Effect of Anti-Hypertensive Medication on Plasma Concentrations of Lysyl Oxidase: Evidence for Aldosterone-IL-6-Dependent Regulation of Lysyl Oxidase Blood Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. ELISA
2.3. Endothelial Cells and Fibroblasts
2.4. PCR
2.5. Statistics
3. Results
3.1. Drug Screening in Cohort
3.2. Effect of ACE/AT1 Blockade, ASA, and Statins
3.3. Validation of Aldosterone Antagonism Effect in Cohort 2
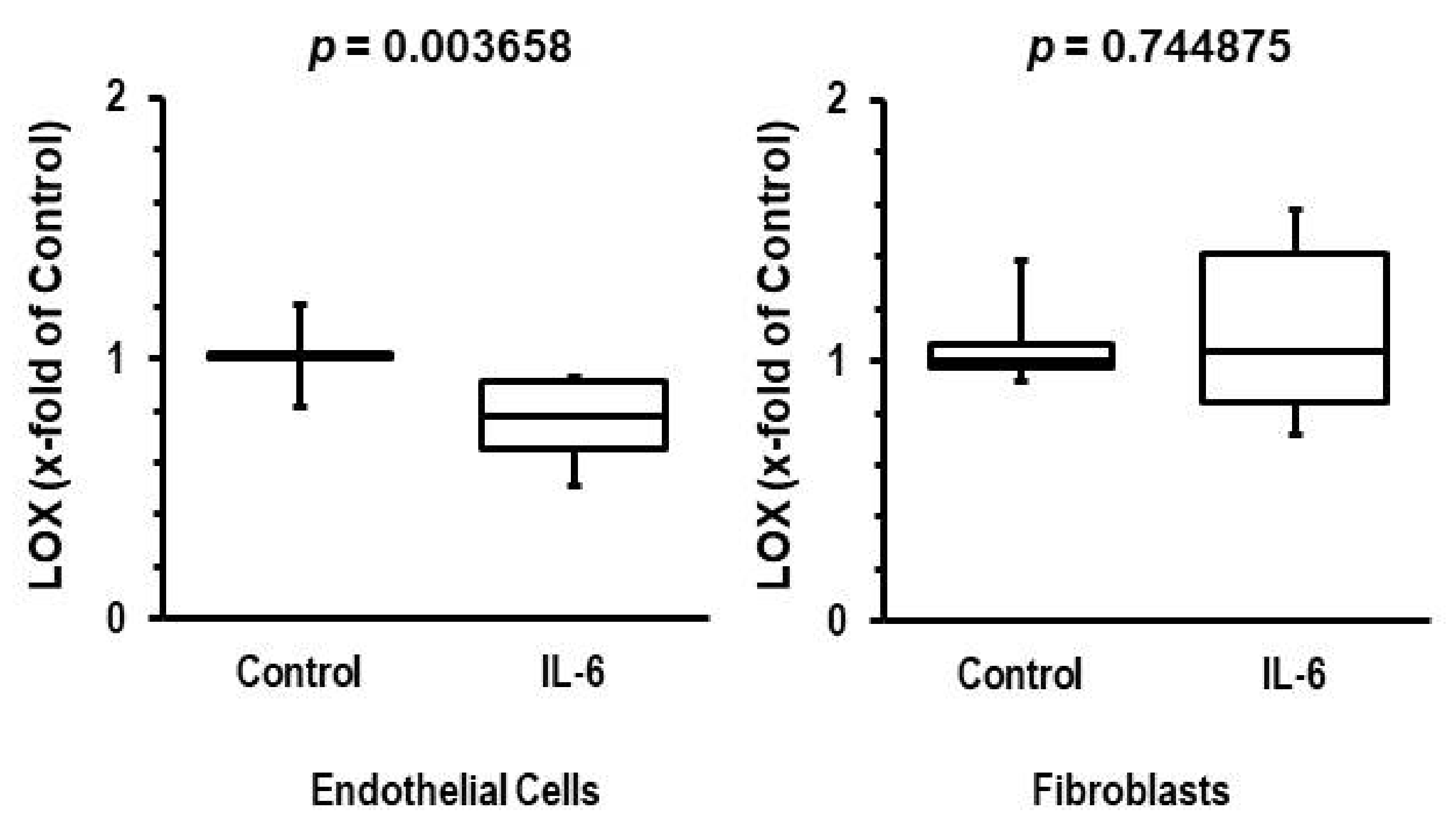
3.4. Participation of IL-6 in Reduction of LOX Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakamuri, S.S.V.P.; Valente, A.J.; Siddesha, J.M.; Delafontaine, P.; Siebenlist, U.; Gardner, J.D.; Bysani, C. TRAF3IP2 mediates aldosterone/salt-induced cardiac hypertrophy and fibrosis. Mol. Cell. Endocrinol. 2016, 429, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreckenberg, R.; Horn, A.-M.; da Costa Rebelo, R.M.; Simsekyilmaz, S.; Niemann, B.; Li, L.; Rohrbach, S.; Schlüter, K.-D. Effects of 6 months‘ exercise on cardiac function, structure, and metabolism in female hypertensive rats–the decisive role of lysyl oxidase and collagen III. Front. Physiol. 2017, 8, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesarwi, O.A.; Shin, K.-M.; Drager, L.F.; Bevans-Fonti, S.; Jun, J.C.; Putcha, N.; Torbenson, M.S.; Pedrose, R.P.; Lorenzi-Filho, G.; Steel, K.E.; et al. Lysyl oxidase as a biomarker of liver fibrosis in patients with severe obesity and obstructive sleep apnea. Sleep 2015, 38, 1583–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberson, L.S.; Sanchez, P.A.; Majeed, B.A.; Tawinwung, S.; Secomb, T.W.; Larson, D.F. Effect of lysyl oxidase inhibition on angiotensin II-induced arterial hypertension, remodeling, and stiffness. PLoS ONE 2015, 10, e012413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anzai, A.; Shimoda, M.; Endo, J.; Kohno, T.; Katsumata, Y.; Matsuhashi, T.; Yamamoto, T.; Ito, K.; Yan, X.; Shirakawa, K.; et al. Adventitial CXCL1/G-CSF expression in response to acute aortic dissection triggers local neutrophil recruitment and activation leading to aortic rupture. Circ. Res. 2015, 116, 612–623. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Takayanagi, T.; Forrester, S.J.; Preston, K.J.; Obama, T.; Tsuji, T.; Kobayashi, T.; Boyer, M.J.; Cooper, H.A.; Kwok, H.F.; et al. Vascular ADAM17 (a disintegrin and metalloproteinase domain 17) is required for angiotensin II/β-aminopropionitrile-induced abdominal aortic aneurysm. Hypertension 2017, 70, 959–963. [Google Scholar] [CrossRef]
- Remus, E.W.; O’Donnell, R.E.; Rafferty, K.; Weiss, D.; Joseph, G.; Csiszar, K.; Fong, S.F.T.; Taylor, W.R. The role of lysyl oxidase family members in the stabilization of abdominal aortic aneurysm. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1067–H1075. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Martinez-Gonzalez, J.; Raposo, B.; Alcudia, J.F.; Guadall, A.; Badimon, L. Regulation of lysyl oxidase in vascular cells: Lysyl oxidase as a new player in cardiovascular disease. Cardiovasc. Res. 2008, 79, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Katsma, M.S.; Patel, S.H.; Eldon, E.; Corbell, K.A.; Shimkus, K.L.; Fluckey, J.D.; Carroll, C.C. The influence of chronic IL-6 exposure, in vivo, on rat achilles tendon extracellular matrix. Cytokine 2017, 93, 10–14. [Google Scholar] [CrossRef]
- Shetty, R.; Sathyanaranamoorthy, A.; Ramachandra, R.A.; Arora, V.; Ghosh, A.; Srivatsa, P.R.; Pahuja, N.; Nuijts, R.M.M.A.; Sinha-Roy, A.; Mohan, R.R.; et al. Attenuation of lysyl oxidase and collagen gene expression in keratoconus patient corneal epithelium corresponds to disease severity. Mol. Vis. 2015, 21, 12–25. [Google Scholar]
- Alcudia, J.F.; Martinez-Gonzalez, J.; Guadall, A.; Gonzalez-Diez, M.; Badimon, L.; Rodriguez, C. Lysyl oxidase and endothelial dysfunction: Mechanimsm of lysly oxidase down-regulation by pro-inflammatory cytokines. Front. Biosci. 2008, 13, 2721–2727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-I.; Lee, J.-H.; Chang, H.-J.; Youn, T.J.; Chung, W.-Y.; Chae, I.-H.; Choi, D.-J.; Park, K.U.; Kim, C.-H. Association between blood pressure variability and inflammatory marker in hypertensive patients. Circ. J. 2008, 72, 293–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.L.; Leite, R.; Fleming, C.; Pollock, J.S.; Webb, R.C.; Brands, M.W. Hypertensive response to acute stress is attenuated in interleukin-6 knockout mice. Hypertension 2004, 44, 259–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennermo, M.; Nordin, M.; Lundman, P.; Boqvist, S.; Held, C.; Samnegard, A.; Ericsson, C.-G.; Silveira, A.; Hamsten, A.; Nastase, M.M.; et al. Genetic and environmental influences on the plasma interleukin-6 concentration in patients with a recent myocardial infarction: A case control study. J. Interferon Cytokine Res. 2011, 31, 259–264. [Google Scholar] [CrossRef]
- Okura, T.; Jotoku, M.; Irita, J.; Enomoto, D.; Nagao, T.; Desilva, V.R.; Yamane, S.; Pei, Z.; Kojima, S.; Hamano, Y.; et al. Association between cytostatin C and inflammation in patients with essential hypertension. Clin. Exp. Nephrol. 2010, 14, 585–588. [Google Scholar] [CrossRef]
- Sanz-Rosa, D.; Oubina, M.P.; Cediel, E.; de Las Heras, N.; Vegazo, O.; Jimenez, J.; Lahera, V.; Cachofeiro, V. Effect of AT1 receptor antagonism on vascular and circulating inflammatory mediators in SHR: Role of NFkappaB/IkappaB system. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H111–H115. [Google Scholar] [CrossRef] [Green Version]
- Bautista, L.E. Inflammation, endothelial dysfunction, and the risk of high blood pressure: Epidemiologic and biological evidence. J. Hum. Hypertens. 2013, 17, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, G.E.; Rhaleb, N.-E.; Nakagawa, P.; Liao, T.-D.; Liu, Y.; Leung, P.; Dai, X.; Yang, X.P.; Carretero, O.A. N-Acetyl-Seryl-Aspartyl-Lysyl-Proline reduces cardiac collagen cross-linking and inflammation in angiotensin II induced hypertensive rats. Clin. Sci. 2014, 126, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Fogelgren, B.; Polgar, N.; Szauter, K.M.; Ujfaludi, Z.; Laczko, R.; Fong, K.S.; Csiszar, K. Cellular fibronectin binds to lysyl oxidase with high affinity and is critical for its proteolytic activation. J. Biol. Chem. 2005, 280, 24690–24697. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Noll, T.; Muhs, A.; Besselmann, M.; Watanabe, H.; Piper, H.M. Initiation of hyperpermeability in energy-depleted coronary endothelial monolayers. Am. J. Physiol. 1995, 268, H1462–H1470. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, M.; Jiang, W.; Javidan, A.; Chen, J.Z.; Howatt, D.A.; Yang, L.; Hamaguchi, M.; Yasugi, T.; Aono, J.; Vazquez-Padron, R.I.; et al. Lysyl oxidase inhibition ablates sexual dimorphism of abdominal aortic aneurysm formation in mice. Circulation 2020, 142, 1993–1995. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xiong, Z.-Q.; Mao, S.-H.; Hu, J.-M.; Wang, J.-Q.; Jiang, H.-W.; Ding, Q. Aldosterone induces inflammatory cytokines in penile corpus cavernosum by activating the NFkB pathway. Asian J. Androl. 2018, 20, 24–29. [Google Scholar]
- Chou, C.-H.; Hug, C.-S.; Liao, C.-W.; Wei, L.-H.; Chen, C.-W.; Shun, C.-T.; Wen, W.-F.; Wan, C.-H.; Wu, X.-M.; Chang, Y.-Y.; et al. IL-6 trans-signalling contributes to aldosterone-induced cardiac fibrosis. Cardiovasc. Res. 2018, 114, 690–702. [Google Scholar] [CrossRef]
- Somanna, N.K.; Yariswamy, M.; Garagliano, J.M.; Siebenlist, U.; Mummidi, S.; Valente, A.J.; Chandrasekar, B. Aldosterone-induced cardiomyocytes growth, and fibroblast migration and proliferation are mediated by TRAF3IP2. Cell Signal. 2015, 27, 1928–1938. [Google Scholar] [CrossRef]
- Rodriguez, C.; Raposo, B.; Martinez-Gonzalez, J.; Casani, L.; Badimon, L. Low density lipoproteins downregulate lysyl oxidase in vascular endothelial cells and the arterial wall. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1409–1414. [Google Scholar] [CrossRef] [Green Version]
- Raposo, B.; Rodriguez, C.; Martinez-Gonzalez, J.; Badimon, L. High levels of homocysteine inhibit lysyl oxidase (LOX) and downregulate LOX expression in vascular endothelial cells. Atherosclerosis 2004, 177, 1–8. [Google Scholar] [CrossRef]
- Lavall, D.; Selzer, C.; Schuster, P.; Lenski, M.; Adam, O.; Schäfer, H.-J.; Böhm, M.; Laufs, U. The mineralocorticoid receptor promotes fibrotic remodeling in atrial fibrillation. J. Biol. Chem. 2014, 289, 6656–6668. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.-H.; Trackman, P.C. Cytokine regulation of gingival fibroblast lysyl oxidase, collagen, and elastin. J. Periodontol. 2002, 73, 145–152. [Google Scholar] [CrossRef]
- Senturk, N.; Keles, G.C.; Kaymaz, F.F.; Yildiz, L.; Acikgoz, G.; Turanli, A.Y. The role of ascorbic acid on collagen structure and levels of serum interleukin-6 and tumor necrosis factor-alpha in experimental lathyrism. Clin. Exp. Dermatol. 2004, 29, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Thaler, R.; Agsten, M.; Spitzer, S.; Paschalis, E.P.; Karlic, H.; Klaushofer, K.; Varga, F. Homocysteine suppresses the expression of the collagen cross-linker lysyl oxidase involving IL-6, Fli1, and epigenetic DNA methylation. J. Biol. Chem. 2011, 286, 5578–5588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Li, Y.; Guo, X.; Chen, Y.; Dai, D.; Sun, Y. The prevalence of hypertension accompanied by high homocysteine and its risk factors in a rural population: A croos-sectional study from northeast China. Int. J. Environ. Res. Public Health 2017, 14, 376. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Real, J.M.; Vayreda, M.; Richart, C.; Gutierrez, C.; Broch, M.; Vendrell, J.; Ricart, W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J. Clin. Endocrin. Metab. 2001, 86, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Cavasin, A.M.; Liao, T.D.; Yang, X.P.; Yang, J.J.; Carretero, O.A. Decreased endogenous level of Ac-SDKP promote organ fibrosis. Hypertension 2007, 50, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Alcudia, J.F.; Martinez-Gonzalez, J.; Guadall, A.; Raposo, B.; Sanchez-Gomez, S.; Badimon, L. Statins normalize vascular lysyl oxidase down-regulation induced by pro-artherogenic risk factors. Cardiovasc. Res. 2009, 83, 595–603. [Google Scholar] [CrossRef] [Green Version]



| Cohort of Hypertensive Patients | Cohort of Patients with Dilative Cardiomyopathy (DCM) | |
|---|---|---|
| Total number | 34 | 37 |
| Male/Female | 20/14 (59%/41%) | 20/17 (54%/46%) |
| BMI > 30 | 13 (38%) | |
| Diabetes | 12 (35%) | |
| Current smoking | 5 (15%) | |
| Age | 67 ± 10 (years) | 51 ± 13 (years) |
| BMI | 29.8 ± 4.9 (kg/m2) | |
| P syst | 153 ± 31 mmHg | |
| P diast | 83 ± 19 mmHg |
| Target | Control * (ng/mL) | Treatment (ng/mL) | Difference (ng/mL) | p-Value |
|---|---|---|---|---|
| Aldosterone antagonist | 25.6 ± 15.8 (n = 29) | 44.3 ± 18.5 (n = 5) | 18.7 | 0.027 |
| ACE inhibitor/AT1 blocker | 20.3 ± 11.7 (n = 11) | 32.2 ± 18.5 (n = 23) | 11.9 | 0.067 |
| Statins | 22.6 ± 13.7 (n = 15) | 32.9 ± 18.8 (n = 19) | 10.3 | 0.094 |
| ASA | 23.6 ± 13.6 (n = 18) | 33.7 ± 19.7 (n = 16) | 10.1 | 0.098 |
| Alpha-2-blocker | 26.9 ± 17.6 (n = 23) | 31.5 ± 16.8 (n = 11) | 4.6 | 0.493 |
| Diuretics | 25.9 ± 20.2 (n = 9) | 29.3 ± 16.3 (n = 25) | 3.3 | 0.636 |
| Renin inhibition | 28.1 ± 18.8 (n = 29) | 30.2 ± 5.3 (n = 5) | 2.1 | 0.813 |
| Beta-blocker | 27.0 ± 19.6 (n = 10) | 29.0 ± 16.5 (n = 24) | 2 | 0.773 |
| Calcium antagonists | 28.4 ± 17.2 (n = 21) | 28.3 ± 18.0 (n = 13) | −0.1 | 0.985 |
| Alpha-1-blocker | 28.6 ± 17.6 (n = 26) | 27.7 ± 17.3 (n = 8) | −0.9 | 0.901 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreckenberg, R.; Dörr, O.; Pankuweit, S.; Schieffer, B.; Troidl, C.; Nef, H.; Hamm, C.W.; Rohrbach, S.; Li, L.; Schlüter, K.-D. Effect of Anti-Hypertensive Medication on Plasma Concentrations of Lysyl Oxidase: Evidence for Aldosterone-IL-6-Dependent Regulation of Lysyl Oxidase Blood Concentration. Biomedicines 2022, 10, 1748. https://doi.org/10.3390/biomedicines10071748
Schreckenberg R, Dörr O, Pankuweit S, Schieffer B, Troidl C, Nef H, Hamm CW, Rohrbach S, Li L, Schlüter K-D. Effect of Anti-Hypertensive Medication on Plasma Concentrations of Lysyl Oxidase: Evidence for Aldosterone-IL-6-Dependent Regulation of Lysyl Oxidase Blood Concentration. Biomedicines. 2022; 10(7):1748. https://doi.org/10.3390/biomedicines10071748
Chicago/Turabian StyleSchreckenberg, Rolf, Oliver Dörr, Sabine Pankuweit, Bernhard Schieffer, Christian Troidl, Holger Nef, Christian W. Hamm, Susanne Rohrbach, Ling Li, and Klaus-Dieter Schlüter. 2022. "Effect of Anti-Hypertensive Medication on Plasma Concentrations of Lysyl Oxidase: Evidence for Aldosterone-IL-6-Dependent Regulation of Lysyl Oxidase Blood Concentration" Biomedicines 10, no. 7: 1748. https://doi.org/10.3390/biomedicines10071748
APA StyleSchreckenberg, R., Dörr, O., Pankuweit, S., Schieffer, B., Troidl, C., Nef, H., Hamm, C. W., Rohrbach, S., Li, L., & Schlüter, K.-D. (2022). Effect of Anti-Hypertensive Medication on Plasma Concentrations of Lysyl Oxidase: Evidence for Aldosterone-IL-6-Dependent Regulation of Lysyl Oxidase Blood Concentration. Biomedicines, 10(7), 1748. https://doi.org/10.3390/biomedicines10071748







