser, D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003, 73, 209–212. [Google Scholar] [CrossRef]
- Samadi, N.; Polak, D.; Kitzmuller, C.; Steinberger, P.; Zlabinger, G.J.; Jahn-Schmid, B.; Bohle, B. T-cell-derived cytokines enhance the antigen-presenting capacity of human neutrophils. Eur. J. Immunol. 2019, 49, 1441–1443. [Google Scholar] [CrossRef]
- Wacnik, P.W.; Luhr, K.M.; Hill, R.H.; Ljunggren, H.G.; Kristensson, K.; Svensson, M. Cannabinoids affect dendritic cell (DC) potassium channel function and modulate DC T cell stimulatory capacity. J. Immunol. 2008, 181, 3057–3066. [Google Scholar] [CrossRef] [Green Version]
- Palma, A.; Jarrah, A.S.; Tieri, P.; Cesareni, G.; Castiglione, F. Gene Regulatory Network Modeling of Macrophage Differentiation Corroborates the Continuum Hypothesis of Polarization States. Front. Physiol. 2018, 9, 1659. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.H.; Faunce, D.E.; Stacey, M.; Terajewicz, A.; Nakamura, T.; Zhang-Hoover, J.; Kerley, M.; Mucenski, M.L.; Gordon, S.; Stein-Streilein, J. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. J. Exp. Med. 2005, 201, 1615–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrose, T.; Simmons, A. Cannabis, Cannabinoids, and the Endocannabinoid System-Is there Therapeutic Potential for Inflammatory Bowel Disease? J. Crohns Colitis 2019, 13, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Scheau, C.; Caruntu, C.; Badarau, I.A.; Scheau, A.E.; Docea, A.O.; Calina, D.; Caruntu, A. Cannabinoids and Inflammations of the Gut-Lung-Skin Barrier. J. Pers. Med. 2021, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- Nso, N.; Nyabera, A.; Nassar, M.; Alshamam, M.S.; Sumbly, V.; Vest, M.; Patel, N.; Ojong, G.; Rizzo, V. Cannabis and Its Potential Protective Role Against Inflammatory Bowel Disease: A Scoping Review. Cureus 2021, 13, e18841. [Google Scholar] [CrossRef] [PubMed]
- Aswad, M.; Hamza, H.; Pechkovsky, A.; Zikrach, A.; Popov, T.; Zohar, Y.; Shahar, E.; Louria-Hayon, I. High-CBD extract (CBD-X) downregulates cytokine storm systemically and locally in inflamed lungs. Front. Immunol. 2022, 13, 875546. [Google Scholar] [CrossRef] [PubMed]
| THCE | CBDE | ||
|---|---|---|---|
| Phytocannabinoids (%) | Total THC | 24.58 | 1.3488 |
| HPLC-UV | Total CBD | 9.62 | 36.0906 |
| Total CBG | 0.3 | 0.4412 | |
| Terpenoids (ppm) | Linalool | 346.1 | 1087.0 |
| SHS-GC/MS/MS | Fenchyl alcohol | 850.9 | 924.1 |
| α-Terpineol | 825.8 | 992.0 | |
| β-Caryophyllene | 1548.7 | 695.3 | |
| α-Humulene | 406.9 | 265.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yekhtin, Z.; Khuja, I.; Meiri, D.; Or, R.; Almogi-Hazan, O. Differential Effects of D9 Tetrahydrocannabinol (THC)- and Cannabidiol (CBD)-Based Cannabinoid Treatments on Macrophage Immune Function In Vitro and on Gastrointestinal Inflammation in a Murine Model. Biomedicines 2022, 10, 1793. https://doi.org/10.3390/biomedicines10081793
Yekhtin Z, Khuja I, Meiri D, Or R, Almogi-Hazan O. Differential Effects of D9 Tetrahydrocannabinol (THC)- and Cannabidiol (CBD)-Based Cannabinoid Treatments on Macrophage Immune Function In Vitro and on Gastrointestinal Inflammation in a Murine Model. Biomedicines. 2022; 10(8):1793. https://doi.org/10.3390/biomedicines10081793
Chicago/Turabian StyleYekhtin, Zhanna, Iman Khuja, David Meiri, Reuven Or, and Osnat Almogi-Hazan. 2022. "Differential Effects of D9 Tetrahydrocannabinol (THC)- and Cannabidiol (CBD)-Based Cannabinoid Treatments on Macrophage Immune Function In Vitro and on Gastrointestinal Inflammation in a Murine Model" Biomedicines 10, no. 8: 1793. https://doi.org/10.3390/biomedicines10081793
APA StyleYekhtin, Z., Khuja, I., Meiri, D., Or, R., & Almogi-Hazan, O. (2022). Differential Effects of D9 Tetrahydrocannabinol (THC)- and Cannabidiol (CBD)-Based Cannabinoid Treatments on Macrophage Immune Function In Vitro and on Gastrointestinal Inflammation in a Murine Model. Biomedicines, 10(8), 1793. https://doi.org/10.3390/biomedicines10081793







