Vaccine mRNA Can Be Detected in Blood at 15 Days Post-Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Collection and RNA Extraction
2.2. Reverse Transcription and qPCR
2.3. Cell Culture
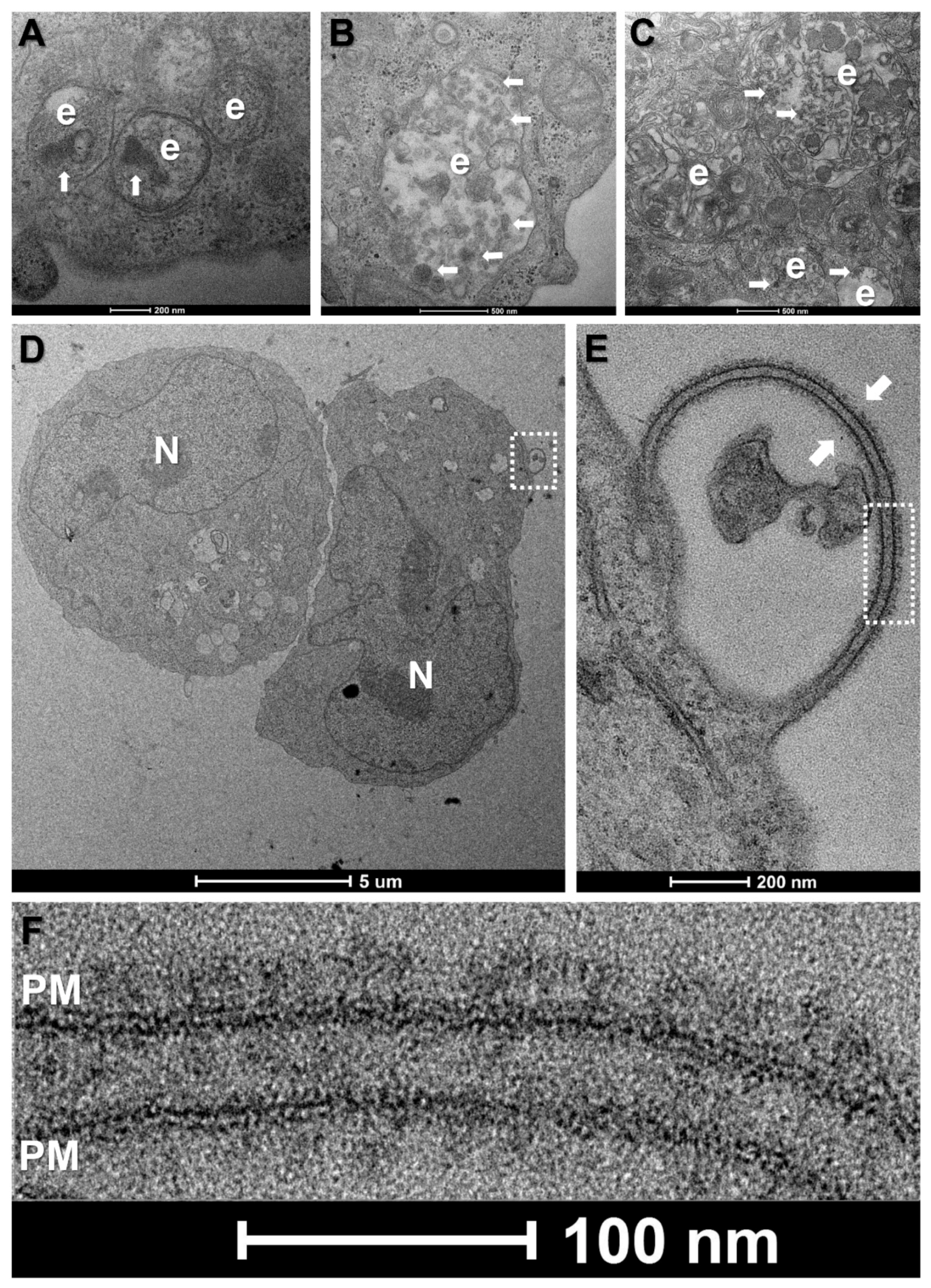
2.4. Transmission Electron Microscopy
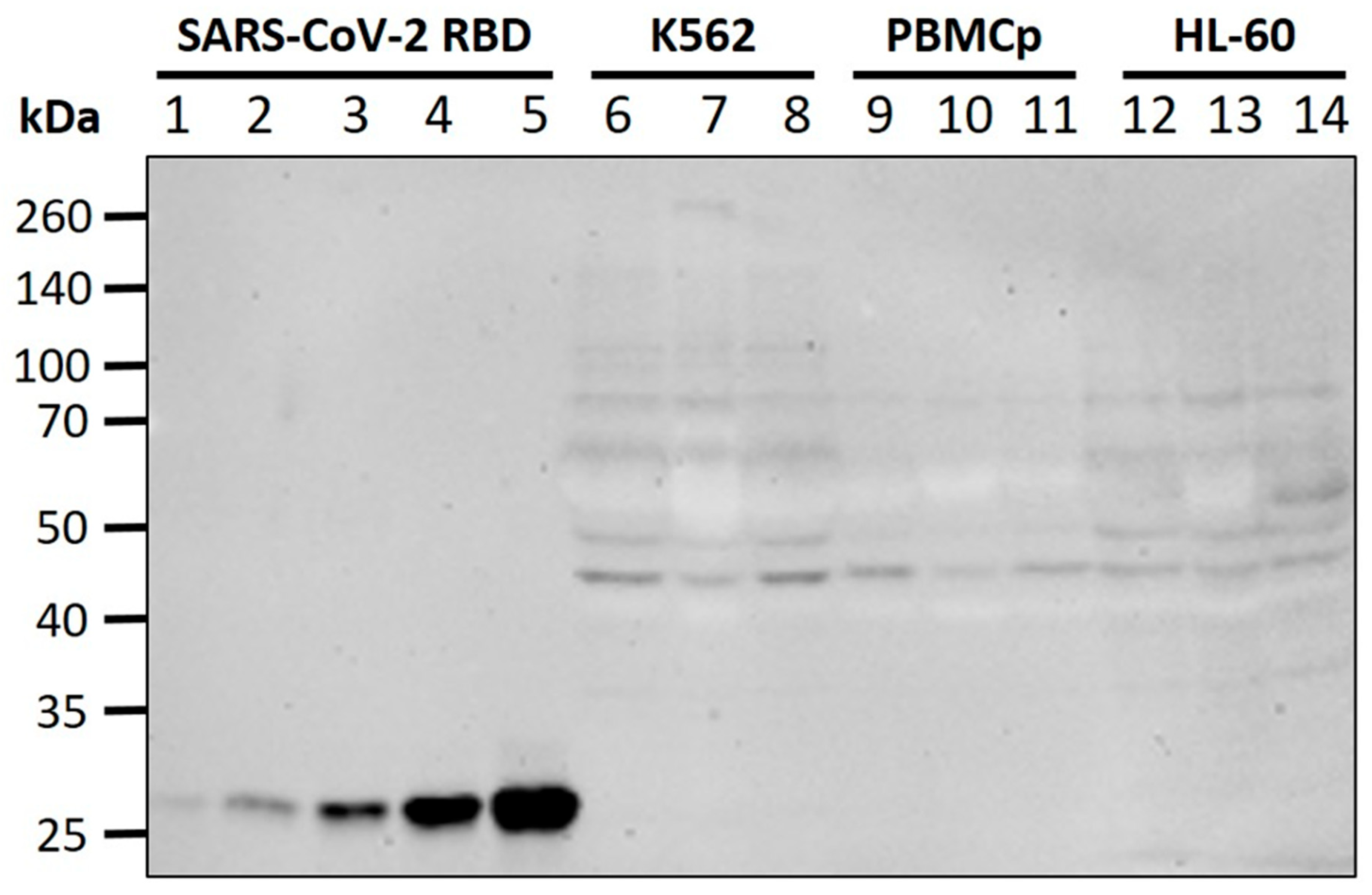
2.5. Western Blot
3. Results
3.1. Study Participants
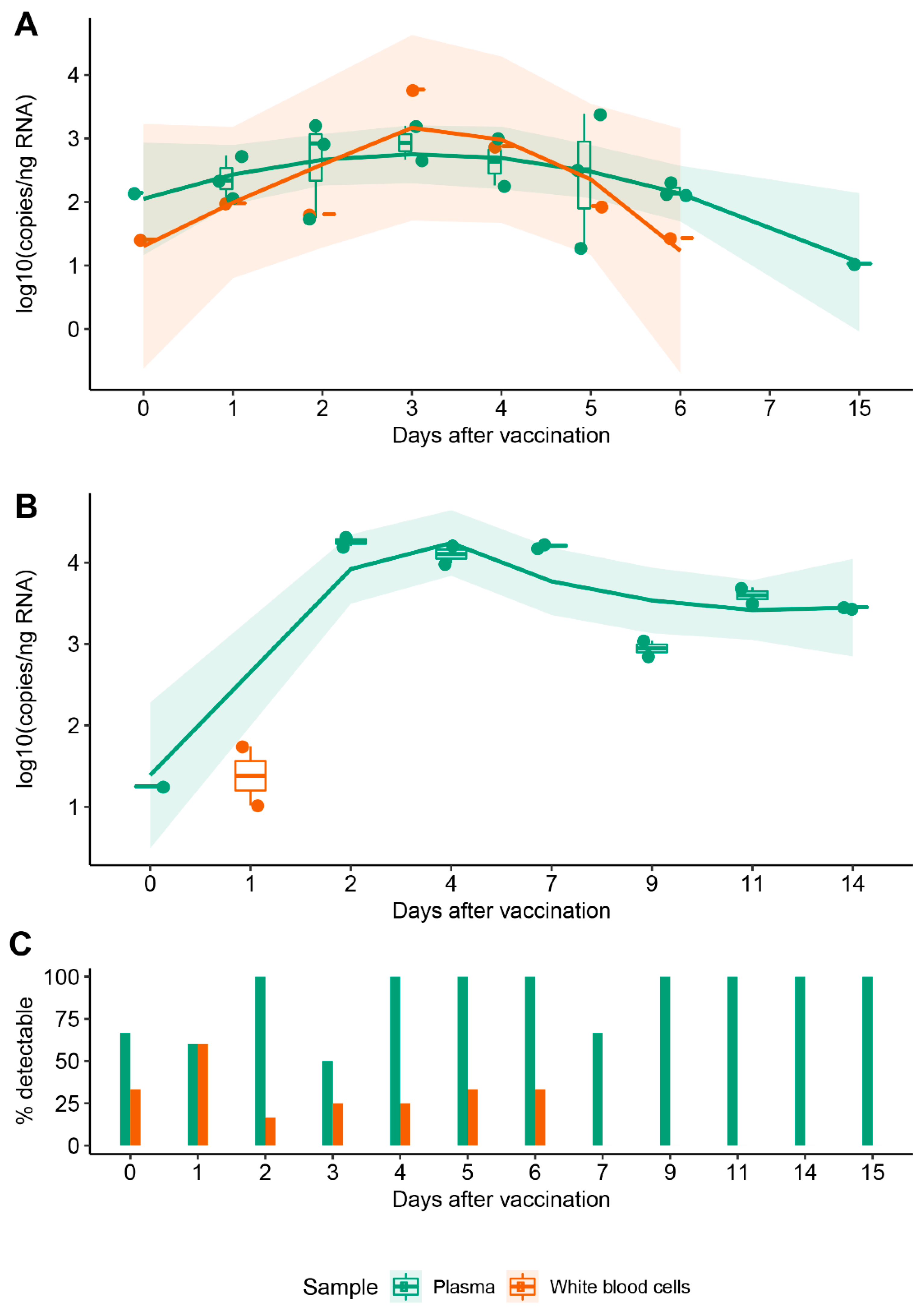
3.2. Vaccine mRNA Remains in Circulation for at Least 15 Days
3.3. WBCs Are Unlikely Candidates for S-Protein Expression In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vilches, T.N.; Moghadas, S.M.; Sah, P.; Fitzpatrick, M.C.; Shoukat, A.; Pandey, A.; Galvani, A.P. Estimating COVID-19 Infections, Hospitalizations, and Deaths Following the US Vaccination Campaigns during the Pandemic. JAMA Netw. Open 2022, 797, 2020–2023. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. MRNA Vaccines for Infectious Diseases: Principles, Delivery and Clinical Translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An MRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, L.; Park, Y.J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 2020, 183, 1024–1042.e21. [Google Scholar] [CrossRef] [PubMed]
- Bahl, K.; Senn, J.J.; Yuzhakov, O.; Bulychev, A.; Brito, L.A.; Hassett, K.J.; Laska, M.E.; Smith, M.; Almarsson, Ö.; Thompson, J.; et al. Preclinical and Clinical Demonstration of Immunogenicity by MRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol. Ther. 2017, 25, 1316–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, F.; Lindgren, G.; Lin, A.; Thompson, E.A.; Ols, S.; Röhss, J.; John, S.; Hassett, K.; Yuzhakov, O.; Bahl, K.; et al. Efficient Targeting and Activation of Antigen-Presenting Cells In Vivo after Modified MRNA Vaccine Administration in Rhesus Macaques. Mol. Ther. 2017, 25, 2635–2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency. Assessment Report EMA/707383/2020 Corr.1*: Comirnaty COVID-19 MRNA Vaccine (Nucleoside-Modified); Assessment Report; European Medicines Agency: Amsterdam, The Netherlands, 2021; Volume 31, pp. 1–140. [Google Scholar]
- European Medicines Agency. Assessment Report EMA/15689/2021 Corr.1*: Covid-19 Vaccine Moderna; European Medicines Agency: Amsterdam, The Netherlands, 2021; Volume 11, p. 169. [Google Scholar]
- An, D.; Schneller, J.L.; Frassetto, A.; Liang, S.; Zhu, X.; Park, J.S.; Theisen, M.; Hong, S.J.; Zhou, J.; Rajendran, R.; et al. Systemic Messenger RNA Therapy as a Treatment for Methylmalonic Acidemia. Cell Rep. 2017, 21, 3548–3558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberli, M.A.; Reichmuth, A.M.; Dorkin, J.R.; Mitchell, M.J.; Fenton, O.S.; Jaklenec, A.; Anderson, D.G.; Langer, R.; Blankschtein, D. Lipid Nanoparticle Assisted MRNA Delivery for Potent Cancer Immunotherapy. Nano Lett. 2017, 17, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Ogata, A.F.; Cheng, C.-A.; Desjardins, M.; Senussi, Y.; Sherman, A.C.; Powell, M.; Novack, L.; Von, S.; Li, X.; Baden, L.R.; et al. Circulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Antigen Detected in the Plasma of MRNA-1273 Vaccine Recipients. Clin. Infect. Dis. 2022, 74, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Röltgen, K.; Nielsen, S.C.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022, 185, 1025–1040. [Google Scholar] [CrossRef]
- Bansal, S.; Perincheri, S.; Fleming, T.; Poulson, C.; Tiffany, B.; Bremner, R.M.; Mohanakumar, T. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer–BioNTech) Vaccination Prior to Development of Antibodies: A Novel Mechanism for Immune Activation by MRNA Vaccines. J. Immunol. 2021, 207, 2405–2410. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.E.; Mccoy, M.; Artiles, K.; Ilbay, O.; Fire, A.; Nadeau, K.; Park, H.; Betts, B.; Boyd, S.; Hoh, R.; et al. Assemblies of Putative SARS-CoV2-Spike-Encoding MRNA Sequences for Vaccines BNT-162b2 and MRNA-1273. Available online: https://virological.org/t/assemblies-of-putative-sars-cov2-spike-encoding-mrna-sequences-for-vaccines-bnt-162b2-and-mrna-1273/663 (accessed on 23 June 2022).
- Pfaffl, M.W. Quantification strategies in real-time PCR. In A-Z of Quantitative PCR; Bustin, S.A., Ed.; California International University: La Jolla, CA, USA, 2004; pp. 87–112. [Google Scholar]
- Forootan, A.; Sjöback, R.; Björkman, J.; Sjögreen, B.; Linz, L.; Kubista, M. Methods to Determine Limit of Detection and Limit of Quantification in Quantitative Real-Time PCR (QPCR). Biomol. Detect. Quantif. 2017, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; ISBN 3-900051-07-0. Available online: http://www.r-project.org (accessed on 23 June 2022).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Fertig, E.T.; Gherghiceanu, M.; Popescu, L.M. Extracellular Vesicles Release by Cardiac Telocytes: Electron Microscopy and Electron Tomography. J. Cell. Mol. Med. 2014, 18, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gao, G.F. MRNA Vaccines: A Matter of Delivery. EClinicalMedicine 2021, 32, 100746. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Beerli, R.R.; Agnellini, P.; Wolinit, P.; Schwarz, K.; Oxenius, A. Long-Lived Memory CD8+ T Cells Are Programmed by Prolonged Antigen Exposure and Low Levels of Cellular Activation. Eur. J. Immunol. 2006, 36, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Glienke, W.; Maute, L.; Koehl, U.; Esser, R.; Milz, E.; Bergmann, L. Effective Treatment of Leukemic Cell Lines with Wt1 SiRNA. Leukemia 2007, 21, 2164–2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paramasivam, P.; Franke, C.; Stöter, M.; Höijer, A.; Bartesaghi, S.; Sabirsh, A.; Lindfors, L.; Arteta, M.Y.; Dahlén, A.; Bak, A.; et al. Endosomal Escape of Delivered MRNA from Endosomal Recycling Tubules Visualized at the Nanoscale. J. Cell Biol. 2022, 221, e202110137. [Google Scholar] [CrossRef] [PubMed]
| Participant ID | Age (Years) | Sex | Vaccine | Doses Received | Time (Days) | |||
|---|---|---|---|---|---|---|---|---|
| Between Doses | Blood Collection after 1st Dose | Blood Collection after 2nd Dose | Blood Collection after 3rd Dose | |||||
| A1.1 | 37 | M | BNT162b2 | 1 | 1 | |||
| B1.1 | 49 | F | BNT162b2 | 1 | 1 | |||
| C1.2 | 37 | M | BNT162b2 | 1 | 2 | |||
| D1.3 | 21 | F | BNT162b2 | 1 | 3 | |||
| E1.3 | 21 | M | BNT162b2 | 1 | 3 | |||
| F1.4 | 21 | F | BNT162b2 | 1 | 4 | |||
| G1.4 | 21 | M | BNT162b2 | 1 | 4 | |||
| H1.5 | 33 | F | BNT162b2 | 1 | 5 | |||
| I1.6 | 21 | F | BNT162b2 | 1 | 6 | |||
| G1.6 | 21 | M | BNT162b2 | 1 | 6 | |||
| H2.0 | 33 | F | BNT162b2 | 2 | 21 | 0 | ||
| J2.1 | 37 | F | BNT162b2 | 2 | 21 | 1 | ||
| B2.2 | 49 | F | BNT162b2 | 2 | 21 | 2 | ||
| K2.2 | 50 | F | BNT162b2 | 2 | 21 | 2 | ||
| J2.2 | 37 | F | BNT162b2 | 2 | 21 | 2 | ||
| C2.3 | 37 | M | BNT162b2 | 2 | 21 | 3 | ||
| L2.3 | 37 | M | BNT162b2 | 2 | 21 | 3 | ||
| M2.5 | 25 | F | BNT162b2 | 2 | 21 | 5 | ||
| N2.5 | 34 | F | BNT162b2 | 2 | 21 | 5 | ||
| O2.6 | 35 | F | BNT162b2 | 2 | 21 | 6 | ||
| P2.7 | 21 | F | BNT162b2 | 2 | 21 | 7 | ||
| B2.15 | 49 | F | BNT162b2 | 2 | 21 | 15 | ||
| M2.27 | 25 | F | BNT162b2 | 2 | 21 | 27 | ||
| CTRL1 | 31 | M | ||||||
| CTRL2 | 68 | F | ||||||
| CTRL3 | 39 | M | ||||||
| B3 | 49 | F | BNT162b2 | 3 | 21/272 | 0, 1, 2, 4, 7, 9, 11, 14 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fertig, T.E.; Chitoiu, L.; Marta, D.S.; Ionescu, V.-S.; Cismasiu, V.B.; Radu, E.; Angheluta, G.; Dobre, M.; Serbanescu, A.; Hinescu, M.E.; et al. Vaccine mRNA Can Be Detected in Blood at 15 Days Post-Vaccination. Biomedicines 2022, 10, 1538. https://doi.org/10.3390/biomedicines10071538
Fertig TE, Chitoiu L, Marta DS, Ionescu V-S, Cismasiu VB, Radu E, Angheluta G, Dobre M, Serbanescu A, Hinescu ME, et al. Vaccine mRNA Can Be Detected in Blood at 15 Days Post-Vaccination. Biomedicines. 2022; 10(7):1538. https://doi.org/10.3390/biomedicines10071538
Chicago/Turabian StyleFertig, Tudor Emanuel, Leona Chitoiu, Daciana Silvia Marta, Victor-Stefan Ionescu, Valeriu Bogdan Cismasiu, Eugen Radu, Giulia Angheluta, Maria Dobre, Ana Serbanescu, Mihail Eugen Hinescu, and et al. 2022. "Vaccine mRNA Can Be Detected in Blood at 15 Days Post-Vaccination" Biomedicines 10, no. 7: 1538. https://doi.org/10.3390/biomedicines10071538
APA StyleFertig, T. E., Chitoiu, L., Marta, D. S., Ionescu, V.-S., Cismasiu, V. B., Radu, E., Angheluta, G., Dobre, M., Serbanescu, A., Hinescu, M. E., & Gherghiceanu, M. (2022). Vaccine mRNA Can Be Detected in Blood at 15 Days Post-Vaccination. Biomedicines, 10(7), 1538. https://doi.org/10.3390/biomedicines10071538







