Cellular and Humoral Responses Follow-up for 8 Months after Vaccination with mRNA-Based Anti-SARS-CoV-2 Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Blood Extraction
2.2. Measurement of Specific Humoral and Cellular Responses
2.3. Statistics
3. Results
3.1. Cohort Characteristics
3.2. Levels of Specific Anti-S Antibodies Were Higher with mRNA-1273, but Both mRNA Vaccines Presented a High Degree of Wane over the Months
3.3. Cellular Responses to SARS-CoV-2-Derived Spike Stimulation
3.4. Specific Cytokines Produced after the Cellular Stimulation Were Higher with mRNA-1273
3.5. Anti-SARS-CoV-2 Spike-Specific Cellular Immunity Is Lost over Time Post-Vaccination
3.6. Correlation between Humoral and Cellular Responses
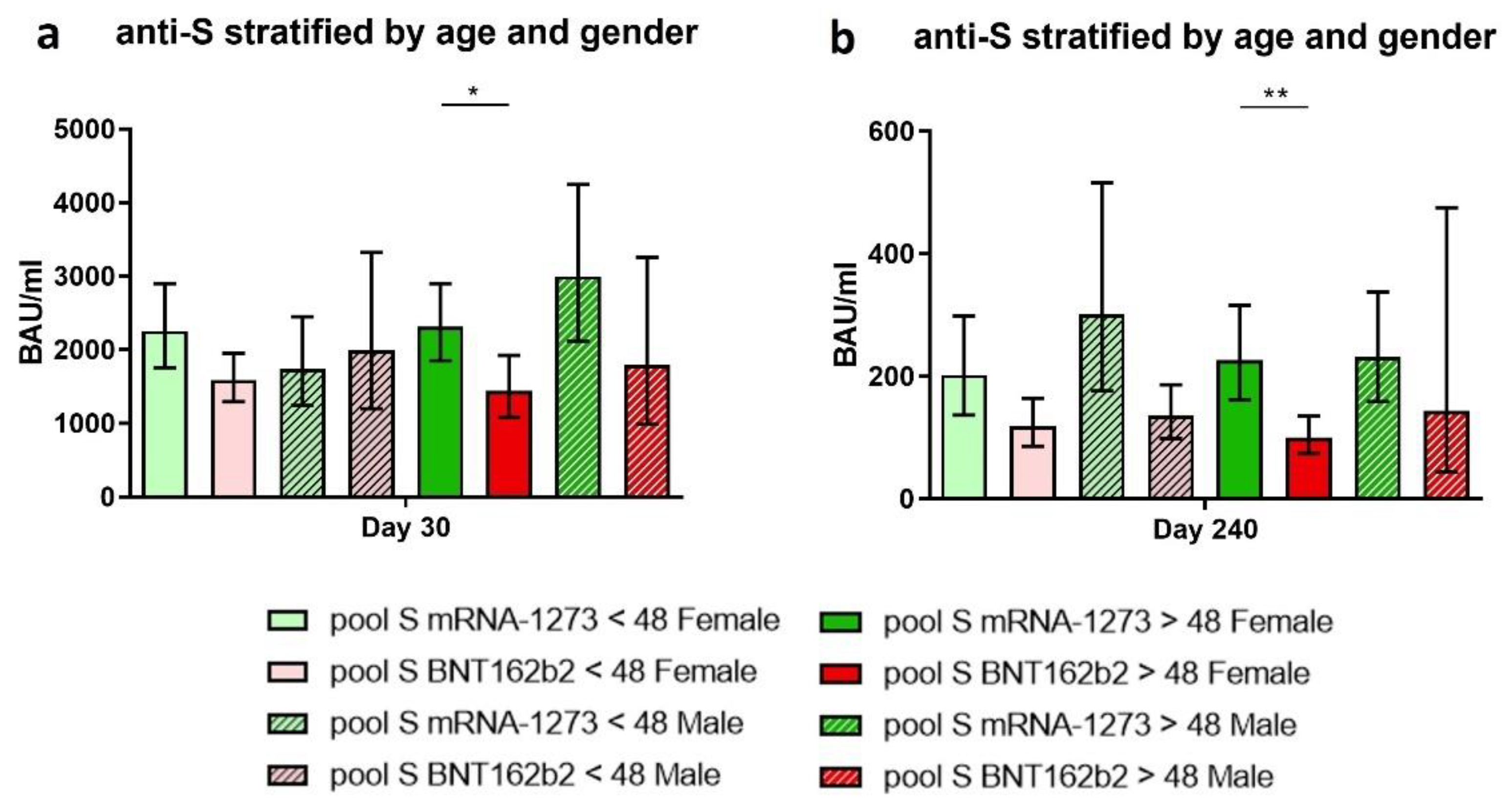
3.7. The Implication of Age and Gender on the Immune Response Achieved after Vaccination
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines-a new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magne, R.; Gomard, E.; Guillet, J.G.; Levy, J.P.; Meulien, P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993, 23, 1719–1722. [Google Scholar] [CrossRef]
- Hess, P.R.; Boczkowski, D.; Nair, S.K.; Snyder, D.; Gilboa, E. Vaccination with mRNAs encoding tumor-associated antigens and granulocyte-macrophage colony-stimulating factor efficiently primes CTL responses, but is insufficient to overcome tolerance to a model tumor/self antigen. Cancer Immunol. Immunother. 2006, 55, 672–683. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2-Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Shen, C.; Peng, W.; Li, D.; Zhao, C.; Li, Z.; Li, S.; Bi, Y.; Yang, Y.; et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020, 368, 1274–1278. [Google Scholar] [CrossRef]
- Parry, H.; Bruton, R.; Stephens, C.; Bentley, C.; Brown, K.; Amirthalingam, G.; Hallis, B.; Otter, A.; Zuo, J.; Moss, P. Extended interval BNT162b2 vaccination enhances peak antibody generation. NPJ Vaccines 2022, 7, 1–5. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for COVID-19. N. Engl. J. Med. 2021, 384, 2259–2261. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef] [PubMed]
- Sokal, A.; Barba-Spaeth, G.; Fernandez, I.; Broketa, M.; Azzaoui, I.; de La Selle, A.; Vandenberghe, A.; Fourati, S.; Roeser, A.; Meola, A.; et al. mRNA vaccination of naive and COVID-19-recovered individuals elicits potent memory B cells that recognize SARS-CoV-2 variants. Immunity 2021, 54, 2893–2907.e5. [Google Scholar] [CrossRef]
- Cho, A.; Muecksch, F.; Schaefer-Babajew, D.; Wang, Z.; Finkin, S.; Gaebler, C.; Ramos, V.; Cipolla, M.; Mendoza, P.; Agudelo, M.; et al. Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. Nature 2021, 600, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Gil-Manso, S.; Carbonell, D.; Lopez-Fernandez, L.; Miguens, I.; Alonso, R.; Buno, I.; Munoz, P.; Ochando, J.; Pion, M.; Correa-Rocha, R. Induction of High Levels of Specific Humoral and Cellular Responses to SARS-CoV-2 After the Administration of COVID-19 mRNA Vaccines Requires Several Days. Front. Immunol. 2021, 12, 726960. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef]
- Jalkanen, P.; Kolehmainen, P.; Hakkinen, H.K.; Huttunen, M.; Tahtinen, P.A.; Lundberg, R.; Maljanen, S.; Reinholm, A.; Tauriainen, S.; Pakkanen, S.H.; et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Coppeta, L.; Ferrari, C.; Somma, G.; Mazza, A.; D’Ancona, U.; Marcuccilli, F.; Grelli, S.; Aurilio, M.T.; Pietroiusti, A.; Magrini, A.; et al. Reduced Titers of Circulating Anti-SARS-CoV-2 Antibodies and Risk of COVID-19 Infection in Healthcare Workers during the Nine Months after Immunization with the BNT162b2 mRNA Vaccine. Vaccines 2022, 10, 141. [Google Scholar] [CrossRef]
- Belda, F.; Mora, O.; Christie, R.; Crowley, M. A longitudinal seroconversion panel shows anti-SARS-CoV-2 antibody levels up to 6.5 months after vaccination with mRNA-1273 (Moderna). medRxiv 2022. [Google Scholar] [CrossRef]
- Keshavarz, B.; Richards, N.E.; Workman, L.J.; Patel, J.; Muehling, L.M.; Canderan, G.; Murphy, D.D.; Brovero, S.G.; Ailsworth, S.M.; Eschenbacher, W.H.; et al. Trajectory of IgG to SARS-CoV-2 After Vaccination With BNT162b2 or mRNA-1273 in an Employee Cohort and Comparison With Natural Infection. Front. Immunol. 2022, 13, 850987. [Google Scholar] [CrossRef]
- Gallagher, K.M.E.; Leick, M.B.; Larson, R.C.; Berger, T.R.; Katsis, K.; Yam, J.Y.; Maus, M.V. Differential T cell immunity to SARS-CoV-2 in mRNA-1273 and BNT162b2 vaccinated individuals. Clin. Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Naranbhai, V.; Garcia-Beltran, W.F.; Chang, C.C.; Mairena, C.B.; Thierauf, J.C.; Kirkpatrick, G.; Onozato, M.L.; Cheng, J.; St Denis, K.J.; Lam, E.C.; et al. Comparative immunogenicity and effectiveness of mRNA-1273, BNT162b2 and Ad26.COV2.S COVID-19 vaccines. J. Infect. Dis. 2021, 225, 1141–1150. [Google Scholar] [CrossRef]
- Le Bert, N.; Clapham, H.E.; Tan, A.T.; Chia, W.N.; Tham, C.Y.L.; Lim, J.M.; Kunasegaran, K.; Tan, L.W.L.; Dutertre, C.A.; Shankar, N.; et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J. Exp. Med. 2021, 218, e20202617. [Google Scholar] [CrossRef]
- Wei, J.; Pouwels, K.B.; Stoesser, N.; Matthews, P.C.; Diamond, I.; Studley, R.; Rourke, E.; Cook, D.; Bell, J.I.; Newton, J.N.; et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat. Med. 2022, 28, 1072–1082. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef]
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, V.; Gadi, N.; Spihlman, A.P.; Wu, S.C.; Choi, C.H.; Moulton, V.R. Aging, Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections? Front. Physiol. 2020, 11, 571416. [Google Scholar] [CrossRef]
- Mohammed, I.; Nauman, A.; Paul, P.; Ganesan, S.; Chen, K.H.; Jalil, S.M.S.; Jaouni, S.H.; Kawas, H.; Khan, W.A.; Vattoth, A.L.; et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum. Vaccin. Immunother. 2022, 20, 2027160. [Google Scholar] [CrossRef] [PubMed]
- Puranik, A.; Lenehan, P.J.; Silvert, E.; Niesen, M.J.M.; Corchado-Garcia, J.; O’Horo, J.C.; Virk, A.; Swift, M.D.; Gordon, J.E.; Speicher, L.L.; et al. Comparative effectiveness of mRNA-1273 and BNT162b2 against symptomatic SARS-CoV-2 infection. Med 2022, 3, 28–41.e8. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadeq, D.W.; Shurrab, F.M.; Ismail, A.; Amanullah, F.H.; Thomas, S.; Aldewik, N.; Yassine, H.M.; Abdul Rahim, H.F.; Abu-Raddad, L.; Nasrallah, G.K. Comparison of antibody immune responses between BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines in naive and previously infected individuals. J. Travel. Med. 2021, 28, 1–5. [Google Scholar] [CrossRef]
- Gray, A.N.; Martin-Blais, R.; Tobin, N.H.; Wang, Y.; Brooker, S.L.; Li, F.; Gadoth, A.; Elliott, J.; Faure-Kumar, E.; Halbrook, M.; et al. Humoral responses to SARS-CoV-2 mRNA vaccines: Role of past infection. PLoS ONE 2021, 16, e0259703. [Google Scholar] [CrossRef] [PubMed]
- Markewitz, R.; Pauli, D.; Dargvainiene, J.; Steinhagen, K.; Engel, S.; Herbst, V.; Zapf, D.; Kruger, C.; Sharifzadeh, S.; Schomburg, B.; et al. B-cell-responses to vaccination with BNT162b2 and mRNA-1273 six months after second dose. Clin. Microbiol. Infect. 2022, 1024, e1–e6. [Google Scholar] [CrossRef]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273. JAMA 2021, 326, 1533–1535. [Google Scholar] [CrossRef]
- Islam, N.; Sheils, N.E.; Jarvis, M.S.; Cohen, K. Comparative effectiveness over time of the mRNA-1273 (Moderna) vaccine and the BNT162b2 (Pfizer-BioNTech) vaccine. Nat. Commun. 2022, 13, 2377. [Google Scholar] [CrossRef]
- Wang, L.; Davis, P.B.; Kaelber, D.C.; Volkow, N.D.; Xu, R. Comparison of mRNA-1273 and BNT162b2 Vaccines on Breakthrough SARS-CoV-2 Infections, Hospitalizations, and Death During the Delta-Predominant Period. JAMA 2022, 327, 678–680. [Google Scholar] [CrossRef]
- Aldén, M.; Olofsson Falla, F.; Yang, D.; Barghouth, M.; Luan, C.; Rasmussen, M.; De Marinis, Y. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr. Issues Mol. Biol. 2022, 44, 1115–1126. [Google Scholar] [CrossRef]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. COVID-19 Vaccine Cominarty: Summary of Product Characteristics. 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf (accessed on 21 March 2022).
- European Medicines Agency. COVID-19 Vaccine Spikevax: Summary of Product Characteristics. 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/spikevax-previously-covid-19-vaccine-moderna-epar-product-information_en.pdf (accessed on 21 March 2022).
- Hall, V.G.; Ferreira, V.H.; Wood, H.; Ierullo, M.; Majchrzak-Kita, B.; Manguiat, K.; Robinson, A.; Kulasingam, V.; Humar, A.; Kumar, D. Delayed-interval BNT162b2 mRNA COVID-19 vaccination enhances humoral immunity and induces robust T cell responses. Nat. Immunol. 2022, 23, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Cueto-Manzano, A.M.; Espinel-Bermudez, M.C.; Hernandez-Gonzalez, S.O.; Rojas-Campos, E.; Nava-Zavala, A.H.; Fuentes-Orozco, C.; Balderas-Pena, L.M.A.; Gonzalez-Ojeda, A.; Cortes-Sanabria, L.; Mireles-Ramirez, M.A.; et al. Risk factors for mortality of adult patients with COVID-19 hospitalised in an emerging country: A cohort study. BMJ Open 2021, 11, e050321. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.K.; Petermann-Rocha, F.; Gray, S.R.; Jani, B.D.; Katikireddi, S.V.; Niedzwiedz, C.L.; Foster, H.; Hastie, C.E.; Mackay, D.F.; Gill, J.M.R.; et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS ONE 2020, 15, e0241824. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Y.; He, Y.; Liu, X.; Liu, M.; Tang, Y.; Li, X.; Yang, G.; Liang, G.; Xu, S.; et al. Age-Related Risk Factors and Complications of Patients With COVID-19: A Population-Based Retrospective Study. Front. Med. 2021, 8, 757459. [Google Scholar] [CrossRef]
- Brockman, M.A.; Mwimanzi, F.; Lapointe, H.R.; Sang, Y.; Agafitei, O.; Cheung, P.; Ennis, S.; Ng, K.; Basra, S.; Lim, L.Y.; et al. Reduced magnitude and durability of humoral immune responses to COVID-19 mRNA vaccines among older adults. J. Infect. Dis. 2021, 225, 1125–1140. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Nanishi, E.; Levy, O.; Ozonoff, A. Waning effectiveness of SARS-CoV-2 mRNA vaccines in older adults: A rapid review. Hum. Vaccin. Immunother. 2022, 18, 2045857. [Google Scholar] [CrossRef]
- Shirazi, S.; Stanford, C.M.; Cooper, L.F. Testing for COVID-19 in dental offices: Mechanism of action, application, and interpretation of laboratory and point-of-care screening tests. J. Am. Dent. Assoc. 2021, 152, 514–525.e8. [Google Scholar] [CrossRef]
- Shirazi, S.; Stanford, C.M.; Cooper, L.F. Characteristics and Detection Rate of SARS-CoV-2 in Alternative Sites and Specimens Pertaining to Dental Practice: An Evidence Summary. J. Clin. Med. 2021, 10, 1158. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; St Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466.e4. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | mRNA-1273 | BNT162b2 | p-Value |
|---|---|---|---|
| Volunteers, n | 68 | 82 | |
| Age (years), mean (SEM) | 47.85 (± 1.38) | 46.52 (± 1.36) | 0.495 |
| Days between 2nd dose and first extraction, median (range) | 27 (24–28) | 31 (23–40) | |
| Days between 2nd dose and second extraction, median (range) | 241 (227–266) | 246 (225–279) | |
| Gender, n (%) | 0.275 | ||
| Male | 14 (20.6) | 11 (13.4) | |
| Female | 54 (79.4) | 71 (86.6) | |
| Ethnicity, n (%) | 1.000 | ||
| Caucasian | 65 (95.6) | 78 (95.1) | |
| Latin-American | 3 (4.4) | 4 (4.9) | |
| Comorbidities, n (%) | |||
| Hyperthyroidism | 0 (0.0) | 1 (1.2) | 0.469 |
| Hypothyroidism | 5 (7.4) | 1 (1.2) | 0.091 |
| Hypertension | 5 (7.4) | 3 (3.6) | 1.000 |
| Dyslipidemia | 3 (4.4) | 4 (4.8) | 1.000 |
| Hypercholesterolemia | 0 (0.0) | 2 (2.4) | 0.501 |
| Diabetes | 0 (0.0) | 1 (1.2) | 1.000 |
| Pulmonary disease | 5 (7.4) | 2 (2.4) | 0.245 |
| Characteristics | mRNA-1273 < 48 Female | BNT162b2 < 48 Female | mRNA-1273 < 48 Male | BNT162b2 < 48 Male | mRNA-1273 > 48 Female | BNT162b2 > 48 Female | mRNA-1273 > 48 Male | BNT162b2 > 48 Male |
|---|---|---|---|---|---|---|---|---|
| Volunteers, n | 26 | 37 | 6 | 3 | 28 | 34 | 8 | 8 |
| Ethnicity, n (%) | ||||||||
| Caucasian | 24 (92.3) | 35 (94.6) | 6 | 3 | 27 (96.4) | 32 (94.1) | 8 | 8 |
| Latin-American | 2 (7.7) | 2 (5.4) | 0 | 0 | 1 (3.6) | 2 (5.9) | 0 | 0 |
| Comorbidities, n (%) | ||||||||
| Hyperthyroidism | 0 | 1 (2.7) | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypothyroidism | 2 (7.7) | 0 | 0 | 0 | 3 (10.7) | 1 (2.9) | 0 | 0 |
| Hypertension | 0 | 0 | 0 | 1 (33.3) | 4 (14.3) | 2 (5.9) | 1 (12.5) | 0 |
| Dyslipidemia | 0 | 0 | 0 | 0 | 2 (7.1) | 3 (8.8) | 1 (12.5) | 1 (12.5) |
| Hypercholesterolemia | 0 | 1 (2.7) | 0 | 0 | 0 | 1 (2.9) | 0 | 0 |
| Diabetes | 0 | 0 | 0 | 0 | 0 | 1 (2.9) | 0 | 0 |
| Pulmonary disease | 2 (7.7) | 1 (2.7) | 0 | 0 | 2 (7.1) | 1 (2.9) | 1 (12.5) | 0 |
| Patient | Gender | Age Years | Anti-S IgG (BAU/mL) | Days between 2nd Dose and Positive Test | Days between 2nd Dose and Anti-S IgG Measurement | Days between Anti-S IgG Measurement and Positive Test | Anti-SARS-CoV-2 Test (Variant) |
|---|---|---|---|---|---|---|---|
| Patient 1 | Female | 63 | 964.24 | 66 | 40 | 26 | PCR test (B.1.1.7) |
| Patient 2 | Female | 62 | 3909.64 | 170 | 31 | 139 | PCR test |
| Patient 3 | Female | 26 | 2121.22 | 183 | 31 | 152 | Antigen test |
| Patient 4 | Female | 54 | 4332.11 | 185 | 31 | 154 | PCR test (B.1.617) |
| Rest of BNT162b2 volunteers | Male n = 11 Female n = 67 | 46 [22–65] | 1939.42 | - | 32 | - | - |
| Total Non-Protected at Day 240 Post-Vaccination N (%) | |
|---|---|
| Number of volunteers vaccinated with mRNA-1273 with IgG (<100 BAU/mL // >100 BAU/mL) | 7 // 56 (12.5%) |
| Number of volunteers vaccinated with BNT162b2 with IgG (<100 BAU/mL // >100 BAU/mL) | 33 // 74 (45%) |
| Day 30 Post-Vaccination | r for mRNA-1273 | p-Value | r for BNT162b2 | p-Value |
| Anti-S IgG–IFN-γ | 0.1728 | 0.162 | 0.4169 | <0.001 |
| Anti-S IgG–IL-2 | 0.2717 | 0.026 | 0.2659 | 0.016 |
| Anti-S IgG–IL-4 | 0.0390 | 0.753 | 0.0304 | 0.799 |
| Anti-S IgG–IL-10 | 0.2111 | 0.086 | 0.0974 | 0.386 |
| Day 240 Post-Vaccination | r for mRNA-1273 | p-Value | r for BNT162b2 | p-Value |
| Anti-S IgG–IFN-γ | 0.1904 | 0.159 | 0.2577 | 0.026 |
| Anti-S IgG–IL-2 | 0.2367 | 0.084 | 0.1902 | 0.104 |
| Anti-S IgG–IL-4 | 0.1882 | 0.177 | 0.1025 | 0.478 |
| Anti-S IgG–IL-10 | 0.0073 | 0.957 | 0.1691 | 0.149 |
| Day 30 Post-Vaccination | r for mRNA-1273 | p-Value | r for BNT162b2 | p-Value |
| IFN-γ–IL-2 | 0.4041 | <0.001 | 0.7708 | <0.001 |
| IFN-γ–IL-4 | 0.1683 | 0.173 | 0.5305 | <0.001 |
| IFN-γ–IL-10 | 0.3577 | 0.003 | 0.4533 | <0.001 |
| Day 240 Post-Vaccination | r for mRNA-1273 | p-Value | r for BNT162b2 | p-Value |
| IFN-γ–IL-2 | 0.6332 | <0.001 | 0.8145 | <0.001 |
| IFN-γ–IL-4 | 0.5723 | <0.001 | 0.5442 | <0.001 |
| IFN-γ–IL-10 | 0.3486 | 0.008 | 0.4464 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Manso, S.; Carbonell, D.; Pérez-Fernández, V.A.; López-Esteban, R.; Alonso, R.; Muñoz, P.; Ochando, J.; Sánchez-Arcilla, I.; Bellón, J.M.; Correa-Rocha, R.; et al. Cellular and Humoral Responses Follow-up for 8 Months after Vaccination with mRNA-Based Anti-SARS-CoV-2 Vaccines. Biomedicines 2022, 10, 1676. https://doi.org/10.3390/biomedicines10071676
Gil-Manso S, Carbonell D, Pérez-Fernández VA, López-Esteban R, Alonso R, Muñoz P, Ochando J, Sánchez-Arcilla I, Bellón JM, Correa-Rocha R, et al. Cellular and Humoral Responses Follow-up for 8 Months after Vaccination with mRNA-Based Anti-SARS-CoV-2 Vaccines. Biomedicines. 2022; 10(7):1676. https://doi.org/10.3390/biomedicines10071676
Chicago/Turabian StyleGil-Manso, Sergio, Diego Carbonell, Verónica Astrid Pérez-Fernández, Rocío López-Esteban, Roberto Alonso, Patricia Muñoz, Jordi Ochando, Ignacio Sánchez-Arcilla, Jose M Bellón, Rafael Correa-Rocha, and et al. 2022. "Cellular and Humoral Responses Follow-up for 8 Months after Vaccination with mRNA-Based Anti-SARS-CoV-2 Vaccines" Biomedicines 10, no. 7: 1676. https://doi.org/10.3390/biomedicines10071676
APA StyleGil-Manso, S., Carbonell, D., Pérez-Fernández, V. A., López-Esteban, R., Alonso, R., Muñoz, P., Ochando, J., Sánchez-Arcilla, I., Bellón, J. M., Correa-Rocha, R., & Pion, M. (2022). Cellular and Humoral Responses Follow-up for 8 Months after Vaccination with mRNA-Based Anti-SARS-CoV-2 Vaccines. Biomedicines, 10(7), 1676. https://doi.org/10.3390/biomedicines10071676







