Gut Bless Your Pain—Roles of the Gut Microbiota, Sleep, and Melatonin in Chronic Orofacial Pain and Depression
Abstract
1. Introduction
2. Search Strategy and Selection Criteria
3. Sleep–Psycho–Pain Axis
4. Inflammation–Psycho–Pain Axis
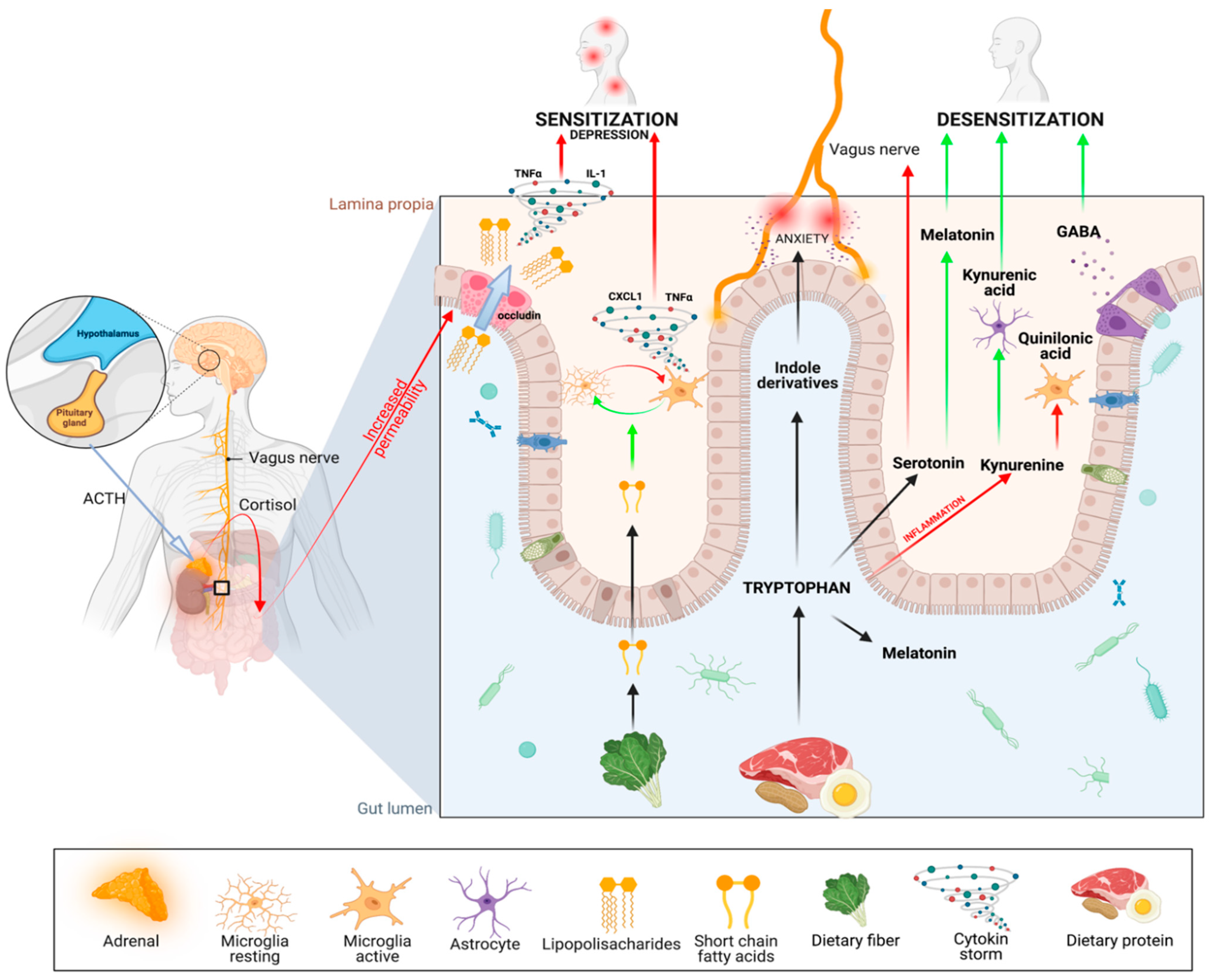
5. Gut–Psycho–Pain Axis
- Production of serotonin (5-HT). Surprisingly, 90% of serotonin is produced by Enterococcus spp. in the gut. Decreased serotonin levels are associated with depression and, as recently hypothesized, with severe sleep bruxism [101]. It is also directly connected with a lack of its derivative—melatonin—as discussed in the following section. It turns out that inflammatory processes in the guts, as well as stress, cause the depletion of serotonin by changing the metabolic pathway of Trp [102] to the second one—the kynurenine pathway (KP) [103].
- The third metabolic pathway for Trp involves the production of indoxyl sulfate, which is associated with anxiety. Strikingly, the gut microbiota is exclusively responsible for the conversion of tryptophan to indole derivatives [106]. This provides further evidence that gut microbiome disruption can directly contribute to neuropsychiatric disorders [107], and that the link between our emotional state and microbiota is bidirectional [108,109]: stress disrupts the gut microbiome which, in turn, can cause stress.
- 4.
- Probiotics (e.g., Bacteroides fragilis) can correct some of the changes related to increased gut permeability [156]. Probiotic supplementation has also shown promising results, with reductions in anxiety and depression [157,158,159,160] through direct and indirect mechanisms of action; for example, local stimulation of the vagus nerve [161,162,163,164,165], which reduces the activity of the sympathetic nervous system. Considering the potential beneficial effects of probiotics on mental health, they are also referred to as “psychobiotics” [83].
- 5.
- Omega-3 fatty acids show anti-inflammatory effects on LPS-stimulated microglia [166], induce an increase in several short-chain fatty acid-producing bacteria species [167], and help to maintain intestinal wall integrity. Thanks to those properties, they have been shown to be effective in the treatment of gut dysbiosis [168] (disruption to the microbiota homeostasis), depression [169], neuropathic pain [170] after neurotrauma [171], joint pain associated with rheumatoid arthritis, and inflammatory bowel disease [172].
- 6.
- Resveratrol is antioxidative, anti-inflammatory [173], and improves the gut microbiota [174]. Recently, resveratrol has been used to alleviate temporomandibular joint inflammatory pain by recovering disturbed gut microbiota. An interesting observation is that the systemic administration of resveratrol restored reduced Bacteroidetes and Lachnospiraceae while attenuating nociception in TMJ-inflamed mice, where the antinociceptive effect was mimicked by fecal transplantation from inflamed animals receiving resveratrol treatment [175].
- 7.
- Short-chain fatty acids (SCFAs), which are derived from bacterial fermentation of dietary fiber in the gut [176], play important roles in regulating microglia morphology and function. SCFAs may act as important mediators derived from the gut microbiota for regulation of pain through receptor-mediated mechanisms, epigenetic regulation mechanisms, or both [177,178,179,180].
- 8.
- The last therapeutic that should be discussed in this review is melatonin, which deserves a separate section.
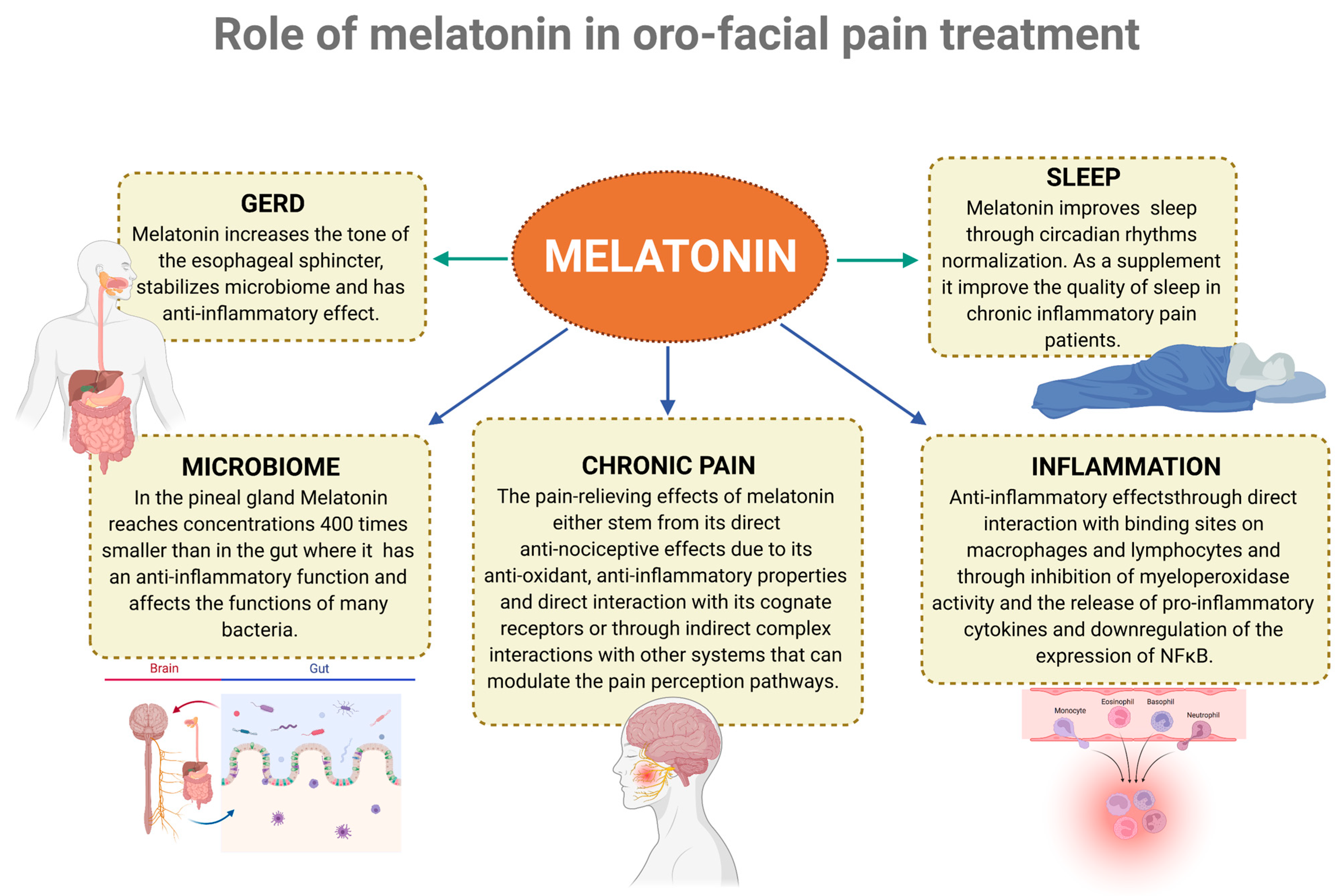
6. Role of Melatonin in Pain, Sleep, and Inflammation
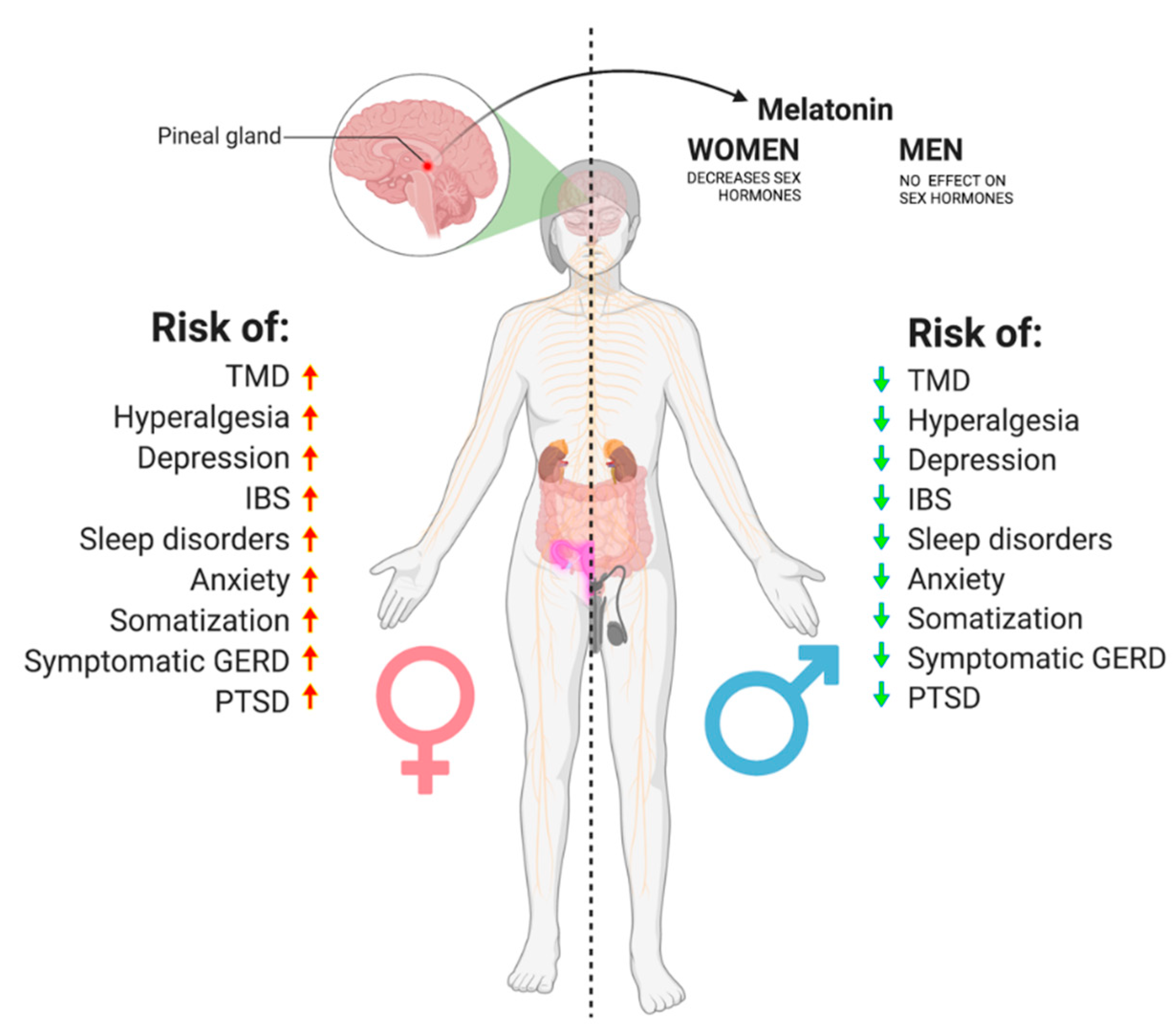
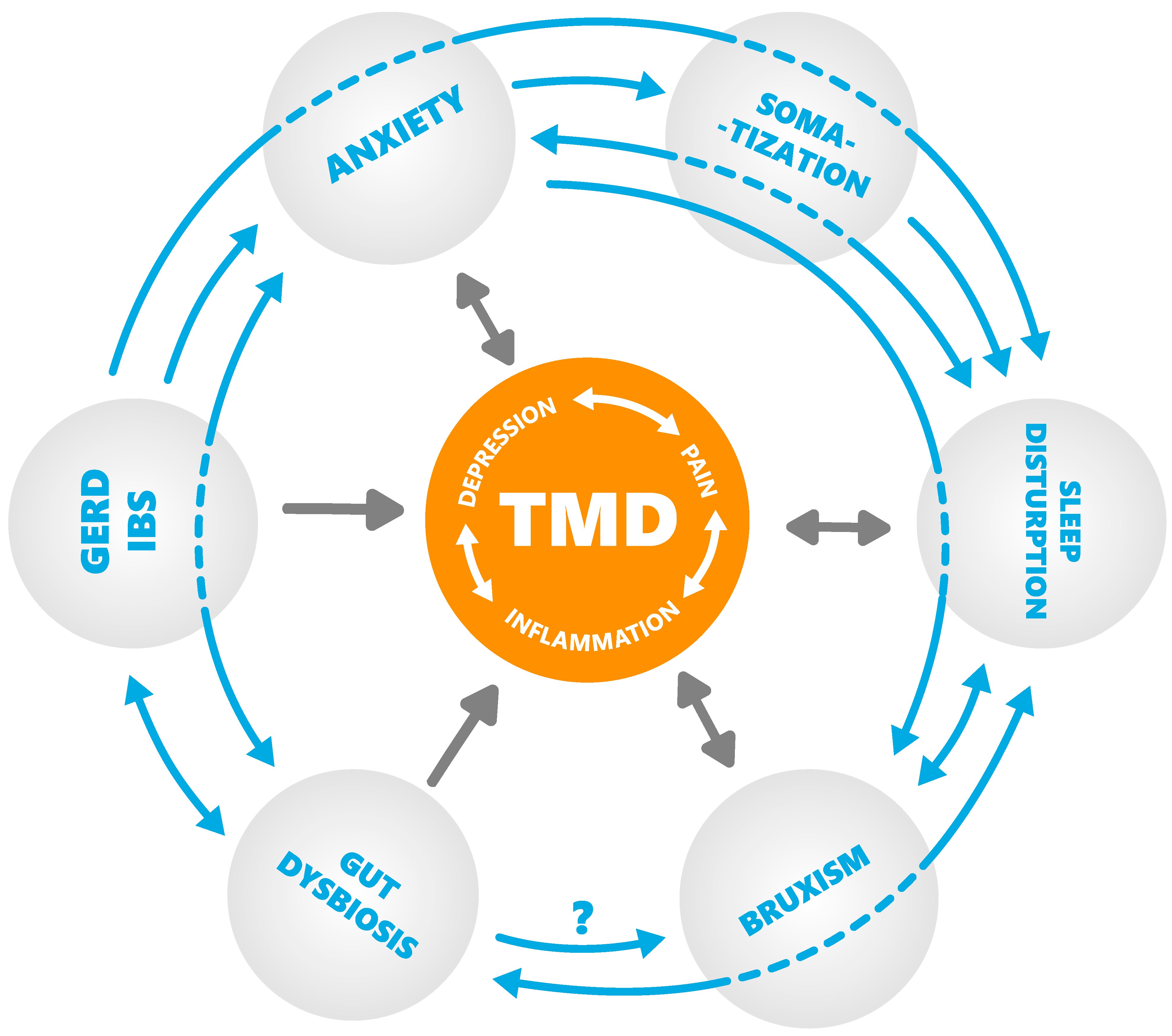
7. A proposed Cascade of Events Leading to Orofacial Pain and Gender Predisposition
8. Conclusions
- Inflammation negatively influences the sleep cycle, leading to a pre-disposition to higher pain sensitivity, anxiety, and stress and, as consequence, to intestinal permeability and disrupted microbiota;
- Bacterial dysbiosis leads (directly and indirectly) to sensitization of the central nervous system, possibly contributing to many types of chronic pain;
- Inflammation and disruption of the intestinal microbiome alter the metabolism of serotonin and melatonin;
- GERD, IBS, sleep disorders, anxiety, depression, PTSD, hyperalgesia, and somatization are all more prevalent among women than men, and so are TMDs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TMD | Temporomandibular disorder |
| TMJ | Temporomandibular joint |
| CNS | Central Nervous System |
| GERD | Gastroesophageal reflux disease |
| IBS | Irritable Bowel Syndrome |
| SIBO | Small intestinal bacterial overgrowth |
| PTSD | Post-traumatic stress disorder |
| REM | Rapid eye movement |
| Non-REM SWS | Non-rapid eye movement slow-wave sleep |
| PPI | Proton pump inhibitor |
| SCFA | Short-Chain Fatty Acids |
| LPS | Lipopolysaccharides |
| GABA | Gamma-aminobutyric acid |
| LH | Luteinizing hormone |
| FSH | Follicle-stimulating hormone |
| HPA | Hypothalamic–pituitary–adrenal |
| MDD | Major depressive disorder |
| gdTcells | Gamma delta T-cells |
| CRP | C-reactive protein |
| ACTH | Adrenocorticotropic hormone |
| KP | Kynurenine pathway |
| Kyna | Kynurenic acid |
| Quin | Quinolinic acid |
| PRR | Pattern recognition receptors |
| Trp | Tryptophan |
| 5-HT | serotonin |
References
- Scrivani, S.J.; Keith, D.A.; Kaban, L.B. Temporomandibular Disorders. N. Engl. J. Med. 2008, 359, 2693–2705. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, T.V.; Glenny, A.-M.; Worthington, H.V. Systematic Review of Population-Based Epidemiological Studies of Oro-Facial Pain. J. Dent. 2001, 29, 451–467. [Google Scholar] [CrossRef]
- Truelove, E.L.; Sommers, E.E.; LeResche, L.; Dworkin, S.F.; von Korff, M. Clinical Diagnostic Criteria for TMD New Classification Permits Multiple Diagnoses. J. Am. Dent. Assoc. 1992, 123, 47–54. [Google Scholar] [CrossRef]
- Dworkin, S.F.; LeResche, L. Research Diagnostic Criteria for Temporomandibular Disorders: Review, Criteria, Examinations and Specifications, Critique. J. Craniomandib. Disord. 1992, 6, 301–355. [Google Scholar] [PubMed]
- Munzenmaier, D.H.; Wilentz, J.; Cowley, A.W. Genetic, Epigenetic, and Mechanistic Studies of Temporomandibular Disorders and Overlapping Pain Conditions. Mol. Pain 2014, 10, 72. [Google Scholar] [CrossRef]
- Gatchel, R.J.; Reuben, D.B.; Dagenais, S.; Turk, D.C.; Chou, R.; Hershey, A.D.; Hicks, G.E.; Licciardone, J.C.; Horn, S.D. Research Agenda for the Prevention of Pain and Its Impact: Report of the Work Group on the Prevention of Acute and Chronic Pain of the Federal Pain Research Strategy. J. Pain 2018, 19, 837–851. [Google Scholar] [CrossRef]
- Gallotta, S.; Bruno, V.; Catapano, S.; Mobilio, N.; Ciacci, C.; Iovino, P. High Risk of Temporomandibular Disorder in Irritable Bowel Syndrome: Is There a Correlation with Greater Illness Severity? World J. Gastroenterol. 2017, 23, 103. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, R.I.; Serlie, M.J.; Kalsbeek, A.; la Fleur, S.E. Serotonin, a Possible Intermediate between Disturbed Circadian Rhythms and Metabolic Disease. Neuroscience 2015, 301, 155–167. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis during Early Life Regulates the Hippocampal Serotonergic System in a Sex-Dependent Manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Ridaura, V.; Belkaid, Y. Gut Microbiota: The Link to Your Second Brain. Cell 2015, 161, 193–194. [Google Scholar] [CrossRef]
- Guo, R.; Chen, L.-H.; Xing, C.; Liu, T. Pain Regulation by Gut Microbiota: Molecular Mechanisms and Therapeutic Potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef]
- Staffe, A.T.; Bech, M.W.; Clemmensen, S.L.K.; Nielsen, H.T.; Larsen, D.B.; Petersen, K.K. Total Sleep Deprivation Increases Pain Sensitivity, Impairs Conditioned Pain Modulation and Facilitates Temporal Summation of Pain in Healthy Participants. PLoS ONE 2019, 14, e0225849. [Google Scholar] [CrossRef]
- Herrero Babiloni, A.; de Koninck, B.P.; Beetz, G.; de Beaumont, L.; Martel, M.O.; Lavigne, G.J. Sleep and Pain: Recent Insights, Mechanisms, and Future Directions in the Investigation of This Relationship. J. Neural Transm. 2020, 127, 647–660. [Google Scholar] [CrossRef]
- Stroemel-Scheder, C.; Kundermann, B.; Lautenbacher, S. The Effects of Recovery Sleep on Pain Perception: A Systematic Review. Neurosci. Biobehav. Rev. 2020, 113, 408–425. [Google Scholar] [CrossRef]
- Al-Jewair, T.; Shibeika, D.; Ohrbach, R. Temporomandibular Disorders and Their Association with Sleep Disorders in Adults: A Systematic Review. J. Oral Facial Pain Headache 2021, 35, 41–53. [Google Scholar] [CrossRef]
- Rener-Sitar, K.; John, M.T.; Pusalavidyasagar, S.S.; Bandyopadhyay, D.; Schiffman, E.L. Sleep Quality in Temporomandibular Disorder Cases. Sleep Med. 2016, 25, 105–112. [Google Scholar] [CrossRef]
- Sommer, I.; Lavigne, G.; Ettlin, D.A. Review of Self-Reported Instruments That Measure Sleep Dysfunction in Patients Suffering from Temporomandibular Disorders and/or Orofacial Pain. Sleep Med. 2015, 16, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; Wickwire, E.M.; Grace, E.G.; Edwards, R.R.; Buenaver, L.F.; Peterson, S.; Klick, B.; Haythornthwaite, J.A. Sleep Disorders and Their Association with Laboratory Pain Sensitivity in Temporomandibular Joint Disorder. Sleep 2009, 32, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Benoliel, R.; Zini, A.; Zakuto, A.; Slutzky, H.; Haviv, Y.; Sharav, Y.; Almoznino, G. Subjective Sleep Quality in Temporomandibular Disorder Patients and Association with Disease Characteristics and Oral Health–Related Quality of Life. J. Oral Facial Pain Headache 2017, 31, 313–322. [Google Scholar] [CrossRef]
- van der Helm, E.; Yao, J.; Dutt, S.; Rao, V.; Saletin, J.M.; Walker, M.P. REM Sleep Depotentiates Amygdala Activity to Previous Emotional Experiences. Curr. Biol. 2011, 21, 2029–2032. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Wu, S.-P.; Hu, Y.; Smith, D.E.; Wiley, J.W.; Hong, S. Corticosterone Mediates Stress-Related Increased Intestinal Permeability in a Region-Specific Manner. Neurogastroenterol. Motil. 2013, 25, e127–e139. [Google Scholar] [CrossRef]
- Cussotto, S.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front. Neuroendocrinol. 2018, 51, 80–101. [Google Scholar] [CrossRef]
- Neckelmann, D.; Mykletun, A.; Dahl, A.A. Chronic Insomnia as a Risk Factor for Developing Anxiety and Depression. Sleep 2007, 30, 873–880. [Google Scholar] [CrossRef]
- Breslau, N.; Roth, T.; Rosenthal, L.; Andreski, P. Sleep Disturbance and Psychiatric Disorders: A Longitudinal Epidemiological Study of Young Adults. Biol. Psychiatry 1996, 39, 411–418. [Google Scholar] [CrossRef]
- Papadimitriou, G.N.; Linkowski, P. Sleep Disturbance in Anxiety Disorders. Int. Rev. Psychiatry 2005, 17, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Aguilar-Gaxiola, S.; Alonso, J.; Chatterji, S.; Lee, S.; Ormel, J.; Üstün, T.B.; Wang, P.S. The Global Burden of Mental Disorders: An Update from the WHO World Mental Health (WMH) Surveys. Epidemiol. Psichiatr. Soc. 2009, 18, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Dang-Vu, T.T.; Schabus, M.; Desseilles, M.; Sterpenich, V.; Bonjean, M.; Maquet, P. Functional Neuroimaging Insights into the Physiology of Human Sleep. Sleep 2010, 33, 1589–1603. [Google Scholar] [CrossRef]
- Miyauchi, S.; Misaki, M.; Kan, S.; Fukunaga, T.; Koike, T. Human Brain Activity Time-Locked to Rapid Eye Movements during REM Sleep. Exp. Brain Res. 2009, 192, 657–667. [Google Scholar] [CrossRef]
- Nofzinger, E.A. Functional Neuroimaging of Sleep. Semin. Neurol. 2005, 25, 9–18. [Google Scholar] [CrossRef]
- McGinty, D.J.; Harper, R.M. Dorsal Raphe Neurons: Depression of Firing during Sleep in Cats. Brain Res. 1976, 101, 569–575. [Google Scholar] [CrossRef]
- Marrosu, F.; Portas, C.; Mascia, M.S.; Casu, M.A.; Fà, M.; Giagheddu, M.; Imperato, A.; Gessa, G.L. Microdialysis Measurement of Cortical and Hippocampal Acetylcholine Release during Sleep-Wake Cycle in Freely Moving Cats. Brain Res. 1995, 671, 329–332. [Google Scholar] [CrossRef]
- Kametani, H.; Kawamura, H. Alterations in Acetylcholine Release in the Rat Hippocampus during Sleep-Wakefulness Detected by Intracerebral Dialysis. Life Sci. 1990, 47, 421–426. [Google Scholar] [CrossRef]
- Shouse, M.N.; Staba, R.J.; Saquib, S.F.; Farber, P.R. Monoamines and Sleep: Microdialysis Findings in Pons and Amygdala. Brain Res. 2000, 860, 181–189. [Google Scholar] [CrossRef]
- Park, S.P. In Vivo Microdialysis Measures of Extracellular Norepinephrine in the Rat Amygdala during Sleep-Wakefulness. J. Korean Med. Sci. 2002, 17, 395. [Google Scholar] [CrossRef]
- Ouyang, M.; Hellman, K.; Abel, T.; Thomas, S.A. Adrenergic Signaling Plays a Critical Role in the Maintenance of Waking and in the Regulation of REM Sleep. J. Neurophysiol. 2004, 92, 2071–2082. [Google Scholar] [CrossRef]
- Walker, M.P.; van der Helm, E. Overnight Therapy? The Role of Sleep in Emotional Brain Processing. Psychol. Bull. 2009, 135, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.; Nielsen, T.A. Disturbed Dreaming, Posttraumatic Stress Disorder, and Affect Distress: A Review and Neurocognitive Model. Psychol. Bull. 2007, 133, 482–528. [Google Scholar] [CrossRef]
- ben Simon, E.; Rossi, A.; Harvey, A.G.; Walker, M.P. Overanxious and Underslept. Nat. Hum. Behav 2020, 4, 100–110. [Google Scholar] [CrossRef]
- Goldstein, A.N.; Walker, M.P. The Role of Sleep in Emotional Brain Function. Annu. Rev. Clin. Psychol. 2014, 10, 679–708. [Google Scholar] [CrossRef]
- Ekman, P.; Davidson, R.J. (Eds.) The Nature of Emotion: Fundamental Questions; Series in Affective Science; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Lautenbacher, S.; Kundermann, B.; Krieg, J. Sleep Deprivation and Pain Perception. Sleep Med. Rev. 2006, 10, 357–369. [Google Scholar] [CrossRef]
- Schuh-Hofer, S.; Wodarski, R.; Pfau, D.B.; Caspani, O.; Magerl, W.; Kennedy, J.D.; Treede, R.-D. One Night of Total Sleep Deprivation Promotes a State of Generalized Hyperalgesia: A Surrogate Pain Model to Study the Relationship of Insomnia and Pain. Pain 2013, 154, 1613–1621. [Google Scholar] [CrossRef]
- Faraut, B.; Léger, D.; Medkour, T.; Dubois, A.; Bayon, V.; Chennaoui, M.; Perrot, S. Napping Reverses Increased Pain Sensitivity Due to Sleep Restriction. PLoS ONE 2015, 10, e0117425. [Google Scholar] [CrossRef]
- Roehrs, T.; Hyde, M.; Blaisdell, B.; Greenwald, M.; Roth, T. Sleep Loss and REM Sleep Loss Are Hyperalgesic. Sleep 2006, 29, 145–151. [Google Scholar] [CrossRef]
- Lentz, M.J.; Landis, C.A.; Rothermel, J.; Shaver, J.L. Effects of Selective Slow Wave Sleep Disruption on Musculoskeletal Pain and Fatigue in Middle Aged Women. J. Rheumatol. 1999, 26, 1586–1592. [Google Scholar]
- Azevedo, E.; Manzano, G.M.; Silva, A.; Martins, R.; Andersen, M.L.; Tufik, S. The Effects of Total and REM Sleep Deprivation on Laser-Evoked Potential Threshold and Pain Perception. Pain 2011, 152, 2052–2058. [Google Scholar] [CrossRef] [PubMed]
- Schrimpf, M.; Liegl, G.; Boeckle, M.; Leitner, A.; Geisler, P.; Pieh, C. The Effect of Sleep Deprivation on Pain Perception in Healthy Subjects: A Meta-Analysis. Sleep Med. 2015, 16, 1313–1320. [Google Scholar] [CrossRef]
- Cheatle, M.D.; Foster, S.; Pinkett, A.; Lesneski, M.; Qu, D.; Dhingra, L. Assessing and Managing Sleep Disturbance in Patients with Chronic Pain. Anesthesiol. Clin. 2016, 34, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Finan, P.H.; Goodin, B.R.; Smith, M.T. The Association of Sleep and Pain: An Update and a Path Forward. J. Pain 2013, 14, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, G.J.; Sessle, B.J. The Neurobiology of Orofacial Pain and Sleep and Their Interactions. J. Dent. Res. 2016, 95, 1109–1116. [Google Scholar] [CrossRef]
- Andersen, M.L.; Araujo, P.; Frange, C.; Tufik, S. Sleep Disturbance and Pain. Chest 2018, 154, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.M.; Obál, F.; Fang, J.; Kubota, T.; Taishi, P. The Role of Cytokines in Physiological Sleep Regulation. Ann. N. Y. Acad. Sci. 2006, 933, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Opp, M.R. Cytokines and Sleep. Sleep Med. Rev. 2005, 9, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Olivadoti, M.D.; Opp, M.R. Effects of i.c.v. Administration of Interleukin-1 on Sleep and Body Temperature of Interleukin-6-Deficient Mice. Neuroscience 2008, 153, 338–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krueger, J.M.; Majde, J.A. Microbial Products and Cytokines in Sleep and Fever Regulation. Crit. Rev. Immunol. 1994, 14, 355–379. [Google Scholar] [CrossRef]
- de Oliveira, C.M.B.; Sakata, R.K.; Issy, A.M.; Gerola, L.R.; Salomão, R. Cytokines and Pain. Braz. J. Anesthesiol. 2011, 61, 255–265. [Google Scholar] [CrossRef]
- Anisman, H.; Merali, Z.; Hayley, S. Neurotransmitter, Peptide and Cytokine Processes in Relation to Depressive Disorder: Comorbidity between Depression and Neurodegenerative Disorders. Prog. Neurobiol. 2008, 85, 1–74. [Google Scholar] [CrossRef]
- Tabatabaeizadeh, S.-A.; Abdizadeh, M.F.; Meshkat, Z.; Khodashenas, E.; Darroudi, S.; Fazeli, M.; Ferns, G.A.; Avan, A.; Ghayour-Mobarhan, M. There Is an Association between Serum High-Sensitivity C-Reactive Protein (Hs-CRP) Concentrations and Depression Score in Adolescent Girls. Psychoneuroendocrinology 2018, 88, 102–104. [Google Scholar] [CrossRef]
- Zalli, A.; Jovanova, O.; Hoogendijk, W.J.G.; Tiemeier, H.; Carvalho, L.A. Low-Grade Inflammation Predicts Persistence of Depressive Symptoms. Psychopharmacology 2016, 233, 1669–1678. [Google Scholar] [CrossRef]
- Parkinson, J.T.; Foley, É.M.; Jadon, D.R.; Khandaker, G.M. Depression in Patients with Spondyloarthritis: Prevalence, Incidence, Risk Factors, Mechanisms and Management. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X2097002. [Google Scholar] [CrossRef]
- Karlović, D.; Serretti, A.; Vrkić, N.; Martinac, M.; Marčinko, D. Serum Concentrations of CRP, IL-6, TNF-α and Cortisol in Major Depressive Disorder with Melancholic or Atypical Features. Psychiatry Res. 2012, 198, 74–80. [Google Scholar] [CrossRef]
- Kaestner, F.; Hettich, M.; Peters, M.; Sibrowski, W.; Hetzel, G.; Ponath, G.; Arolt, V.; Cassens, U.; Rothermundt, M. Different Activation Patterns of Proinflammatory Cytokines in Melancholic and Non-Melancholic Major Depression Are Associated with HPA Axis Activity. J. Affect. Disord. 2005, 87, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Dunjic-Kostic, B.; Ivkovic, M.; Radonjic, N.V.; Petronijevic, N.D.; Pantovic, M.; Damjanovic, A.; Poznanovic, S.T.; Jovanovic, A.; Nikolic, T.; Jasovic-Gasic, M. Melancholic and Atypical Major Depression--Connection between Cytokines, Psychopathology and Treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral Cytokine and Chemokine Alterations in Depression: A Meta-Analysis of 82 Studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ho, R.C.-M.; Mak, A. Interleukin (IL)-6, Tumour Necrosis Factor Alpha (TNF-α) and Soluble Interleukin-2 Receptors (SIL-2R) Are Elevated in Patients with Major Depressive Disorder: A Meta-Analysis and Meta-Regression. J. Affect. Disord. 2012, 139, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of Depression with C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Stewart, J.C.; Rand, K.L.; Muldoon, M.F.; Kamarck, T.W. A Prospective Evaluation of the Directionality of the Depression-Inflammation Relationship. Brain Behav. Immun. 2009, 23, 936–944. [Google Scholar] [CrossRef]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef]
- Maes, M.; Yirmyia, R.; Noraberg, J.; Brene, S.; Hibbeln, J.; Perini, G.; Kubera, M.; Bob, P.; Lerer, B.; Maj, M. The Inflammatory & Neurodegenerative (I&ND) Hypothesis of Depression: Leads for Future Research and New Drug Developments in Depression. Metab. Brain Dis. 2009, 24, 27–53. [Google Scholar] [CrossRef]
- Alves de Lima, K.; Rustenhoven, J.; da Mesquita, S.; Wall, M.; Salvador, A.F.; Smirnov, I.; Martelossi Cebinelli, G.; Mamuladze, T.; Baker, W.; Papadopoulos, Z.; et al. Meningeal Γδ T Cells Regulate Anxiety-like Behavior via IL-17a Signaling in Neurons. Nat. Immunol. 2020, 21, 1421–1429. [Google Scholar] [CrossRef]
- Liao, C.-H.; Chang, C.-S.; Chang, S.-N.; Lane, H.-Y.; Lyu, S.-Y.; Morisky, D.E.; Sung, F.-C. The Risk of Temporomandibular Disorder in Patients with Depression: A Population-Based Cohort Study. Community Dent. Oral Epidemiol. 2011, 39, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Slade, G.D.; Diatchenko, L.; Bhalang, K.; Sigurdsson, A.; Fillingim, R.B.; Belfer, I.; Max, M.B.; Goldman, D.; Maixner, W. Influence of Psychological Factors on Risk of Temporomandibular Disorders. J. Dent. Res. 2007, 86, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, N.; Lähdesmäki, R.; Mäki, P.; Ek, E.; Taanila, A.; Pesonen, P.; Sipilä, K. Association of Stress and Depression with Chronic Facial Pain: A Case-Control Study Based on the Northern Finland 1966 Birth Cohort. CRANIO® 2017, 35, 187–191. [Google Scholar] [CrossRef]
- Sipilä, K.; Mäki, P.; Laajala, A.; Taanila, A.; Joukamaa, M.; Veijola, J. Association of Depressiveness with Chronic Facial Pain: A Longitudinal Study. Acta Odontol. Scand. 2013, 71, 644–649. [Google Scholar] [CrossRef]
- Korszun, A.; Hinderstein, B.; Wong, M.; Peterson, L.J. Comorbidity of Depression with Chronic Facial Pain and Temporomandibular Disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1996, 82, 496–500. [Google Scholar] [CrossRef]
- Banafa, A.; Sipilä, K.; Suvisaari, J.; Suominen, A.L. Low-Grade Inflammation as a Potential Mediator between Depressive Symptoms and Temporomandibular Pain: An 11-Year Follow-up Study on Finnish Adults. Acta Odontol. Scand. 2021, 79, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Engel, G.L. The Need for a New Medical Model: A Challenge for Biomedicine. Science 1977, 196, 129–136. [Google Scholar] [CrossRef]
- Manfredini, D.; Ahlberg, J.; Winocur, E.; Guarda-Nardini, L.; Lobbezoo, F. Correlation of RDC/TMD Axis I Diagnoses and Axis II Pain-Related Disability. A Multicenter Study. Clin. Oral Investig. 2011, 15, 749–756. [Google Scholar] [CrossRef]
- Manfredini, D.; Favero, L.; del Giudice, A.; Masiero, S.; Stellini, E.; Guarda-Nardini, L. Axis II Psychosocial Findings Predict Effectiveness of TMJ Hyaluronic Acid Injections. Int. J. Oral Maxillofac. Surg. 2013, 42, 364–368. [Google Scholar] [CrossRef]
- Slade, G.D.; Ohrbach, R.; Greenspan, J.D.; Fillingim, R.B.; Bair, E.; Sanders, A.E.; Dubner, R.; Diatchenko, L.; Meloto, C.B.; Smith, S.; et al. Painful Temporomandibular Disorder. J. Dent. Res. 2016, 95, 1084–1092. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Kasper, L.H. The Second Brain: Is the Gut Microbiota a Link Between Obesity and Central Nervous System Disorders? Curr. Obes. Rep. 2016, 5, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Schwarz, M.J. The Immune-Mediated Alteration of Serotonin and Glutamate: Towards an Integrated View of Depression. Mol. Psychiatry 2007, 12, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, R.; Zhan, G.; Wang, D.; Tan, X.; Xu, H. Enterochromaffin Cells: Sentinels to Gut Microbiota in Hyperalgesia? Front. Cell. Infect. Microbiol. 2021, 11, 760076. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Du, X.; Hao, H.; Yang, Y.; Huang, S.; Wang, C.; Gigout, S.; Ramli, R.; Li, X.; Jaworska, E.; Edwards, I.; et al. Local GABAergic Signaling within Sensory Ganglia Controls Peripheral Nociceptive Transmission. J. Clin. Investig. 2017, 127, 1741–1756. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Johnson, C.; Luk, B.; Uribe, G.; Fu, Y.; Oezguen, N.; Matsunami, R.K.; Lugo, M.; Major, A.; Mori-Akiyama, Y.; et al. GABA-Producing Bifidobacterium Dentium Modulates Visceral Sensitivity in the Intestine. Neurogastroenterol. Motil. 2017, 29, e12904. [Google Scholar] [CrossRef]
- Bear, T.L.K.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The Role of the Gut Microbiota in Dietary Interventions for Depression and Anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef]
- Gareau, M.; Silva, M.; Perdue, M. Pathophysiological Mechanisms of Stress-Induced Intestina Damage. Curr. Mol. Med. 2008, 8, 274–281. [Google Scholar] [CrossRef]
- Whitehead, W.E.; Palsson, O.; Jones, K.R. Systematic Review of the Comorbidity of Irritable Bowel Syndrome with Other Disorders: What Are the Causes and Implications? Gastroenterology 2002, 122, 1140–1156. [Google Scholar] [CrossRef]
- Durham, J.; Newton-John, T.R.O.; Zakrzewska, J.M. Temporomandibular Disorders. BMJ 2015, 350, h1154. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, X.-D.; Miao, W.-Y.; Sun, Y.-J.; Xiong, G.; Wu, Q.; Li, G.; Yang, P.; Yu, H.; Li, H.; et al. PDGFRβ Cells Rapidly Relay In-flammatory Signal from the Circulatory System to Neurons via Chemokine CCL2. Neuron 2018, 100, 183–200.e8. [Google Scholar] [CrossRef] [PubMed]
- Rafalski, V.A.; Merlini, M.; Akassoglou, K. Pericytes: The Brain’s Very First Responders? Neuron 2018, 100, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.T.; Choi, J.P.; Kotzin, J.J.; Yang, Y.; Hong, C.C.; Hobson, N.; Girard, R.; Zeineddine, H.A.; Lightle, R.; Moore, T.; et al. Endothelial TLR4 and the Microbiome Drive Cerebral Cavernous Malformations. Nature 2017, 545, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M. Modulation of Pain and Itch by Spinal Glia. Neurosci. Bull. 2018, 34, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Xu, Z.-Z.; Gao, Y.-J. Emerging Targets in Neuroinflammation-Driven Chronic Pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef]
- Das, N.; Dewan, V.; Grace, P.M.; Gunn, R.J.; Tamura, R.; Tzarum, N.; Watkins, L.R.; Wilson, I.A.; Yin, H. HMGB1 Activates Proinflammatory Signaling via TLR5 Leading to Allodynia. Cell Rep. 2016, 17, 1128–1140. [Google Scholar] [CrossRef]
- Miller, R.E.; Ishihara, S.; Tran, P.B.; Golub, S.B.; Last, K.; Miller, R.J.; Fosang, A.J.; Malfait, A.-M. An Aggrecan Fragment Drives Osteoarthritis Pain through Toll-like Receptor 2. JCI Insight 2018, 3, e95704. [Google Scholar] [CrossRef]
- Liu, T.; Gao, Y.-J.; Ji, R.-R. Emerging Role of Toll-like Receptors in the Control of Pain and Itch. Neurosci. Bull. 2012, 28, 131–144. [Google Scholar] [CrossRef]
- Ramos-Chávez, L.A.; Lugo Huitrón, R.; González Esquivel, D.; Pineda, B.; Ríos, C.; Silva-Adaya, D.; Sánchez-Chapul, L.; Roldán-Roldán, G.; Pérez de la Cruz, V. Relevance of Alternative Routes of Kynurenic Acid Production in the Brain. Oxidative Med. Cell. Longev. 2018, 2018, 5272741. [Google Scholar] [CrossRef]
- Smardz, J.; Martynowicz, H.; Wojakowska, A.; Wezgowiec, J.; Danel, D.; Mazur, G.; Wieckiewicz, M. Lower Serotonin Levels in Severe Sleep Bruxism and Its Association with Sleep, Heart Rate, and Body Mass Index. J. Oral Rehabil. 2022, 49, 422–429. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s Metabolites in Exercise, Inflammation, and Mental Health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Stone, T.W.; Stoy, N.; Darlington, L.G. An Expanding Range of Targets for Kynurenine Metabolites of Tryptophan. Trends Pharmacol. Sci. 2013, 34, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Resta, F.; Masi, A.; Sili, M.; Laurino, A.; Moroni, F.; Mannaioni, G. Kynurenic Acid and Zaprinast Induce Analgesia by Modulating HCN Channels through GPR35 Activation. Neuropharmacology 2016, 108, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Cosi, C.; Mannaioni, G.; Cozzi, A.; Carlà, V.; Sili, M.; Cavone, L.; Maratea, D.; Moroni, F. G-Protein Coupled Receptor 35 (GPR35) Activation and Inflammatory Pain: Studies on the Antinociceptive Effects of Kynurenic Acid and Zaprinast. Neuropharmacology 2011, 60, 1227–1231. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics Analysis Reveals Large Effects of Gut Microflora on Mammalian Blood Metabolites. Proc. Natl. Acad. Sci. USA. 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [PubMed]
- Brydges, C.R.; Fiehn, O.; Mayberg, H.S.; Schreiber, H.; Dehkordi, S.M.; Bhattacharyya, S.; Cha, J.; Choi, K.S.; Craighead, W.E.; Krishnan, R.R.; et al. Indoxyl Sulfate, a Gut Microbiome-Derived Uremic Toxin, Is Associated with Psychic Anxiety and Its Functional Magnetic Resonance Imaging-Based Neurologic Signature. Sci. Rep. 2021, 11, 21011. [Google Scholar] [CrossRef]
- Allen, R.G.; Lafuse, W.P.; Galley, J.D.; Ali, M.M.; Ahmer, B.M.M.; Bailey, M.T. The Intestinal Microbiota Are Necessary for Stressor-Induced Enhancement of Splenic Macrophage Microbicidal Activity. Brain Behav. Immun. 2012, 26, 371–382. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.v.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.v.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Abdel-Haq, R.; Schlachetzki, J.C.M.; Glass, C.K.; Mazmanian, S.K. Microbiome–Microglia Connections via the Gut–Brain Axis. J. Exp. Med. 2019, 216, 41–59. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Prinz, M. Communicating Systems in the Body: How Microbiota and Microglia Cooperate. Immunology 2017, 150, 7–15. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a Microglial Disease. Trends Neurosci. 2015, 38, 637–658. [Google Scholar] [CrossRef] [PubMed]
- Stephan, A.H.; Barres, B.A.; Stevens, B. The Complement System: An Unexpected Role in Synaptic Pruning during Development and Disease. Annu. Rev. Neurosci. 2012, 35, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Elmer, B.M.; McAllister, A.K. Major Histocompatibility Complex Class I Proteins in Brain Development and Plasticity. Trends Neurosci. 2012, 35, 660–670. [Google Scholar] [CrossRef]
- Deverman, B.E.; Patterson, P.H. Cytokines and CNS Development. Neuron 2009, 64, 61–78. [Google Scholar] [CrossRef]
- Gao, Y.-J.; Ji, R.-R. Chemokines, Neuronal–Glial Interactions, and Central Processing of Neuropathic Pain. Pharmacol. Ther. 2010, 126, 56–68. [Google Scholar] [CrossRef]
- Gao, Y.-J.; Ji, R.-R. Targeting Astrocyte Signaling for Chronic Pain. Neurotherapeutics 2010, 7, 482–493. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.-Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.-R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Matsuda, M.; Huh, Y.; Ji, R.-R. Roles of Inflammation, Neurogenic Inflammation, and Neuroinflammation in Pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Steiner, J.; Bielau, H.; Brisch, R.; Danos, P.; Ullrich, O.; Mawrin, C.; Bernstein, H.-G.; Bogerts, B. Immunological Aspects in the Neurobiology of Suicide: Elevated Microglial Density in Schizophrenia and Depression Is Associated with Suicide. J. Psychiatr. Res. 2008, 42, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Rock, R.B.; Gekker, G.; Hu, S.; Sheng, W.S.; Cheeran, M.; Lokensgard, J.R.; Peterson, P.K. Role of Microglia in Central Nervous System Infections. Clin. Microbiol. Rev. 2004, 17, 942–964. [Google Scholar] [CrossRef] [PubMed]
- Attwells, S.; Setiawan, E.; Wilson, A.A.; Rusjan, P.M.; Miler, L.; Xu, C.; Hutton, C.; Husain, M.I.; Kish, S.; Vasdev, N.; et al. Replicating Predictive Serum Correlates of Greater Translocator Protein Distribution Volume in Brain. Neuropsychopharmacology 2020, 45, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Nakatani, E.; Yoshikawa, H.; Kanno, T.; Nariai, Y.; Yoshino, A.; Vieth, M.; Kinoshita, Y.; Sekine, J. Oral Soft Tissue Disorders Are Associated with Gastroesophageal Reflux Disease: Retrospective Study. BMC Gastroenterol. 2017, 17, 92. [Google Scholar] [CrossRef]
- Ohmure, H.; Kanematsu-Hashimoto, K.; Nagayama, K.; Taguchi, H.; Ido, A.; Tominaga, K.; Arakawa, T.; Miyawaki, S. Evaluation of a Proton Pump Inhibitor for Sleep Bruxism. J. Dent. Res. 2016, 95, 1479–1486. [Google Scholar] [CrossRef]
- Mengatto, C.M.; da Silveira Dalberto, C.; Scheeren, B.; Silva de Barros, S.G. Association between Sleep Bruxism and Gastroesophageal Reflux Disease. J. Prosthet. Dent. 2013, 110, 349–355. [Google Scholar] [CrossRef]
- Li, Y.; Yu, F.; Niu, L.; Hu, W.; Long, Y.; Tay, F.; Chen, J. Associations among Bruxism, Gastroesophageal Reflux Disease, and Tooth Wear. J. Clin. Med. 2018, 7, 417. [Google Scholar] [CrossRef]
- Li, Y.; Yu, F.; Niu, L.; Long, Y.; Tay, F.R.; Chen, J. Association between Bruxism and Symptomatic Gastroesophageal Reflux Disease: A Case-Control Study. J. Dent. 2018, 77, 51–58. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Ishimura, N.; Ishihara, S. Advantages and Disadvantages of Long-Term Proton Pump Inhibitor Use. J. Neurogastroenterol. Motil. 2018, 24, 182–196. [Google Scholar] [CrossRef]
- William, J.H.; Danziger, J. Proton-Pump Inhibitor-Induced Hypomagnesemia: Current Research and Proposed Mechanisms. World J. Nephrol. 2016, 5, 152. [Google Scholar] [CrossRef]
- Hoorn, E.J.; van der Hoek, J.; de Man, R.A.; Kuipers, E.J.; Bolwerk, C.; Zietse, R. A Case Series of Proton Pump Inhibitor–Induced Hypomagnesemia. Am. J. Kidney Dis. 2010, 56, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; Kittanamongkolchai, W.; Srivali, N.; Edmonds, P.J.; Ungprasert, P.; O’Corragain, O.A.; Korpaisarn, S.; Erickson, S.B. Proton Pump Inhibitors Linked to Hypomagnesemia: A Systematic Review and Meta-Analysis of Observational Studies. Ren. Fail. 2015, 37, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Hartman, B.; Donnelly-VanderLoo, M.; Watson, T.; O’Connor, C.; Madill, J. Proton-Pump Inhibitor Therapy and Vitamin B 12 Status in an Inpatient Hospital Setting. Appl. Physiol. Nutr. Metab. 2016, 41, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.R.; Schneider, J.L.; Zhao, W.; Corley, D.A. Proton Pump Inhibitor and Histamine 2 Receptor Antagonist Use and Vitamin B 12 Deficiency. JAMA 2013, 310, 2435. [Google Scholar] [CrossRef] [PubMed]
- Hirschowitz, B.I.; Worthington, J.; Mohnen, J. Vitamin B12 Deficiency in Hypersecretors during Long-Term Acid Suppression with Proton Pump Inhibitors. Aliment. Pharmacol. Ther. 2008, 27, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N. Neurologic Aspects of Cobalamin (B12) Deficiency. Handb. Clin. Neurol. 2014, 120, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, D.E.; Lebwohl, B.; Abrams, J.A. The Impact of Proton Pump Inhibitors on the Human Gastrointestinal Microbiome. Clin. Lab. Med. 2014, 34, 771–785. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Cai, S.-T.; Tian, Y.-P.; Zhao, H.-J.; Zhang, Y.-B.; Chen, J.; Ren, R.-R.; Luo, X.; Peng, L.-H.; Sun, G.; et al. Effects of Proton Pump Inhibitors on the Gastrointestinal Microbiota in Gastroesophageal Reflux Disease. Genom. Proteom. Bioinform. 2019, 17, 52–63. [Google Scholar] [CrossRef]
- Takagi, T.; Naito, Y.; Inoue, R.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Tsuchiya, S.; Okayama, T.; Dohi, O.; Yoshida, N.; et al. The Influence of Long-Term Use of Proton Pump Inhibitors on the Gut Microbiota: An Age-Sex-Matched Case-Control Study. J. Clin. Biochem. Nutr. 2018, 62, 100–105. [Google Scholar] [CrossRef]
- Freedberg, D.E.; Toussaint, N.C.; Chen, S.P.; Ratner, A.J.; Whittier, S.; Wang, T.C.; Wang, H.H.; Abrams, J.A. Proton Pump Inhibitors Alter Specific Taxa in the Human Gastrointestinal Microbiome: A Crossover Trial. Gastroenterology 2015, 149, 883–885.e9. [Google Scholar] [CrossRef]
- Tsuda, A.; Suda, W.; Morita, H.; Takanashi, K.; Takagi, A.; Koga, Y.; Hattori, M. Influence of Proton-Pump Inhibitors on the Luminal Microbiota in the Gastrointestinal Tract. Clin. Transl. Gastroenterol. 2015, 6, e89. [Google Scholar] [CrossRef] [PubMed]
- Lucas López, R.; Grande Burgos, M.J.; Gálvez, A.; Pérez Pulido, R. The Human Gastrointestinal Tract and Oral Microbiota in Inflammatory Bowel Disease: A State of the Science Review. APMIS 2017, 125, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Fourie, N.H.; Wang, D.; Abey, S.K.; Sherwin, L.B.; Joseph, P.V.; Rahim-Williams, B.; Ferguson, E.G.; Henderson, W.A. The Microbiome of the Oral Mucosa in Irritable Bowel Syndrome. Gut Microbes 2016, 7, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.; Chan, W.W. Proton Pump Inhibitor Use and the Risk of Small Intestinal Bacterial Overgrowth: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, B.M.R.; Chey, W.D.; Chang, L. Bacterial Overgrowth and Irritable Bowel Syndrome: Unifying Hypothesis or a Spurious Consequence of Proton Pump Inhibitors? Am. J. Gastroenterol. 2008, 103, 2972–2976. [Google Scholar] [CrossRef]
- Fujimori, S. What Are the Effects of Proton Pump Inhibitors on the Small Intestine? World J. Gastroenterol. 2015, 21, 6817–6819. [Google Scholar] [CrossRef]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Wong, I.C.K.; Leung, W.K. Long-Term Proton Pump Inhibitors and Risk of Gastric Cancer Development after Treatment for Helicobacter Pylori: A Population-Based Study. Gut 2018, 67, 28–35. [Google Scholar] [CrossRef]
- Brusselaers, N.; Wahlin, K.; Engstrand, L.; Lagergren, J. Maintenance Therapy with Proton Pump Inhibitors and Risk of Gastric Cancer: A Nationwide Population-Based Cohort Study in Sweden. BMJ Open 2017, 7, e017739. [Google Scholar] [CrossRef]
- Ahn, J.S. Acid Suppressive Drugs and Gastric Cancer: A Meta-Analysis of Observational Studies. World J. Gastroenterol. 2013, 19, 2560. [Google Scholar] [CrossRef]
- Ahn, J.; Chen, C.Y.; Hayes, R.B. Oral Microbiome and Oral and Gastrointestinal Cancer Risk. Cancer Causes Control 2012, 23, 399–404. [Google Scholar] [CrossRef]
- Reiter, R.J. Antioxidative Capacity of Melatonin. In Handbook of Antioxidants; Marcel Dekker: New York, NY, USA, 2002; Volume 2, pp. 565–613. [Google Scholar]
- Konturek, S.J.; Zayachkivska, O.; Havryluk, X.O.; Brzozowski, T.; Sliwowski, Z.; Pawlik, M.; Konturek, P.C.; Cześnikiewicz-Guzik, M.; Gzhegotsky, M.R.; Pawlik, W.W. Protective Influence of Melatonin against Acute Esophageal Lesions Involves Prostaglandins, Nitric Oxide and Sensory Nerves. J. Physiol. Pharm. 2007, 58, 361–377. [Google Scholar]
- Pereira, R.d.S. Regression of Gastroesophageal Reflux Disease Symptoms Using Dietary Supplementation with Melatonin, Vitamins and Aminoacids: Comparison with Omeprazole. J. Pineal Res. 2006, 41, 195–200. [Google Scholar] [CrossRef]
- Werbach, M.R. Melatonin for the Treatment of Gastroesophageal Reflux Disease. Altern. Ther. Health Med. 2008, 14, 54–58. [Google Scholar] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the Probiotic Bifidobacterium Infantis in the Maternal Separation Model of Depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus Helveticus NS8 Improves Behavioral, Cognitive, and Biochemical Aberrations Caused by Chronic Restraint Stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of Psychotropic-like Properties of a Probiotic Formulation (Lactobacillus Helveticus R0052 and Bifidobacterium Longum R0175) in Rats and Human Subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Rao, A.V.; Bested, A.C.; Beaulne, T.M.; Katzman, M.A.; Iorio, C.; Berardi, J.M.; Logan, A.C. A Randomized, Double-Blind, Placebo-Controlled Pilot Study of a Probiotic in Emotional Symptoms of Chronic Fatigue Syndrome. Gut Pathog. 2009, 1, 6. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The Probiotic Bifidobacteria Infantis: An Assessment of Potential Antidepressant Properties in the Rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Auteri, M.; Zizzo, M.G.; Serio, R. GABA and GABA Receptors in the Gastrointestinal Tract: From Motility to Inflammation. Pharmacol. Res. 2015, 93, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Baganz, N.L.; Blakely, R.D. A Dialogue between the Immune System and Brain, Spoken in the Language of Serotonin. ACS Chem. Neurosci. 2013, 4, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Tanaka, M.; Masuda, S.; Ohue-Kitano, R.; Yamakage, H.; Muranaka, K.; Wada, H.; Kusakabe, T.; Shimatsu, A.; Hasegawa, K.; et al. Omega-3 Polyunsaturated Fatty Acids Suppress the Inflammatory Responses of Lipopolysaccharide-Stimulated Mouse Microglia by Activating SIRT1 Pathways. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2017, 1862, 552–560. [Google Scholar] [CrossRef]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A Randomised Trial of the Effect of Omega-3 Polyunsaturated Fatty Acid Supplements on the Human Intestinal Microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramanieapillai, M.; Fan, B.; Lu, C.; McIntyre, R.S. Efficacy of Omega-3 PUFAs in Depression: A Meta-Analysis. Transl. Psychiatry 2019, 9, 190. [Google Scholar] [CrossRef]
- Ko, G.D.; Nowacki, N.B.; Arseneau, L.; Eitel, M.; Hum, A. Omega-3 Fatty Acids for Neuropathic Pain. Clin. J. Pain 2010, 26, 168–172. [Google Scholar] [CrossRef]
- Galán-Arriero, I.; Serrano-Muñoz, D.; Gómez-Soriano, J.; Goicoechea, C.; Taylor, J.; Velasco, A.; Ávila-Martín, G. The Role of Omega-3 and Omega-9 Fatty Acids for the Treatment of Neuropathic Pain after Neurotrauma. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 1629–1635. [Google Scholar] [CrossRef]
- Goldberg, R.J.; Katz, J. A Meta-Analysis of the Analgesic Effects of Omega-3 Polyunsaturated Fatty Acid Supplementation for Inflammatory Joint Pain. Pain 2007, 129, 210–223. [Google Scholar] [CrossRef]
- Frémont, L. Biological Effects of Resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Alrafas, H.R.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol Modulates the Gut Microbiota to Prevent Murine Colitis Development through Induction of Tregs and Suppression of Th17 Cells. J. Leukoc. Biol. 2019, 106, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, S.; Shu, H.; Crawford, J.; Xing, Y.; Tao, F. Resveratrol Alleviates Temporomandibular Joint Inflammatory Pain by Recovering Disturbed Gut Microbiota. Brain Behav. Immun. 2020, 87, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; de Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Banasiewicz, T.; Krokowicz, Ł.; Stojcev, Z.; Kaczmarek, B.F.; Kaczmarek, E.; Maik, J.; Marciniak, R.; Krokowicz, P.; Walkowiak, J.; Drews, M. Microencapsulated Sodium Butyrate Reduces the Frequency of Abdominal Pain in Patients with Irritable Bowel Syndrome. Colorectal Dis. 2013, 15, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Huda-Faujan, N.; Abdulamir, A.S.; Fatimah, A.B.; Anas, O.M.; Shuhaimi, M.; Yazid, A.M.; Loong, Y.Y. The Impact of the Level of the Intestinal Short Chain Fatty Acids in Inflammatory Bowel Disease Patients Versus Healthy Subjects. Open Biochem. J. 2010, 4, 53–58. [Google Scholar] [CrossRef]
- Kukkar, A.; Singh, N.; Jaggi, A.S. Attenuation of Neuropathic Pain by Sodium Butyrate in an Experimental Model of Chronic Constriction Injury in Rats. J. Formos. Med. Assoc. 2014, 113, 921–928. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and Inflammation-Story of a Double-Edged Blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef]
- Chen, C.-Q. Distribution, Function and Physiological Role of Melatonin in the Lower Gut. World J. Gastroenterol. 2011, 17, 3888. [Google Scholar] [CrossRef]
- Huether, G. Melatonin Synthesis in the Gastrointestinal Tract and the Impact of Nutritional Factors on Circulating Melatonin. Ann. N. Y. Acad. Sci 1994, 719, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Grigorov, I.; Bogojević, D.; Jovanović, S.; Petrović, A.; Ivanović-Matić, S.; Zolotarevski, L.; Poznanović, G.; Martinović, V. Hepatoprotective Effects of Melatonin against Pronecrotic Cellular Events in Streptozotocin-Induced Diabetic Rats. J. Physiol. Biochem. 2014, 70, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Welin, A.-K.; Svedin, P.; Lapatto, R.; Sultan, B.; Hagberg, H.; Gressens, P.; Kjellmer, I.; Mallard, C. Melatonin Reduces Inflammation and Cell Death in White Matter in the Mid-Gestation Fetal Sheep Following Umbilical Cord Occlusion. Pediatric Res. 2007, 61, 153–158. [Google Scholar] [CrossRef]
- Ohashi, N.; Ishigaki, S.; Isobe, S. The Pivotal Role of Melatonin in Ameliorating Chronic Kidney Disease by Suppression of the Renin–Angiotensin System in the Kidney. Hypertens. Res. 2019, 42, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Brennan, R.; Jan, J.E.; Lyons, C.J. Light, Dark, and Melatonin: Emerging Evidence for the Importance of Melatonin in Ocular Physiology. Eye 2007, 21, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a Master Regulator of Cell Death and Inflammation: Molecular Mechanisms and Clinical Implications for Newborn Care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, J.; Reiter, R.J.; Ma, X. Melatonin Mediates Mucosal Immune Cells, Microbial Metabolism, and Rhythm Crosstalk: A Therapeutic Target to Reduce Intestinal Inflammation. Med. Res. Rev. 2020, 40, 606–632. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Weng, S.-J.; Sun, X.-H.; Yang, X.-L. Neuromodulatory Role of Melatonin in Retinal Information Processing. Prog. Retin. Eye Res. 2013, 32, 64–87. [Google Scholar] [CrossRef]
- Chen, X.; Eslamfam, S.; Fang, L.; Qiao, S.; Ma, X. Maintenance of Gastrointestinal Glucose Homeostasis by the Gut-Brain Axis. Curr. Protein Pept. Sci. 2017, 18, 541–547. [Google Scholar] [CrossRef]
- Wilhelmsen, M.; Amirian, I.; Reiter, R.J.; Rosenberg, J.; Gögenur, I. Analgesic Effects of Melatonin: A Review of Current Evidence from Experimental and Clinical Studies. J. Pineal Res. 2011, 51, 270–277. [Google Scholar] [CrossRef]
- Danilov, A.; Kurganova, J. Melatonin in Chronic Pain Syndromes. Pain Ther. 2016, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin in Aging and Disease -Multiple Consequences of Reduced Secretion, Options and Limits of Treatment. Aging Dis. 2012, 3, 194–225. [Google Scholar] [PubMed]
- Ambriz-Tututi, M.; Rocha-González, H.I.; Cruz, S.L.; Granados-Soto, V. Melatonin: A Hormone That Modulates Pain. Life Sci. 2009, 84, 489–498. [Google Scholar] [CrossRef]
- Esposito, E.; Paterniti, I.; Mazzon, E.; Bramanti, P.; Cuzzocrea, S. Melatonin Reduces Hyperalgesia Associated with Inflammation. J. Pineal Res. 2010, 49, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Acuna-Castroviejo, D.; Escames, G.; Reiter, R.J. Melatonin Therapy in Fibromyalgia. J. Pineal Res. 2006, 40, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Citera, G.; Arias, M.A.; Maldonado-Cocco, J.A.; La´zaro, M.A.; Rosemffet, M.G.; Brusco, L.I.; Scheines, E.J.; Cardinalli, D.P. The Effect of Melatonin in Patients with Fibromyalgia: A Pilot Study. Clin. Rheumatol. 2000, 19, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Lauterbach, E.C.; Yu Ho, K.; Acuna-Castroviejo, D.; Zakaria, R.; Brzezinski, A. Melatonin in Antinociception: Its Therapeutic Applications. Curr. Neuropharmacol. 2012, 10, 167–178. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, Y.; Duan, Y.; Li, W.; Zhang, L.; Huang, Y.; Zhao, W.; Wang, Y.; Li, J.; Feng, T.; et al. Exogenous Melatonin in the Treatment of Pain: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 100582–100592. [Google Scholar] [CrossRef]
- Myung, S.-K. Analgesic Efficacy of Melatonin: A Meta-Analysis of Randomized, Double-Blind, Placebo-Controlled Trials. J. Clin. Med. 2020, 9, 1553. [Google Scholar] [CrossRef]
- Xie, S.; Fan, W.; He, H.; Huang, F. Role of Melatonin in the Regulation of Pain. J. Pain Res. 2020, 13, 331–343. [Google Scholar] [CrossRef]
- Huang, C.-T.; Chiang, R.P.-Y.; Chen, C.-L.; Tsai, Y.-J. Sleep Deprivation Aggravates Median Nerve Injury-Induced Neuropathic Pain and Enhances Microglial Activation by Suppressing Melatonin Secretion. Sleep 2014, 37, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Caumo, W.; Hidalgo, M.P.; Souza, A.; Torres, I.L.d.S.; Conceicao Antunes, L. Melatonin Is a Biomarker of Circadian Dysregulation and Is Correlated with Major Depression and Fibromyalgia Symptom Severity. J. Pain Res. 2019, 12, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Vidor, L.P.; Torres, I.L.S.; Custódio de Souza, I.C.; Fregni, F.; Caumo, W. Analgesic and Sedative Effects of Melatonin in Temporomandibular Disorders: A Double-Blind, Randomized, Parallel-Group, Placebo-Controlled Study. J. Pain Symptom Manag. 2013, 46, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Erden, S. Sleep-Related Bruxism Response to Melatonin Treatment. J. Child Adolesc. Psychopharmacol. 2020, 30, 201. [Google Scholar] [CrossRef]
- Zarezadeh, M.; Khorshidi, M.; Emami, M.; Janmohammadi, P.; Kord-varkaneh, H.; Mousavi, S.M.; Mohammed, S.H.; Saedisomeolia, A.; Alizadeh, S. Melatonin Supplementation and Pro-Inflammatory Mediators: A Systematic Review and Meta-Analysis of Clinical Trials. Eur. J. Nutr. 2020, 59, 1803–1813. [Google Scholar] [CrossRef]
- MacInnis, M.J.; Dziedzic, C.E.; Wood, E.; Oikawa, S.Y.; Phillips, S.M. Presleep α-Lactalbumin Consumption Does Not Improve Sleep Quality or Time-Trial Performance in Cyclists. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 197–202. [Google Scholar] [CrossRef]
- Besag, F.M.C.; Vasey, M.J.; Lao, K.S.J.; Wong, I.C.K. Adverse Events Associated with Melatonin for the Treatment of Primary or Secondary Sleep Disorders: A Systematic Review. CNS Drugs 2019, 33, 1167–1186. [Google Scholar] [CrossRef]
- Seabra, M.d.L.v.; Bignotto, M.; Pinto, L.R., Jr.; Tufik, S. Randomized, Double-Blind Clinical Trial, Controlled with Placebo, of the Toxicology of Chronic Melatonin Treatment. J. Pineal Res. 2000, 29, 193–200. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef]
- Rajaratnam, S.M.W.; Dijk, D.-J.; Middleton, B.; Stone, B.M.; Arendt, J. Melatonin Phase-Shifts Human Circadian Rhythms with No Evidence of Changes in the Duration of Endogenous Melatonin Secretion or the 24-Hour Production of Reproductive Hormones. J. Clin. Endocrinol. Metab. 2003, 88, 4303–4309. [Google Scholar] [CrossRef][Green Version]
- Luboshitzky, R.; Levi, M.; Shen-Orr, Z.; Blumenfeld, Z.; Herer, P.; Lavie, P. Long-Term Melatonin Administration Does Not Alter Pituitary-Gonadal Hormone Secretion in Normal Men. Hum. Reprod. 2000, 15, 60–65. [Google Scholar] [CrossRef]
- Voordouw, B.C.; Euser, R.; Verdonk, R.E.; Alberda, B.T.; de Jong, F.H.; Drogendijk, A.C.; Fauser, B.C.; Cohen, M. Melatonin and Melatonin-Progestin Combinations Alter Pituitary-Ovarian Function in Women and Can Inhibit Ovulation. J. Clin. Endocrinol. Metab. 1992, 74, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Aleem, F.A.; Weitzman, E.D.; Weinberg, U. Suppression of Basal Luteinizing Hormone Concentrations by Melatonin in Postmenopausal Women. Fertil. Steril. 1984, 42, 923–925. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.; Soares, J.J.M.; Gallo, C.C.; Furtado, A.; Cavaco, J.E.; Gonçalves, I.; Santos, C.R.A.; Quintela, T. The Crosstalk between Melatonin and Sex Steroid Hormones. Neuroendocrinology 2022, 112, 115–129. [Google Scholar] [CrossRef]
- Gonzalez, A.; Cos, S.; Martinez-Campa, C.; Alonso-Gonzalez, C.; Sanchez-Mateos, S.; Mediavilla, M.D.; Sanchez-Barcelo, E.J. Selective Estrogen Enzyme Modulator Actions of Melatonin in Human Breast Cancer Cells. J. Pineal Res. 2008, 45, 86–92. [Google Scholar] [CrossRef]
- Cos, S.; Gonzalez, A.; Martinez-Campa, C.; Mediavilla, M.; Alonso-Gonzalez, C.; Sanchez-Barcelo, E. Melatonin as a Selective Estrogen Enzyme Modulator. Curr. Cancer Drug Targets 2008, 8, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.S.; Manfredini, D. Transitioning to Chronic Temporomandibular Disorder Pain: A Combination of Patient Vulnerabilities and Iatrogenesis. J. Oral Rehabil. 2021, 48, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Kindler, S.; Schwahn, C.; Bernhardt, O.; Söhnel, A.; Mksoud, M.; Biffar, R.; Meyer, G.; Völzke, H.; Metelmann, H.; Grabe, H. Association Between Symptoms of Posttraumatic Stress Disorder and Signs of Temporomandibular Disorders in the General Population. J. Oral Facial Pain Headache 2019, 33, 67–76. [Google Scholar] [CrossRef]
- Najavits, L.M.; Weiss, R.D.; Shaw, S.R. The Link between Substance Abuse and Posttraumatic Stress Disorder in Women. A Research Review. Am. J. Addict. 1997, 6, 273–283. [Google Scholar]
- Pietrzak, R.H.; Goldstein, R.B.; Southwick, S.M.; Grant, B.F. Prevalence and Axis I Comorbidity of Full and Partial Posttraumatic Stress Disorder in the United States: Results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J. Anxiety Disord. 2011, 25, 456–465. [Google Scholar] [CrossRef]
- Salk, R.H.; Hyde, J.S.; Abramson, L.Y. Gender Differences in Depression in Representative National Samples: Meta-Analyses of Diagnoses and Symptoms. Psychol. Bull. 2017, 143, 783–822. [Google Scholar] [CrossRef] [PubMed]
- Prather, A.A.; Carroll, J.E.; Fury, J.M.; McDade, K.K.; Ross, D.; Marsland, A.L. Gender Differences in Stimulated Cytokine Production Following Acute Psychological Stress. Brain Behav. Immun. 2009, 23, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Palma-Gudiel, H.; Peralta, V.; Deuschle, M.; Navarro, V.; Fañanás, L. Epigenetics-by-Sex Interaction for Somatization Conferred by Methylation at the Promoter Region of SLC6A4 Gene. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 89, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, D.; Akhtar, R.; Ciufolini, S.; Pariante, C.M.; Mondelli, V. Childhood Trauma and Adulthood Inflammation: A Meta-Analysis of Peripheral C-Reactive Protein, Interleukin-6 and Tumour Necrosis Factor-α. Mol. Psychiatry 2016, 21, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wing, Y.-K. Sex Differences in Insomnia: A Meta-Analysis. Sleep 2006, 29, 85–93. [Google Scholar] [CrossRef]
- Irwin, M.R. Sleep Deprivation and Activation of Morning Levels of Cellular and Genomic Markers of Inflammation. Arch. Intern. Med. 2006, 166, 1756. [Google Scholar] [CrossRef]
- Irwin, M.R.; Wang, M.; Ribeiro, D.; Cho, H.J.; Olmstead, R.; Breen, E.C.; Martinez-Maza, O.; Cole, S. Sleep Loss Activates Cellular Inflammatory Signaling. Biol. Psychiatry 2008, 64, 538–540. [Google Scholar] [CrossRef]
- Roane, B.M.; Taylor, D.J. Adolescent Insomnia as a Risk Factor for Early Adult Depression and Substance Abuse. Sleep 2008, 31, 1351–1356. [Google Scholar]
- Wong, M.M.; Brower, K.J.; Zucker, R.A. Childhood Sleep Problems, Early Onset of Substance Use and Behavioral Problems in Adolescence. Sleep Med. 2009, 10, 787–796. [Google Scholar] [CrossRef]
- Telzer, E.H.; Fuligni, A.J.; Lieberman, M.D.; Galván, A. The Effects of Poor Quality Sleep on Brain Function and Risk Taking in Adolescence. Neuroimage 2013, 71, 275–283. [Google Scholar] [CrossRef]
- Morgan, P.T.; Pace-Schott, E.F.; Sahul, Z.H.; Coric, V.; Stickgold, R.; Malison, R.T. Sleep, Sleep-Dependent Procedural Learning and Vigilance in Chronic Cocaine Users: Evidence for Occult Insomnia. Drug Alcohol Depend. 2006, 82, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Debell, F.; Fear, N.T.; Head, M.; Batt-Rawden, S.; Greenberg, N.; Wessely, S.; Goodwin, L. A Systematic Review of the Comorbidity between PTSD and Alcohol Misuse. Soc. Psychiatry Psychiatr. Epidemiol. 2014, 49, 1401–1425. [Google Scholar] [CrossRef] [PubMed]
- Colrain, I.M.; Nicholas, C.L.; Baker, F.C. Alcohol and the Sleeping Brain. Handb. Clin. Neurol. 2014, 125, 415–431. [Google Scholar] [CrossRef]
- Scrima, L.; Broudy, M.; Nay, K.N.; Cohn, M.A. Increased Severity of Obstructive Sleep Apnea After Bedtime Alcohol Ingestion: Diagnostic Potential and Proposed Mechanism of Action. Sleep 1982, 5, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Roehrs, T. Ethanol as a Hypnotic in Insomniacs Self Administration and Effects on Sleep and Mood. Neuropsychopharmacology 1999, 20, 279–286. [Google Scholar] [CrossRef]
- Wilson, S.; Argyropoulos, S. Antidepressants and Sleep. Drugs 2005, 65, 927–947. [Google Scholar] [CrossRef]
- Benedict, C.; Brooks, S.J.; O’Daly, O.G.; Almèn, M.S.; Morell, A.; Åberg, K.; Gingnell, M.; Schultes, B.; Hallschmid, M.; Broman, J.-E.; et al. Acute Sleep Deprivation Enhances the Brain’s Response to Hedonic Food Stimuli: An FMRI Study. J. Clin. Endocrinol. Metab. 2012, 97, E443–E447. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, N.; Kim, G.H. Sex and Gender Differences in Gastroesophageal Reflux Disease. J. Neurogastroenterol. Motil. 2016, 22, 575–588. [Google Scholar] [CrossRef]
- Lee, S.P.; Sung, I.-K.; Kim, J.H.; Lee, S.-Y.; Park, H.S.; Shim, C.S. The Effect of Emotional Stress and Depression on the Prevalence of Digestive Diseases. J. Neurogastroenterol. Motil. 2015, 21, 273–282. [Google Scholar] [CrossRef]
- On, Z.X.; Grant, J.; Shi, Z.; Taylor, A.W.; Wittert, G.A.; Tully, P.J.; Hayley, A.C.; Martin, S. The Association between Gastroesophageal Reflux Disease with Sleep Quality, Depression, and Anxiety in a Cohort Study of Australian Men. J. Gastroenterol. Hepatol. 2017, 32, 1170–1177. [Google Scholar] [CrossRef]
- You, Z.-H.; Perng, C.-L.; Hu, L.-Y.; Lu, T.; Chen, P.-M.; Yang, A.C.; Tsai, S.-J.; Huang, Y.-S.; Chen, H.-J. Risk of Psychiatric Disorders Following Gastroesophageal Reflux Disease: A Nationwide Population-Based Cohort Study. Eur. J. Intern. Med. 2015, 26, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.H.; Lieberman, D.; Oehlke, M. Psychological Distress in Patients with Gastroesophageal Reflux Disease. Am. J. Gastroenterol. 1995, 90, 1797–1803. [Google Scholar] [PubMed]
- Fillingim, R.B.; Ohrbach, R.; Greenspan, J.D.; Knott, C.; Diatchenko, L.; Dubner, R.; Bair, E.; Baraian, C.; Mack, N.; Slade, G.D.; et al. Psychological Factors Associated with Development of TMD: The OPPERA Prospective Cohort Study. J. Pain 2013, 14, T75–T90. [Google Scholar] [CrossRef] [PubMed]
- Jaggar, M.; Rea, K.; Spichak, S.; Dinan, T.G.; Cryan, J.F. You’ve Got Male: Sex and the Microbiota-Gut-Brain Axis across the Lifespan. Front. Neuroendocrinol. 2020, 56, 100815. [Google Scholar] [CrossRef] [PubMed]
- d’Hennezel, E.; Abubucker, S.; Murphy, L.O.; Cullen, T.W. Total Lipopolysaccharide from the Human Gut Microbiome Silences Toll-Like Receptor Signaling. mSystems 2017, 2, e00046-17. [Google Scholar] [CrossRef]
- Santos-Marcos, J.A.; Rangel-Zuñiga, O.A.; Jimenez-Lucena, R.; Quintana-Navarro, G.M.; Garcia-Carpintero, S.; Malagon, M.M.; Landa, B.B.; Tena-Sempere, M.; Perez-Martinez, P.; Lopez-Miranda, J.; et al. Influence of Gender and Menopausal Status on Gut Microbiota. Maturitas 2018, 116, 43–53. [Google Scholar] [CrossRef]
- Sinha, T.; Vich Vila, A.; Garmaeva, S.; Jankipersadsing, S.A.; Imhann, F.; Collij, V.; Bonder, M.J.; Jiang, X.; Gurry, T.; Alm, E.J.; et al. Analysis of 1135 Gut Metagenomes Identifies Sex-Specific Resistome Profiles. Gut Microbes 2019, 10, 358–366. [Google Scholar] [CrossRef]
- Mohlin, B.; Axelsson, S.; Paulin, G.; Pietilä, T.; Bondemark, L.; Brattström, V.; Hansen, K.; Holm, A.-K. TMD in Relation to Malocclusion and Orthodontic Treatment. Angle Orthod. 2007, 77, 542–548. [Google Scholar] [CrossRef]
- Bueno, C.H.; Pereira, D.D.; Pattussi, M.P.; Grossi, P.K.; Grossi, M.L. Gender Differences in Temporomandibular Disorders in Adult Populational Studies: A Systematic Review and Meta-Analysis. J. Oral Rehabil. 2018, 45, 720–729. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lassmann, Ł.; Pollis, M.; Żółtowska, A.; Manfredini, D. Gut Bless Your Pain—Roles of the Gut Microbiota, Sleep, and Melatonin in Chronic Orofacial Pain and Depression. Biomedicines 2022, 10, 1528. https://doi.org/10.3390/biomedicines10071528
Lassmann Ł, Pollis M, Żółtowska A, Manfredini D. Gut Bless Your Pain—Roles of the Gut Microbiota, Sleep, and Melatonin in Chronic Orofacial Pain and Depression. Biomedicines. 2022; 10(7):1528. https://doi.org/10.3390/biomedicines10071528
Chicago/Turabian StyleLassmann, Łukasz, Matteo Pollis, Agata Żółtowska, and Daniele Manfredini. 2022. "Gut Bless Your Pain—Roles of the Gut Microbiota, Sleep, and Melatonin in Chronic Orofacial Pain and Depression" Biomedicines 10, no. 7: 1528. https://doi.org/10.3390/biomedicines10071528
APA StyleLassmann, Ł., Pollis, M., Żółtowska, A., & Manfredini, D. (2022). Gut Bless Your Pain—Roles of the Gut Microbiota, Sleep, and Melatonin in Chronic Orofacial Pain and Depression. Biomedicines, 10(7), 1528. https://doi.org/10.3390/biomedicines10071528





