Clinical Characteristics and Outcome of Hospitalized COVID-19 Patients Treated with Standard Dose of Dexamethasone or High Dose of Methylprednisolone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Steroid Dosage
2.3. Outcome and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bohn, M.K.; Hall, A.; Sepiashvili, L.; Jung, B.; Steele, S.; Adeli, K. Pathophysiology of COVID-19: Mechanisms Underlying Disease Severity and Progression. Physiology 2020, 35, 288–301. [Google Scholar] [CrossRef]
- Bime, C.; Casanova, N.G.; Nikolich-Zugich, J.; Knox, K.S.; Camp, S.M.; Garcia, J.G.N. Strategies to DAMPen COVID-19-mediated lung and systemic inflammation and vascular injury. Transl. Res. 2021, 232, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Parasher, A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, K.; Moghadami, M.; Mirahmadizadeh, A.; Fallahi, M.J.; Khaloo, V.; Shahriarirad, R.; Erfani, A.; Khodamoradi, Z.; Gholampoor Saadi, M.H. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: A triple-blinded randomized controlled trial. BMC Infect. Dis. 2021, 21, 337. [Google Scholar] [CrossRef]
- Pinzón, M.A.; Ortiz, S.; Holguín, H.; Betancur, J.F.; Cardona Arango, D.; Laniado, H.; Arias Arias, C.; Muñoz, B.; Quiceno, J.; Jaramillo, D.; et al. Dexamethasone vs methylprednisolone high dose for COVID-19 pneumonia. PLoS ONE 2021, 16, e0252057. [Google Scholar] [CrossRef]
- Mehta, J.; Rolta, R.; Mehta, B.B.; Kaushik, N.; Choi, E.H.; Kaushik, N.K. Role of Dexamethasone and Methylprednisolone Corticosteroids in Coronavirus Disease 2019 Hospitalized Patients: A Review. Front. Microbiol. 2022, 13, 813358. [Google Scholar] [CrossRef]
- World Health Organization. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance. Available online: https://apps.who.int/iris/handle/10665/331329 (accessed on 2 May 2022).
- Russo, A.; Bellelli, V.; Ceccarelli, G.; Marincola Cattaneo, F.; Bianchi, L.; Pierro, R.; Russo, R.; Steffanina, A.; Pugliese, F.; Mastroianni, C.M.; et al. Comparison Between Hospitalized Patients Affected or Not Affected by Coronavirus Disease 2019. Clin. Infect. Dis. 2021, 72, e1158–e1159. [Google Scholar] [CrossRef]
- Corticosteroids for COVID-19. Living Guidance. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 (accessed on 24 June 2022).
- Braude, A.C.; Rebuck, A.S. Prednisone and methylprednisolone disposition in the lung. Lancet 1983, 2, 995–997. [Google Scholar] [CrossRef]
- Hirano, T.; Homma, M.; Oka, K.; Tsushima, H.; Niitsuma, T.; Hayashi, T. Individual variations in lymphocyte-responses to glucocorticoids in patients with bronchial asthma: Comparison of potencies for five glucocorticoids. Immunopharmacology 1998, 40, 57–66. [Google Scholar] [CrossRef]
- Vichyanond, P.; Irvin, C.G.; Larsen, G.L.; Szefler, S.J.; Hill, M.R. Penetration of corticosteroids into the lung: Evidence for a difference between methylprednisolone and prednisolone. J. Allergy Clin. Immunol. 1989, 84, 867–873. [Google Scholar] [CrossRef]
- Torres, A.; Sibila, O.; Ferrer, M.; Polverino, E.; Menendez, R.; Mensa, J.; Gabarrús, A.; Sellarés, J.; Restrepo, M.I.; Anzueto, A.; et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: A randomized clinical trial. JAMA 2015, 313, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Zha, L.; Li, S.; Pan, L.; Tefsen, B.; Li, Y.; French, N.; Chen, L.; Yang, G.; Villanueva, E.V. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19). Med. J. Aust. 2020, 212, 416–420. [Google Scholar] [CrossRef]
- Fadel, R.; Morrison, A.R.; Vahia, A.; Smith, Z.R.; Chaudhry, Z.; Bhargava, P.; Miller, J.; Kenney, R.M.; Alangaden, G.; Ramesh, M.S. Henry Ford COVID-19 Management Task Force. Early Short-Course Corticosteroids in Hospitalized Patients With COVID-19. Clin. Infect. Dis. 2020, 71, 2114–2120. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Liu, Y.; Tian, D.; Wang, C.; Wang, S.; Cheng, J.; Hu, M.; Fang, M.; Gao, Y. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct. Target. Ther. 2020, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.; Xu, S.B.; Lin, Y.X.; Tian, D.; Zhu, Z.Q.; Dai, F.H.; Wu, F.; Song, Z.G.; Huang, W.; Chen, J.; et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin. Med. J. 2020, 133, 1039–1043. [Google Scholar] [CrossRef]
- Stockman, L.J.; Bellamy, R.; Garner, P. SARS: Systematic review of treatment effects. PLoS Med. 2006, 3, e343. [Google Scholar] [CrossRef] [Green Version]
- Bhimraj, A.; Morgan, R.L.; Shumaker, A.H.; Lavergne, V.; Baden, L.; Cheng, V.C.; Edwards, K.M.; Gandhi, R.; Muller, W.J.; O’Horo, J.C.; et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin. Infect. Dis. 2020, ciaa478. [Google Scholar] [CrossRef]
- Bartoletti, M.; Azap, O.; Barac, A.; Bussini, L.; Ergonul, O.; Krause, R.; Paño-Pardo, J.R.; Power, N.R.; Sibani, M.; Szabo, B.G.; et al. ESCMID COVID-19 living guidelines: Drug treatment and clinical management. Clin. Microbiol. Infect. 2022, 28, 222–238. [Google Scholar] [CrossRef]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Diaz, J.; Villar, J.; Murthy, S.; Slutsky, A.S.; Perner, A.; Jüni, P.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; et al. Corticosteroid therapy for critically ill patients with COVID-19: A structured summary of a study protocol for a prospective meta-analysis of randomized trials. Trials 2020, 21, 734. [Google Scholar] [CrossRef] [PubMed]
- Jeronimo, C.M.P.; Farias, M.E.L.; Val, F.F.A.; Sampaio, V.S.; Alexandre, M.A.A.; Melo, G.C.; Safe, I.P.; Borba, M.G.S.; Netto, R.L.A.; Maciel, A.B.S.; et al. Methylprednisolone as Adjunctive Therapy for Patients Hospitalized with Coronavirus Disease 2019 (COVID-19; Metcovid): A Randomized, Double-blind, Phase IIb, Placebo-controlled Trial. Clin. Infect. Dis. 2021, 72, e373–e381. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.A.M.; Mohamed, A.H.; Owaynat, A.H. Comparison between methylprednisolone infusion and dexamethasone in COVID-19 ARDS mechanically ventilated patients. Egypt. J. Intern. Med. 2022, 34, 19. [Google Scholar] [CrossRef]
| Variables | All Patients (n = 990) |
|---|---|
| Demographic information | |
| Age (years), median [IQR 25–75%] | 69.3 (58–81) |
| Male sex | 447 (45.1) |
| Comorbidities [n (%)] | |
| Arterial hypertension | 205 (20.7) |
| Cardiovascular disease other than arterial hypertension | 340 (34.3) |
| Obesity | 108 (10.9) |
| Diabetes mellitus type 2 | 125 (12.6) |
| Chronic kidney disease | 151 (15.2) |
| Chronic obstructive pulmonary disease | 211 (21.3) |
| Oncologic disease | 87 (8.7) |
| Psychiatric disorders | 48 (4.8) |
| Neurologic disorders | 60 (6) |
| Clinical features[n (%)] | |
| Fever | 586 (59.1) |
| Cough | 401 (40.5) |
| Dyspnoea | 443 (44.7) |
| Headache | 16 (1.6) |
| Diarrhoea | 111 (11.1) |
| Asthenia | 337 (34) |
| Ageusia | 77 (7.7) |
| Anosmia | 89 (9) |
| Lung function parameters [median (IQR 25–75%)] | |
| Respiratory rate (acts/min) | 21 (17–26) |
| PaO2/FiO2 ratio | 314 (216–374) |
| Laboratory values [median (IQR 25–75%)] | |
| Interleukin-6 (pg/mL) | 22 (9–45.3) |
| D-dimer (mg/L) | 0.75 (0.4–1.3) |
| Fibrinogen (mg/dL) | 520 (451–580) |
| Lactate dehydrogenase (UI/L) | 357 (267–474) |
| Ferritin (ng/mL) | 507 (207.3–867.5) |
| Platelets count (cells/µL) | 213 (163–268.8) |
| Lymphocytes count (cells/µL) | 930 (625–1400) |
| C-reactive protein (mg/L) | 11 (4.8–27) |
| Procalcitonin (ng/mL) | 0.08 (0.1–0.2) |
| Pharmacological Therapy | |
| Antibiotic | 683 (69) |
| Standard-dose steroid | 695 (70.2) |
| High-dose steroid | 295 (29.8) |
| Remdesivir | 270 (27.2) |
| Tocilizumab | 17 (1.7) |
| Low-weight molecular heparin | 479 (48.3) |
| Oxygen therapy | |
| Low-flow oxygen | 671 (67.7) |
| HFNC/CPAP/NIV | 319 (32.3) |
| Outcomes | |
| 30-day mortality | 198 (20) |
| Variables | Standard-Dose Steroid (n = 695) | High-Dose Steroid (n = 295) | p-Value |
|---|---|---|---|
| Demographic information | |||
| Age (years), median [IQR 25–75%] | 71.7 (58.5–82.8) | 66.3 (55.3–80) | 0.001 |
| Male sex | 364 (52.3) | 83 (28.1) | <0.001 |
| Comorbidities [n (%)] | |||
| Arterial hypertension | 142 (20.4) | 63 (21.3) | 0.255 |
| Cardiovascular disease other than Arterial hypertension | 285 (41) | 55 (18.6) | <0.001 |
| Obesity | 75 (10.8) | 33 (11.1) | 0.577 |
| Diabetes mellitus type 2 | 67 (9.6) | 58 (19.6) | <0.001 |
| Chronic kidney disease | 127 (18.2) | 24 (8.1) | <0.001 |
| Chronic obstructive pulmonary disease | 178 (25.6) | 33 (11.1) | <0.001 |
| Oncologic disease | 77 (11) | 10 (3.3) | 0.052 |
| Psychiatric disorders | 30 (4.3) | 18 (6.1) | 0.158 |
| Neurologic disorders | 42 (6) | 18 (6.1) | 0.953 |
| Clinical features[n (%)] | |||
| Fever | 390 (56.1) | 196 (66.4) | 0.001 |
| Cough | 280 (40.2) | 121 (41) | 0.885 |
| Dyspnoea | 285 (41) | 158 (53.5) | <0.001 |
| Headache | 15 (2.1) | 1 (0.3) | 0.122 |
| Diarrhoea | 76 (10.9) | 35 (11.8) | 0.822 |
| Asthenia | 270 (38.8) | 67 (22.7) | <0.001 |
| Ageusia | 57 (8.2) | 20 (6.7) | 0.681 |
| Anosmia | 71 (10.2) | 18 (6.1) | 0.165 |
| Lung function parameters [median (IQR 25–75%)] | |||
| Respiratory rate (acts/min) | 18 (16–24) | 24 (19.5–28.5) | <0.001 |
| PaO2/FiO2 ratio | 320 (229–386) | 286 (172–357) | <0.001 |
| Laboratory values [median (IQR 25–75%)] | |||
| Interleukin-6 (pg/mL) | 17.6 (8.4–40.8) | 35.3 (15.9–64.4) | 0.41 |
| D-dimer (mg/L) | 0.7 (0.4–1.3) | 0.71 (0.37–1.31) | 0.914 |
| Fibrinogen (mg/dL) | 512 (436–574) | 546 (467.8–614.5) | 0.005 |
| Lactate dehydrogenase (UI/L) | 373 (278–473.5) | 334 (239–476) | 0.233 |
| Ferritin (ng/mL) | 492 (195–857) | 569 (305–947) | 0.007 |
| Platelets count (cells/µL) | 215 (164–268) | 206 (159–270) | 0.177 |
| Lymphocytes count (cells/µL) | 1000 (670–1490) | 815 (552.5–1147.5) | 0.078 |
| C-reactive protein (mg/L) | 11.9 (6.7–21.9) | 7.9 (2.3–37.5) | 0.009 |
| Procalcitonin (ng/mL) | 0.1 (0.1–0.2) | 0.00 (0.00–0.10) | 0.105 |
| Variables | Standard-Dose Steroid (n = 695) | High-Dose Steroid (n = 295) | p-Value |
|---|---|---|---|
| Pharmacological Therapy | |||
| Antibiotic | 452 (65) | 231 (78.3) | 0.001 |
| Remdesivir | 106 (15.2) | 164 (55.5) | <0.001 |
| Tocilizumab | 5 (0.7) | 12 (4) | 0.064 |
| Low-weight molecular heparin | 252 (36.2) | 227 (76.9) | <0.001 |
| Oxygen therapy | |||
| Low-flow oxygen | 430 (61.8) | 241 (81.6) | <0.001 |
| HFNC/CPAP/NIV | 239 (34.3) | 80 (27.1) | 0.246 |
| Outcomes | |||
| 30-day mortality | 166 (23.8) | 32 (10.8) | <0.001 |
| Variables | Survivors (n = 792) | Non-Survivors (n = 198) | p-Value |
|---|---|---|---|
| Demographic information | |||
| Age (years), median [IQR 25–75%] | 65 (56–80) | 82 (73–88) | <0.001 |
| Male sex [n (%)] | 347 (43.8) | 100 (50.5) | 0.381 |
| Comorbidities [n (%)] | |||
| Arterial hypertension | 175 (22.1) | 30 (15.1) | 0.345 |
| Cardiovascular disease other than Arterial hypertension | 209 (26.4) | 131 (66.1) | <0.001 |
| Obesity | 91 (11.4) | 17 (8.6) | 0.675 |
| Diabetes mellitus type 2 | 88 (11.1) | 37 (18.7) | 0.355 |
| Chronic kidney disease | 100 (12.6) | 51 (25.7) | 0.002 |
| Chronic obstructive pulmonary disease | 100 (12.6) | 111 (56) | <0.001 |
| Oncologic disease | 50 (6.3) | 37 (18.7) | 0.002 |
| Psychiatric disorders | 43 (5.4) | 5 (2.5) | 0.401 |
| Neurologic disorders | 50 (6.3) | 10 (5) | 0.891 |
| Clinical features [n (%)] | |||
| Fever | 516 (65.1) | 70 (35.3) | <0.001 |
| Cough | 361 (45.5) | 40 (20.2) | <0.001 |
| Dyspnoea | 370 (46.7) | 73 (36.8) | 0.03 |
| Headache | 16 (2) | 0 | 0.633 |
| Diarrhoea | 90 (11.3) | 21 (10.6) | 0.987 |
| Asthenia | 217 (27.4) | 120 (60.6) | <0.001 |
| Ageusia | 66 (8.3) | 11 (5.5) | 0.482 |
| Anosmia | 74 (9.3) | 15 (7.5) | 0.633 |
| Lung function parameters [median (IQR 25–75%)] | |||
| Respiratory rate (acts/min) | 20 (17–24) | 27 (20–30) | 0.009 |
| PaO2/FiO2 ratio | 316 (228.8–376.4) | 310 (142.8–362.5) | 0.001 |
| Laboratory values [median (IQR 25–75%)] | |||
| Interleukin-6 (pg/mL) | 18.9 (8.3–43) | 49.8 (24.6–112.6) | 0.032 |
| D-dimer (mg/L) | 0.7 (0.4–1.3) | 0.9 (0.4–1.5) | 0.043 |
| Fibrinogen (mg/dL) | 517.5 (448.3–575) | 560.5 (447.8–657.5) | 0.089 |
| Lactate dehydrogenase (UI/L) | 336 (252–457) | 457 (332–614.5) | <0.001 |
| Ferritin (ng/mL) | 506 (209–852) | 551 (214–1078) | 0.144 |
| Platelets count (cells/µL) | 210 (162–268) | 226 (166.8–270.8) | 0.147 |
| Lymphocytes count (cells/µL) | 950 (640–1380) | 900 (600–1470) | 0.323 |
| C-reactive protein (mg/L) | 10.5 (3.7–29) | 13.1 (8.8–26.7) | 0.160 |
| Procalcitonin (ng/mL) | 0.1 (0.0–0.2) | 0.1 (0.1–0.6) | 0.202 |
| Variables | Survivors (n = 792) | Non-Survivors (n = 198) | p-Value |
|---|---|---|---|
| Pharmacological Therapy | |||
| Antibiotic | 551 (69.5) | 132 (66.6) | 0.359 |
| Remdesivir | 250 (31.5) | 20 (10.1) | <0.001 |
| Tocilizumab | 12 (1.5) | 5 (2.5) | 0.572 |
| Low-weight molecular heparin | 420 (53) | 59 (29.8) | <0.001 |
| Standard-dose steroid therapy | 539 (68) | 156 (78.8) | 0.122 |
| High-dose steroid therapy | 262 (33) | 33 (16.6) | <0.001 |
| Oxygen therapy | |||
| Low-flow oxygen | 583 (73.6) | 88 (44.4) | <0.001 |
| HFNC/CPAP/NIV | 180 (22.7) | 139 (70.2) | <0.001 |
| Variables | Hazard Ratio | CI95%-Lower | CI95%-Upper | p-Value |
|---|---|---|---|---|
| Chronic kidney disease | 5.21 | 1.48 | 22.23 | 0.001 |
| Oncologic disease | 2.81 | 1.45 | 19.8 | 0.005 |
| Chronic obstructive pulmonary disease | 1.98 | 1.34 | 9.81 | 0.002 |
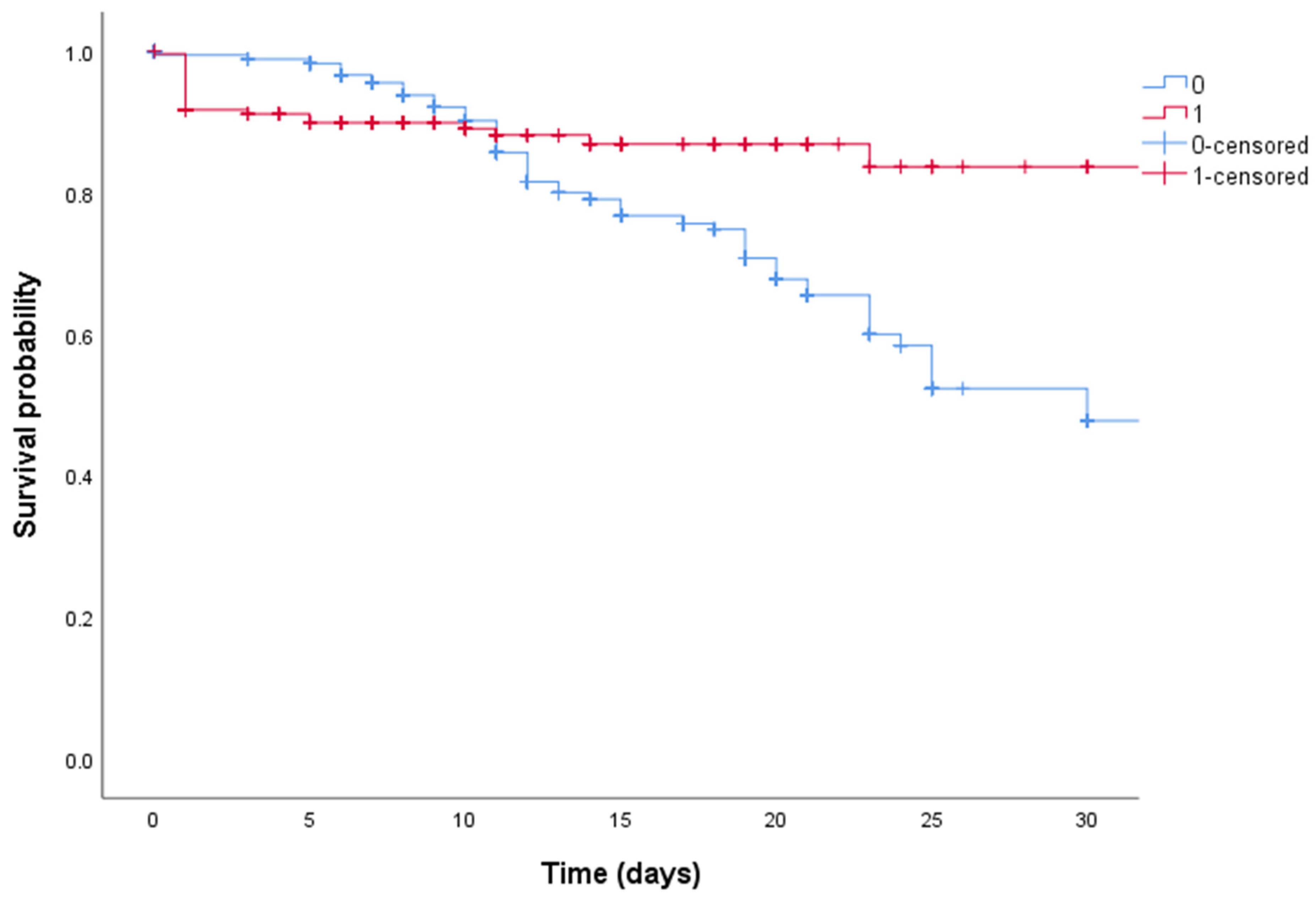
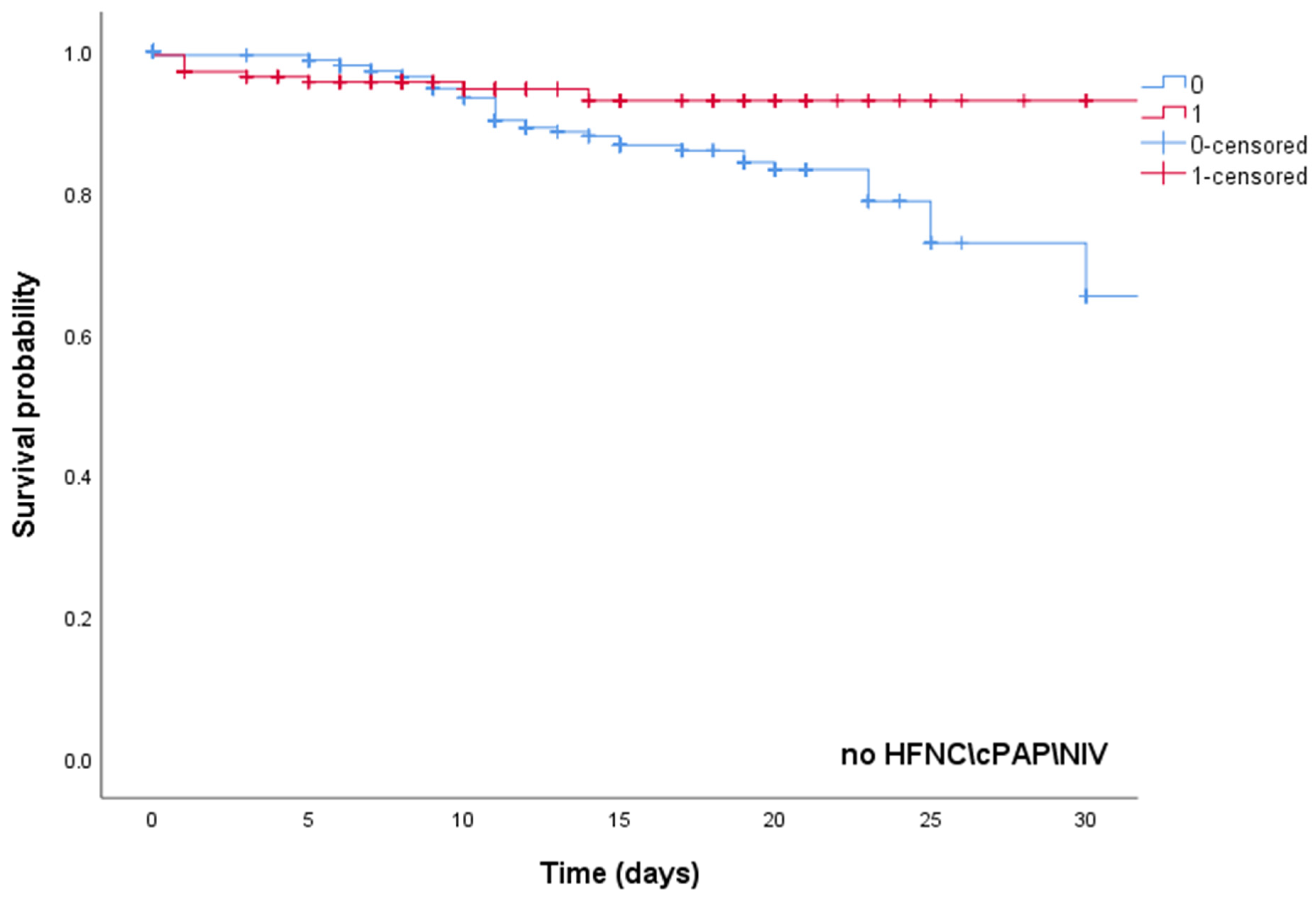
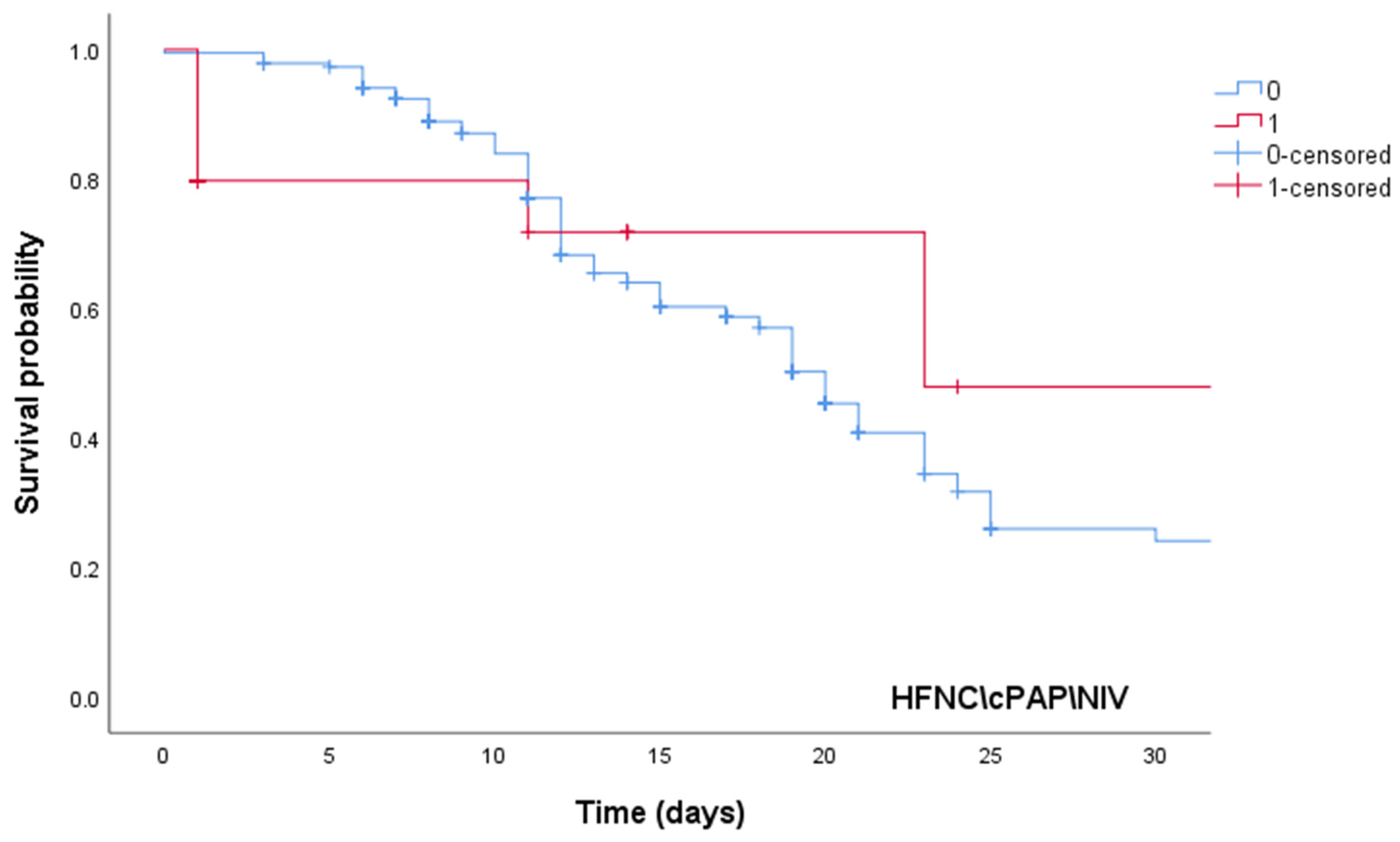
| High-dose steroid therapy | 0.42 | 0.38 | 0.86 | 0.002 |
| HFNC/CPAP/NIV | 61.1 | 5.12 | 511.1 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, A.; Davoli, C.; Borrazzo, C.; Olivadese, V.; Ceccarelli, G.; Fusco, P.; Lazzaro, A.; Lionello, R.; Ricchio, M.; Serapide, F.; et al. Clinical Characteristics and Outcome of Hospitalized COVID-19 Patients Treated with Standard Dose of Dexamethasone or High Dose of Methylprednisolone. Biomedicines 2022, 10, 1548. https://doi.org/10.3390/biomedicines10071548
Russo A, Davoli C, Borrazzo C, Olivadese V, Ceccarelli G, Fusco P, Lazzaro A, Lionello R, Ricchio M, Serapide F, et al. Clinical Characteristics and Outcome of Hospitalized COVID-19 Patients Treated with Standard Dose of Dexamethasone or High Dose of Methylprednisolone. Biomedicines. 2022; 10(7):1548. https://doi.org/10.3390/biomedicines10071548
Chicago/Turabian StyleRusso, Alessandro, Chiara Davoli, Cristian Borrazzo, Vincenzo Olivadese, Giancarlo Ceccarelli, Paolo Fusco, Alessandro Lazzaro, Rosaria Lionello, Marco Ricchio, Francesca Serapide, and et al. 2022. "Clinical Characteristics and Outcome of Hospitalized COVID-19 Patients Treated with Standard Dose of Dexamethasone or High Dose of Methylprednisolone" Biomedicines 10, no. 7: 1548. https://doi.org/10.3390/biomedicines10071548
APA StyleRusso, A., Davoli, C., Borrazzo, C., Olivadese, V., Ceccarelli, G., Fusco, P., Lazzaro, A., Lionello, R., Ricchio, M., Serapide, F., Tassone, B., Gentilini Cacciola, E., Mastroianni, C. M., Torti, C., d’Ettorre, G., & Trecarichi, E. M. (2022). Clinical Characteristics and Outcome of Hospitalized COVID-19 Patients Treated with Standard Dose of Dexamethasone or High Dose of Methylprednisolone. Biomedicines, 10(7), 1548. https://doi.org/10.3390/biomedicines10071548









