Cross-Protection Induced by Virus-like Particles Derived from the Influenza B Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice, Cells, and Viruses
2.2. Generation of Recombinant Baculovirus and Virus-like Particles
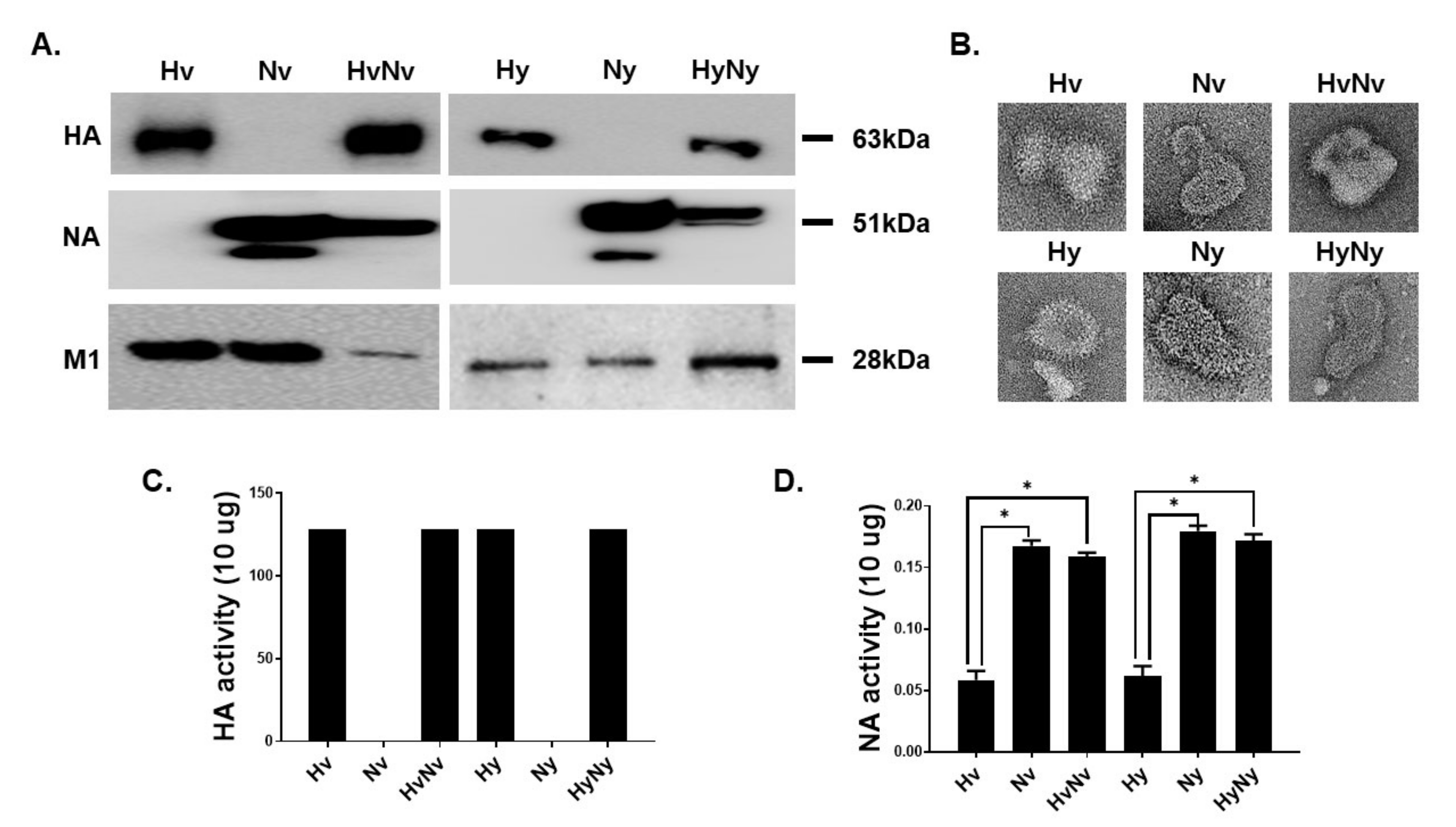
2.3. Western Blot
2.4. Hemagglutinin Activity
2.5. Neuraminidase Activity
2.6. Transmission Electron Microscopy (TEM)
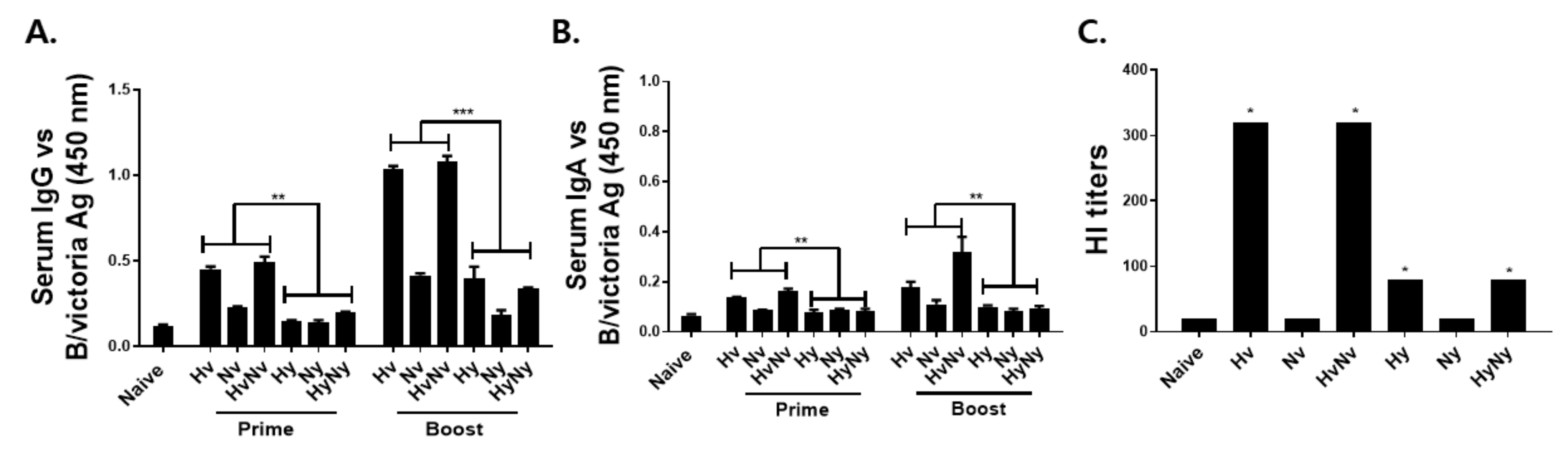
2.7. Hemagglutinin Inhibition Assay
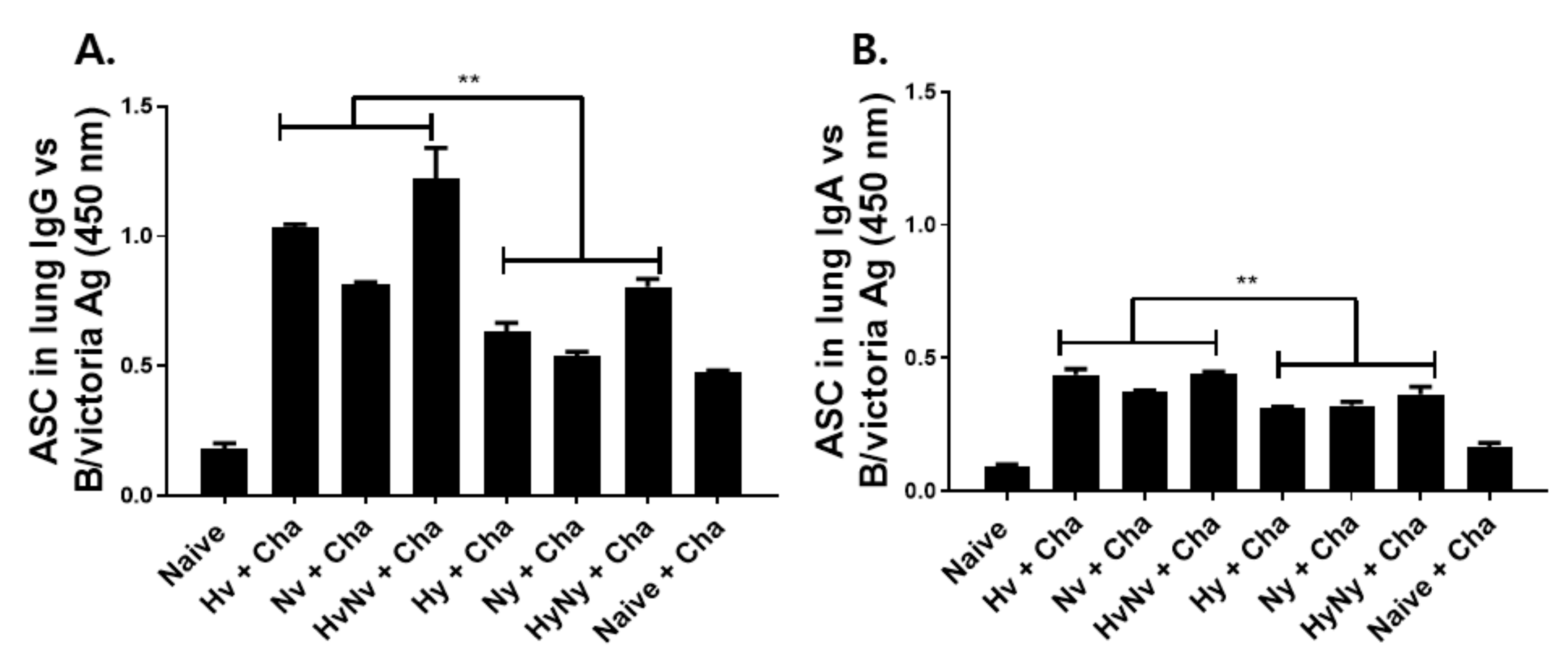
2.8. Antibody Secreting Cells (ASC) Assay
2.9. Antibody Secreting Cells (ASC) Assay
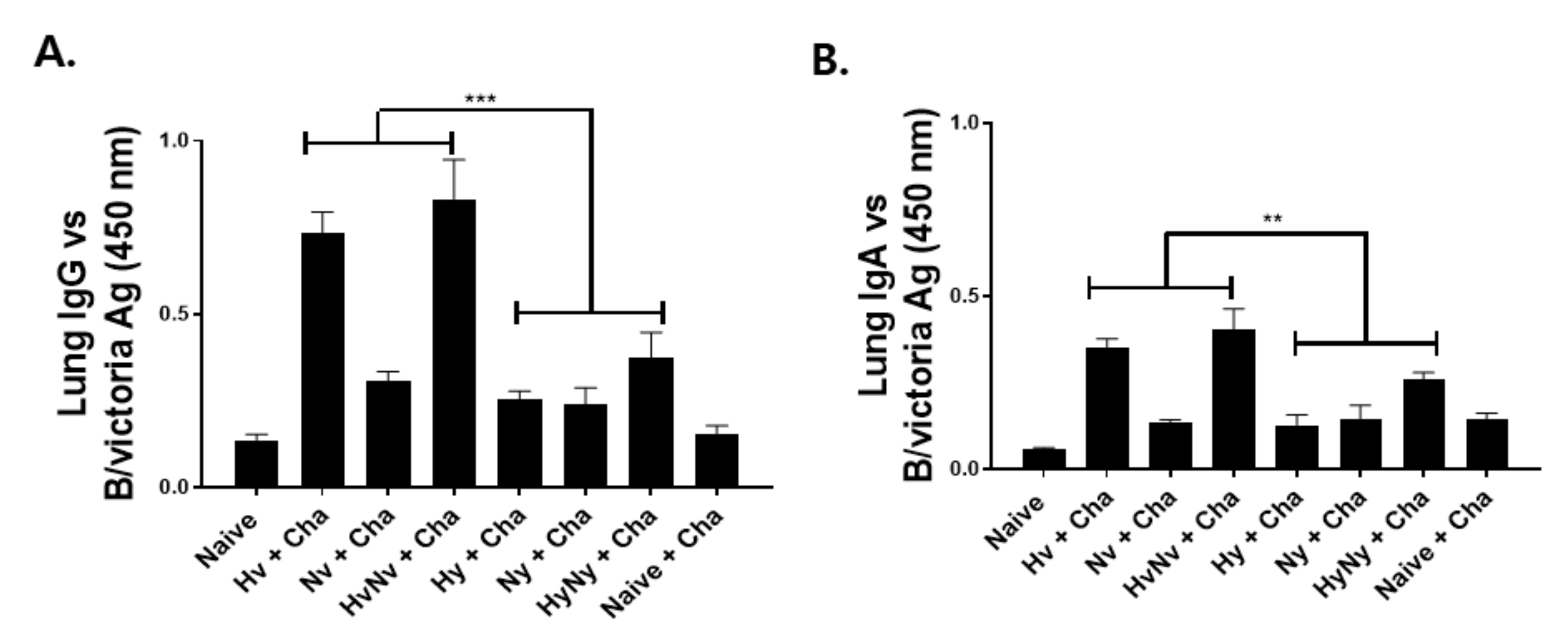
2.10. Enzyme-Linked Immunosorbent Assay (ELISA) for Antibody and Cytokine Production
2.11. Flow Cytometry Assessment of Lung Cell Populations
2.12. Lung Virus Titer
2.13. Statistical Analysis
3. Results
3.1. Characterization of the Influenza B VLPs Containing HA and NA
3.2. Immunization with Influenza B VLPs Induced Antibody Response in Serum
3.3. Immunization with Influenza B VLPs Induced Antibody Responses in the Lung
3.4. Influenza B VLPs Immunization Induces Effective ASC Responses
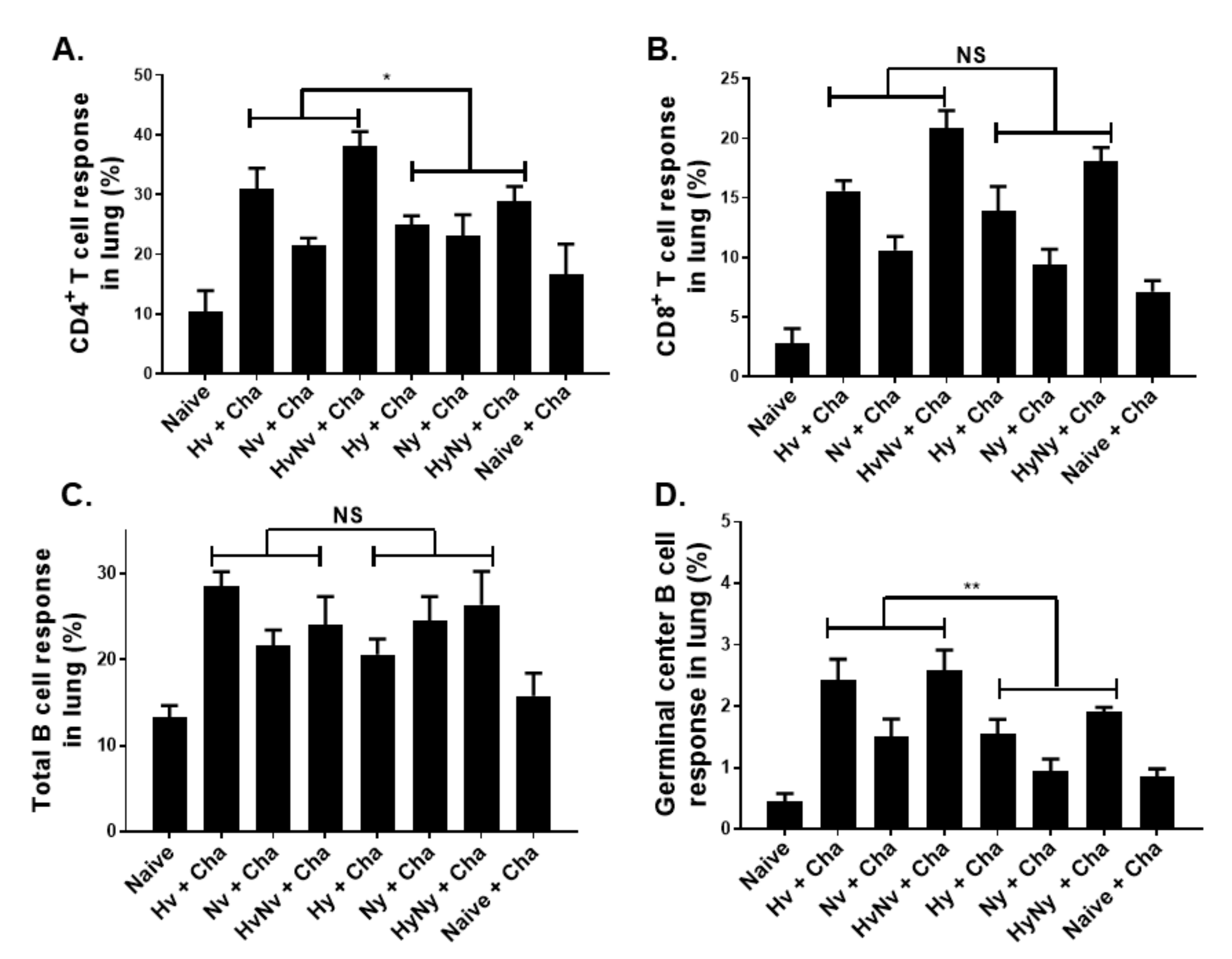
3.5. Influenza B VLPs Induced a Higher Level of Cellular Immune Cell Response
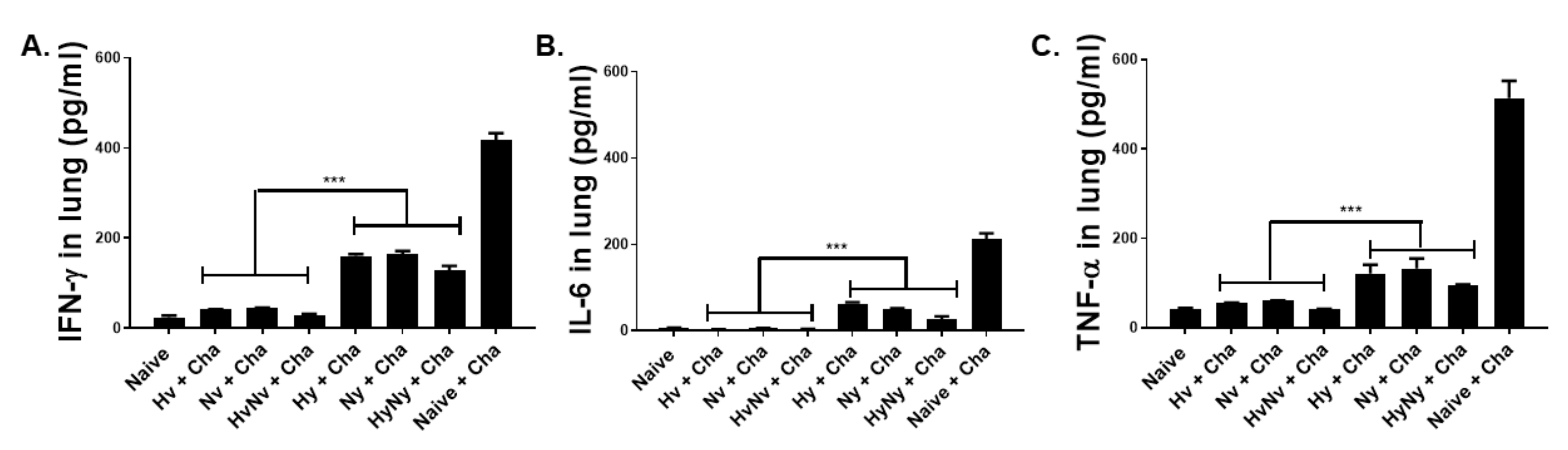
3.6. Influenza B VLPs Immunization Inhibits Lung Inflammatory Cytokine Expressions
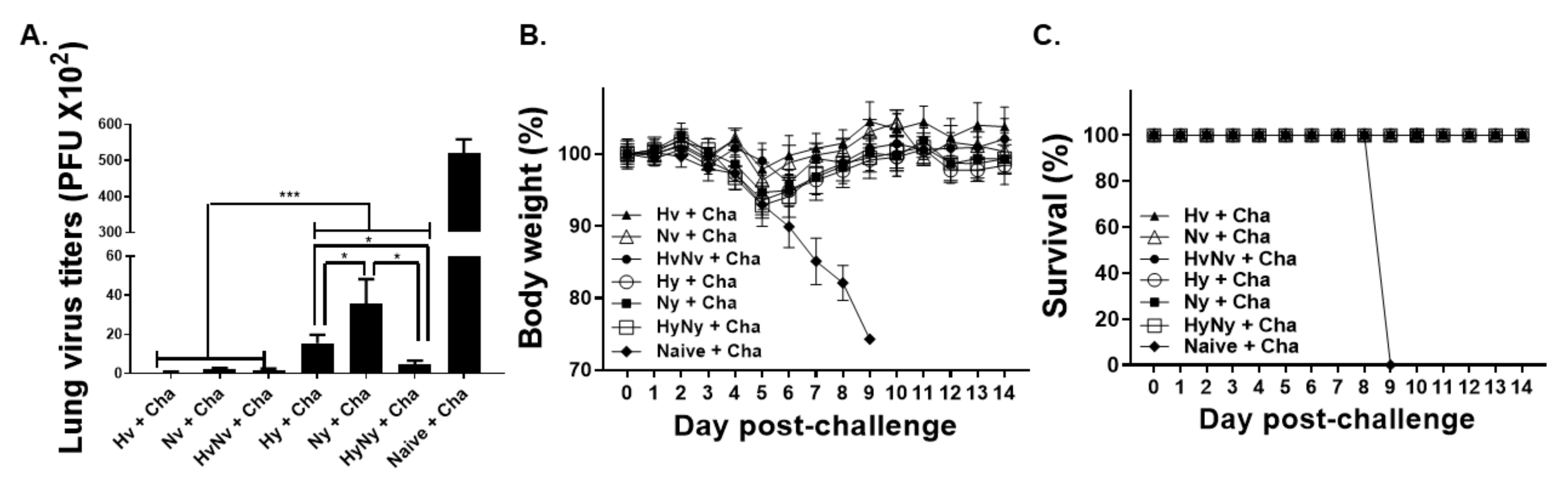
3.7. Influenza B VLP Immunization Contributes to Protecting Mice from Lethal Influenza B Virus Challenge
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaitonde, D.Y.; Moore, F.C.; Morgan, M.K. Influenza: Diagnosis and treatment. Am. Fam. Fam. Physician 2019, 100, 751–758. [Google Scholar]
- Taubenberger, J.K.; Morens, D.M. Influenza: The once and future pandemic. Public Health Rep. 2010, 125, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Virk, R.K.; Jayakumar, J.; Mendenhall, I.H.; Moorthy, M.; Lam, P.; Linster, M.; Lim, J.; Lin, C.; Oon, L.L.; Lee, H.K. Divergent evolutionary trajectories of influenza B viruses underlie their contemporaneous epidemic activity. Proc. Natl. Acad. Sci. USA 2020, 117, 619–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul Glezen, W.; Schmier, J.K.; Kuehn, C.M.; Ryan, K.J.; Oxford, J. The burden of influenza B: A structured literature review. Am. J. Public Health 2013, 103, e43–e51. [Google Scholar] [CrossRef]
- Zaraket, H.; Hurt, A.C.; Clinch, B.; Barr, I.; Lee, N. Burden of influenza B virus infection and considerations for clinical management. Antivir. Res. 2021, 185, 104970. [Google Scholar] [CrossRef]
- Terebuh, P.; Uyeki, T.; Fukuda, K. Impact of influenza on young children and the shaping of United States influenza vaccine policy. Pediatr. Infect. Dis. J. 2003, 22, S231–S235. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Adlhoch, C.; Snacken, R.; Melidou, A.; Ionescu, S.; Penttinen, P. Dominant influenza A (H3N2) and B/Yamagata virus circulation in EU/EEA, 2016/17 and 2017/18 seasons, respectively. Eurosurveillance 2018, 23, 18-00146. [Google Scholar] [CrossRef] [Green Version]
- Morbidity and Mortality Weekly Report. Never their hand. Also discuss the impor-tance of adequate hydration with patients who are sick. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 634–642. [Google Scholar]
- Borchering, R.K.; Gunning, C.E.; Gokhale, D.V.; Weedop, K.B.; Saeidpour, A.; Brett, T.S.; Rohani, P. Anomalous influenza seasonality in the United States and the emergence of novel influenza B viruses. Proc. Natl. Acad. Sci. USA 2021, 118, e2012327118. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevntion. FluView Interactive. Available online: https://www.cdc.gov/flu/weekly/fluviewinteractive.htm (accessed on 6 April 2022).
- Ambrose, C.S.; Levin, M.J. The rationale for quadrivalent influenza vaccines. Hum. Vaccines Immunother. 2012, 8, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Heikkinen, T.; Ikonen, N.; Ziegler, T. Impact of influenza B lineage-level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999–2012. Clin. Infect. Dis. 2014, 59, 1519–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Sandt, C.E.; Dou, Y.; Vogelzang-van Trierum, S.E.; Westgeest, K.B.; Pronk, M.R.; Osterhaus, A.D.; Fouchier, R.A.; Rimmelzwaan, G.F.; Hillaire, M.L. Influenza B virus-specific CD8+ T-lymphocytes strongly cross-react with viruses of the opposing influenza B lineage. J. Gen. Virol. 2015, 96, 2061. [Google Scholar] [CrossRef] [PubMed]
- Van De Sandt, C.E.; Bodewes, R.; Rimmelzwaan, G.F.; De Vries, R.D. Influenza B viruses: Not to be discounted. Future Microbiol. 2015, 10, 1447–1465. [Google Scholar] [CrossRef] [PubMed]
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Blanton, L.H.; Fry, A.M.; Jernigan, D.B.; Atmar, R.L. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020–21 influenza season. MMWR Recomm. Rep. 2020, 69, 1. [Google Scholar] [CrossRef] [PubMed]
- Stech, J.; Garn, H.; Herwig, A.; Stech, O.; Dauber, B.; Wolff, T.; Mettenleiter, T.C.; Klenk, H.-D. Influenza B virus with modified hemagglutinin cleavage site as a novel attenuated live vaccine. J. Infect. Dis. 2011, 204, 1483–1490. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.J.; Finch, C.; Sutton, T.; Obadan, A.; Aguirre, I.; Wan, Z.; Lopez, D.; Geiger, G.; Gonzalez-Reiche, A.S.; Ferreri, L. Development of an alternative modified live influenza B virus vaccine. J. Virol. 2017, 91, e00056-17. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Kang, J.-O.; Kim, J.-Y.; Jung, H.E.; Lee, H.K.; Chang, J. Single mucosal vaccination targeting nucleoprotein provides broad protection against two lineages of influenza B virus. Antivir. Res. 2019, 163, 19–28. [Google Scholar] [CrossRef]
- Piepenbrink, M.S.; Nogales, A.; Basu, M.; Fucile, C.F.; Liesveld, J.L.; Keefer, M.C.; Rosenberg, A.F.; Martinez-Sobrido, L.; Kobie, J.J. Broad and protective influenza B virus neuraminidase antibodies in humans after vaccination and their clonal persistence as plasma cells. MBio 2019, 10, e00066-19. [Google Scholar] [CrossRef] [Green Version]
- Zeigler, D.F.; Gage, E.; Clegg, C.H. Epitope-targeting platform for broadly protective influenza vaccines. PLoS ONE 2021, 16, e0252170. [Google Scholar] [CrossRef]
- Belshe, R.B. The need for quadrivalent vaccine against seasonal influenza. Vaccine 2010, 28, D45–D53. [Google Scholar] [CrossRef] [PubMed]
- Stech, J.; Garn, H.; Wegmann, M.; Wagner, R.; Klenk, H. A new approach to an influenza live vaccine: Modification of the cleavage site of hemagglutinin. Nat. Med. 2005, 11, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.M.; Mahmood, K.; Crevar, C.J.; Schneider-Ohrum, K.; Heaton, P.M.; Bright, R.A. A trivalent virus-like particle vaccine elicits protective immune responses against seasonal influenza strains in mice and ferrets. PLoS ONE 2009, 4, e6032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone, L.A.; Ahmad, A.; Veguilla, V.; Lu, X.; Smith, G.; Katz, J.M.; Pushko, P.; Tumpey, T.M. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J. Virol. 2009, 83, 5726–5734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Macías, C. Virus-like particle (VLP)-based vaccines for pandemic influenza: Performance of a VLP vaccine during the 2009 influenza pandemic. Hum. Vaccines Immunother. 2012, 8, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Gwon, Y.-D.; Kim, S.; Cho, Y.; Heo, Y.; Cho, H.; Park, K.; Lee, H.-J.; Choi, J.; Poo, H.; Kim, Y.B. Immunogenicity of virus like particle forming baculoviral DNA vaccine against pandemic influenza H1N1. PLoS ONE 2016, 11, e0154824. [Google Scholar]
- Kang, Y.-M.; Cho, H.-K.; Kim, J.H.; Lee, S.J.; Park, S.-J.; Kim, D.-Y.; Kim, S.Y.; Park, J.-w.; Lee, M.-H.; Kim, M.-C. Single dose of multi-clade virus-like particle vaccine protects chickens against clade 2.3. 2.1 and clade 2.3. 4.4 highly pathogenic avian influenza viruses. Sci. Rep. 2021, 11, 13786. [Google Scholar] [CrossRef]
- Schwartzman, L.M.; Cathcart, A.L.; Pujanauski, L.M.; Qi, L.; Kash, J.C.; Taubenberger, J.K. An intranasal virus-like particle vaccine broadly protects mice from multiple subtypes of influenza A virus. MBio 2015, 6, e01044-15. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-Y.; Yeh, Y.-C.; Chan, J.-T.; Yang, Y.-C.; Yang, J.-R.; Liu, M.-T.; Wu, H.-S.; Hsiao, P.-W. A VLP vaccine induces broad-spectrum cross-protective antibody immunity against H5N1 and H1N1 subtypes of influenza A virus. PLoS ONE 2012, 7, e42363. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.-J.; Chu, K.-B.; Lee, D.-H.; Lee, S.-H.; Park, B.R.; Kim, M.-C.; Kang, S.-M.; Quan, F.-S. Influenza M2 virus-like particle vaccination enhances protection in combination with avian influenza HA VLPs. PLoS ONE 2019, 14, e0216871. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.-J.; Chu, K.-B.; Yoon, K.-W.; Eom, G.-D.; Mao, J.; Kim, M.-J.; Lee, S.-H.; Moon, E.-K.; Quan, F.-S. Neuraminidase in Virus-like Particles Contributes to the Protection against High Dose of Avian Influenza Virus Challenge Infection. Pathogens 2021, 10, 1291. [Google Scholar] [CrossRef]
- Basak, S.; Kang, H.-J.; Lee, S.-H.; Chu, K.-B.; Moon, E.-K.; Quan, F.-S. Influenza vaccine efficacy induced by orally administered recombinant baculoviruses. PLoS ONE 2020, 15, e0233520. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.-S.; Huang, C.; Compans, R.W.; Kang, S.-M. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J. Virol. 2007, 81, 3514–3524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palm, A.-K.E.; Henry, C. Remembrance of things past: Long-term B cell memory after infection and vaccination. Front. Immunol. 2019, 10, 1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horns, F.; Dekker, C.L.; Quake, S.R. Memory B cell activation, broad anti-influenza antibodies, and bystander activation revealed by single-cell transcriptomics. Cell Rep. 2020, 30, 905–913.e906. [Google Scholar] [CrossRef] [Green Version]
- Lam, J.H.; Baumgarth, N. The multifaceted B cell response to influenza virus. J. Immunol. 2019, 202, 351–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manz, R.A.; Hauser, A.E.; Hiepe, F.; Radbruch, A. Maintenance of serum antibody levels. Annu. Rev. Immunol. 2005, 23, 367–386. [Google Scholar] [CrossRef]
- De Vries, R.D.; Nieuwkoop, N.J.; van der Klis, F.R.; Koopmans, M.P.; Krammer, F.; Rimmelzwaan, G.F. Primary human influenza B virus infection induces cross-lineage hemagglutinin stalk–specific antibodies mediating antibody-dependent cellular cytoxicity. J. Infect. Dis. 2018, 217, 3. [Google Scholar] [CrossRef]
- De Vries, R.D.; Nieuwkoop, N.J.; Krammer, F.; Hu, B.; Rimmelzwaan, G.F. Analysis of the vaccine-induced influenza B virus hemagglutinin-specific antibody dependent cellular cytotoxicity response. Virus Res. 2020, 277, 197839. [Google Scholar] [CrossRef]
- Asahi-Ozaki, Y.; Yoshikawa, T.; Iwakura, Y.; Suzuki, Y.; Tamura, S.i.; Kurata, T.; Sata, T. Secretory IgA antibodies provide cross-protection against infection with different strains of influenza B virus. J. Med. Virol. 2004, 74, 328–335. [Google Scholar] [CrossRef]
- Getie-Kebtie, M.; Sultana, I.; Eichelberger, M.; Alterman, M. Label-free mass spectrometry-based quantification of hemagglutinin and neuraminidase in influenza virus preparations and vaccines. Influenza Other Respir. Viruses 2013, 7, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Buffin, S.; Peubez, I.; Barrière, F.; Nicolaï, M.-C.; Tapia, T.; Dhir, V.; Forma, E.; Sève, N.; Legastelois, I. Influenza A and B virus-like particles produced in mammalian cells are highly immunogenic and induce functional antibodies. Vaccine 2019, 37, 6857–6867. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.L.; Lo, C.-Y.; Misplon, J.A.; Bennink, J.R. Mechanism of protective immunity against influenza virus infection in mice without antibodies. J. Immunol. 1998, 160, 322–327. [Google Scholar]
- Rattan, A.; White, C.L.; Nelson, S.; Eismann, M.; Padilla-Quirarte, H.; Glover, M.A.; Dileepan, T.; Marathe, B.M.; Govorkova, E.A.; Webby, R.J. Development of a Mouse Model to Explore CD4 T Cell Specificity, Phenotype, and Recruitment to the Lung after Influenza B Infection. Pathogens 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.E.; Brett, I.C. Recombinant influenza B virus HA and NA antigens administered in equivalent amounts are immunogenically equivalent and induce equivalent homotypic and broader heterovariant protection in mice than conventional and live influenza vaccines. Hum. Vaccines 2008, 4, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Hervas-Stubbs, S.; Rueda, P.; Lopez, L.; Leclerc, C. Insect baculoviruses strongly potentiate adaptive immune responses by inducing type I IFN. J. Immunol. 2007, 178, 2361–2369. [Google Scholar] [CrossRef] [Green Version]
- McMahon, M.; Kirkpatrick, E.; Stadlbauer, D.; Strohmeier, S.; Bouvier, N.M.; Krammer, F. Mucosal immunity against neuraminidase prevents influenza B virus transmission in Guinea Pigs. MBio 2019, 10, e00560-19. [Google Scholar] [CrossRef] [Green Version]
- Gravel, C.; Muralidharan, A.; Duran, A.; Zetner, A.; Pfeifle, A.; Zhang, W.; Hashem, A.; Tamming, L.; Farnsworth, A.; Loemba, H. Synthetic vaccine affords full protection to mice against lethal challenge of influenza B virus of both genetic lineages. iScience 2021, 24, 103328. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.-J.; Chu, K.-B.; Yoon, K.-W.; Eom, G.-D.; Mao, J.; Quan, F.-S. Cross-Protection Induced by Virus-like Particles Derived from the Influenza B Virus. Biomedicines 2022, 10, 1618. https://doi.org/10.3390/biomedicines10071618
Kang H-J, Chu K-B, Yoon K-W, Eom G-D, Mao J, Quan F-S. Cross-Protection Induced by Virus-like Particles Derived from the Influenza B Virus. Biomedicines. 2022; 10(7):1618. https://doi.org/10.3390/biomedicines10071618
Chicago/Turabian StyleKang, Hae-Ji, Ki-Back Chu, Keon-Woong Yoon, Gi-Deok Eom, Jie Mao, and Fu-Shi Quan. 2022. "Cross-Protection Induced by Virus-like Particles Derived from the Influenza B Virus" Biomedicines 10, no. 7: 1618. https://doi.org/10.3390/biomedicines10071618
APA StyleKang, H.-J., Chu, K.-B., Yoon, K.-W., Eom, G.-D., Mao, J., & Quan, F.-S. (2022). Cross-Protection Induced by Virus-like Particles Derived from the Influenza B Virus. Biomedicines, 10(7), 1618. https://doi.org/10.3390/biomedicines10071618





