Effects of Lipid Lowering Therapy Optimization by PCSK9 Inhibitors on Circulating CD34+ Cells and Pulse Wave Velocity in Familial Hypercholesterolemia Subjects without Atherosclerotic Cardiovascular Disease: Real-World Data from Two Lipid Units
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Biochemical Analysis
2.3. CD34+ Cell Count
2.4. Pulse Wave Velocity Evaluation
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, C.; Kanaganayagam, G.S.; Jiang, B.; Chowienczyk, P.J.; Zbinden, R.; Saha, M.; Rahman, S.; Shah, A.M.; Marber, M.S.; Kearney, M.T. Vascular Dysfunction and Reduced Circulating Endothelial Progenitor Cells in Young Healthy UK South Asian Men. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 936–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basili, S.; Loffredo, L.; Pastori, D.; Proieti, M.; Farcomeni, A.; Vesti, A.R.; Pignatelli, P.; Davì, G.; Hiatt, W.R.; Lip, G.Y.H.; et al. Carotid plaque detection improves the predictve value of CHA2DS2VASc score in patients with non-valvular atrial fibrilation: The ARAPACIS Study. Int. J. Cardiol. 2017, 231, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Dart, A.M.; Chin-Dusting, J.P.F. Lipids and the endothelium. Cardiovasc. Res. 1999, 43, 308–322. [Google Scholar] [CrossRef] [Green Version]
- Cookson, F.B. The Origin of Foam Cells in Atherosclerosis. Br. J. Exp. Pathol. 1971, 52, 62. [Google Scholar]
- Sen, S.; McDonald, S.P.; Coates, P.T.H.; Bonder, C.S. Endothelial progenitor cells: Novel biomarker and promising cell therapy for cardiovascular disease. Clin. Sci. 2011, 120, 263–283. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wei, J.; Da Fonseca Ferreira, A.; Wang, H.; Zhang, L.; Zhang, Q.; Bellio, M.A.; Chu, X.M.; Khan, A.; Jayaweera, D.; et al. Rejuvenation of Senescent Endothelial Progenitor Cells by Extracellular Vesicles Derived From Mesenchymal Stromal Cells. JACC Basic Transl. Sci. 2020, 5, 1127–1141. [Google Scholar] [CrossRef]
- Tilling, L.; Chowienczyk, P.; Clapp, B. Progenitors in motion: Mechanisms of mobilization of endothelial progenitor cells. Br. J. Clin. Pharmacol. 2009, 68, 484. [Google Scholar] [CrossRef] [Green Version]
- Muggeridge, D.; Dodd, J.; Ross, M.D. CD34+ progenitors are predictive of mortality and are associated with physical activity in cardiovascular disease patients. Atherosclerosis 2021, 333, 108–115. [Google Scholar] [CrossRef]
- Patel, R.S.; Li, Q.; Ghasemzadeh, N.; Eapen, D.J.; Moss, L.D.; Janjua, A.U.; Manocha, P.; Al Kassem, H.; Veledar, E.; Samady, H.; et al. Circulating CD34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circ. Res. 2015, 116, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Elahi, M.M.; Matata, B.M. Effects of maternal high-fat diet and statin treatment on bone marrow endothelial progenitor cells and cardiovascular risk factors in female mice offspring fed a similar diet. Nutrition 2017, 35, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Chantzichristos, V.G.; Agouridis, A.P.; Moutzouri, E.; Stellos, K.; Elisaf, M.S.; Tselepis, A.D. Effect of rosuvastatin or its combination with omega-3 fatty acids on circulating CD34(+) progenitor cells and on endothelial colony formation in patients with mixed dyslipidaemia. Atherosclerosis 2016, 251, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.N.; Low Wang, C.C.; Hiatt, W.R. PCSK9 Inhibitors: Mechanisms of Action, Metabolic Effects, and Clinical Outcomes. Annu. Rev. Med. 2018, 69, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Basiak, M.; Kosowski, M.; Cyrnek, M.; Bułdak, Ł.; Maligłówka, M.; Machnik, G.; Okopień, B. Pleiotropic Effects of PCSK-9 Inhibitors. Int. J. Mol. Sci. 2021, 22, 3144. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Scicali, R.; Di Pino, A.; Ferrara, V.; Rabuazzo, A.M.; Purrello, F.; Piro, S. Effect of PCSK9 inhibitors on pulse wave velocity and monocyte-to-HDL-cholesterol ratio in familial hypercholesterolemia subjects: Results from a single-lipid-unit real-life setting. Acta Diabetol. 2021, 58, 949–957. [Google Scholar] [CrossRef]
- Pirillo, A.; Garlaschelli, K.; Arca, M.; Averna, M.; Bertolini, S.; Calandra, S.; Tarugi, P.; Catapano, A.L.; LIPIGEN Group, M.; Averna, M.; et al. Spectrum of mutations in Italian patients with familial hypercholesterolemia: New results from the LIPIGEN study. Atheroscler. Suppl. 2017, 29, 17–24. [Google Scholar] [CrossRef]
- Mandraffino, G.; Scicali, R.; Rodríguez-Carrio, J.; Savarino, F.; Mamone, F.; Scuruchi, M.; Cinquegrani, M.; Imbalzano, E.; Di Pino, A.; Piro, S.; et al. Arterial stiffness improvement after adding on PCSK9 inhibitors or ezetimibe to high-intensity statins in patients with familial hypercholesterolemia: A Two–Lipid Center Real-World Experience. J. Clin. Lipidol. 2020, 14, 231–240. [Google Scholar] [CrossRef]
- Barnett, D.; Janossy, G.; Lubenko, A.; Matutes, E.; Newland, A.; Reilly, J.T.; Bain, B.J.; Amos, R.; Cavill, I.; Chapman, C.; et al. Guideline for the flow cytometric enumeration of CD34+ haematopoietic stem cells. Prepared by the CD34+ haematopoietic stem cell working party. General Haematology Task Force of the British Committee for Standards in Haematology. Clin. Lab. Haematol. 1999, 21, 301–308. [Google Scholar] [CrossRef]
- Mandraffino, G.; Aragona, C.O.; Basile, G.; Cairo, V.; Mamone, F.; Morace, C.; D’Ascola, A.; Alibrandi, A.; Lo Gullo, A.; Loddo, S.; et al. CD34+ cell count predicts long lasting life in the oldest old. Mech. Ageing Dev. 2017, 164, 139–145. [Google Scholar] [CrossRef]
- Gleissner, C.A. Translational atherosclerosis research: From experimental models to coronary artery disease in humans. Atherosclerosis 2016, 248, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Zhou, J.; Gong, R.; Huang, X.; Pansuria, M.; Virtue, A.; Li, X.; Wang, H.; Yang, X.F. Endothelial progenitor cells in atherosclerosis. Front. Biosci. (Landmark Ed.) 2012, 17, 2327–2349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadini, G.P.; de Kreutzenberg, S.; Agostini, C.; Boscaro, E.; Tiengo, A.; Dimmeler, S.; Avogaro, A. Low CD34+ cell count and metabolic syndrome synergistically increase the risk of adverse outcomes. Atherosclerosis 2009, 207, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Mandraffino, G.; Imbalzano, E.; Sardo, M.A.; D’Ascola, A.; Mamone, F.; Lo Gullo, A.; Alibrandi, A.; Loddo, S.; Mormina, E.; David, A.; et al. Circulating progenitor cells in hypertensive patients with different degrees of cardiovascular involvement. J. Hum. Hypertens. 2014, 28, 543–550. [Google Scholar] [CrossRef]
- Goldberg, L.R. Extracellular Vesicles and Hematopoietic Stem Cell Aging. Arterioscler. Thromb. Vasc. Biol. 2021, 41, E399–E416. [Google Scholar] [CrossRef]
- Daub, K.; Langer, H.; Seizer, P.; Stellos, K.; May, A.E.; Goyal, P.; Bigalke, B.; Schönberger, T.; Geisler, T.; Siegel-Axel, D.; et al. Platelets induce differentiation of human CD34+ progenitor cells into foam cells and endothelial cells. FASEB J. 2006, 20, 2559–2561. [Google Scholar] [CrossRef] [Green Version]
- Lo Gullo, A.; Mandraffino, G.; Sardo, M.A.; D’Ascola, A.; Mamone, F.; Loddo, S.; Alibrandi, A.; Imbalzano, E.; Mandraffino, R.; Mormina, E.; et al. Circulating progenitor cells in rheumatoid arthritis: Association with inflammation and oxidative stress. Scand. J. Rheumatol. 2014, 43, 184–193. [Google Scholar] [CrossRef]
- Mandraffino, G.; Sardo, M.A.; Riggio, S.; Loddo, S.; Imbalzano, E.; Alibrandi, A.; Saitta, C.; Cinquegrani, M.; Mormina, E.M.; Saitta, A. Circulating progenitor cells are increased in newly diagnosed untreated hypertensive patients with arterial stiffening but normal carotid intima-media thickness. Hypertens. Res. 2011, 34, 876–883. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kawashiri, S.Y.; Kiyoura, K.; Koyamatsu, J.; Fukui, S.; Tamai, M.; Nobusue, K.; Yamanashi, H.; Nagata, Y.; Maeda, T. Circulating CD34+ cells and active arterial wall thickening among elderly men: A prospective study. Sci. Rep. 2020, 10, 4656. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, Z.; Yan, B.; Yin, H.; Tai, S.; Peng, J.; Cui, Y.; Gui, Y.; Belke, D.; Zhou, S.; et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) Triggers Vascular Smooth Muscle Cell Senescence and Apoptosis: Implication of Its Direct Role in Degenerative Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 67–86. [Google Scholar] [CrossRef]
- Tripaldi, R.; Lanuti, P.; Simeone, P.G.; Liani, R.; Bologna, G.; Ciotti, S.; Simeone, P.; Di Castelnuovo, A.; Marchisio, M.; Cipollone, F.; et al. Endogenous PCSK9 may influence circulating CD45neg/CD34bright and CD45neg/CD34bright/CD146neg cells in patients with type 2 diabetes mellitus. Sci. Rep. 2021, 11, 9659. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.H.; Chen, I.C.; Li, Y.H.; Lee, P.T.; Tseng, S.Y. Plasma Levels of Proprotein Convertase Subtilisin/Kexin Type 9 Are Elevated in Patients With Peripheral Artery Disease and Associated With Metabolic Disorders and Dysfunction in Circulating Progenitor Cells. J. Am. Heart Assoc. 2016, 5, e003497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, S.; Rubil, S.; Endres, M.; Princen, H.M.G.; Boeckel, J.N.; Winter, K.; Werner, C.; Laufs, U. Anti-PCSK9 antibodies inhibit pro-atherogenic mechanisms in APOE*3Leiden.CETP mice. Sci. Rep. 2019, 9, 11079. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.X.; Liu, H.H.; Li, S.; Li, J.J. A Meta-Analysis of the Effect of PCSK9-Monoclonal Antibodies on Circulating Lipoprotein (a) Levels. Am. J. Cardiovasc. Drugs 2019, 19, 87–97. [Google Scholar] [CrossRef]
| HeFH | Controls | p | |
|---|---|---|---|
| N | 30 | 30 | |
| Age, years | 54.5 (17) | 52 (13) | 0.456 |
| Men, n [%] | 17 [56.7] | 13 [43.3] | 0.302 |
| BMI Kg/m2 | 26.4 (7.7) | 24 (3.7) | 0.09 |
| SBP mmHg | 135 (10) | 120 (20) | <0.05 |
| DBP mmHg | 80 (13) | 70 (10) | <0.05 |
| PWV m/s | 10.7 (3.7) | 4.9 (0.75) | <0.001 |
| FH genotype | |||
| LDLR, n [%] | 30 [100] | ||
| Mutation class | |||
| Amino acid change, n [%] | 18 [60.0] | ||
| Null allele, n [%] | 12 [40.0] | ||
| FH phenotype | |||
| Heterozygous FH, n [%] | 30 [100] | ||
| Statin therapy | |||
| Rosuvastatin 20 mg, n [%] | 12 [40] | ||
| Rosuvastatin 10 mg, n [%] | 10 [33.3] | ||
| Atorvastatin 40 mg, n [%] | 4 [13.3] | ||
| Atorvastatin 20 mg, n [%] | 4 [13.3] | ||
| Ezetimibe 10 mg n [%] | 30 [100] |
| HeFH Subjects (n = 30) | HeFH Subjects (n = 30) | HeFH Subjects (n = 30) | ∆ T1-T0 (p) | ∆ T2-T1 (p) | |
|---|---|---|---|---|---|
| Baseline | Six-Month Statin + Ezetimibe | Six-Month Add-on PCSK9-i | |||
| Risk factors | |||||
| Hypertension | 0 | 0 | 0 | - | - |
| Type 2 diabetes, n | 0 | 0 | 0 | - | - |
| ASCVD | 0 | 0 | 0 | - | - |
| Lipid Profile | |||||
| TC, mg/dL | 350 (80) | 222.5 (85) | 140.5 (64) | <0.001 | <0.001 |
| HDL, mg/dL | 50.5 (19) | 49.5 (22) | 51 (19) | 0.861 | 0.512 |
| TG, mg/dL | 98.5 (52) | 105.5 (83) | 88 (40) | 0.212 | 0.109 |
| LDL-C, mg/dL | 299 (98) | 149.3 (65.5) | 66.1 (49) | <0.001 | <0.001 |
| CV risk markers | |||||
| CD34+ cells (cells/μL) | - | 1.7 (3.55) | 2.2 (1.7) | - | 0.709 |
| PWV (m/s) | 10.72 (3.7) | 8.71 (2.6) | 7.66 (2.1) | <0.001 | <0.001 |
| CD34+ Cells | (p) | ∆ | (p) | ||||
|---|---|---|---|---|---|---|---|
| Controls | 2.12 (1.95) | ||||||
| HeFH | T0 | - | T0 vs. ctrls | - | |||
| T1 | 1.77 (3.55) | T1 vs. ctrls | =0.639 | - | - | - | |
| T2 | 2.2 (0.7) | T2 vs. ctrls | =0.685 | +22.57% | T2 vs. T1 | =0.709 | |
| PWV | (p) | ∆ | (p) | ||||
| Controls | 4.9 (0.75) | ||||||
| T0 | 10.7 (3.7) | T0 vs. ctrls | <0.001 | ||||
| HeFH | T1 | 8.7 (2.5) | T1 vs. ctrls | <0.001 | −14.80% | T1 vs. T0 | <0.001 |
| T2 | 7.6 (1.9) | T2 vs. ctrls | <0.001 | −10.95% | T2 vs. T1 | <0.001 | |
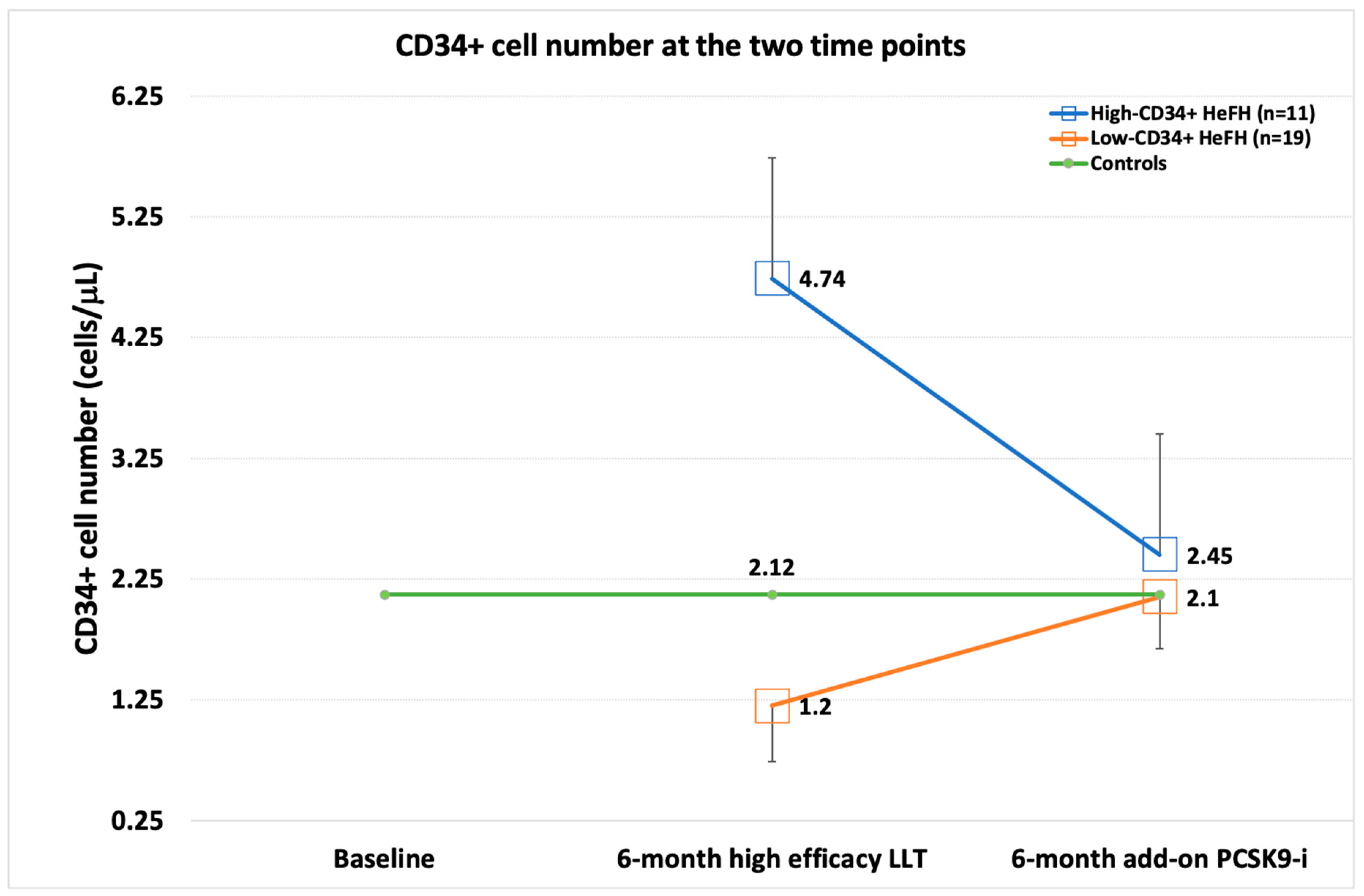
| CD34+ Cells | (p) | ∆ | (p) | (p) Low vs. High | |||
|---|---|---|---|---|---|---|---|
| Controls | 2.12 (1.95) | ||||||
| L-CD34+ | T0 | - | T0 vs. ctrls | - | |||
| T1 | 1.2 (0.46) | T1 vs. ctrls | <0.001 | - | - | ||
| T2 | 2.1 (0.43) | T2 vs. ctrls | 0.952 | +67.45% | <0.001 vs. T1 | ||
| H-CD34+ | T0 | - | T0 vs. ctrls | - | |||
| T1 | 4.74 (1.92) | T1 vs. ctrls | <0.001 | - | - | p < 0.001 | |
| T2 | 2.45 (0.83) | T2 vs. ctrls | 0.419 | −39.24% | =0.008 vs. T1 | p = 0.164 | |
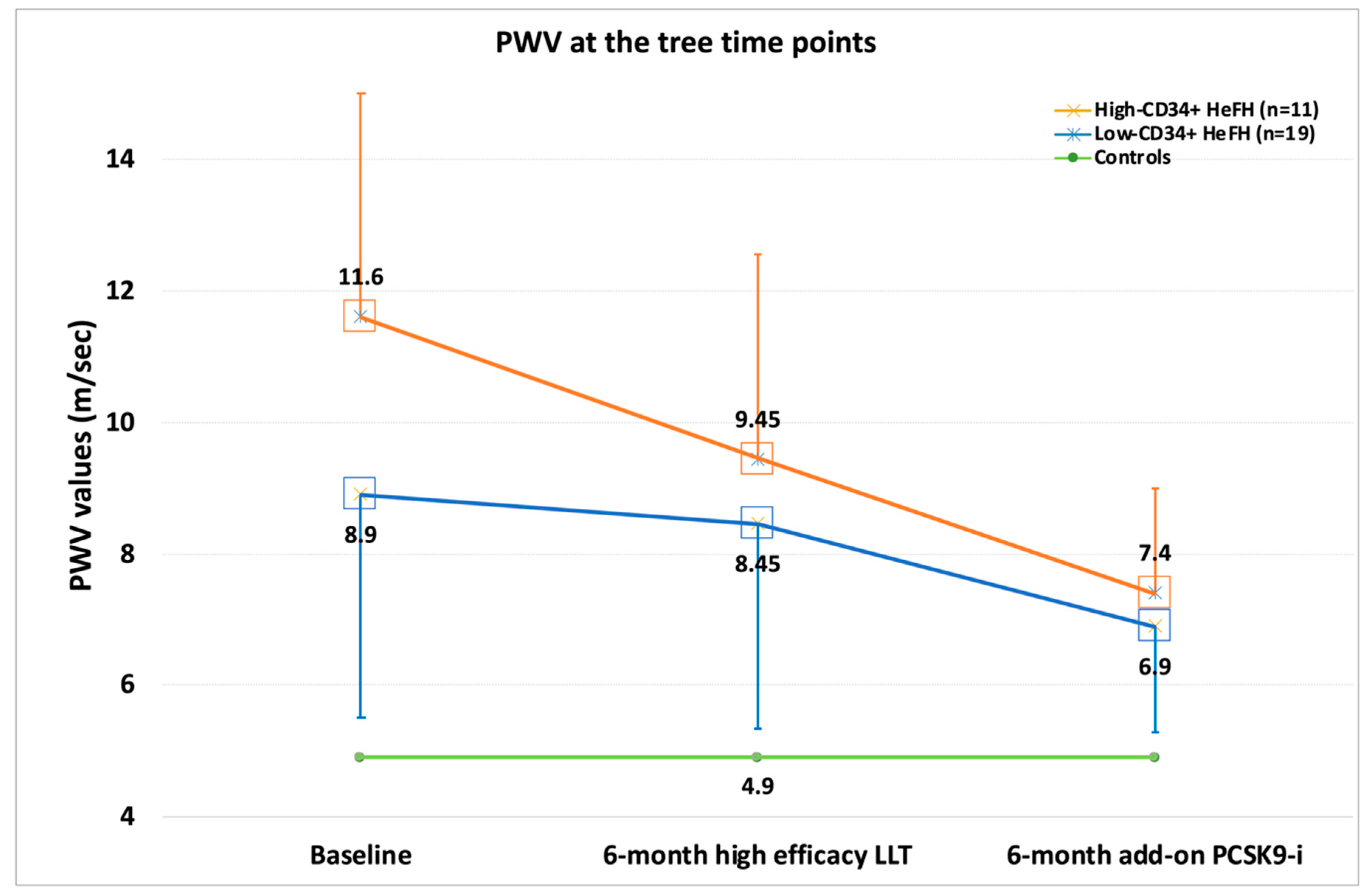
| PWV | (p) | ∆ | (p) low vs. high | ||||
| Controls | 4.9 (0.75) | ||||||
| T0 | 11.6 (3.9) | T0 vs. ctrls | <0.001 | ||||
| L-CD34+ | T1 | 9.45 (2.1) | T1 vs. ctrls | <0.001 | −15.5% | <0.001 vs. T0 | |
| T2 | 8.32 (1.9) | T2 vs. ctrls | <0.001 | −11.9% | <0.001 vs. T1 | ||
| T0 | 8.9 (3.4)) | T0 vs. ctrls | <0.001 | =0.075 | |||
| H-CD34+ | T1 | 7.4 (2.3) | T1 vs. ctrls | <0.001 | −13.5% | <0.001 vs. T0 | <0.05 |
| T2 | 6.9 (1.6) | T2 vs. ctrls | <0.001 | −9.2% | <0.001 vs. T1 | =0.055 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scicali, R.; Mandraffino, G.; Scuruchi, M.; Lo Gullo, A.; Di Pino, A.; Ferrara, V.; Morace, C.; Aragona, C.O.; Squadrito, G.; Purrello, F.; et al. Effects of Lipid Lowering Therapy Optimization by PCSK9 Inhibitors on Circulating CD34+ Cells and Pulse Wave Velocity in Familial Hypercholesterolemia Subjects without Atherosclerotic Cardiovascular Disease: Real-World Data from Two Lipid Units. Biomedicines 2022, 10, 1715. https://doi.org/10.3390/biomedicines10071715
Scicali R, Mandraffino G, Scuruchi M, Lo Gullo A, Di Pino A, Ferrara V, Morace C, Aragona CO, Squadrito G, Purrello F, et al. Effects of Lipid Lowering Therapy Optimization by PCSK9 Inhibitors on Circulating CD34+ Cells and Pulse Wave Velocity in Familial Hypercholesterolemia Subjects without Atherosclerotic Cardiovascular Disease: Real-World Data from Two Lipid Units. Biomedicines. 2022; 10(7):1715. https://doi.org/10.3390/biomedicines10071715
Chicago/Turabian StyleScicali, Roberto, Giuseppe Mandraffino, Michele Scuruchi, Alberto Lo Gullo, Antonino Di Pino, Viviana Ferrara, Carmela Morace, Caterina Oriana Aragona, Giovanni Squadrito, Francesco Purrello, and et al. 2022. "Effects of Lipid Lowering Therapy Optimization by PCSK9 Inhibitors on Circulating CD34+ Cells and Pulse Wave Velocity in Familial Hypercholesterolemia Subjects without Atherosclerotic Cardiovascular Disease: Real-World Data from Two Lipid Units" Biomedicines 10, no. 7: 1715. https://doi.org/10.3390/biomedicines10071715
APA StyleScicali, R., Mandraffino, G., Scuruchi, M., Lo Gullo, A., Di Pino, A., Ferrara, V., Morace, C., Aragona, C. O., Squadrito, G., Purrello, F., & Piro, S. (2022). Effects of Lipid Lowering Therapy Optimization by PCSK9 Inhibitors on Circulating CD34+ Cells and Pulse Wave Velocity in Familial Hypercholesterolemia Subjects without Atherosclerotic Cardiovascular Disease: Real-World Data from Two Lipid Units. Biomedicines, 10(7), 1715. https://doi.org/10.3390/biomedicines10071715











