Tocilizumab Use among Patients Who Developed Pulmonary Embolism in the Course of Cytokine Release Storm and COVID-19 Pneumonia—A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Groups
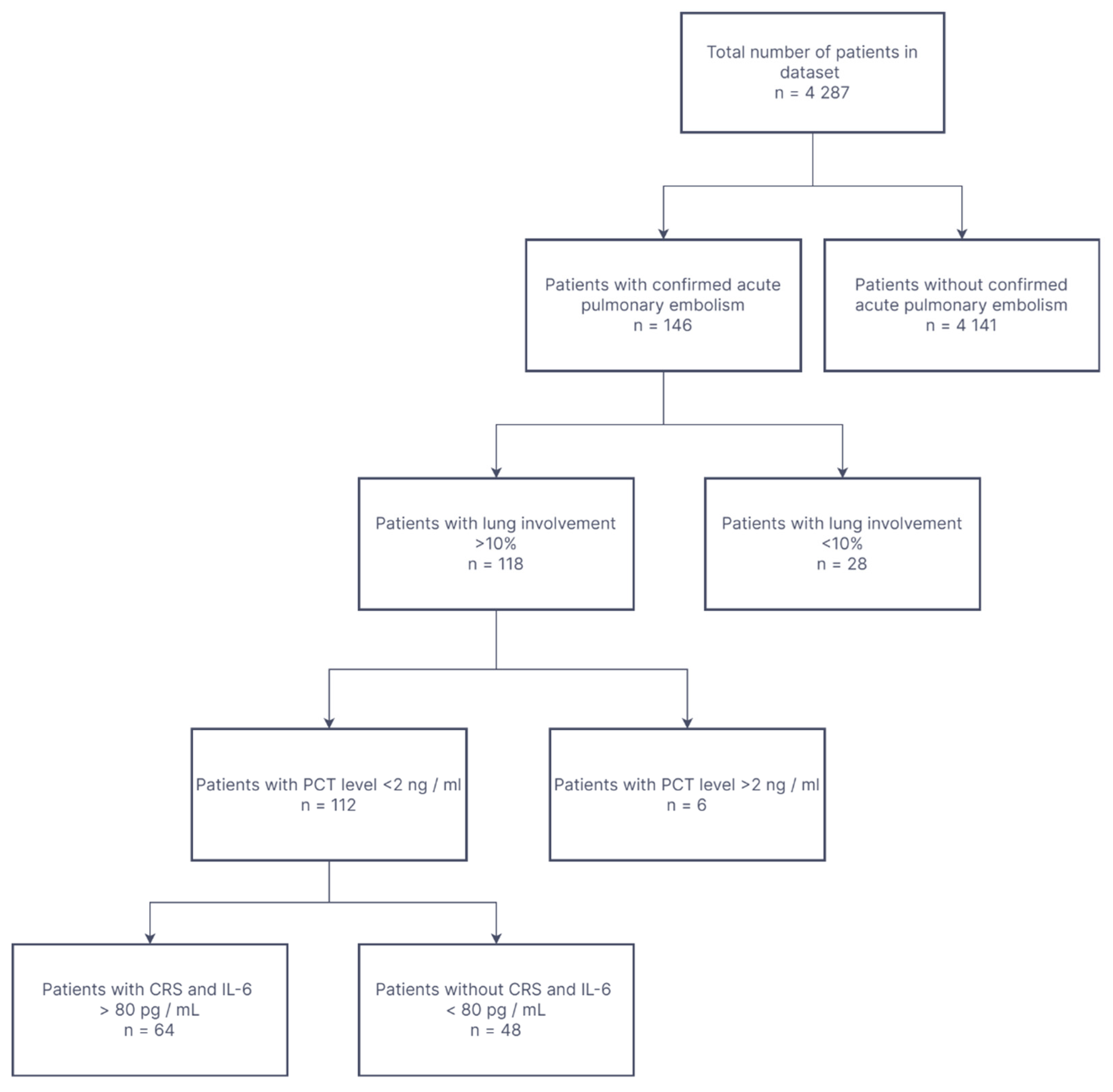
- Inclusion criteria for this study were as follows:
- -
- CT confirmed COVID-19-pneumonia with at least 10% lung involvement;
- -
- CT and clinically proven acute PE;
- -
- CRS with IL-6 levels exceeding 80 pg/mL;
- -
- Age > 18 years;
- -
- Fever > 38 °C at admission to hospital;
- -
- Oxygen saturation less or equal to 90%;
- -
- Confirmed ongoing SARS-CoV-2 infection.
- Exclusion criteria:
- -
- Procalcitonin level > 2 ng/mL-as a predictor of septic complications where TCZ is contraindicated;
- -
- Chloroquine was not used.
- -
- Supportive treatment was applied to each of the patients.The supportive treatment included:
- -
- antibiotic therapy (Ceftriaxon was drug of choice but could vary depending on the patient’s condition);
- -
- Oxygen therapy (Low-/high-flow oxygen therapy or mechanical ventilation were used. No ECMO were used);
- -
- Intravenous rehydration;
- -
- Dexamethasone administered intravenously in a dose of at least 6 mg per day;
- -
- Low-molecular-weight or non-fractionated heparin in therapeutic doses.All patients, in accordance with the applicable standards and guidelines, received:
- -
- Prophylactic antibiotic (preferably ceftriaxone in a dose of at least 2 g per day);
- -
- Glucocorticoids (dexamethasone in a dose of at least 6 mg per day iv);
- -
- Supportive oxygen supplementary therapy;
- -
- Intravenous rehydration.
2.2. Ethical Issues
2.3. Sampling and Data Collection Methodology
2.4. Statistics
3. Results
3.1. Clinical Characteristics of Patients with COVID-19
3.2. Clinical and Laboratory Data on TCZ Treated vs. Control Group
3.3. Overall Mortality Risk
3.4. Tocilizumab-Associated Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis: Epidemiologic Aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Koupenova, M.; Kehrel, B.E.; Corkrey, H.A.; Freedman, J.E. Thrombosis and platelets: An update. Eur. Heart J. 2017, 38, 785–791. [Google Scholar] [CrossRef]
- Wiener, R.S.; Schwartz, L.M.; Woloshin, S. Time trends in pulmonary embolism in the United States: Evidence of overdiagnosis. Arch. Intern. Med. 2011, 171, 831–836. [Google Scholar] [CrossRef] [Green Version]
- Kröger, K.; Moerchel, C.; Moysidis, T.; Santosa, F. Incidence rate of pulmonary embolism in Germany: Data from the federal statistical office. J. Thromb. Thrombolysis 2010, 29, 349–353. [Google Scholar] [CrossRef]
- Aujesky, D.; Hughes, R.; Jiménez, D. Short-term prognosis of pulmonary embolism. J. Thromb. Haemost. 2009, 7 (Suppl. 1), 318–321. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Kayali, F.; Olson, R.E. Estimated case fatality rate of pulmonary embolism, 1979 to 1998. Am. J. Cardiol. 2004, 93, 1197–1199. [Google Scholar] [CrossRef] [PubMed]
- Laporte, S.; Mismetti, P.; Décousus, H.; Uresandi, F.; Otero, R.; Lobo, J.L.; Monreal, M.; RIETE Investigators. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: Findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation 2008, 117, 1711–1716. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/ (accessed on 12 March 2022).
- Elezkurtaj, S.; Greuel, S.; Ihlow, J.; Michaelis, E.G.; Bischoff, P.; Kunze, C.A.; Sinn, B.V.; Gerhold, M.; Hauptmann, K. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci. Rep. 2021, 11, 4263. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Mainbourg, S.; Friggeri, A.; Bertoletti, L.; Douplat, M.; Dargaud, Y.; Grange, C.; Lobbes, H.; Provencher, S.; Lega, J.C. Arterial and venous thromboembolism in COVID-19: A study-level meta-analysis. Thorax 2021, 76, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Kollias, A.; Kyriakoulis, K.G.; Lagou, S.; Kontopantelis, E.; Stergiou, G.S.; Syrigos, K. Venous thromboembolism in COVID-19: A systematic review and meta-analysis. Vasc. Med. 2021, 26, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Hanff, T.C.; Mohareb, A.M.; Giri, J.; Cohen, J.B.; Chirinos, J.A. Thrombosis in COVID-19. Am. J. Hematol. 2020, 95, 1578. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Ceccarelli, G.; Cangemi, R.; Cipollone, F.; D’Ardes, D.; Oliva, A.; Pirro, M.; Rocco, M.; Alessandri, F.; D’Ettorre, G.; et al. Arterial and venous thrombosis in coronavirus 2019 disease (COVID-19): Relationship with mortality. Intern. Emerg. Med. 2021, 16, 1231–1237. [Google Scholar] [CrossRef]
- Suh, Y.J.; Hong, H.; Ohana, M.; Bompard, F.; Revel, M.P.; Valle, C.; Gervaise, A.; Poissy, J.; Susen, S.; Hékimian, G.; et al. Pulmonary Embolism and Deep Vein Thrombosis in COVID-19: A Systematic Review and Meta-Analysis. Radiology 2021, 298, E70–E80. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Yang, P.; Zhang, S. COVID-19 complicated by acute pulmonary embolism. Radiol. Cardiothorac. Imaging 2020, 2, e200067. [Google Scholar] [CrossRef] [Green Version]
- Cheruiyot, I.; Kipkorir, V.; Ngure, B.; Misiani, M.; Munguti, J.; Ogeng’o, J. Arterial Thrombosis in Coronavirus Disease 2019 Patients: A Rapid Systematic Review. Ann. Vasc. Surg. 2021, 70, 273. [Google Scholar] [CrossRef]
- Zakeri, A.; Jadhav, A.P.; Sullenger, B.A.; Nimjee, S.M. Ischemic stroke in COVID-19-positive patients: An overview of SARS-CoV-2 and thrombotic mechanisms for the neurointerventionalist. J. Neurointerv. Surg. 2021, 13, 202–206. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Violi, F.; Pignatelli, P.; Cammisotto, V.; Bartimoccia, S.; Carnevale, R.; Nocella, C. COVID-19 and thrombosis: Clinical features, mechanism of disease, and therapeutic implications. Kardiol. Pol. (Pol. Heart J.) 2021, 79, 1197–1205. [Google Scholar] [CrossRef]
- Libby, P.; Lüscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.M.; Gonagle, D.M.; Ward, S.E.; Preston, R.J.S.; O’Donnell, J.S. Endothelial cells orchestrate COVID-19 coagulopathy. Lancet Haematol. 2020, 7, e553–e555. [Google Scholar] [CrossRef]
- Fiore, J.R.; Ciarallo, M.; Di Stefano, M.; Sica, S.; Scarale, M.; D’Errico, M.; Corallo, F.; Lo Caputo, S.; Margaglione, M.; Santantonio, T. Severe systemic thrombosis in a young COVID-19 patient with a rare homozygous prothrombin G20210A mutation. Infez Med. 2021, 29, 259–262. [Google Scholar] [PubMed]
- Abani, O.; Abbas, A.; Abbas, F.; Abbas, M.; Abbasi, S.; Abbass, H.; Abbott, A.; Abdallah, N.; Abdelaziz, A.; Abdelfattah, M.; et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- Chober, D.; Aksak-Wąs, B.; Bobrek-Lesiakowska, K.; Budny-Finster, A.; Hołda, E.; Mieżyńska-Kurtycz, J.; Jamro, G.; Parczewski, M. Effectiveness of Tocilizumab in Patients with Severe or Critical Lung Involvement in COVID-19: A Retrospective Study. J. Clin. Med. 2022, 11, 2286. [Google Scholar] [CrossRef]
- Herold, T.; Jurinovic, V.; Arnreich, C.; Lipworth, B.J.; Hellmuth, J.C.; von Bergwelt-Baildon, M.; Klein, M.; Weinberger, T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020, 146, 128–136.e4. [Google Scholar] [CrossRef]
- Atallah, B.; El Nekidy, W.; Mallah, S.I.; Cherfan, A.; AbdelWareth, L.; Mallat, J.; Hamed, F. Thrombotic events following tocilizumab therapy in critically ill COVID-19 patients: A Façade for prognostic markers. Thromb. J. 2020, 18, 22. [Google Scholar] [CrossRef]
- Chan, K.H.; Patel, B.; Podel, B.; Szablea, M.E.; Shaaban, H.S.; Guron, G.; Slim, J. Tocilizumab and Thromboembolism in COVID-19: A Retrospective Hospital-Based Cohort Analysis. Cureus 2021, 15, e15208. [Google Scholar] [CrossRef]
- Warrior, S.; Behrens, E.; Thomas, J.; Rajakumar, P.; Gezer, S.; Venugopal, P.; Jain, S. Impact of Treatment and Anticoagulation on Thrombosis in COVID-19 Patients. Blood 2020, 136 (Suppl. 1), 6–7. [Google Scholar] [CrossRef]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Mastalerz-Migas, A.; Owczuk, R.; Parczewski, M.; Pawłowska, M.; Piekarska, A.; Simon, K.; et al. Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of April 26, 2021. Polish Arch. Intern. Med. 2021, 131, 487–496. [Google Scholar] [CrossRef]
- Flisiak, R.; Hor-ban, A.; Jaroszewicz, J.; Kozielewicz, D.; Mastalerz-Migas, A.; Owczuk, R.; Parczewski, M.; Pawlowska, M.; Piek-arska, A.; Simon, K.; et al. Diagnosis and therapy of SARS-CoV-2 infection: Recommendations of the polish association of epidemiologists and infectiologists as of November 12, 2021. Polish Arch. Intern. Med. 2021, 131, 16140. [Google Scholar] [CrossRef]
- Revel, M.P.; Parkar, A.P.; Prosch, H.; Silva, M.; Sverzellati, N.; Gleeson, F.; Brady, A. COVID-19 patients and the radiology department—Advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). Eur. Radiol. 2020, 30, 4903–4909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalaber, C.; Lapotre, T.; Morcet-Delattre, T.; Ribet, F.; Jouneau, S.; Lederlin, M. Chest CT in COVID-19 pneumonia: A review of current knowledge. Diagn. Interv. Imaging 2020, 101, 431–437. [Google Scholar] [CrossRef]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Pawłowska, M.; Parczewski, M.; Piekarska, A.; Simon, K.; Tomasiewicz, K.; Zarębska-Michaluk, D. Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of March 31, 2020. Polish Arch. Intern. Med. 2020, 130, 352–357. [Google Scholar] [CrossRef] [Green Version]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef]
- Middeldorp, S.; Coppens, M.; van Haaps, T.F.; Foppen, M.; Vlaar, A.P.; Müller, M.C.; Bouman, C.C.; Beenen, L.F.; Kootte, R.S.; Heijmans, J.; et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1995–2002. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Llitjos, J.F.; Leclerc, M.; Chochois, C.; Monsallier, J.M.; Ramakers, M.; Auvray, M.; Merouani, K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020, 18, 1743–1746. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.J.H.A.; Van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 2020, 191, 148–150. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, X.; Zhang, D.; Jiang, C.; Mei, H.; Wang, J.; Zhang, C.; Li, H.; Xia, X.; Kong, S.; et al. Deep Vein Thrombosis in Hospitalized Patients with COVID-19 in Wuhan, China: Prevalence, Risk Factors, and Outcome. Circulation 2020, 142, 114–128. [Google Scholar] [CrossRef]
- Marietta, M.; Vandelli, P.; Mighali, P.; Vicini, R.; Coluccio, V.; D’Amico, R. Randomised controlled trial comparing efficacy and safety of high versus low Low-Molecular Weight Heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): A structured summary of a study protocol. Trials 2020, 21, 574. [Google Scholar] [CrossRef] [PubMed]
- Di Castelnuovo, A.; Costanzo, S.; Antinori, A.; Berselli, N.; Blandi, L.; Bonaccio, M.; Cauda, R.; Guaraldi, G.; Menicanti, L.; Mennuni, M.; et al. Heparin in COVID-19 Patients Is Associated with Reduced In-Hospital Mortality: The Multicenter Italian CORIST Study. Thromb. Haemost. 2021, 121, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Patel, D.; Odeh, T.; Rojas, E.; Sakhuja, A.; Meersman, M.; Dalton, D.; Nanchal, R.; Guddati, A.K. Incidence of Venous Thromboembolism and Effect of Anticoagulant Dosing in Hospitalized COVID-19 Patients. J. Hematol. 2021, 10, 162–170. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics of All Patients Included in the Study | ||||
|---|---|---|---|---|
| N | Median | Lower Quartile | Upper Quartile | |
| Age, years | 64 | 68.00 | 57.00 | 75.00 |
| Percentage of Lung Involvement,% | 64 | 38.27 | 24.77 | 50.34 |
| WBC, ×103/μL | 64 | 8.81 | 6.72 | 10.91 |
| RBC, ×106/μL | 64 | 4.66 | 4.25 | 5.06 |
| HGB, g/dL | 64 | 13.95 | 12.65 | 15.10 |
| HCT,% | 64 | 40.15 | 36.85 | 43.45 |
| Platelets, ×103/μL | 64 | 227.50 | 179.00 | 302.50 |
| Procalcitonin, ng/mL | 63 | 0.17 | 0.10 | 0.31 |
| CRP, mg/L | 64 | 146.40 | 104.68 | 205.53 |
| IL-6, pg/mL | 64 | 138.50 | 113.00 | 206.50 |
| LDH, U/L | 58 | 499.50 | 349.00 | 638.00 |
| D-dimer, ug/L | 64 | 1611.50 | 631.50 | 8481.50 |
| Creatinine, mg/dL | 64 | 0.94 | 0.82 | 1.22 |
| eGFR, mL/min | 64 | 80.25 | 57.61 | 96.21 |
| AST, U/L | 57 | 49.00 | 35.00 | 72.00 |
| ALT, U/L | 57 | 36.00 | 22.00 | 62.00 |
| Glucose, mg/dL | 61 | 123.00 | 113.00 | 145.00 |
| Bilirubin total, mg/dL | 59 | 0.63 | 0.41 | 0.85 |
| TCZ N = 28 (44%) | Non-TCZ N = 36 (56%) | p Value | |
|---|---|---|---|
| Age, years | 68.5 (54.5–75) | 67 (61.5–74) | 0.538 |
| Percentage of Lung Involvement,% | 33.82 (24.28–49.22) | 43.67 (28.2–53.34) | 0.211 |
| WBC, ×103/μL | 8.43 (6.65–10.78) | 8.81 (7.39–10.96) | 0.461 |
| RBC, ×106/μL | 4.58 (4.13–5.01) | 4.76 (4.45–5.13) | 0.206 |
| HGB, g/dL | 13.6 (11.75–15.2) | 14.05 (13.05–15.05) | 0.218 |
| HCT,% | 39.95 (35–43.45) | 41.3 (38.85–43.65) | 0.307 |
| Platelets, ×103/μL | 227.5 (176–316.5) | 232 (180–290) | 0.898 |
| Procalcitonin, ng/mL | 0.17 (0.1–0.35) | 0.2 (0.12–0.3) | 0.429 |
| CRP, mg/L | 132.54 (97.64–227.02) | 153.93 (132.29–189.51) | 0.201 |
| IL-6, pg/mL | 137.5 (104.55–209) | 146.5 (116–206.5) | 0.844 |
| LDH, U/L | 481 (355–638) | 515 (345–674) | 0.919 |
| D-dimer, ug/L | 2301.05 (695–8481.5) | 912.5 (586–6799) | 0.486 |
| Creatinine, mg/dL | 0.95 (0.74–1.23) | 0.94 (0.88–1.22) | 0.477 |
| eGFR, mL/min | 80.39 (56.66–103.63) | 79.47 (58.98–90.25) | 0.574 |
| AST, U/L | 53 (41–82) | 47.5 (34–69) | 0.378 |
| ALT, U/L | 43 (22–89) | 33 (25–55) | 0.665 |
| Glucose, mg/dL | 123 (114–145) | 123.5 (112–141.5) | 0.739 |
| Bilirubin total, mg/dL | 0.7 (0.45–0.98) | 67 (61.5–74) | 0.205 |
| Gender, n (%) Male (reference) | 24 (86%) | 23 (64%) | Chi-square Pearson p = 0.049 |
| Diabetes, n (%) Yes (reference) | 2 (7%) | 0 (0%) | Chi-square Pearson p = 0.103 |
| Hypertension, n (%) Yes (reference) | 5 (18%) | 8 (22%) | Chi-square Pearson p = 0.666 |
| Cancer, n (%) Yes (reference) | 0 (0%) | 1 (3%) | Chi-square Pearson p = 0.374 |
| Remdesivir admission, n (%) Yes (reference) | 23 (82%) | 28 (78%) | Chi-square Pearson p = 0.666 |
| ICU admission, n (%) Yes (reference) | 4 (14%) | 2 (6%) | Chi-square Pearson p = 0.234 |
| Mortality, n (%) Died (reference) | 12 (43%) | 7 (19%) | Chi-square Pearson p = 0.041 |
| Survived N = 45(70%) | Died N = 19 (30%) | p Value | |
|---|---|---|---|
| Age, years | 67 (54–71) | 76 (63–82) | 0.002 |
| Percentage of Lung Involvement,% | 35.67 (23.88–49.49) | 44.77 (30.49–60.57) | 0.113 |
| WBC, ×103/μL | 8.59 (6.67–10.93) | 9.34 (7.09–10.77) | 0.730 |
| RBC, ×106/μL | 4.78 (4.32–5.15) | 4.46 (4.14–4.81) | 0.092 |
| HGB, g/dL | 14.1 (12.9–15.2) | 13.3 (12.1–14.4) | 0.123 |
| HCT,% | 40.9 (37.3–43.6) | 39.1 (34.4–42.4) | 0.169 |
| Platelets, ×103/μL | 242 (183–315) | 224 (178–288) | 0.370 |
| Procalcitonin, ng/mL | 0.16 (0.1–0.25) | 0.27 (0.14–0.49) | 0.028 |
| CRP, mg/L | 138.6 (101.63–188.16) | 163.83 (117.35–207.23) | 0.258 |
| IL-6, pg/mL | 129 (95.7–176) | 168 (123–242) | 0.071 |
| LDH, U/L | 503.5 (355–636) | 474 (313–678) | 0.608 |
| D-dimer, ug/L | 1223 (603–8463) | 2145 (805–8861) | 0.436 |
| Creatinine, mg/dL | 0.94 (0.8–1.1) | 1.03 (0.83–1.55) | 0.246 |
| eGFR, mL/min | 83.11 (66.38–96.55) | 72.94 (41.61–93.58) | 0.146 |
| AST, U/L | 53 (41–77) | 38 (30–69) | 0.160 |
| ALT, U/L | 43.5 (28–79) | 26 (16–55) | 0.084 |
| Glucose, mg/dL | 121.5 (111–136) | 127 (114–173) | 0.327 |
| Bilirubin total, mg/dL | 0.64 (0.41–0.83) | 0.62 (0.36–0.96) | 0.651 |
| Gender, n (%) Male (reference) | 34 (75%) | 13 (68%) | Chi-square Pearson p = 0.554 |
| Diabetes, n (%) Yes (reference) | 2 (4%) | 0 (0%) | Chi-square Pearson p = 0.350 |
| Hypertension, n (%) Yes (reference) | 12 (27%) | 1 (5%) | Chi-square Pearson p = 0.052 |
| Cancer, n (%) Yes (reference) | 1 (2%) | 0 (0%) | Chi-square Pearson p = 0.512 |
| Remdesivir admission, n (%) Yes (reference) | 34 (76%) | 17 (90%) | Chi-square Pearson p = 0.206 |
| ICU admission, n (%) Yes (reference) | 2 (4%) | 4 (21%) | Chi-square Pearson p = 0.037 |
| Cox Proportional-Hazards Model for Mortality | ||||
|---|---|---|---|---|
| p Value | Hazard Ratio (HR) | Lower 95%CI HR Value | Upper 95%CI HR Value | |
| Age, years | 0.001 | 1.119 | 1.055 | 1.187 |
| Percentage of Lung Involvement,% | 0.511 | 1.009 | 0.980 | 1.039 |
| Procalcitonin, ng/mL | 0.084 | 2.039 | 0.906 | 4.586 |
| Administration of Remdesivir (reference) vs. no Remdesivir | 0.132 | 0.261 | 0.045 | 1.502 |
| Administration of TCZ (reference) vs. no TCZ | 0.036 | 3.342 | 1.077 | 10.370 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chober, D.; Aksak-Wąs, B.; Niścigorska-Olsen, J.; Niekrasz, M.; Parczewski, M. Tocilizumab Use among Patients Who Developed Pulmonary Embolism in the Course of Cytokine Release Storm and COVID-19 Pneumonia—A Retrospective Study. Biomedicines 2022, 10, 1581. https://doi.org/10.3390/biomedicines10071581
Chober D, Aksak-Wąs B, Niścigorska-Olsen J, Niekrasz M, Parczewski M. Tocilizumab Use among Patients Who Developed Pulmonary Embolism in the Course of Cytokine Release Storm and COVID-19 Pneumonia—A Retrospective Study. Biomedicines. 2022; 10(7):1581. https://doi.org/10.3390/biomedicines10071581
Chicago/Turabian StyleChober, Daniel, Bogusz Aksak-Wąs, Jolanta Niścigorska-Olsen, Małgorzata Niekrasz, and Miłosz Parczewski. 2022. "Tocilizumab Use among Patients Who Developed Pulmonary Embolism in the Course of Cytokine Release Storm and COVID-19 Pneumonia—A Retrospective Study" Biomedicines 10, no. 7: 1581. https://doi.org/10.3390/biomedicines10071581
APA StyleChober, D., Aksak-Wąs, B., Niścigorska-Olsen, J., Niekrasz, M., & Parczewski, M. (2022). Tocilizumab Use among Patients Who Developed Pulmonary Embolism in the Course of Cytokine Release Storm and COVID-19 Pneumonia—A Retrospective Study. Biomedicines, 10(7), 1581. https://doi.org/10.3390/biomedicines10071581






