NMDA and AMPA Receptors at Synapses: Novel Targets for Tau and α-Synuclein Proteinopathies
Abstract
1. Introduction
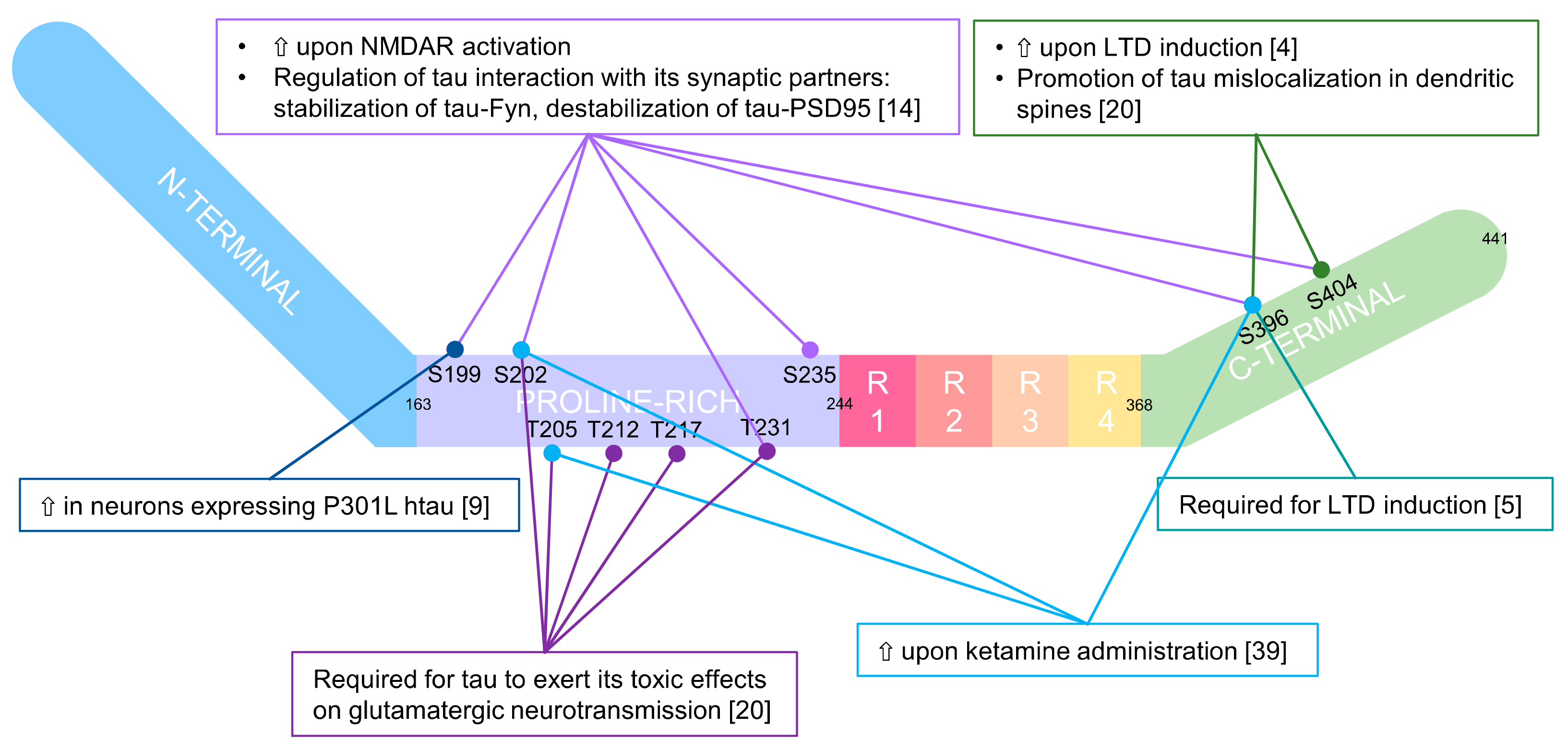
2. Tau
2.1. Physiological Function of Synaptic Tau
2.1.1. Tau as a Mediator of Synaptic Plasticity
2.1.2. A Physiological Role in the Spine for Tau Phosphorylation
2.2. The Pathological Counterpart of Synaptic Tau
2.2.1. In Vitro Models
2.2.2. In Vivo Models
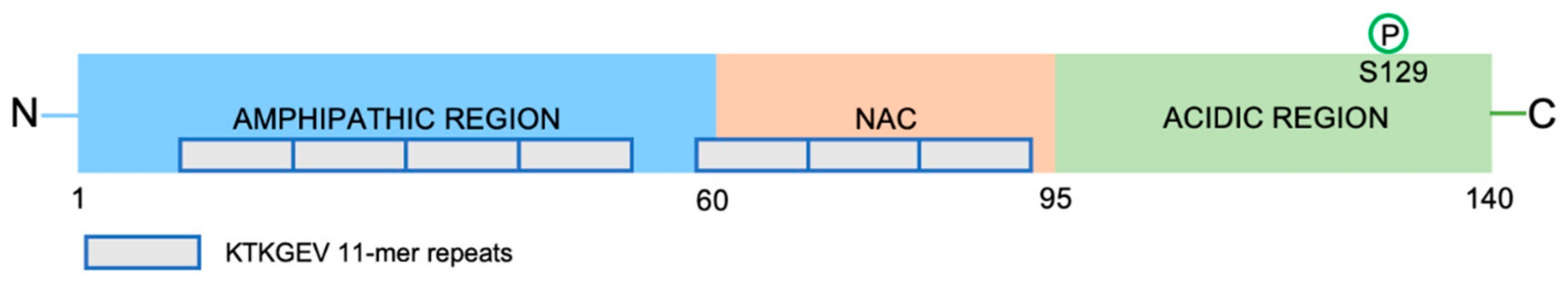
3. α-Synuclein
3.1. α-Synuclein Conformations
3.2. α-Synuclein Synaptic Functions
3.3. Role of α-Synuclein Aggregates in Functional Alterations of Glutamatergic Synapses
3.3.1. In Vitro Models
Effect of α-Synuclein Monomers
Effect of Oligomers and Fibrils
3.3.2. In Vivo Studies
4. α-Synuclein and Tau: Additional Molecular Mechanisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovacs, G.G. Invited Review: Neuropathology of Tauopathies: Principles and Practice: Neuropathology of Tauopathies. Neuropathol. Appl. Neurobiol. 2015, 41, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Regan, P.; Whitcomb, D.J.; Cho, K. Physiological and Pathophysiological Implications of Synaptic Tau. Neuroscientist 2017, 23, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Tanaka, T.; Soeda, Y.; Almeida, O.F.X.; Takashima, A. Local Somatodendritic Translation and Hyperphosphorylation of Tau Protein Triggered by AMPA and NMDA Receptor Stimulation. EBioMedicine 2017, 20, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Whitcomb, D.J.; Jo, J.; Regan, P.; Piers, T.; Heo, S.; Brown, C.; Hashikawa, T.; Murayama, M.; Seok, H.; et al. Microtubule-Associated Protein Tau Is Essential for Long-Term Depression in the Hippocampus. Philo. Trans. R. Soc. B 2014, 369, 20130144. [Google Scholar] [CrossRef]
- Regan, P.; Piers, T.; Yi, J.-H.; Kim, D.-H.; Huh, S.; Park, S.J.; Ryu, J.H.; Whitcomb, D.J.; Cho, K. Tau Phosphorylation at Serine 396 Residue Is Required for Hippocampal LTD. J. Neurosci. 2015, 35, 4804–4812. [Google Scholar] [CrossRef]
- Morris, M.; Maeda, S.; Vossel, K.; Mucke, L. The Many Faces of Tau. Neuron 2011, 70, 410–426. [Google Scholar] [CrossRef]
- Dong, Z.; Bai, Y.; Wu, X.; Li, H.; Gong, B.; Howland, J.G.; Huang, Y.; He, W.; Li, T.; Wang, Y.T. Hippocampal Long-Term Depression Mediates Spatial Reversal Learning in the Morris Water Maze. Neuropharmacology 2013, 64, 65–73. [Google Scholar] [CrossRef]
- Nicholls, R.E.; Alarcon, J.M.; Malleret, G.; Carroll, R.C.; Grody, M.; Vronskaya, S.; Kandel, E.R. Transgenic Mice Lacking NMDAR-Dependent LTD Exhibit Deficits in Behavioral Flexibility. Neuron 2008, 58, 104–117. [Google Scholar] [CrossRef]
- Hoover, B.R.; Reed, M.N.; Su, J.; Penrod, R.D.; Kotilinek, L.A.; Grant, M.K.; Pitstick, R.; Carlson, G.A.; Lanier, L.M.; Yuan, L.-L.; et al. Tau Mislocalization to Dendritic Spines Mediates Synaptic Dysfunction Independently of Neurodegeneration. Neuron 2010, 68, 1067–1081. [Google Scholar] [CrossRef]
- Suzuki, M.; Kimura, T. Microtubule-Associated Tau Contributes to Intra-Dendritic Trafficking of AMPA Receptors in Multiple Ways. Neurosci. Lett. 2017, 653, 276–282. [Google Scholar] [CrossRef]
- Ittner, L.M.; Ke, Y.D.; Delerue, F.; Bi, M.; Gladbach, A.; van Eersel, J.; Wölfing, H.; Chieng, B.C.; Christie, M.J.; Napier, I.A.; et al. Dendritic Function of Tau Mediates Amyloid-β Toxicity in Alzheimer’s Disease Mouse Models. Cell 2010, 142, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Mondragón-Rodríguez, S.; Trillaud-Doppia, E.; Dudilot, A.; Bourgeois, C.; Lauzon, M.; Leclerc, N.; Boehm, J. Interaction of Endogenous Tau Protein with Synaptic Proteins Is Regulated by N-Methyl-d-Aspartate Receptor-Dependent Tau Phosphorylation. J. Biol. Chem. 2012, 287, 32040–32053. [Google Scholar] [CrossRef] [PubMed]
- Sydow, A.; Van der Jeugd, A.; Zheng, F.; Ahmed, T.; Balschun, D.; Petrova, O.; Drexler, D.; Zhou, L.; Rune, G.; Mandelkow, E.; et al. Tau-Induced Defects in Synaptic Plasticity, Learning, and Memory Are Reversible in Transgenic Mice after Switching Off the Toxic Tau Mutant. J. Neurosci. 2011, 31, 2511–2525. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, K.; Hasegawa, M.; Ihara, Y.; Iwatsubo, T. Somatodendritic Localization of Phosphorylated Tau in Neonatal and Adult Rat Cerebral Cortex. NeuroReport 1997, 8, 2797–2801. [Google Scholar] [CrossRef] [PubMed]
- Peineau, S.; Taghibiglou, C.; Bradley, C.; Wong, T.P.; Liu, L.; Lu, J.; Lo, E.; Wu, D.; Saule, E.; Bouschet, T.; et al. LTP Inhibits LTD in the Hippocampus via Regulation of GSK3β. Neuron 2007, 53, 703–717. [Google Scholar] [CrossRef]
- Du, J.; Wei, Y.; Liu, L.; Wang, Y.; Khairova, R.; Blumenthal, R.; Tragon, T.; Hunsberger, J.G.; Machado-Vieira, R.; Drevets, W.; et al. A Kinesin Signaling Complex Mediates the Ability of GSK-3β to Affect Mood-Associated Behaviors. Proc. Natl. Acad. Sci. USA 2010, 107, 11573–11578. [Google Scholar] [CrossRef]
- Yagishita, S.; Murayama, M.; Ebihara, T.; Maruyama, K.; Takashim, A. Glycogen Synthase Kinase 3β-Mediated Phosphorylation in the Most C-Terminal Region of Protein Interacting with C Kinase 1 (PICK1) Regulates the Binding of PICK1 to Glutamate Receptor Subunit GluA2. J. Biol. Chem. 2015, 290, 29438–29448. [Google Scholar] [CrossRef]
- Draffin, J.E.; Sánchez-Castillo, C.; Fernández-Rodrigo, A.; Sánchez-Sáez, X.; Ávila, J.; Wagner, F.F.; Esteban, J.A. GSK3α, Not GSK3β, Drives Hippocampal NMDAR-dependent LTD via Tau-mediated Spine Anchoring. EMBO J. 2021, 40, e105513. [Google Scholar] [CrossRef]
- Miller, E.C.; Teravskis, P.J.; Dummer, B.W.; Zhao, X.; Huganir, R.L.; Liao, D. Tau Phosphorylation and Tau Mislocalization Mediate Soluble Aβ Oligomer-Induced AMPA Glutamate Receptor Signaling Deficits. Eur. J. Neurosci. 2014, 39, 1214–1224. [Google Scholar] [CrossRef]
- Teravskis, P.J.; Oxnard, B.R.; Miller, E.C.; Kemper, L.; Ashe, K.H.; Liao, D. Phosphorylation in Two Discrete Tau Domains Regulates a Stepwise Process Leading to Postsynaptic Dysfunction. J. Physiol. 2021, 599, 2483–2498. [Google Scholar] [CrossRef]
- Braun, N.J.; Yao, K.R.; Alford, P.W.; Liao, D. Mechanical Injuries of Neurons Induce Tau Mislocalization to Dendritic Spines and Tau-Dependent Synaptic Dysfunction. Proc. Natl. Acad. Sci. USA 2020, 117, 29069–29079. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.; Lucas, J.J.; Pérez, M.; Hernández, F. Role of Tau Protein in Both Physiological and Pathological Conditions. Physiol. Rev. 2004, 84, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Gendron, T.F.; Petrucelli, L. The Role of Tau in Neurodegeneration. Mol. Neurodegener. 2009, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-Y.; Hudry, E.; Hashimoto, T.; Kuchibhotla, K.; Rozkalne, A.; Fan, Z.; Spires-Jones, T.; Xie, H.; Arbel-Ornath, M.; Grosskreutz, C.L.; et al. Amyloid Induces the Morphological Neurodegenerative Triad of Spine Loss, Dendritic Simplification, and Neuritic Dystrophies through Calcineurin Activation. J. Neurosci. 2010, 30, 2636–2649. [Google Scholar] [CrossRef]
- Zhao, X.; Kotilinek, L.A.; Smith, B.; Hlynialuk, C.; Zahs, K.; Ramsden, M.; Cleary, J.; Ashe, K.H. Caspase-2 Cleavage of Tau Reversibly Impairs Memory. Nat. Med. 2016, 22, 1268–1276. [Google Scholar] [CrossRef]
- Shrivastava, A.N.; Redeker, V.; Pieri, L.; Bousset, L.; Renner, M.; Madiona, K.; Mailhes-Hamon, C.; Coens, A.; Buée, L.; Hantraye, P.; et al. Clustering of Tau Fibrils Impairs the Synaptic Composition of A3-Na+/K+-ATP Ase and AMPA Receptors. EMBO J. 2019, 38, e99871. [Google Scholar] [CrossRef]
- Clavaguera, F.; Bolmont, T.; Crowther, R.A.; Abramowski, D.; Frank, S.; Probst, A.; Fraser, G.; Stalder, A.K.; Beibel, M.; Staufenbiel, M.; et al. Transmission and Spreading of Tauopathy in Transgenic Mouse Brain. Nat. Cell Biol. 2009, 11, 909–913. [Google Scholar] [CrossRef]
- Dujardin, S.; Lécolle, K.; Caillierez, R.; Bégard, S.; Zommer, N.; Lachaud, C.; Carrier, S.; Dufour, N.; Aurégan, G.; Winderickx, J.; et al. Neuron-to-Neuron Wild-Type Tau Protein Transfer through a Trans-Synaptic Mechanism: Relevance to Sporadic Tauopathies. Acta Neuropathol. Commun. 2014, 2, 14. [Google Scholar] [CrossRef]
- Sanders, D.W.; Kaufman, S.K.; DeVos, S.L.; Sharma, A.M.; Mirbaha, H.; Li, A.; Barker, S.J.; Foley, A.C.; Thorpe, J.R.; Serpell, L.C.; et al. Distinct Tau Prion Strains Propagate in Cells and Mice and Define Different Tauopathies. Neuron 2014, 82, 1271–1288. [Google Scholar] [CrossRef]
- Iba, M.; McBride, J.D.; Guo, J.L.; Zhang, B.; Trojanowski, J.Q.; Lee, V.M.-Y. Tau Pathology Spread in PS19 Tau Transgenic Mice Following Locus Coeruleus (LC) Injections of Synthetic Tau Fibrils Is Determined by the LC’s Afferent and Efferent Connections. Acta Neuropathol. 2015, 130, 349–362. [Google Scholar] [CrossRef]
- Markesbery, W.R.; Schmitt, F.A.; Kryscio, R.J.; Davis, D.G.; Smith, C.D.; Wekstein, D.R. Neuropathologic Substrate of Mild Cognitive Impairment. Arch. Neurol. 2006, 63, 38. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s Disease Is a Synaptic Failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; Manczak, M.; Yin, X.; Wang, R.; Reddy, P.H. Hippocampal Phosphorylated Tau Induced Cognitive Decline, Dendritic Spine Loss and Mitochondrial Abnormalities in a Mouse Model of Alzheimer’s Disease. Hum. Mol. Genet. 2018, 27, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Cullen, W.K.; Suh, Y.-H.; Anwyl, R.; Rowan, M.J. Block of LTP in Rat Hippocampus in Vivo by β-Amyloid Precursor Protein Fragments. NeuroReport 1997, 8, 3213–3217. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Klyubin, I.; Fadeeva, J.V.; Cullen, W.K.; Anwyl, R.; Wolfe, M.S.; Rowan, M.J.; Selkoe, D.J. Naturally Secreted Oligomers of Amyloid β Protein Potently Inhibit Hippocampal Long-Term Potentiation in Vivo. Nature 2002, 416, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q. Block of Long-Term Potentiation by Naturally Secreted and Synthetic Amyloid -Peptide in Hippocampal Slices Is Mediated via Activation of the Kinases c-Jun N-Terminal Kinase, Cyclin-Dependent Kinase 5, and P38 Mitogen-Activated Protein Kinase as Well as Metabotropic Glutamate Receptor Type 5. J. Neurosci. 2004, 24, 3370–3378. [Google Scholar] [CrossRef]
- Townsend, M.; Shankar, G.M.; Mehta, T.; Walsh, D.M.; Selkoe, D.J. Effects of Secreted Oligomers of Amyloid β-Protein on Hippocampal Synaptic Plasticity: A Potent Role for Trimers: Amyloid β-Protein and Hippocampal Synaptic Plasticity. J. Physiol. 2006, 572, 477–492. [Google Scholar] [CrossRef]
- Shipton, O.A.; Leitz, J.R.; Dworzak, J.; Acton, C.E.J.; Tunbridge, E.M.; Denk, F.; Dawson, H.N.; Vitek, M.P.; Wade-Martins, R.; Paulsen, O.; et al. Tau Protein Is Required for Amyloid -Induced Impairment of Hippocampal Long-Term Potentiation. J. Neurosci. 2011, 31, 1688–1692. [Google Scholar] [CrossRef]
- Monteiro-Fernandes, D.; Silva, J.M.; Soares-Cunha, C.; Dalla, C.; Kokras, N.; Arnaud, F.; Billiras, R.; Zhuravleva, V.; Waites, C.; Bretin, S.; et al. Allosteric Modulation of AMPA Receptors Counteracts Tau-Related Excitotoxic Synaptic Signaling and Memory Deficits in Stress- and Aβ-Evoked Hippocampal Pathology. Mol. Psychiatry 2021, 26, 5899–5911. [Google Scholar] [CrossRef]
- Li, Y.; Ding, R.; Ren, X.; Wen, G.; Dong, Z.; Yao, H.; Tan, Y.; Yu, H.; Wang, X.; Zhan, X.; et al. Long-Term Ketamine Administration Causes Tau Protein Phosphorylation and Tau Protein-Dependent AMPA Receptor Reduction in the Hippocampus of Mice. Toxicol. Lett. 2019, 315, 107–115. [Google Scholar] [CrossRef]
- Tao, G.; Zhang, J.; Zhang, L.; Dong, Y.; Yu, B.; Crosby, G.; Culley, D.J.; Zhang, Y.; Xie, Z. Sevoflurane Induces Tau Phosphorylation and Glycogen Synthase Kinase 3β Activation in Young Mice. Anesthesiology 2014, 121, 510–527. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Liu, F.; Ji, F.; Liang, J.; Liu, L.; Wu, Q.; Wang, T. Effect of C-Jun N-Terminal Kinase (JNK)/P38 Mitogen-Activated Protein Kinase (P38 MAPK) in Morphine-Induced Tau Protein Hyperphosphorylation. Behav. Brain Res. 2013, 237, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Maroteaux, L.; Campanelli, J.T.; Scheller, R.H. Synuclein: A Neuron-Specific Protein Localized to the Nucleus and Presynaptic Nerve Terminal. J. Neurosci. 1988, 8, 2804–2815. [Google Scholar] [CrossRef] [PubMed]
- Bussell, R.; Eliezer, D. A Structural and Functional Role for 11-Mer Repeats in α-Synuclein and Other Exchangeable Lipid Binding Proteins. J. Mol. Biol. 2003, 329, 763–778. [Google Scholar] [CrossRef]
- Bussell, R. Helix Periodicity, Topology, and Dynamics of Membrane-Associated-Synuclein. Protein Sci. 2005, 14, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.S.; Jonas, A.; Clayton, D.F.; George, J.M. Stabilization of Alpha-Synuclein Secondary Structure upon Binding to Synthetic Membranes. J. Biol. Chem. 1998, 273, 9443–9449. [Google Scholar] [CrossRef]
- George, J.M.; Jin, H.; Woods, W.S.; Clayton, D.F. Characterization of a Novel Protein Regulated during the Critical Period for Song Learning in the Zebra Finch. Neuron 1995, 15, 361–372. [Google Scholar] [CrossRef]
- Xu, L.; Nussinov, R.; Ma, B. Coupling of the Non-Amyloid-Component (NAC) Domain and the KTK(E/Q)GV Repeats Stabilize the α-Synuclein Fibrils. Eur. J. Med. Chem. 2016, 121, 841–850. [Google Scholar] [CrossRef]
- Burai, R.; Ait-Bouziad, N.; Chiki, A.; Lashuel, H.A. Elucidating the Role of Site-Specific Nitration of α-Synuclein in the Pathogenesis of Parkinson’s Disease via Protein Semisynthesis and Mutagenesis. J. Am. Chem. Soc. 2015, 137, 5041–5052. [Google Scholar] [CrossRef]
- Fujiwara, H.; Hasegawa, M.; Dohmae, N.; Kawashima, A.; Masliah, E.; Goldberg, M.S.; Shen, J.; Takio, K.; Iwatsubo, T. α-Synuclein Is Phosphorylated in Synucleinopathy Lesions. Nat. Cell Biol. 2002, 4, 160–164. [Google Scholar] [CrossRef]
- Souza, J.M.; Giasson, B.I.; Chen, Q.; Lee, V.M.-Y.; Ischiropoulos, H. Dityrosine Cross-Linking Promotes Formation of Stable α-Synuclein Polymers Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J. Biol. Chem. 2000, 275, 18344–18349. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, W.; Cherny, D.; Subramaniam, V.; Jovin, T.M. Impact of the Acidic C-Terminal Region Comprising Amino Acids 109−140 on α-Synuclein Aggregation in Vitro. Biochemistry 2004, 43, 16233–16242. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.S.; Vorum, H.; Lindersson, E.; Jensen, P.H. Ca2+ Binding to α-Synuclein Regulates Ligand Binding and Oligomerization. J. Biol. Chem. 2001, 276, 22680–22684. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, A.; Fournier, M.; Lashuel, H.A. Role of Post-Translational Modifications in Modulating the Structure, Function and Toxicity of α-Synuclein. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2010; Volume 183, pp. 115–145. ISBN 978-0-444-53614-3. [Google Scholar]
- Burré, J.; Vivona, S.; Diao, J.; Sharma, M.; Brunger, A.T.; Südhof, T.C. Properties of Native Brain α-Synuclein. Nature 2013, 498, E4–E6; discussion E6–E7. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Evidence That the Precursor Protein of Non-A Beta Component of Alzheimer’s Disease Amyloid (NACP) Has an Extended Structure Primarily Composed of Random-Coil. Mol. Cells 1997, 7, 78–83. [Google Scholar]
- Weinreb, P.H.; Zhen, W.; Poon, A.W.; Conway, K.A.; Lansbury, P.T. NACP, A Protein Implicated in Alzheimer’s Disease and Learning, Is Natively Unfolded. Biochemistry 1996, 35, 13709–13715. [Google Scholar] [CrossRef]
- Bartels, T.; Choi, J.G.; Selkoe, D.J. α-Synuclein Occurs Physiologically as a Helically Folded Tetramer That Resists Aggregation. Nature 2011, 477, 107–110. [Google Scholar] [CrossRef]
- Burre, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Sudhof, T.C. -Synuclein Promotes SNARE-Complex Assembly in Vivo and in Vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. Alpha-Synuclein in Filamentous Inclusions of Lewy Bodies from Parkinson’s Disease and Dementia with Lewy Bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef]
- Totterdell, S.; Hanger, D.; Meredith, G.E. The Ultrastructural Distribution of Alpha-Synuclein-like Protein in Normal Mouse Brain. Brain Res. 2004, 1004, 61–72. [Google Scholar] [CrossRef]
- Pieri, L.; Madiona, K.; Melki, R. Structural and Functional Properties of Prefibrillar α-Synuclein Oligomers. Sci. Rep. 2016, 6, 24526. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.W.; Drakulic, S.; Deas, E.; Ouberai, M.; Aprile, F.A.; Arranz, R.; Ness, S.; Roodveldt, C.; Guilliams, T.; De-Genst, E.J.; et al. Structural Characterization of Toxic Oligomers That Are Kinetically Trapped during α-Synuclein Fibril Formation. Proc. Natl. Acad. Sci. USA 2015, 112, E1994–E2003. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Functional Amyloid, and Human Disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Jappelli, R.; Maji, S.K.; Desplats, P.A.; Boyer, L.; Aigner, S.; Hetzer, C.; Loher, T.; Vilar, M.; Campioni, S.; et al. In Vivo Demonstration That Alpha-Synuclein Oligomers Are Toxic. Proc. Natl. Acad. Sci. USA 2011, 108, 4194–4199. [Google Scholar] [CrossRef]
- Brehme, M.; Voisine, C.; Rolland, T.; Wachi, S.; Soper, J.H.; Zhu, Y.; Orton, K.; Villella, A.; Garza, D.; Vidal, M.; et al. A Chaperome Subnetwork Safeguards Proteostasis in Aging and Neurodegenerative Disease. Cell Rep. 2014, 9, 1135–1150. [Google Scholar] [CrossRef]
- Morimoto, R.I. The Heat Shock Response: Systems Biology of Proteotoxic Stress in Aging and Disease. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 91–99. [Google Scholar] [CrossRef]
- Bousset, L.; Pieri, L.; Ruiz-Arlandis, G.; Gath, J.; Jensen, P.H.; Habenstein, B.; Madiona, K.; Olieric, V.; Böckmann, A.; Meier, B.H.; et al. Structural and Functional Characterization of Two Alpha-Synuclein Strains. Nat. Commun. 2013, 4, 2575. [Google Scholar] [CrossRef]
- Heise, H.; Hoyer, W.; Becker, S.; Andronesi, O.C.; Riedel, D.; Baldus, M. Molecular-Level Secondary Structure, Polymorphism, and Dynamics of Full-Length α-Synuclein Fibrils Studied by Solid-State NMR. Proc. Natl. Acad. Sci. USA 2005, 102, 15871–15876. [Google Scholar] [CrossRef]
- Tuttle, M.D.; Comellas, G.; Nieuwkoop, A.J.; Covell, D.J.; Berthold, D.A.; Kloepper, K.D.; Courtney, J.M.; Kim, J.K.; Barclay, A.M.; Kendall, A.; et al. Solid-State NMR Structure of a Pathogenic Fibril of Full-Length Human α-Synuclein. Nat. Struct. Mol. Biol. 2016, 23, 409–415. [Google Scholar] [CrossRef]
- Verasdonck, J.; Bousset, L.; Gath, J.; Melki, R.; Böckmann, A.; Meier, B.H. Further Exploration of the Conformational Space of α-Synuclein Fibrils: Solid-State NMR Assignment of a High-PH Polymorph. Biomol. NMR Assign. 2016, 10, 5–12. [Google Scholar] [CrossRef]
- Melki, R. Role of Different Alpha-Synuclein Strains in Synucleinopathies, Similarities with Other Neurodegenerative Diseases. J. Parkinson’s Dis. 2015, 5, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Peelaerts, W.; Bousset, L.; Van der Perren, A.; Moskalyuk, A.; Pulizzi, R.; Giugliano, M.; Van den Haute, C.; Melki, R.; Baekelandt, V. α-Synuclein Strains Cause Distinct Synucleinopathies after Local and Systemic Administration. Nature 2015, 522, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Burré, J.; Sharma, M.; Südhof, T.C. Cell Biology and Pathophysiology of α-Synuclein. Cold Spring Harb. Perspect. Med. 2018, 8, a024091. [Google Scholar] [CrossRef] [PubMed]
- Withers, G.S.; George, J.M.; Banker, G.A.; Clayton, D.F. Delayed Localization of Synelfin (Synuclein, NACP) to Presynaptic Terminals in Cultured Rat Hippocampal Neurons. Dev. Brain Res. 1997, 99, 87–94. [Google Scholar] [CrossRef]
- Greten-Harrison, B.; Polydoro, M.; Morimoto-Tomita, M.; Diao, L.; Williams, A.M.; Nie, E.H.; Makani, S.; Tian, N.; Castillo, P.E.; Buchman, V.L.; et al. -Synuclein Triple Knockout Mice Reveal Age-Dependent Neuronal Dysfunction. Proc. Natl. Acad. Sci. USA 2010, 107, 19573–19578. [Google Scholar] [CrossRef]
- Burré, J. The Synaptic Function of α-Synuclein. J. Parkinson’s Dis. 2015, 5, 699–713. [Google Scholar] [CrossRef]
- Yu, S.; Uéda, K.; Chan, P. α-Synuclein and Dopamine Metabolism. Mol. Neurobiol. 2005, 31, 243–254. [Google Scholar] [CrossRef]
- Cheng, F.; Vivacqua, G.; Yu, S. The Role of Alpha-Synuclein in Neurotransmission and Synaptic Plasticity. J. Chem. Neuroanat. 2011, 42, 242–248. [Google Scholar] [CrossRef]
- Longhena, F.; Faustini, G.; Spillantini, M.G.; Bellucci, A. Living in Promiscuity: The Multiple Partners of Alpha-Synuclein at the Synapse in Physiology and Pathology. Int. J. Mol. Sci. 2019, 20, 141. [Google Scholar] [CrossRef]
- Chen, R.H.C.; Wislet-Gendebien, S.; Samuel, F.; Visanji, N.P.; Zhang, G.; Marsilio, D.; Langman, T.; Fraser, P.E.; Tandon, A. α-Synuclein Membrane Association Is Regulated by the Rab3a Recycling Machinery and Presynaptic Activity. J. Biol. Chem. 2013, 288, 7438–7449. [Google Scholar] [CrossRef]
- Diao, J.; Burré, J.; Vivona, S.; Cipriano, D.J.; Sharma, M.; Kyoung, M.; Südhof, T.C.; Brunger, A.T. Native α-Synuclein Induces Clustering of Synaptic-Vesicle Mimics via Binding to Phospholipids and Synaptobrevin-2/VAMP2. eLife 2013, 2, e00592. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, M.; Chieregatti, E. Mechanisms of Alpha-Synuclein Action on Neurotransmission: Cell-Autonomous and Non-Cell Autonomous Role. Biomolecules 2015, 5, 865–892. [Google Scholar] [CrossRef] [PubMed]
- Somayaji, M.; Cataldi, S.; Choi, S.J.; Edwards, R.H.; Mosharov, E.V.; Sulzer, D. A Dual Role for α-Synuclein in Facilitation and Depression of Dopamine Release from Substantia Nigra Neurons in Vivo. Proc. Natl. Acad. Sci. USA 2020, 117, 32701–32710. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.; Gonçalves, N.P.; Jan, A.; Jensen, N.M.; van der Laan, A.; Mohseni, S.; Vægter, C.B.; Jensen, P.H. Trans-Synaptic Spreading of Alpha-Synuclein Pathology through Sensory Afferents Leads to Sensory Nerve Degeneration and Neuropathic Pain. Acta Neuropathol. Commun. 2021, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, W.; Li, X.; Li, X.; Yang, H.; Xu, Z.; Yu, S. α-Synuclein-Induced Internalization of NMDA Receptors in Hippocampal Neurons Is Associated with Reduced Inward Current and Ca2+ Influx upon NMDA Stimulation. Neuroscience 2015, 300, 297–306. [Google Scholar] [CrossRef]
- Yu, W.; Yang, W.; Li, X.; Li, X.; Yu, S. Alpha-Synuclein Oligomerization Increases Its Effect on Promoting NMDA Receptor Internalization. Int. J. Clin. Exp. Pathol. 2019, 12, 87–100. [Google Scholar]
- Durante, V.; de Iure, A.; Loffredo, V.; Vaikath, N.; De Risi, M.; Paciotti, S.; Quiroga-Varela, A.; Chiasserini, D.; Mellone, M.; Mazzocchetti, P.; et al. Alpha-Synuclein Targets GluN2A NMDA Receptor Subunit Causing Striatal Synaptic Dysfunction and Visuospatial Memory Alteration. Brain 2019, 142, 1365–1385. [Google Scholar] [CrossRef]
- Tozzi, A.; Sciaccaluga, M.; Loffredo, V.; Megaro, A.; Ledonne, A.; Cardinale, A.; Federici, M.; Bellingacci, L.; Paciotti, S.; Ferrari, E.; et al. Dopamine-Dependent Early Synaptic and Motor Dysfunctions Induced by α-Synuclein in the Nigrostriatal Circuit. Brain 2021, 144, 3477–3491. [Google Scholar] [CrossRef]
- Navarria, L.; Zaltieri, M.; Longhena, F.; Spillantini, M.G.; Missale, C.; Spano, P.; Bellucci, A. Alpha-Synuclein Modulates NR2B-Containing NMDA Receptors and Decreases Their Levels after Rotenone Exposure. Neurochem. Int. 2015, 85–86, 14–23. [Google Scholar] [CrossRef]
- Emanuele, M.; Esposito, A.; Camerini, S.; Antonucci, F.; Ferrara, S.; Seghezza, S.; Catelani, T.; Crescenzi, M.; Marotta, R.; Canale, C.; et al. Exogenous Alpha-Synuclein Alters Pre- and Post-Synaptic Activity by Fragmenting Lipid Rafts. EBioMedicine 2016, 7, 191–204. [Google Scholar] [CrossRef][Green Version]
- Shrivastava, A.N.; Bousset, L.; Renner, M.; Redeker, V.; Savistchenko, J.; Triller, A.; Melki, R. Differential Membrane Binding and Seeding of Distinct α-Synuclein Fibrillar Polymorphs. Biophys. J. 2020, 118, 1301–1320. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.G.; Temido-Ferreira, M.; Vicente Miranda, H.; Batalha, V.L.; Coelho, J.E.; Szegö, É.M.; Marques-Morgado, I.; Vaz, S.H.; Rhee, J.S.; Schmitz, M.; et al. α-Synuclein Interacts with PrPC to Induce Cognitive Impairment through MGluR5 and NMDAR2B. Nat. Neurosci. 2017, 20, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, A.; de Iure, A.; Bagetta, V.; Tantucci, M.; Durante, V.; Quiroga-Varela, A.; Costa, C.; Di Filippo, M.; Ghiglieri, V.; Latagliata, E.C.; et al. Alpha-Synuclein Produces Early Behavioral Alterations via Striatal Cholinergic Synaptic Dysfunction by Interacting With GluN2D N -Methyl-D-Aspartate Receptor Subunit. Biol. Psychiatry 2016, 79, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Trudler, D.; Sanz-Blasco, S.; Eisele, Y.S.; Ghatak, S.; Bodhinathan, K.; Akhtar, M.W.; Lynch, W.P.; Piña-Crespo, J.C.; Talantova, M.; Kelly, J.W.; et al. α-Synuclein Oligomers Induce Glutamate Release from Astrocytes and Excessive Extrasynaptic NMDAR Activity in Neurons, Thus Contributing to Synapse Loss. J. Neurosci. 2021, 41, 2264–2273. [Google Scholar] [CrossRef]
- Diogenes, M.J.; Dias, R.B.; Rombo, D.M.; Vicente Miranda, H.; Maiolino, F.; Guerreiro, P.; Nasstrom, T.; Franquelim, H.G.; Oliveira, L.M.A.; Castanho, M.A.R.B.; et al. Extracellular Alpha-Synuclein Oligomers Modulate Synaptic Transmission and Impair LTP Via NMDA-Receptor Activation. J. Neurosci. 2012, 32, 11750–11762. [Google Scholar] [CrossRef]
- Hüls, S.; Högen, T.; Vassallo, N.; Danzer, K.M.; Hengerer, B.; Giese, A.; Herms, J. AMPA-Receptor-Mediated Excitatory Synaptic Transmission Is Enhanced by Iron-Induced α-Synuclein Oligomers: α-Synuclein Oligomers Alter Synaptic Transmission. J. Neurochem. 2011, 117, 868–878. [Google Scholar] [CrossRef]
- Hering, H.; Lin, C.-C.; Sheng, M. Lipid Rafts in the Maintenance of Synapses, Dendritic Spines, and Surface AMPA Receptor Stability. J. Neurosci. 2003, 23, 3262–3271. [Google Scholar] [CrossRef]
- Chung, H.J. Regulation of the NMDA Receptor Complex and Trafficking by Activity-Dependent Phosphorylation of the NR2B Subunit PDZ Ligand. J. Neurosci. 2004, 24, 10248–10259. [Google Scholar] [CrossRef]
- Miñano-Molina, A.J.; España, J.; Martín, E.; Barneda-Zahonero, B.; Fadó, R.; Solé, M.; Trullás, R.; Saura, C.A.; Rodríguez-Alvarez, J. Soluble Oligomers of Amyloid-β Peptide Disrupt Membrane Trafficking of α-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid Receptor Contributing to Early Synapse Dysfunction. J. Biol. Chem. 2011, 286, 27311–27321. [Google Scholar] [CrossRef]
- Mellone, M.; Stanic, J.; Hernandez, L.F.; Iglesias, E.; Zianni, E.; Longhi, A.; Prigent, A.; Picconi, B.; Calabresi, P.; Hirsch, E.C.; et al. NMDA Receptor GluN2A/GluN2B Subunit Ratio as Synaptic Trait of Levodopa-Induced Dyskinesias: From Experimental Models to Patients. Front. Cell. Neurosci. 2015, 9, 245. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Zhu, J. Kainic Acid-Induced Neurotoxicity: Targeting Glial Responses and Glia-Derived Cytokines. Curr. Neuropharmacol. 2011, 9, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Bhardwaj, R.; Dhawan, D.K.; Kaur, T. Exploring the Effect of Endoplasmic Reticulum Stress Inhibition by 4-Phenylbutyric Acid on AMPA-Induced Hippocampal Excitotoxicity in Rat Brain. Neurotox. Res. 2019, 35, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Sokka, A.-L.; Putkonen, N.; Mudo, G.; Pryazhnikov, E.; Reijonen, S.; Khiroug, L.; Belluardo, N.; Lindholm, D.; Korhonen, L. Endoplasmic Reticulum Stress Inhibition Protects against Excitotoxic Neuronal Injury in the Rat Brain. J. Neurosci. 2007, 27, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kim, M.H.; Lee, H.J.; Huh, J.-W.; Lee, H.-S.; Lee, D.-S. Peroxiredoxin 4 Attenuates Glutamate-Induced Neuronal Cell Death through Inhibition of Endoplasmic Reticulum Stress. Free. Radic. Res. 2020, 54, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Resende, R.; Ferreiro, E.; Pereira, C.; Oliveira, C.R. ER Stress Is Involved in Aβ-induced GSK-3β Activation and Tau Phosphorylation. J. Neurosci. Res. 2008, 86, 2091–2099. [Google Scholar] [CrossRef]
- Shi, C.; Zeng, J.; Li, Z.; Chen, Q.; Hang, W.; Xia, L.; Wu, Y.; Chen, J.; Shi, A. Melatonin Mitigates Kainic Acid-Induced Neuronal Tau Hyperphosphorylation and Memory Deficits through Alleviating ER Stress. Front. Mol. Neurosci. 2018, 11, 5. [Google Scholar] [CrossRef]
- Liu, Z.-C.; Fu, Z.-Q.; Song, J.; Zhang, J.-Y.; Wei, Y.-P.; Chu, J.; Han, L.; Qu, N.; Wang, J.-Z.; Tian, Q. Bip Enhanced the Association of GSK-3β with Tau During ER Stress Both in vivo and in vitro. J. Alzheimers Dis. 2012, 29, 727–740. [Google Scholar] [CrossRef]
- Jiang, P.; Gan, M.; Ebrahim, A.S.; Lin, W.-L.; Melrose, H.L.; Yen, S.-H.C. ER Stress Response Plays an Important Role in Aggregation of α-Synuclein. Mol. Neurodegener. 2010, 5, 56. [Google Scholar] [CrossRef]
- Cóppola-Segovia, V.; Cavarsan, C.; Maia, F.G.; Ferraz, A.C.; Nakao, L.S.; Lima, M.M.; Zanata, S.M. ER Stress Induced by Tunicamycin Triggers α-Synuclein Oligomerization, Dopaminergic Neurons Death and Locomotor Impairment: A New Model of Parkinson’s Disease. Mol. Neurobiol. 2017, 54, 5798–5806. [Google Scholar] [CrossRef]
- Tsai, S.-Y.A.; Pokrass, M.J.; Klauer, N.R.; Nohara, H.; Su, T.-P. Sigma-1 Receptor Regulates Tau Phosphorylation and Axon Extension by Shaping P35 Turnover via Myristic Acid. Proc. Natl. Acad. Sci. USA 2015, 112, 6742–6747. [Google Scholar] [CrossRef]
- Lahmy, V.; Meunier, J.; Malmström, S.; Naert, G.; Givalois, L.; Kim, S.H.; Villard, V.; Vamvakides, A.; Maurice, T. Blockade of Tau Hyperphosphorylation and Aβ1–42 Generation by the Aminotetrahydrofuran Derivative ANAVEX2-73, a Mixed Muscarinic and Σ1 Receptor Agonist, in a Nontransgenic Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology 2013, 38, 1706–1723. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.; Bezprozvanny, I.; Wu, L.; Ryskamp, D.A.; Bar-Ner, N.; Natan, N.; Brandeis, R.; Elkon, H.; Nahum, V.; Gershonov, E.; et al. AF710B, a Novel M1/Σ1 Agonist with Therapeutic Efficacy in Animal Models of Alzheimer’s Disease. Neurodegener. Dis. 2016, 16, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Wang, L.; Zhang, T.; Zhang, B.; Chen, L. Sigma-1 Receptor Knockout Increases α-Synuclein Aggregation and Phosphorylation with Loss of Dopaminergic Neurons in Substantia Nigra. Neurobiol. Aging 2017, 59, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Limegrover, C.S.; Yurko, R.; Izzo, N.J.; LaBarbera, K.M.; Rehak, C.; Look, G.; Rishton, G.; Safferstein, H.; Catalano, S.M. Sigma-2 Receptor Antagonists Rescue Neuronal Dysfunction Induced by Parkinson’s Patient Brain-derived A-synuclein. J. Neurosci. Res. 2021, 99, 1161–1176. [Google Scholar] [CrossRef]

| Targeted iGluR | α-Synuclein Species | Model | Mechanism | Ref. |
|---|---|---|---|---|
| GluN1 | Overexpression, monomers | Primary hippocampal neurons | ⇧ internalisation, impaired calcium influx | [86] |
| Oligomers | MES23.5 dopaminergic cells | ⇧ internalisation | [87] | |
| GluN2A | Small oligomers | Corticostriatal slices | LTP blockade in SPNs, ⇩ GluN2A currents, ⇩ GluN2A expression | [88] |
| Protofibrils | Striatal injection (rats) | LTP, LTD impairments (SPNs), behavioral alterations | [89] | |
| GluN2B | Overexpression (wt/αsyn1-120) | SK-N-SH, primary cortical neurons, Tg-αsyn1-120-mice | ⇧ internalisation, impaired calcium influx | [90] |
| Monomers | Corticostriatal slices | Mislocalisation, LTP blockade | [91] | |
| Fibrils | Primary hippocampal neurons hippocampal organotypic slices | ⇧ synaptic expression, network activity impairment | [92] | |
| Oligomers | Hippocampal slices primary hippocampal neurons tg-αsyn-mice | LTP impairments, PrPC/Fyn/GluN2B deregulation | [93] | |
| GluN2D | In vivo overexpression | Tg-αsyn1-120-mice AAV-αsyn-rats | LTP-blockade (ChIs), Memory and motor alterations | [94] |
| Extra-synaptic NMDARs | Soluble aggregates (oligomers+fibrils) | Primary astrocytes tg-αsyn-mice cerebrocortical cultures hippocampal slices | Astrocytes-mediated glutamate release, direct activation of extra-synaptic NMDARs | [95] |
| GluA1 | HNE-stabilised oligomers | Hippocampal slices | ⇧ AMPAR rectification currents, impaired LTP | [96] |
| GluA2 | Fibrils-91 | Primary hippocampal neurons hippocampal organotypic slices | ⇧ synaptic expression, network activity impairment | [92] |
| AMPARs | Iron-induced oligomers | Autaptic cultures of primary neurons | ⇧ AMPAR-mEPSCs ⇧ responsiveness/AMPAR n° | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Italia, M.; Ferrari, E.; Diluca, M.; Gardoni, F. NMDA and AMPA Receptors at Synapses: Novel Targets for Tau and α-Synuclein Proteinopathies. Biomedicines 2022, 10, 1550. https://doi.org/10.3390/biomedicines10071550
Italia M, Ferrari E, Diluca M, Gardoni F. NMDA and AMPA Receptors at Synapses: Novel Targets for Tau and α-Synuclein Proteinopathies. Biomedicines. 2022; 10(7):1550. https://doi.org/10.3390/biomedicines10071550
Chicago/Turabian StyleItalia, Maria, Elena Ferrari, Monica Diluca, and Fabrizio Gardoni. 2022. "NMDA and AMPA Receptors at Synapses: Novel Targets for Tau and α-Synuclein Proteinopathies" Biomedicines 10, no. 7: 1550. https://doi.org/10.3390/biomedicines10071550
APA StyleItalia, M., Ferrari, E., Diluca, M., & Gardoni, F. (2022). NMDA and AMPA Receptors at Synapses: Novel Targets for Tau and α-Synuclein Proteinopathies. Biomedicines, 10(7), 1550. https://doi.org/10.3390/biomedicines10071550






