Susceptibility of Drug Resistant Hepatitis B Virus Mutants to Besifovir
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinically Isolated HBV Mutant Clones and Construction of Artificial HBV Mutants
2.2. Cell Culture, Transfection and Drug Treatment
2.3. ELISA
2.4. Southern Blot
2.5. Statistically Analysis
3. Results
3.1. The LMV-Resistant MV Mutant Is Resistant to BFV
3.2. The ADV-Resistant RT Mutants Are Susceptible to BFV
3.3. The ETV-Resistant RT Mutants Are partially Resistant to BFV
3.4. The HBV Mutants Harboring Primary Mutations to TFV Resistance Are Susceptible to BFV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, M.H.; Wong, G.; Gane, E.; Kao, J.H.; Dusheiko, G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin. Microbiol. Rev. 2020, 33, e00046-19. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Budzinska, M.A.; Vondran, F.W.R.; Shackel, N.A.; Urban, S. Hepatitis B Virus DNA Integration Occurs Early in the Viral Life Cycle in an In Vitro Infection Model via Sodium Taurocholate Cotransporting Polypeptide-Dependent Uptake of Enveloped Virus Particles. J. Virol. 2018, 92, e02007-17. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.; Chakraborty, A.; Chou, W.M.; Hasreiter, J.; Wettengel, J.M.; Stadler, D.; Bester, R.; Asen, T.; Zhang, K.; Wisskirchen, K.; et al. Hepatitis B virus genome recycling and de novo secondary infection events maintain stable cccDNA levels. J. Hepatol. 2018, 69, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Dusseaux, M.; Masse-Ranson, G.; Darche, S.; Ahodantin, J.; Li, Y.; Fiquet, O.; Beaumont, E.; Moreau, P.; Riviere, L.; Neuveut, C.; et al. Viral Load Affects the Immune Response to HBV in Mice With Humanized Immune System and Liver. Gastroenterology 2017, 153, 1647–1661. [Google Scholar] [CrossRef]
- Konig, A.; Yang, J.; Jo, E.; Park, K.H.P.; Kim, H.; Than, T.T.; Song, X.; Qi, X.; Dai, X.; Park, S.; et al. Efficient long-term amplification of hepatitis B virus isolates after infection of slow proliferating HepG2-NTCP cells. J. Hepatol. 2019, 71, 289–300. [Google Scholar] [CrossRef]
- Yasutake, Y.; Hattori, S.I.; Tamura, N.; Matsuda, K.; Kohgo, S.; Maeda, K.; Mitsuya, H. Structural features in common of HBV and HIV-1 resistance against chirally-distinct nucleoside analogues entecavir and lamivudine. Sci. Rep. 2020, 10, 3021. [Google Scholar] [CrossRef]
- Seifer, M.; Patty, A.; Serra, I.; Li, B.; Standring, D.N. Telbivudine, a nucleoside analog inhibitor of HBV polymerase, has a different in vitro cross-resistance profile than the nucleotide analog inhibitors adefovir and tenofovir. Antivir. Res. 2009, 81, 147–155. [Google Scholar] [CrossRef]
- Lopatin, U. Drugs in the Pipeline for HBV. Clin. Liver Dis. 2019, 23, 535–555. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Park, Y.K.; Ahn, S.H.; Cho, E.S.; Choe, W.H.; Lee, C.H.; Kim, B.K.; Ko, S.Y.; Choi, H.S.; Park, E.S.; et al. Identification and characterization of clevudine-resistant mutants of hepatitis B virus isolated from chronic hepatitis B patients. J. Virol. 2010, 84, 4494–4503. [Google Scholar] [CrossRef]
- Hoofnagle, J.H.; Doo, E.; Liang, T.J.; Fleischer, R.; Lok, A.S. Management of hepatitis B: Summary of a clinical research workshop. Hepatology 2007, 45, 1056–1075. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, Y.K.; Park, E.S.; Kim, K.H. Molecular diagnosis and treatment of drug-resistant hepatitis B virus. World J. Gastroenterol. 2014, 20, 5708–5720. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Dienstag, J.; Schiff, E.; Leung, N.W.; Atkins, M.; Hunt, C.; Brown, N.; Woessner, M.; Boehme, R.; Condreay, L. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2003, 36, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.F.; Seto, W.K.; Chow, D.H.; Tsui, K.; Wong, D.K.; Ngai, V.W.; Wong, B.C.; Fung, J.; Yuen, J.C.; Lai, C.L. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antivir. Ther. 2007, 12, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Park, E.S.; Lee, A.R.; Kim, D.H.; Lee, J.H.; Yoo, J.J.; Ahn, S.H.; Sim, H.; Park, S.; Kang, H.S.; Won, J.; et al. Identification of a quadruple mutation that confers tenofovir resistance in chronic hepatitis B patients. J. Hepatol. 2019, 70, 1093–1102. [Google Scholar] [CrossRef]
- Fung, J.; Lai, C.L.; Yuen, M.F. LB80380: A promising new drug for the treatment of chronic hepatitis B. Expert Opin. Investig. Drugs 2008, 17, 1581–1588. [Google Scholar] [CrossRef]
- Ahn, S.H.; Kim, W.; Jung, Y.K.; Yang, J.M.; Jang, J.Y.; Kweon, Y.O.; Cho, Y.K.; Kim, Y.J.; Hong, G.Y.; Kim, D.J.; et al. Efficacy and Safety of Besifovir Dipivoxil Maleate Compared With Tenofovir Disoproxil Fumarate in Treatment of Chronic Hepatitis B Virus Infection. Clin. Gastroenterol. Hepatol. 2019, 17, 1850–1859. [Google Scholar] [CrossRef]
- Lai, C.L.; Ahn, S.H.; Lee, K.S.; Um, S.H.; Cho, M.; Yoon, S.K.; Lee, J.W.; Park, N.H.; Kweon, Y.O.; Sohn, J.H.; et al. Phase IIb multicentred randomised trial of besifovir (LB80380) versus entecavir in Asian patients with chronic hepatitis B. Gut 2014, 63, 996–1004. [Google Scholar] [CrossRef]
- Jung, J.A.; Kim, S.R.; Kim, T.E.; Kim, J.R.; Lee, S.Y.; Huh, W.; Ko, J.W. Pharmacokinetic comparison of the maleate and free base formulations of LB80380, a novel nucleotide analog, in healthy male volunteers. Int. J. Clin. Pharmacol. Ther. 2012, 50, 657–664. [Google Scholar] [CrossRef]
- Yuen, M.F.; Kim, J.; Kim, C.R.; Ngai, V.; Yuen, J.C.; Min, C.; Kang, H.M.; Shin, B.S.; Yoo, S.D.; Lai, C.L. A randomized placebo-controlled, dose-finding study of oral LB80380 in HBeAg-positive patients with chronic hepatitis B. Antivir. Ther. 2006, 11, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Korean Association for the Study of the Liver. KASL clinical practice guidelines for management of chronic hepatitis B. Clin. Mol. Hepatol. 2019, 25, 93–159. [Google Scholar] [CrossRef] [PubMed]
- Song, D.S.; Kim, W.; Ahn, S.H.; Yim, H.J.; Jang, J.Y.; Kweon, Y.O.; Cho, Y.K.; Kim, Y.J.; Hong, G.Y.; Kim, D.J.; et al. Continuing besifovir dipivoxil maleate versus switching from tenofovir disoproxil fumarate for treatment of chronic hepatitis B: Results of 192-week phase 3 trial. Clin. Mol. Hepatol. 2021, 27, 346–359. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, H.Y.; Lee, A.R.; Dezhbord, M.; Lee, D.R.; Kim, S.H.; Won, J.; Park, S.; Kim, N.Y.; Shin, J.J.; et al. Identification and Characterization of Besifovir-Resistant Hepatitis B Virus Isolated from a Chronic Hepatitis B Patient. Biomedicines 2022, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Park, Y.K.; Park, E.S.; Kim, J.H.; Kim, D.H.; Lim, K.H.; Jang, M.S.; Choe, W.H.; Ko, S.Y.; Sung, I.K.; et al. The impact of the hepatitis B virus polymerase rtA181T mutation on replication and drug resistance is potentially affected by overlapping changes in surface gene. J. Virol. 2014, 88, 6805–6818. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, E.S.; Koo, J.E.; Park, Y.K.; Lee, A.R.; Dezhbord, M.; Cho, E.S.; Ahn, S.H.; Kim, D.H.; Lee, J.H.; et al. Entecavir-resistant hepatitis B virus decreases surface antigenicity: A full genome and functional characterization. Liver Int. 2020, 40, 1564–1577. [Google Scholar] [CrossRef]
- Lee, A.R.; Cho, J.Y.; Kim, J.C.; Dezhbord, M.; Choo, S.Y.; Ahn, C.H.; Kim, N.Y.; Shin, J.J.; Park, S.; Park, E.S.; et al. Distinctive HBV Replication Capacity and Susceptibility to Tenofovir Induced by a Polymerase Point Mutation in Hepatoma Cell Lines and Primary Human Hepatocytes. Int. J. Mol. Sci. 2021, 22, 1606. [Google Scholar] [CrossRef]
- Zoulim, F.; Locarnini, S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology 2009, 137, 1593–1608. [Google Scholar] [CrossRef]
- Nakajima, S.; Watashi, K.; Kato, T.; Muramatsu, M.; Wakita, T.; Tamura, N.; Hattori, S.I.; Maeda, K.; Mitsuya, H.; Yasutake, Y.; et al. Biochemical and Structural Properties of Entecavir-Resistant Hepatitis B Virus Polymerase with L180M/M204V Mutations. J. Virol. 2021, 95, e0240120. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Chen, Y.U.; Zheng, S.; Zhou, L.I.; Lu, F.; Duan, Z. Lamivudine-resistant rtL180M and rtM204I/V are persistently dominant during combination rescue therapy with entecavir and adefovir for hepatitis B. Exp. Ther. Med. 2016, 11, 2293–2299. [Google Scholar] [CrossRef][Green Version]
- Lin, C.L.; Yang, H.C.; Kao, J.H. Hepatitis B virus: New therapeutic perspectives. Liver Int. Off. J. Int. Assoc. Study Liver 2016, 36 (Suppl. S1), 85–92. [Google Scholar] [CrossRef]
- Scruggs, E.R.; Dirks Naylor, A.J. Mechanisms of zidovudine-induced mitochondrial toxicity and myopathy. Pharmacology 2008, 82, 83–88. [Google Scholar] [CrossRef]
- Delaney, W.E.T.; Yang, H.; Westland, C.E.; Das, K.; Arnold, E.; Gibbs, C.S.; Miller, M.D.; Xiong, S. The hepatitis B virus polymerase mutation rtV173L is selected during lamivudine therapy and enhances viral replication in vitro. J. Virol. 2003, 77, 11833–11841. [Google Scholar] [CrossRef]
- Pal, A.; Sarkar, N.; Saha, D.; Guha, S.K.; Saha, B.; Chakrabarti, S.; Chakravarty, R. High incidence of lamivudine-resistance-associated vaccine-escape HBV mutants among HIV-coinfected patients on prolonged antiretroviral therapy. Antivir. Ther. 2015, 20, 545–554. [Google Scholar] [CrossRef]
- Yin, F.; Wu, Z.; Fang, W.; Wu, C.; Rayner, S.; Han, M.; Deng, F.; Du, R.; Liu, J.; Wang, M.; et al. Resistant mutations and quasispecies complexity of hepatitis B virus during telbivudine treatment. J. Gen. Virol. 2015, 96, 3302–3312. [Google Scholar] [CrossRef]
- Yuen, M.F.; Han, K.H.; Um, S.H.; Yoon, S.K.; Kim, H.R.; Kim, J.; Kim, C.R.; Lai, C.L. Antiviral activity and safety of LB80380 in hepatitis B e antigen-positive chronic hepatitis B patients with lamivudine-resistant disease. Hepatology 2010, 51, 767–776. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, N.D.; Seong, B.L. Discovery and development of anti-HBV agents and their resistance. Molecules 2010, 15, 5878–5908. [Google Scholar] [CrossRef]
- Song, J.E.; Park, J.Y. Besifovir dipivoxil maleate: A novel antiviral agent with low toxicity and high genetic barriers for chronic hepatitis B. Expert Opin. Pharmacother. 2021, 22, 2427–2433. [Google Scholar] [CrossRef]
- Yim, H.J.; Kim, W.; Ahn, S.H.; Yang, J.M.; Jang, J.Y.; Kweon, Y.O.; Cho, Y.K.; Kim, Y.J.; Hong, G.Y.; Kim, D.J.; et al. Besifovir Dipivoxil Maleate 144-Week Treatment of Chronic Hepatitis B: An Open-Label Extensional Study of a Phase 3 Trial. Am. J. Gastroenterol. 2020, 115, 1217–1225. [Google Scholar] [CrossRef]
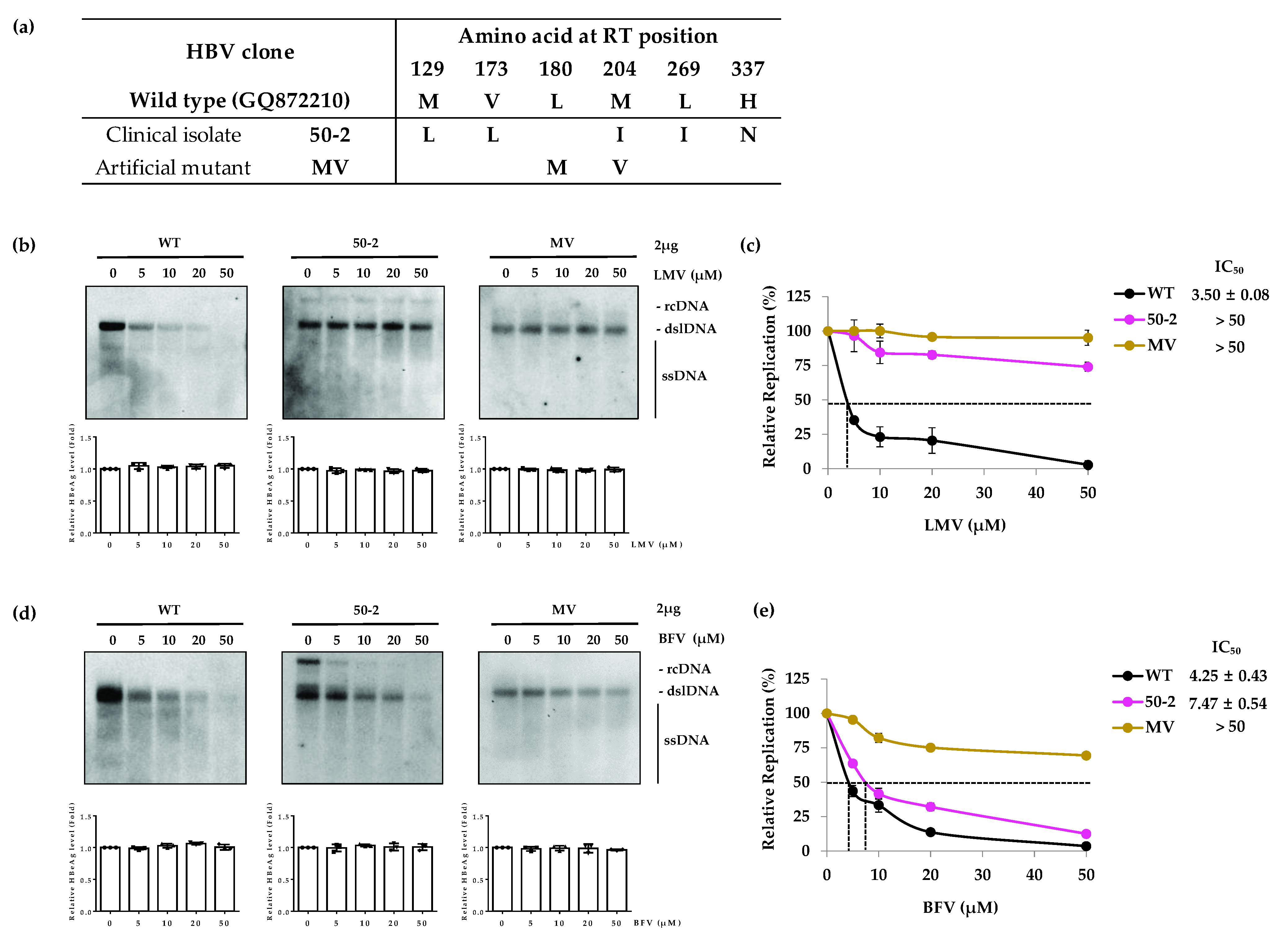
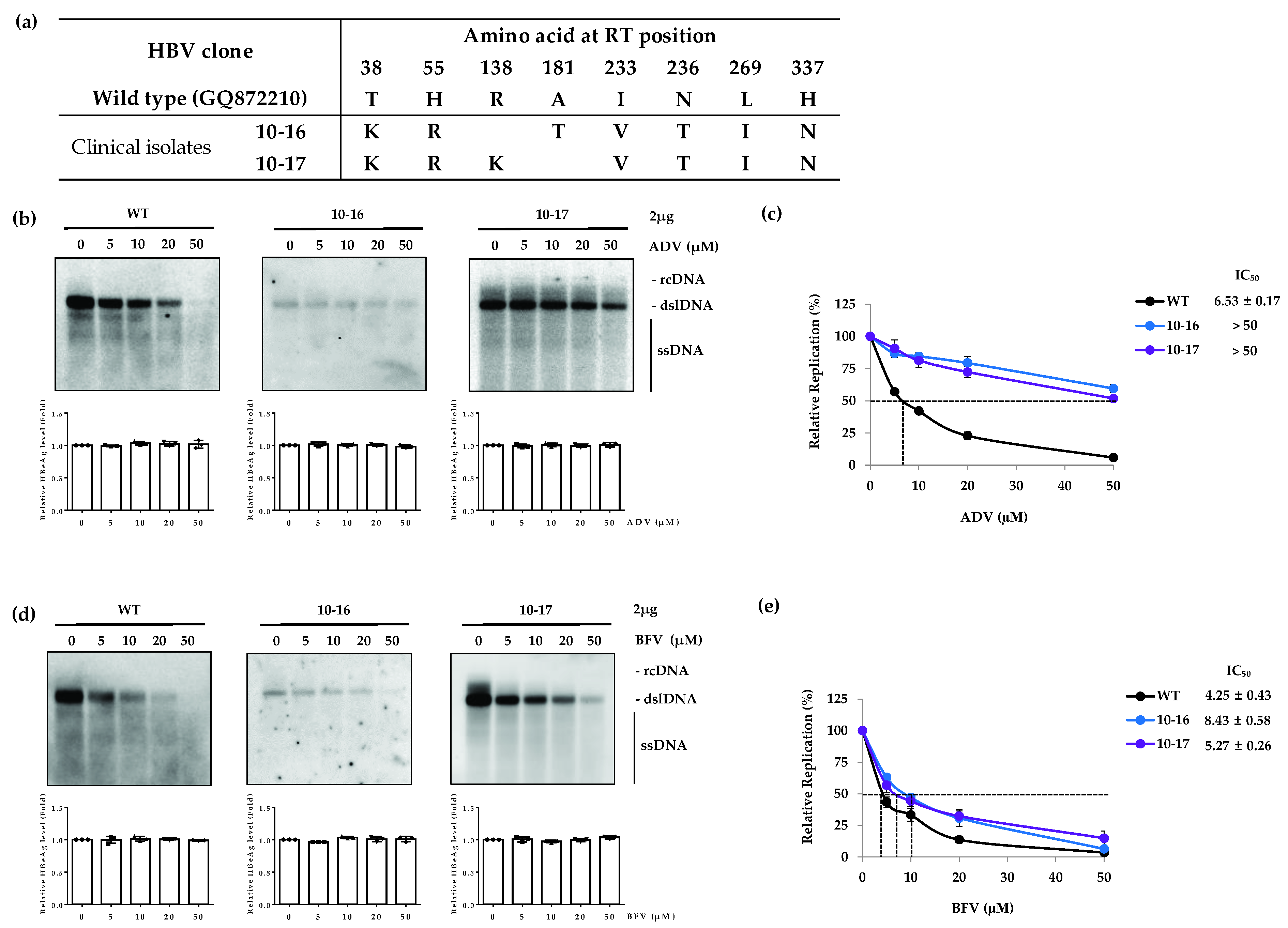
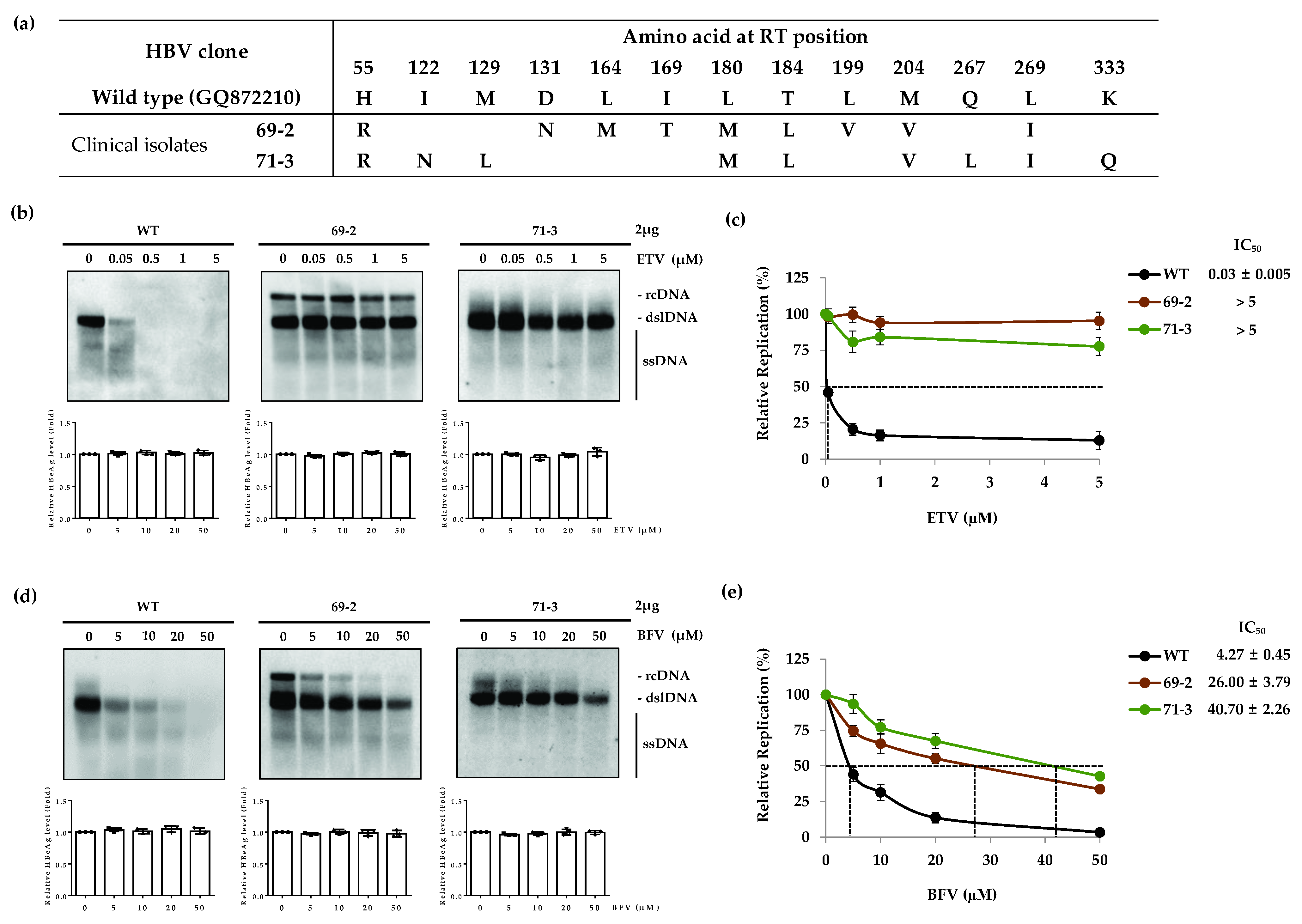
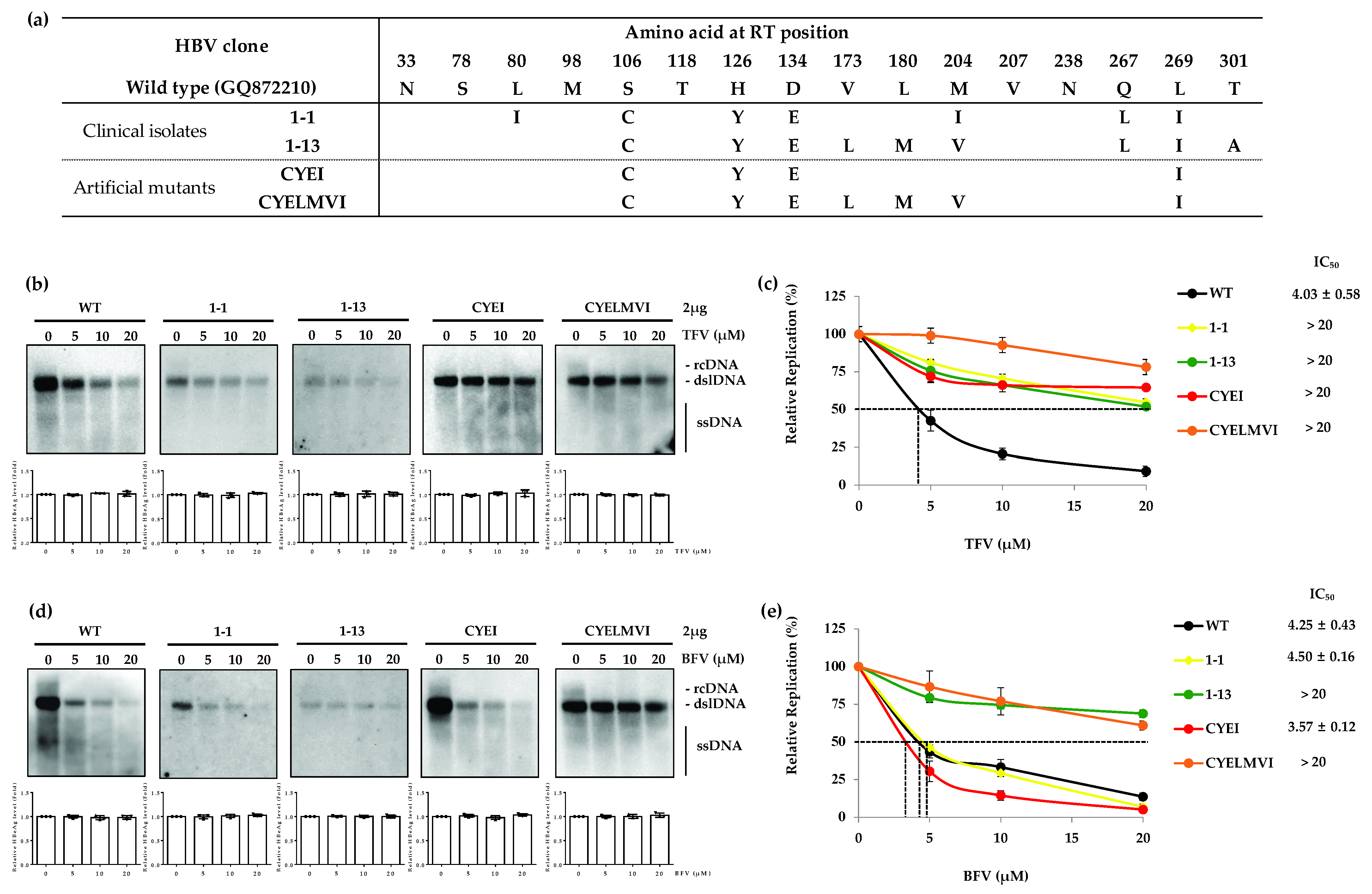
| Drug | Clone | IC50 (µM) (Fold Resistance) | ||||
|---|---|---|---|---|---|---|
| Resistance | BFV | LMV | ADV | ETV | TFV | |
| WT | 4.25 ± 0.43 (1) | 3.50 ± 0.08 (1) | 6.53 ± 0.17 (1) | 0.03 ± 0.005 (1) | 4.03 ± 0.58 (1) | |
| LMV/CLV/ETV | 50-2 | 7.47 ± 0.54 (1.8) | >50 (>14.2) | |||
| MV | >50 (>11.8) | >50 (>14.2) | ||||
| ADV | 10-16 | 8.43 ± 0.58 (2.0) | >50 (>7.6) | |||
| 10-17 | 5.27 ± 0.26 (1.2) | >50 (>7.6) | ||||
| ETV | 69-2 | 26.00 ± 3.79 (6.1) | >5 (>166.7) | |||
| 71-3 | 40.70 ± 2.26 (9.6) | >5 (>166.7) | ||||
| TFV | 1-1 | 4.50 ± 0.16 (1.1) | >20 | |||
| 1-13 | >20 (>4.7) | >20 | ||||
| CYEI | 3.57 ± 0.12 (0.8) | >20 | ||||
| CYELMVI | >20 (>4.7) | >20 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, J.; Lee, A.R.; Dezhbord, M.; Lee, D.R.; Kim, S.H.; Kim, J.C.; Park, S.; Kim, N.; Jae, B.; Kim, K.-H. Susceptibility of Drug Resistant Hepatitis B Virus Mutants to Besifovir. Biomedicines 2022, 10, 1637. https://doi.org/10.3390/biomedicines10071637
Won J, Lee AR, Dezhbord M, Lee DR, Kim SH, Kim JC, Park S, Kim N, Jae B, Kim K-H. Susceptibility of Drug Resistant Hepatitis B Virus Mutants to Besifovir. Biomedicines. 2022; 10(7):1637. https://doi.org/10.3390/biomedicines10071637
Chicago/Turabian StyleWon, Juhee, Ah Ram Lee, Mehrangiz Dezhbord, Da Rae Lee, Seong Ho Kim, Jong Chul Kim, Soree Park, Nayeon Kim, Byengjune Jae, and Kyun-Hwan Kim. 2022. "Susceptibility of Drug Resistant Hepatitis B Virus Mutants to Besifovir" Biomedicines 10, no. 7: 1637. https://doi.org/10.3390/biomedicines10071637
APA StyleWon, J., Lee, A. R., Dezhbord, M., Lee, D. R., Kim, S. H., Kim, J. C., Park, S., Kim, N., Jae, B., & Kim, K.-H. (2022). Susceptibility of Drug Resistant Hepatitis B Virus Mutants to Besifovir. Biomedicines, 10(7), 1637. https://doi.org/10.3390/biomedicines10071637






