Assessing Detection Accuracy of Computerized Sonographic Features and Computer-Assisted Reading Performance in Differentiating Thyroid Cancers
Abstract
:1. Introduction
2. Materials and Methods
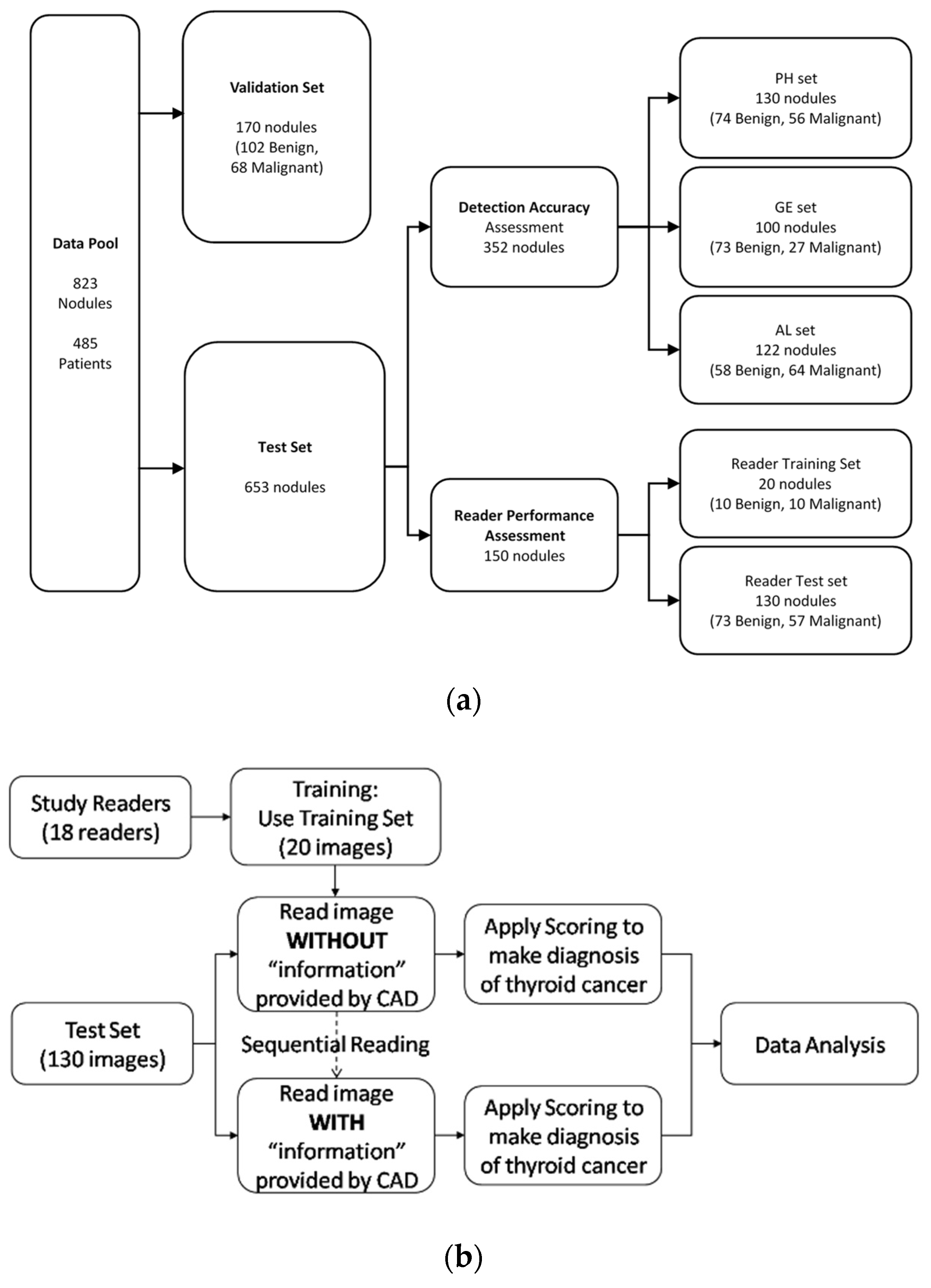
2.1. Database for Computerized Features Testing
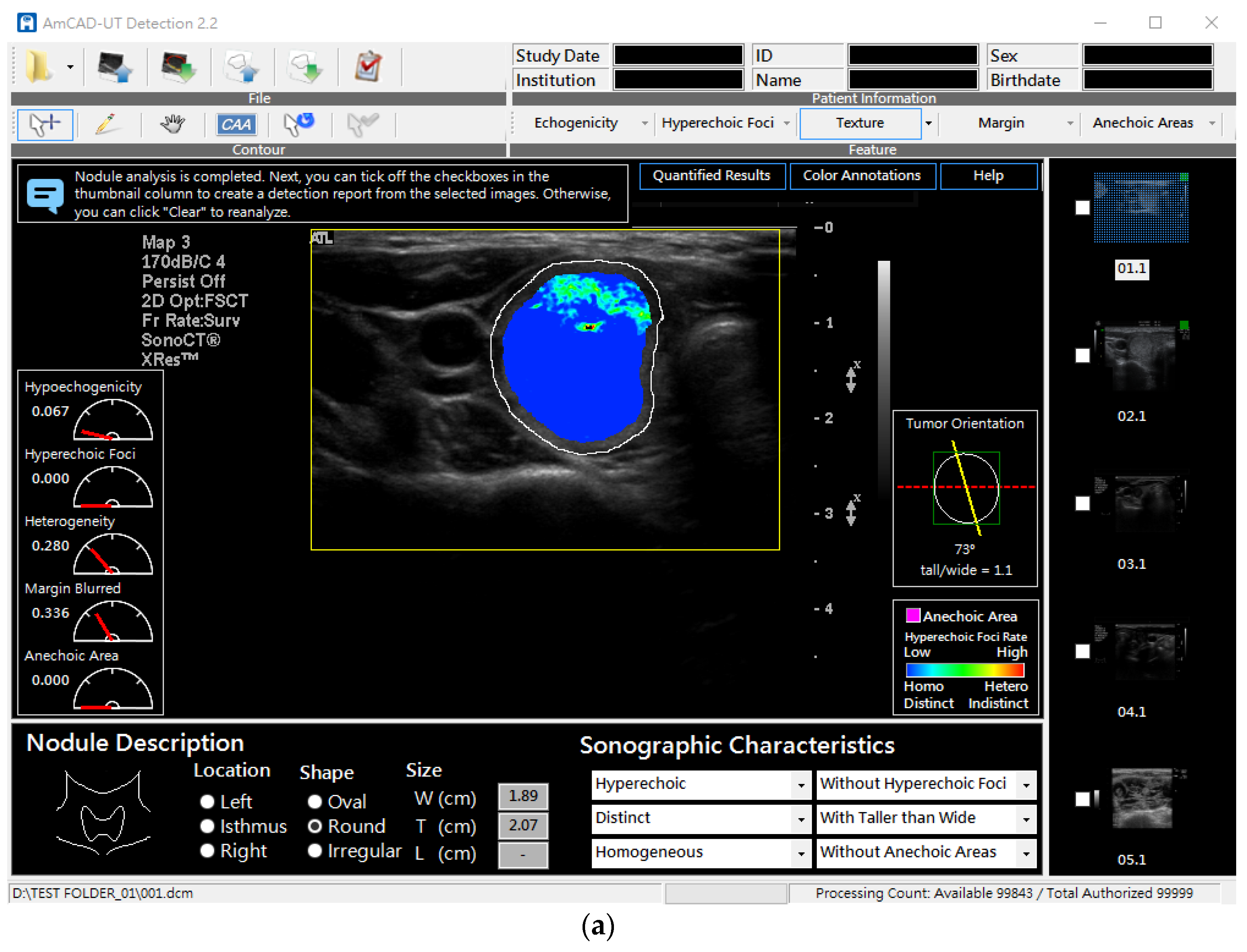
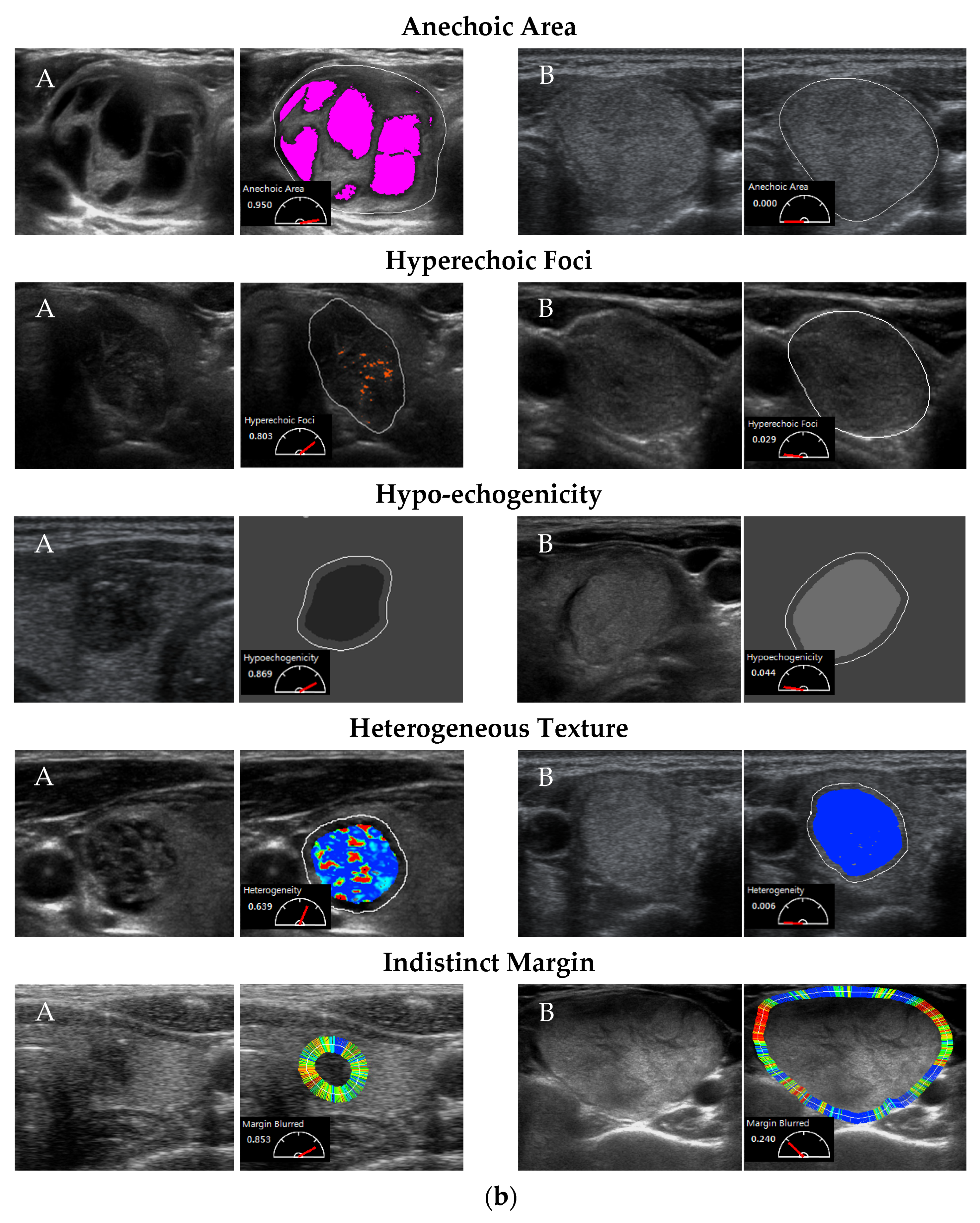
2.2. Testing CSF Detection Accuracy
2.3. Assessing Diagnosis Performance of Readers Assisted with CSF
3. Results
3.1. Detection Accuracy of Computerized Sonographic Features
3.2. Reader Performance Assisted with CSF
4. Discussion
4.1. Population and Features
4.2. CSF Accuracy
4.3. Reader Performance with CSF
4.4. CAD Device in Clinical Practice
4.5. Study Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Desser, T.S.; McDougall, I.R.; Weigel, R.J.; Jeffrey, R.B., Jr. Common and uncommon sonographic features of papillary thyroid carcinoma. J. Ultrasound Med. 2003, 22, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Papini, E.; Guglielmi, R.; Bianchini, A.; Crescenzi, A.; Taccogna, S.; Nardi, F.; Panunzi, C.; Rinaldi, R.; Toscano, V.; Pacella, C.M. Risk of malignancy in nonpalpable thyroid nodules: Predictive value of ultrasound and color-Doppler features. Int. J. Clin. Endocrinol. Metab. 2002, 87, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Sesser, T.S.; Frates, M.C.; et al. ACR thyroid imaging, reporting and data system (TI-RADS): White paper of the ACR TI-RADS committee. J. Am. Coll. Radiol. 2017, 14, 587. [Google Scholar] [CrossRef] [Green Version]
- Horvath, E.; Majlis, S.; Rossi, R.; Franco, C.; Niedmann, J.P.; Castro, A.; Dominguez, M. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. Int. J. Clin. Endocrinol. Metab. 2009, 94, 1748–1751. [Google Scholar] [CrossRef]
- Russ, G. Risk stratification of thyroid nodules on ultrasonography with the French TI-RADS: Description and reflections. Ultrasonography 2016, 35, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Mechanick, J.I.; Harrell, R.M.; Allende-Vigo, M.Z.; Alvayero, C.; Arita-Melzer, O.; Aschner, P.; Camacho, P.M.; Castillo, R.Z.; Cerdas, S.; Coutinho, W.F.; et al. Transculturalization Recommendations for Developing Latin American Clinical Practice Algorithms in Endocrinology--Proceedings of the 2015 Pan-American Workshop by the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr. Pract. 2016, 22, 476–501. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.; Na, D.G.; Kim, J.H.; Kim, K.W.; Yoon, J.W. Ultrasound-Based Risk Stratification for Malignancy in Thyroid Nodules: A Four-Tier Categorization System. Eur. Radiol. 2015, 25, 2153–2162. [Google Scholar] [CrossRef]
- Kwak, J.Y. Indications for fine needle aspiration in thyroid nodules. Endocrinol. Metab. 2013, 28, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Kim, E.K.; Kwak, J.Y.; Kim, M.J.; Son, E.J. Interobserver and intraobserver variations in ultrasound assessment of thyroid nodules. Thyroid 2010, 20, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Kim, S.H.; Jung, S.L.; Kang, B.J.; Kim, J.Y.; Choi, J.J.; Sung, M.S.; Yim, H.W.; Jeong, S.H. Observer variability in the sonographic evaluation of thyroid nodules. J. Clin. Ultrasound. 2010, 38, 287–293. [Google Scholar] [CrossRef] [PubMed]
- van Ginneken, B.; Schaefer-Prokop, C.M.; Prokop, M. Computer-aided diagnosis: How to move from the laboratory to the clinic. Radiology 2011, 261, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Petrick, N.; Sahiner, B.; Armato, S.G.; Bert, A.; Correale, L.; Delsanto, S.; Freedman, M.T.; Fryd, D.; Gur, D.; Hadjiiski, L.; et al. Evaluation of computer-aided detection and diagnosis systems. Med. Phys. 2013, 40, 087001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giger, M.L.; Huo, Z.; Kupinski, M.A.; Vyborny, C.J. Computer-aided diagnosis in mammography. In Handbook of Medical Imaging; Fitzpatrick JMS, M., Ed.; SPIE PRESS: Bellingham, WA, USA, 2000; pp. 915–1004. [Google Scholar]
- Singh, S.; Maxwell, J.; Baker, J.A.; Nicholas, J.L.; Lo, J.Y. Computer-aided classification of breast masses: Performance and interobserver variability of expert radiologists versus residents. Radiology 2011, 258, 73–80. [Google Scholar] [CrossRef]
- Sugimoto, K.; Shiraishi, J.; Moriyasu, F.; Doi, K. Computer-aided diagnosis of focal liver lesions by use of physicians’ subjective classification of echogenic patterns in baseline and contrast-enhanced ultrasonography. Acad. Radiol. 2009, 16, 401–411. [Google Scholar] [CrossRef]
- Doi, K. Computer-aided diagnosis in medical imaging: Historical review, current status and future potential. Comput. Med. Imaging Graph. 2007, 31, 198–211. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Nishikawa, R.M.; Schmidt, R.A.; Metz, C.E. Comparison of independent double readings and computer-aided diagnosis (CAD) for the diagnosis of breast calcifications. Acad. Radiol. 2006, 13, 84–94. [Google Scholar] [CrossRef]
- Chen, K.Y.; Chen, C.N.; Wu, M.H.; Ho, M.C.; Tai, H.C.; Huang, W.C.; Chung, Y.C.; Chen, A.; Chang, K.J. Computerized detection and quantification of microcalcifications in thyroid nodules. Ultrasound Med. Biol. 2011, 37, 870–878. [Google Scholar] [CrossRef]
- Wu, M.H.; Chen, C.N.; Chen, K.Y.; Ho, M.C.; Tai, H.C.; Chung, Y.C.; Lo, C.P.; Chen, A.; Chang, K.J. Quantitative analysis of dynamic power Doppler sonograms for patients with thyroid nodules. Ultrasound Med. Biol. 2013, 39, 1543–1551. [Google Scholar] [CrossRef]
- Chen, K.Y.; Chen, C.N.; Wu, M.H.; Ho, M.C.; Tai, H.C.; Kuo, W.H.; Huang, W.C.; Wang, Y.H.; Chen, A.; Chang, K.J. Computerized quantification of ultrasonic heterogeneity in thyroid nodules. Ultrasound Med. Biol. 2014, 40, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Chen, C.N.; Chen, K.Y.; Ho, M.C.; Tai, H.C.; Wang, Y.H.; Chen, A.; Chang, K.-J. Quantitative analysis of echogenicity for patients with thyroid nodules. Sci. Rep. 2016, 6, 35632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.H.; Chen, K.Y.; Chen, A.; Chen, C.N. Software-Based Analysis of the Taller-Than-Wide Feature of High-Risk Thyroid Nodules. Ann. Surg. Oncol. 2021, 28, 4347–4357. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Faust, O.; Sree, S.V.; Molinari, F.; Suri, J.S. ThyroScreen system: High resolution ultrasound thyroid image characterization into benign and malignant classes using novel combination of texture and discrete wavelet transform. Comput. Methods Programs Biomed. 2012, 107, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Paul, A.K.; Kim, N.; Baek, J.H.; Choi, Y.J.; Ha, E.J.; Lee, K.D.; Lee, H.S.; Shin, D.; Kim, N. Computer-aided diagnosis for classifying benign versus malignant thyroid nodules based on ultrasound images: A comparison with radiologist-based assessments. Med. Phys. 2016, 43, 554. [Google Scholar] [CrossRef]
- Choi, Y.J.; Baek, J.H.; Park, H.S.; Shim, W.H.; Kim, T.Y.; Shong, Y.K.; Lee, J.H. A computer-aided diagnosis system using artificial intelligence for the diagnosis and characterization of thyroid nodules on ultrasound: Initial clinical assessment. Thyroid 2017, 27, 546–552. [Google Scholar] [CrossRef]
- Wu, M.-H.; Chen, K.-Y.; Shih, S.-R.; Ho, M.C.; Tai, H.C.; Chang, K.J.; Chen, A.; Chen, C.N. Multi-reader multi-case study for performance evaluation of high-risk thyroid ultrasound with computer-aided detection. Cancers 2020, 12, 373. [Google Scholar] [CrossRef] [Green Version]
- Hillis, S.L.; Berbaum, K.S. Power estimation for the Dorfman-Berbaum-Metz method. Acad. Radiol. 2004, 11, 1260–1273. [Google Scholar] [CrossRef]
- Clinical Performance Assessment: Considerations for Computer-Assisted Detection Devices Applied to Radiology Images and Radiology Device Data in Premarket Notification (510(k)) Submissions. Available online: https://www.fda.gov/media/77642/download (accessed on 29 May 2022).
- Gallas, B.D.; Chan, H.P.; D’Orsi, C.J.; Dodd, L.E.; Giger, M.L.; Gur, D.; Krupinski, E.A.; Metz, C.E.; Myers, K.J.; Obuchowski, N.A.; et al. Evaluating imaging and computer-aided detection and diagnosis devices at the FDA. Acad. Radiol. 2012, 19, 463–477. [Google Scholar] [CrossRef] [Green Version]
- Metz ROC Software. Available online: http://metz-roc.uchicago.edu/MetzROC/software (accessed on 29 May 2022).
- Xiao-Hua, Z.N.; Donna, K. McClish. In Statistical Methods in Diagnostic Medicine, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Dendumrongsup, T.; Plumb, A.A.; Halligan, S.; Fanshawe, T.R.; Altman, D.G.; Mallett, S. Multi-reader multi-case studies using the area under the receiver operator feature curve as a measure of Diagnosis-Accuracy: Systematic review with a focus on quality of data reporting. PLoS ONE 2014, 9, e116018. [Google Scholar] [CrossRef]
- Cancer Stat Facts: Thyroid Cancer. Available online: https://seer.cancer.gov/statfacts/html/thyro.html (accessed on 29 May 2022).
- Moon, W.J.; Jung, S.L.; Lee, J.H.; Na, D.G.; Baek, J.H.; Lee, Y.H.; Kim, J.; Kim, H.S.; Byun, J.S.; Lee, D.H. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology 2008, 247, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Cancer Registryy. Available online: https://www.hpa.gov.tw/Pages/ashx/File.ashx?FilePath=~/File/Attach/6069/File_5962.pdf (accessed on 29 May 2022).
- Thurfjell, E.L.; Lernevall, K.A.; Taube, A.A. Benefit of independent double reading in a population-based mammography screening program. Radiology 1994, 191, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Krupinski, E.A. Computer-aided detection in clinical environment: Benefits and challenges for radiologists. Radiology 2004, 231, 7–9. [Google Scholar] [CrossRef] [PubMed]



| Detection Accuracy Assessment PH Set | Detection Accuracy Assessment GE Set | |||||
| Patients(n = 113) | Patients(n = 84) | |||||
| Benign (n = 74) | Malignant (n = 56) | Total (n = 130) | Benign (n = 73) | Malignant (n = 27) | Total (n = 100) | |
| Gender No. (%) | ||||||
| Male | 14 (18.9) | 9 (16.1) | 23 (17.7) | 14 (19.2) | 3 (11.1) | 17 (17.0) |
| Female | 60 (81.1) | 47 (83.9) | 107 (82.3) | 59 (80.8) | 24 (88.9) | 83 (83.0) |
| Age (year) | ||||||
| Mean ± SD * | 48.2 ± 13.8 | 48.6 ± 15.4 | 48.3 ± 14.5 | 51.1 ± 13.2 | 50.1 ± 16.6 | 50.5 ± 14.1 |
| Range | 20.9–76.9 | 11.2–71.6 | 11.2–76.9 | 20.9–75.3 | 20.4–84.8 | 20.4–84.8 |
| Size of Nodule (cm) | ||||||
| Mean ± SD * | 2.38 ± 0.88 | 1.87 ± 0.86 | 2.16 ± 0.90 | 2.50 ± 0.94 | 1.86 ± 0.72 | 2.33 ± 0.93 |
| Range | 0.94–4.37 | 0.49–4.09 | 0.49–4.37 | 0.71–4.16 | 0.87–3.66 | 0.71–4.16 |
| (a) Demographics of patients and nodule images acquired from Philips (PH), GE, and Aloka (AL) ultrasound scanners for detection accuracy and reader performance assessment. | ||||||
| Detection Accuracy Assessment AL Set | Reader Performance Assessment | |||||
| Patients(n = 107) | Patients(n = 129) | |||||
| Benign (n = 58) | Malignant (n = 64) | Total (n = 122) | Benign (n = 83) | Malignant (n = 67) | Total (n = 150) | |
| Gender No. (%) | ||||||
| Male | 8 (13.8) | 14 (21.9) | 22 (18.0) | 16 (19.3) | 11 (16.4) | 27 (18.0) |
| Female | 50 (86.2) | 50 (78.1) | 100 (82.0) | 67 (80.7) | 56 (83.6) | 123 (82.0) |
| Age (year) | ||||||
| Mean ± SD * | 45.9 ± 9.5 | 42.7 ± 11.8 | 44.2 ± 10.8 | 48.5 ± 13.9 | 47.8 ± 14.8 | 48.2 ± 14.3 |
| Range | 21.7–64.3 | 1.5–74.0 | 1.5–74.0 | 20.9–76.9 | 11.2–71.6 | 11.2–76.9 |
| Size of Nodule (cm) | ||||||
| Mean ± SD * | 2.45 ± 1.06 | 1.48 ± 0.72 | 1.94 ± 1.02 | 2.38 ± 0.86 | 1.86 ± 0.83 | 2.15 ± 0.88 |
| Range | 0.53–4.29 | 0.53–4.12 | 0.53–4.29 | 0.94–4.37 | 0.49–4.09 | 0.49–4.37 |
| (b) The pathology diagnosis results of the 823 nodules. | ||||||
| Pathology of Nodules | No. (%) | |||||
| Benign (n = 499) | Nodular hyperplasia | 428 (85.8) | ||||
| Follicular adenoma | 70 (14.0) | |||||
| Unidentified adenoma | 1 (0.2) | |||||
| Malignant (n = 324) | Papillary thyroid carcinoma | 296 (91.4) | ||||
| Follicular thyroid carcinoma | 15 (4.6) | |||||
| Medullary thyroid carcinoma | 5 (1.5) | |||||
| Anaplastic carcinoma | 5 (1.5) | |||||
| Others | 3 (1.0) | |||||
| Sonographic Features | Determined by a Panel of Specialists | PH No. (%) | GE No. (%) | AL No. (%) |
|---|---|---|---|---|
| Anechoic Areas | Absence | 68 (52.3%) | 82 (82.0%) | 96 (78.7%) |
| Presence | 62 (47.7%) | 18 (18.0%) | 26 (21.3%) | |
| Hyperechoic Foci | Absence | 97 (74.6%) | 74 (74.0%) | 93 (76.2%) |
| Presence | 33 (25.4%) | 26 (26.0%) | 29 (23.8%) | |
| Hypoechoic Pattern | Absence | 39 (30.0%) | 30 (30.0%) | 21 (17.2%) |
| Presence | 91 (70.0%) | 70 (70.0%) | 101 (82.8) | |
| Heterogeneous Texture | Absence | 11 (8.5%) | 5 (5.0%) | 10 (8.2%) |
| Presence | 119 (91.5%) | 95 (95.0%) | 112 (91.8%) | |
| Indistinct Margin | Absence | 102 (78.5%) | 77 (77.0%) | 49 (44.5%) |
| Presence | 28 (21.5%) | 23 (23.0%) | 61 (55.5%) |
| Anechoic Areas | Hyperechoic Foci | Hypoechoic Pattern | Heterogeneous Texture | Indistinct Margin | ||
|---|---|---|---|---|---|---|
| PH (n = 130) | AUC (95% CI) | 0.902 (0.838–0.947) | 0.913 (0.850–0.955) | 0.837 (0.762–0.896) | 0.701 (0.614–0.778) | 0.702 (0.616–0.779) |
| p-value | <0.0001 | <0.0001 | <0.0001 | 0.0347 | 0.0007 | |
| GE (n = 100) | AUC (95% CI) | 0.888 (0.809–0.942) | 0.825 (0.736–0.894) | 0.847 (0.761–0.911) | 0.627 (0.525–0.722) | 0.766 (0.670–0.845) |
| p-value | 0.0001 | <0.0001 | <0.0001 | 0.0323 | 0.0002 | |
| AL (n = 122, n = 110 for Margin) | AUC (95% CI) | 0.946 (0.890–0.979) | 0.830 (0.751–0.892) | 0.812 (0.732–0.877) | 0.77 (0.685–0.841) | 0.676 (0.580–0.762) |
| p-value | <0.0001 | 0.0002 | 0.0156 | 0.0045 | 0.0002 |
| (a) AUC of Each Reader | ||||
| Seniority** (Year) | AUC | |||
| Without CSF | With CSF | |||
| Senior * Readers | Reader 1 | 25 | 0.761 | 0.805 |
| Reader 2 | 21 | 0.786 | 0.821 | |
| Reader 3 | 21 | 0.766 | 0.804 | |
| Reader 8 # | 12 | 0.807 | 0.810 | |
| Reader 11 ## | 7 | 0.832 | 0.825 | |
| Reader 12 ## | 7 | 0.822 | 0.817 | |
| Reader 13 | 15 | 0.731 | 0.767 | |
| Reader 14 | 10 | 0.614 | 0.738 | |
| Reader 15 | 11 | 0.711 | 0.805 | |
| Junior Readers | Reader 4 | 5 | 0.740 | 0.770 |
| Reader 5 | 5 | 0.609 | 0.749 | |
| Reader 6 | 5 | 0.744 | 0.753 | |
| Reader 7 | 2 | 0.739 | 0.797 | |
| Reader 9 | 1 | 0.514 | 0.642 | |
| Reader 10 | 5 | 0.717 | 0.781 | |
| Reader 16 | 3 | 0.653 | 0.761 | |
| Reader 17 | 2 | 0.633 | 0.716 | |
| Reader 18 | 1 | 0.784 | 0.810 | |
| (b) Mean AUC | ||||
| Without CSF (95% CI) | With CSF (95% CI) | Improvement (95% CI) | p-Value | |
| All Readers | 0.720 (0.661, 0.780) | 0.776 (0.708, 0.844) | 0.056 (0.002, 0.110) | 0.0420 |
| Senior Readers | 0.759 (0.706, 0.812) | 0.799 (0.732, 0.866) | 0.040 (−0.014, 0.094) | 0.1462 |
| Junior Readers | 0.681 (0.608, 0.755) | 0.753 (0.679, 0.827) | 0.072 (0.009, 0.136) | 0.0265 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, H.-C.; Chen, K.-Y.; Wu, M.-H.; Chang, K.-J.; Chen, C.-N.; Chen, A. Assessing Detection Accuracy of Computerized Sonographic Features and Computer-Assisted Reading Performance in Differentiating Thyroid Cancers. Biomedicines 2022, 10, 1513. https://doi.org/10.3390/biomedicines10071513
Tai H-C, Chen K-Y, Wu M-H, Chang K-J, Chen C-N, Chen A. Assessing Detection Accuracy of Computerized Sonographic Features and Computer-Assisted Reading Performance in Differentiating Thyroid Cancers. Biomedicines. 2022; 10(7):1513. https://doi.org/10.3390/biomedicines10071513
Chicago/Turabian StyleTai, Hao-Chih, Kuen-Yuan Chen, Ming-Hsun Wu, King-Jen Chang, Chiung-Nien Chen, and Argon Chen. 2022. "Assessing Detection Accuracy of Computerized Sonographic Features and Computer-Assisted Reading Performance in Differentiating Thyroid Cancers" Biomedicines 10, no. 7: 1513. https://doi.org/10.3390/biomedicines10071513
APA StyleTai, H.-C., Chen, K.-Y., Wu, M.-H., Chang, K.-J., Chen, C.-N., & Chen, A. (2022). Assessing Detection Accuracy of Computerized Sonographic Features and Computer-Assisted Reading Performance in Differentiating Thyroid Cancers. Biomedicines, 10(7), 1513. https://doi.org/10.3390/biomedicines10071513






