The Role of Mitochondrial Metabolism, AMPK-SIRT Mediated Pathway, LncRNA and MicroRNA in Osteoarthritis
Abstract
1. Introduction
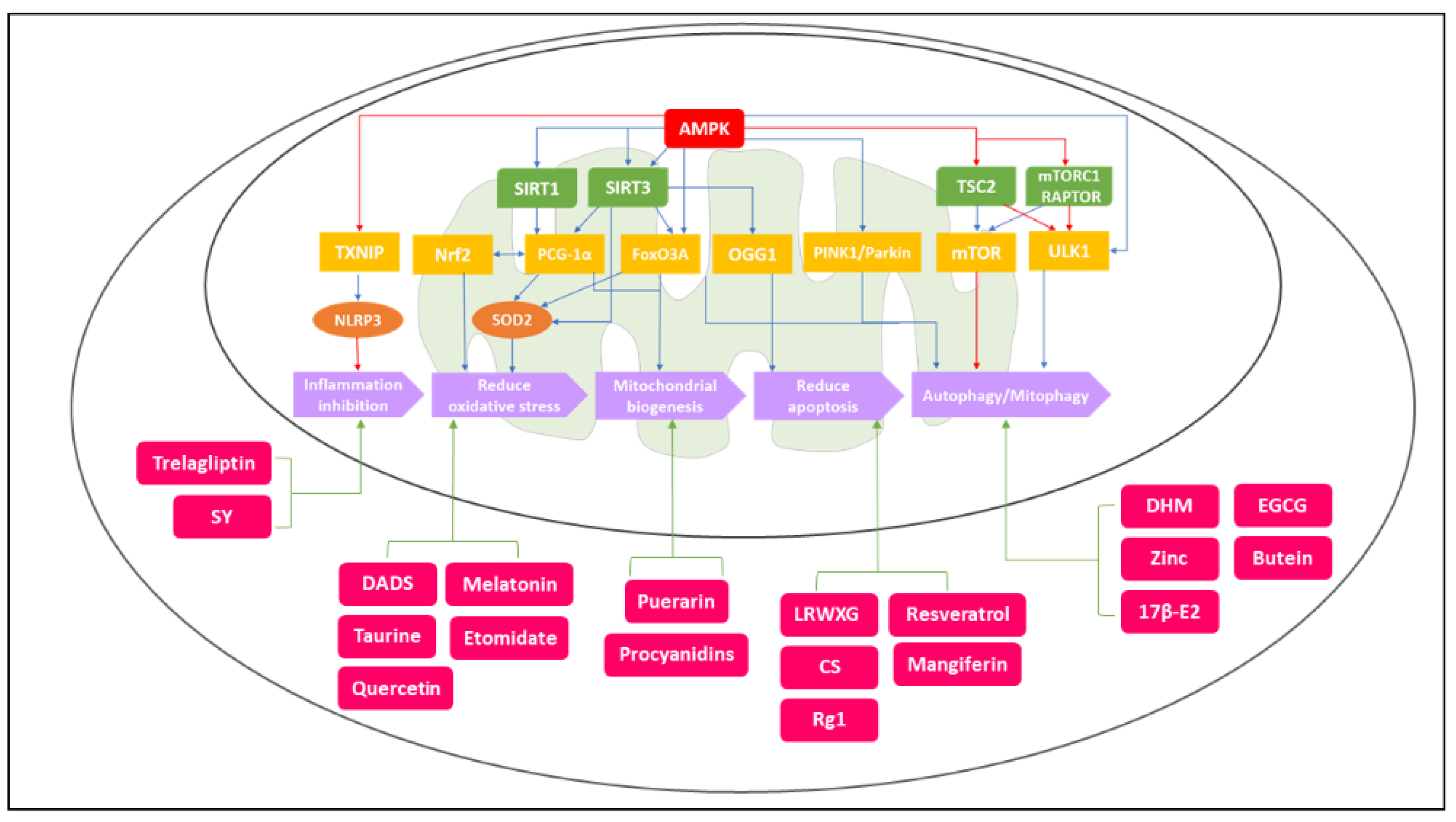
2. Mitochondrial Metabolism Dysfunction to OA Pathogenesis
2.1. Mitochondrial Respiratory Chain (MRC) in Osteoarthritis
2.2. Reactive Oxygen Species (ROS) in Osteoarthritis
| Drugs | Mechanism | Function | Ref. |
|---|---|---|---|
| DADS | Increase Nrf2 nuclear translocation and gene expressions of antioxidant enzymes | Reduce the production of ROS | [62] |
| Taurine | Scavenge excessive ROS | Decrease the H2O2-induced ROS production | [63] |
| Quercetin | Promote the expression of GSH and GSH-PX | Attenuate the generation of ROS | [64] |
| AMPK/SIRT1 signaling pathway | Inhibit cartilage ECM degradation | ||
| Melatonin | Inhibit H2O2 cytotoxicity, iNOS, and COX-2 gene expression | Reduce the oxidative stress and inflammatory responses | [65] |
| LRWXG | Upregulate the expressions of Bcl-2 and downregulate the expressions of caspase-9, caspase-3, and Bax | Inhibit the apoptosis of chondrocytes | [66] |
| CS | Inhibit the expressions of caspase-3 and caspase-9 | Inhibit the apoptosis of chondrocytes | [67] |
| Rg1 | PI3K/Akt/mitochondrial signaling pathway | Inhibit IL-1β-induced chondrocyte apoptosis | [68] |
| DHM | AMPK/SIRT3/PGC-1α signaling pathway | Inhibit cartilage degeneration | [69] |
| Upregulate SIRT3 | Promote mitophagy activity | ||
| Puerarin | AMPK/PGC-1α signaling pathway | Increase mitochondrial biogenesis and attenuate mitochondrial dysfunctions | [70] |
| Zinc | PINK1-dependent mitophagy pathway | Promote mitophagy activity | [71] |
| 17β-E2 | AMPK/SIRT1/mTOR signaling pathway | Induce mitophagy upregulation | [72] |
| Trelagliptin | AMPK/SOX-9 | Ameliorate oxidative stress and inflammatory responses | [73] |
| Etomidate | Upregulate AMPK signaling | Decrease oxidative stress, degradation of ECM, and chondrocyte senescence | [74] |
| SY | AMPK/SIRT1/NF-κB pathway | Inhibit degradation of cartilage ECM and inflammatory responses | [75] |
| Drugs | Mechanism | Function | Ref. |
|---|---|---|---|
| EGCG | Decrease the expressions of mTOR | Reduce the chondrocyte apoptosis and activate autophagy | [76] |
| Decrease expressions of COX-2 and MMP-13 | Attenuate the inflammation on cartilage | ||
| Resveratrol | Suppression of IL-1β, ROS, and p53 production | Inhibit IL-1β-induced degradation of mitochondria and chondrocytes apoptosis | [77,78] |
| Inhibit mitochondrial membrane depolarization, PGE2 synthesis, and ATP depletion | Reduce IL-1β-induced catabolic metabolism and chondrocytes apoptosis | ||
| Activate SIRT1 signaling pathway | Inhibit NO-induced apoptosis | ||
| Procyanidins | AMPK/SIRT1/PGC-1α signaling | Promote mitochondrial biogenesis and proteoglycan homeostasis in chondrocytes | [79] |
| Butein | AMPK/TSC2/ULK1/mTOR signaling pathway | Activate autophagy and inhibit inflammatory responses in OA chondrocytes | [80] |
| Mangiferin | Activate AMPK signaling pathway | Inhibit apoptosis, ECM degradation and enhance autophagy in OA chondrocytes | [81] |
2.3. Chondrocyte Senescence and Mitochondrial Autophagy in Osteoarthritis
2.4. Chondrocyte Apoptosis and Calcium Homeostasis Regulation in Osteoarthritis
2.5. mtDNA Haplogroups in Osteoarthritis
3. AMPK and SIRT Pathway in Related to OA Pathogenesis
3.1. AMPK and SIRT Regulate ECM (Extracellular Matrix) Production and Mitochondrial Biogenesis in Chondrocytes
3.2. AMPK and SIRT Regulate Autophagy and Mitophagy in Chondrocytes
3.3. AMPK and SIRT Regulate Oxidative Stress and Inflammation Inhibition in Chondrocytes
4. Long Non-Coding RNA (lncRNA) and MicroRNA (miRNA) in Related to OA Pathogenesis
4.1. Relationship between lncRNA and OA Pathogenesis
| lncRNA | Target/Signaling Pathway | Function | Ref. |
|---|---|---|---|
| HOTAIR | FUT2/Wnt/β-catenin | Increase ECM degradation, chondrocyte apoptosis | [192,193] |
| ADAMTS-5 | Increase ECM degradation, promoting OA progression | [194] | |
| MALAT1 | miR-150-5p/AKT3 | Inhibit apoptosis and ECM degradation, promote chondrocyte proliferation | [197] |
| IL-8 | Promote chondrocyte proliferation, inhibit inflammatory responses | [198] | |
| GAS5 | miR-21/MMPs | Stimulate apoptosis, inhibit autophagy | [199] |
| H19 lncRNA | miR-675 | Increase inflammatory responses, aggravate chondrocyte injury | [200,201] |
| miR-130a/PTEN/PI3K/Akt | |||
| MEG3 | miR-93/TGFBR2 | Inhibit ECM degradation | [202,203] |
| miR-203/Sirt1/PI3K/AKT | Attenuate inflammatory damage | [204] | |
| FOXD2-AS1 | miR-27a-3p/TLR4 | Increase ECM degradation and inflammation responses | [205,206] |
| miR-206/CCND1 | Promote chondrocyte proliferation | [207] |
4.2. Relationship between miRNA and OA Pathogenesis
| mi-RNA | Target/Signaling Pathway | Function | Ref. |
|---|---|---|---|
| miR-483-5p | Matn3 | Promote ECM degradation and chondrocyte hypertrophy | [210] |
| TIMP2 | |||
| miR-145 | MKK4 | Alleviate cartilage degradation | [211] |
| miR-16-5p | Smad3 | Increase ECM degradation | [212] |
| miR-21 | GDF-5 | Inhibit chondrocyte proliferation | [213] |
| miR-146a | Smad4 | Promote chondrocyte apoptosis | [214] |
| miR-146b | A2M | Promote chondrocyte apoptosis | [215] |
| miR-181a | GPD1L | Promote chondrocyte apoptosis | [216] |
| miR-210 | HIF-3α | Promote chondrocyte proliferation, increase ECM deposition | [217] |
| miR-146 | TNF-α | Inhibit inflammatory responses | [218] |
| miR-9 | NF-κB | Inhibit inflammatory responses | [219] |
| miR-558 | COX-2 | Inhibit inflammatory responses, inhibit ECM degradation | [220] |
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, Y.; Wu, Z.; Xu, L.; Xu, K.; Chen, Z.; Ran, J.; Wu, L. The role of SIRT3-mediated mitochondrial homeostasis in osteoarthritis. Cell. Mol. Life Sci. 2020, 77, 3729–3743. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Hootman, J.M.; Helmick, C.G.; Barbour, K.E.; Theis, K.A.; Boring, M.A. Updated Projected Prevalence of Self-Reported Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation Among US Adults, 2015–2040. Arthritis Rheumatol. 2016, 68, 1582–1587. [Google Scholar] [CrossRef]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.-J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Thomas Aigner, M.; Kurz, B.; Fukui, N.; Sandell, L. Roles of chondrocytes in the pathogenesis of osteoarthritis. Curr. Opin. Reumatol. 2002, 14, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Funck-Brentano, T.; Cohen-Solal, M. Subchondral bone and osteoarthritis. Curr. Opin. Rheumatol. 2015, 27, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Malemud, C.J. Matrix Metalloproteinases and Synovial Joint Pathology. Prog. Mol. Biol. Transl. Sci. 2017, 148, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Chen, D.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis Pathogenesis: A Review of Molecular Mechanisms. Calcif. Tissue Res. 2014, 95, 495–505. [Google Scholar] [CrossRef]
- Martel-Pelletier, J. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef]
- Sacitharan, P.K. Ageing and Osteoarthritis. Subcell Biochem. 2019, 91, 123–159. [Google Scholar]
- Maneiro, E.; Martín, M.A.; De Andres, M.C.; López-Armada, M.J.; Fernández-Sueiro, J.L.; Del Hoyo, P.; Galdo, F.; Arenas, J.; Blanco, F.J. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Care Res. 2003, 48, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.S.; D’Imprima, E.; Vonck, J. Mitochondrial Respiratory Chain Complexes. Subcell Biochem. 2018, 87, 167–227. [Google Scholar] [PubMed]
- Marcus, R.E.; Sokoloff, L. The effect of low oxygen concentration on growth, glycolysis, and sulfate incorporation by articular chondrocytes in monolayer culture. Arthritis Care Res. 1973, 16, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Otte, P. Basic cell metabolism of articular cartilage. Manometric studies. Z. Rheumatol. 1991, 50, 304–312. [Google Scholar] [PubMed]
- Blanco, F.J.; Rego, I.; Ruiz-Romero, C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 161–169. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Cao, Y.; Cui, Y.; Yang, X.; Meng, Z.; Wang, R. Effect of chondrocyte mitochondrial dysfunction on cartilage degeneration: A possible pathway for osteoarthritis pathology at the subcellular level. Mol. Med. Rep. 2019, 20, 3308–3316. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Li, D.; Wang, W.; Xie, G. Reactive Oxygen Species: The 2-Edged Sword of Osteoarthritis. Am. J. Med Sci. 2012, 344, 486–490. [Google Scholar] [CrossRef]
- Blanco, J.F.; June, R.K., 2nd. Cartilage Metabolism, Mitochondria, and Osteoarthritis. J. Am. Acad. Orthop. Surg. 2020, 28, e242–e244. [Google Scholar] [CrossRef]
- He, Y.; Makarczyk, M.J.; Lin, H. Role of mitochondria in mediating chondrocyte response to mechanical stimuli. Life Sci. 2020, 263, 118602. [Google Scholar] [CrossRef]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Henrotin, Y.E.; Bruckner, P.; Pujol, J.-P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr. Cartil. 2003, 11, 747–755. [Google Scholar] [CrossRef]
- Quiros, P.M.; Mottis, A.; Auwerx, J. Mitonuclear communication in homeostasis and stress. Nat. Rev. Mol. Cell Biol. 2016, 17, 213–226. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.C.; Di Benedetto, G.; Scorrano, L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 2011, 13, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Peng, G.; Ning, X.; Wang, J.; Yang, H.; Deng, J. Emerging roles of long noncoding RNA in chondrogenesis, osteogenesis, and osteoarthritis. Am. J. Transl. Res. 2019, 11, 16–30. [Google Scholar] [PubMed]
- Aigner, T.; Söder, S.; Gebhard, P.M.; McAlinden, A.; Haag, J. Mechanisms of disease: Role of chondrocytes in the pathogenesis of osteoarthritis—Structure, chaos and senescence. Nat. Clin. Pract. Rheumatol. 2007, 3, 391–399. [Google Scholar] [CrossRef]
- Le, L.T.T.; Swingler, T.E.; Clark, I.M. Review: The Role of MicroRNAs in Osteoarthritis and Chondrogenesis. Arthritis Care Res. 2013, 65, 1963–1974. [Google Scholar] [CrossRef]
- Swingler, E.T.; Niu, L.; Smith, P.; Paddy, P.; Le, L.; Barter, M.J.; Young, D.; Clark, I.M. The function of microRNAs in cartilage and osteoarthritis. Clin. Exp. Reumatol. 2019, 37, 40–47. [Google Scholar]
- Zorova, L.D. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Cui, Z.; Urban, J.P.G. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: A modeling study. Arthritis Care Res. 2004, 50, 3915–3924. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 14708728. [Google Scholar] [CrossRef] [PubMed]
- Terkeltauba, R.J.K.; Murphy, A.; Ghoshb, S. Invited review the mitochondrion in osteoarthritis. Mitochondrion 2002, 1, 301–319. [Google Scholar] [CrossRef]
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2014, 11, 35–44. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Hardie, D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013, 493, 346–355. [Google Scholar] [CrossRef]
- Tchetina, E.V.; Markova, G.A. Regulation of energy metabolism in the growth plate and osteoarthritic chondrocytes. Rheumatol. Int. 2018, 38, 1963–1974. [Google Scholar] [CrossRef]
- Nishida, T. Impaired glycolytic metabolism causes chondrocyte hypertrophy-like changes via promotion of phospho-Smad1/5/8 translocation into nucleus. Osteoarthr. Cartil. 2013, 21, 700–709. [Google Scholar] [CrossRef]
- Qu, J.; Lu, D.; Guo, H.; Miao, W.; Wu, G.; Zhou, M. PFKFB3 modulates glycolytic metabolism and alleviates endoplasmic reticulum stress in human osteoarthritis cartilage. Clin. Exp. Pharmacol. Physiol. 2016, 43, 312–318. [Google Scholar] [CrossRef]
- Liu, J.; Guo, X.; Ma, W.; Zhang, Y.; Xu, P.; Yao, J.; Bai, Y. Mitochondrial function is altered in articular chondrocytes of an endemic osteoarthritis, Kashin–Beck disease. Osteoarthr. Cartil. 2010, 18, 1218–1226. [Google Scholar] [CrossRef]
- Lopez-Armada, M.J. Mitochondrial activity is modulated by TNFalpha and IL-1beta in normal human chondrocyte cells. Osteoarthr. Cartil. 2006, 14, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Cillero-Pastor, B.; Rego-Perez, I.; Oreiro, N.; Fernández-López, C.; Blanco, F.J. Mitochondrial respiratory chain dysfunction modulates metalloproteases -1, -3 and -13 in human normal chondrocytes in culture. BMC Musculoskelet. Disord. 2013, 14, 235. [Google Scholar] [CrossRef] [PubMed]
- Goetz, J.E.; Coleman, M.C.; Fredericks, U.C.; Petersen, E.; Martin, J.A.; McKinley, T.O.; Tochigi, Y. Time-dependent loss of mitochondrial function precedes progressive histologic cartilage degeneration in a rabbit meniscal destabilization model. J. Orthop. Res. 2016, 35, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Zignego, D.L.; Hilmer, J.K.; June, R.K. Mechanotransduction in primary human osteoarthritic chondrocytes is mediated by metabolism of energy, lipids, and amino acids. J. Biomech. 2015, 48, 4253–4261. [Google Scholar] [CrossRef] [PubMed]
- Bartell, L.R. Mitoprotective therapy prevents rapid, strain-dependent mitochondrial dysfunction after articular cartilage injury. J. Orthop. Res. 2020, 38, 1257–1267. [Google Scholar] [CrossRef]
- Quintana-Cabrera, R.; Mehrotra, A.; Rigoni, G.; Soriano, M. Who and how in the regulation of mitochondrial cristae shape and function. Biochem. Biophys. Res. Commun. 2018, 500, 94–101. [Google Scholar] [CrossRef]
- Sauter, E.; Buckwalter, J.; McKinley, T.O.; Martin, J.A. Cytoskeletal dissolution blocks oxidant release and cell death in injured cartilage. J. Orthop. Res. 2011, 30, 593–598. [Google Scholar] [CrossRef]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Coleman, M.C.; Ramakrishnan, P.S.; Brouillette, M.J.; Martin, J. Injurious Loading of Articular Cartilage Compromises Chondrocyte Respiratory Function. Arthritis Rheumatol. 2015, 68, 662–671. [Google Scholar] [CrossRef]
- Reed, K.N.; Wilson, G.; Pearsall, A.; Grishko, V.I. The role of mitochondrial reactive oxygen species in cartilage matrix destruction. Mol. Cell. Biochem. 2014, 397, 195–201. [Google Scholar] [CrossRef]
- Drevet, S.; Gavazzi, G.; Grange, L.; Dupuy, C.; Lardy, B. Reactive oxygen species and NADPH oxidase 4 involvement in osteoarthritis. Exp. Gerontol. 2018, 111, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Kurz, B.; Aigner, T. Oxygen and reactive oxygen species in cartilage degradation: Friends or foes? Osteoarthr. Cartil. 2005, 13, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.-H. Aging: A revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp. Gerontol. 2007, 42, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Moulton, P.J.; Goldring, M. NADPH oxidase of chondrocytes contains an isoform of the gp91phox subunit. Biochem. J. 1998, 329, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Grange, L.; Nguyen, M.V.C.; Lardy, B.; Derouazi, M.; Campion, Y.; Trocme, C.; Paclet, M.; Gaudin, P.; Morel, F. NAD(P)H Oxidase Activity of Nox4 in Chondrocytes Is Both Inducible and Involved in Collagenase Expression. Antioxid. Redox Signal. 2006, 8, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, H.; Wan, R.; Wu, Y.; Shi, Z.; Huang, W. Mechanisms linking mitochondrial mechanotransduction and chondrocyte biology in the pathogenesis of osteoarthritis. Ageing Res. Rev. 2021, 67, 101315. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, K.A.; Papavassiliou, A.G. Redox and NF-kappaB signaling in osteoarthritis. Free Radic. Biol. Med. 2019, 132, 90–100. [Google Scholar] [CrossRef]
- Sen, C.K. Oxygen toxicity and antioxidants: State of the art. Indian J. Physiol. Pharmacol. 1995, 39, 177–196. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Ruiz-Romero, C. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: A decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol. Cell. Proteom. 2009, 8, 172–189. [Google Scholar] [CrossRef]
- Koike, M.; Nojiri, H.; Ozawa, Y.; Watanabe, K.; Muramatsu, Y.; Kaneko, H.; Morikawa, D.; Kobayashi, K.; Saita, Y.; Sasho, T.; et al. Mechanical overloading causes mitochondrial superoxide and SOD2 imbalance in chondrocytes resulting in cartilage degeneration. Sci. Rep. 2015, 5, 11722. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A. Evaluating the Protective Effects and Mechanisms of Diallyl Disulfide on Interlukin-1beta-Induced Oxidative Stress and Mitochondrial Apoptotic Signaling Pathways in Cultured Chondrocytes. J. Cell. Biochem. 2017, 118, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Kim, E.-K.; Hwang, J.-W.; Kim, J.-S.; Shin, W.-B.; Dong, X.; Nawarathna, W.P.A.S.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Neuroprotective Effect of Taurine-Rich Cuttlefish (Sepia officinalis) Extract Against Hydrogen Peroxide-Induced Oxidative Stress in SH-SY5Y Cells. Single Mol. Single Cell Seq. 2017, 975, 243–254. [Google Scholar] [CrossRef]
- Qiu, L.; Luo, Y.; Chen, X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharmacother. 2018, 103, 1585–1591. [Google Scholar] [CrossRef]
- Lim, H.-D.; Kim, Y.-S.; Ko, S.-H.; Yoon, I.-J.; Cho, S.-G.; Chun, Y.-H.; Choi, B.-J.; Kim, E.-C. Cytoprotective and anti-inflammatory effects of melatonin in hydrogen peroxide-stimulated CHON-001 human chondrocyte cell line and rabbit model of osteoarthritis via the SIRT1 pathway. J. Pineal Res. 2012, 53, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Chen, Q.; Dou, X.; Chen, L.; Wu, J.; Zhang, W.; Shao, H.; Ling, P.; Liu, F.; Wang, F. Lower range of molecular weight of xanthan gum inhibits cartilage matrix destruction via intrinsic bax-mitochondria cytochrome c-caspase pathway. Carbohydr. Polym. 2018, 198, 354–363. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Sun, Y.; Han, S. Chondroitin sulfate from sturgeon bone protects chondrocytes via inhibiting apoptosis in osteoarthritis. Int. J. Biol. Macromol. 2019, 134, 1113–1119. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, D.; Fan, W. Protection of ginsenoside Rg1 on chondrocyte from IL-1β-induced mitochondria-activated apoptosis through PI3K/Akt signaling. Mol. Cell. Biochem. 2014, 392, 249–257. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Huang, C.; Lin, D.; Zhou, Y.; Wu, Y.; Tian, N.; Fan, P.; Pan, X.; Xu, D.; et al. SIRT3 Activation by Dihydromyricetin Suppresses Chondrocytes Degeneration via Maintaining Mitochondrial Homeostasis. Int. J. Biol. Sci. 2018, 14, 1873–1882. [Google Scholar] [CrossRef]
- Salminen, A.; Hyttinen, J.M.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef]
- Huang, L.-W.; Huang, T.-C.; Hu, Y.-C.; Hsieh, B.-S.; Chiu, P.-R.; Cheng, H.-L.; Chang, K.-L. Zinc protects chondrocytes from monosodium iodoacetate-induced damage by enhancing ATP and mitophagy. Biochem. Biophys. Res. Commun. 2019, 521, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Mei, R.; Lou, P.; You, G.; Jiang, T.; Yu, X.; Guo, L. 17beta-Estradiol Induces Mitophagy Upregulation to Protect Chondrocytes via the SIRT1-Mediated AMPK/mTOR Signaling Pathway. Front. Endocrinol. 2020, 11, 615250. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zuo, Q.; Li, Z.; Chen, J.; Liu, F. Trelagliptin ameliorates IL-1β-impaired chondrocyte function via the AMPK/SOX-9 pathway. Mol. Immunol. 2021, 140, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Xu, Y. The protective effects of etomidate against interleukin-1beta (IL-1beta)-induced oxidative stress, extracellular matrix alteration and cellular senescence in chondrocytes. Bioengineered 2022, 13, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gao, Y.; Zhang, Z.; Chi, Q.; Liu, Y.; Yang, L.; Xu, K. Safflower yellow alleviates osteoarthritis and prevents inflammation by inhibiting PGE2 release and regulating NF-kappaB/SIRT1/AMPK signaling pathways. Phytomedicine 2020, 78, 153305. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.T. Intra-Articular Injection of (-)-Epigallocatechin 3-Gallate to Attenuate Articular Cartilage Degeneration by Enhancing Autophagy in a Post-Traumatic Osteoarthritis Rat Model. Antioxidants 2020, 10, 8. [Google Scholar] [CrossRef]
- Csaki, C.; Keshishzadeh, N.; Fischer, K.; Shakibaei, M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem. Pharmacol. 2008, 75, 677–687. [Google Scholar] [CrossRef]
- Dave, M.; Attur, M.; Palmer, G.; Al-Mussawir, H.E.; Kennish, L.; Patel, J.; Abramson, S.B. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Care Res. 2008, 58, 2786–2797. [Google Scholar] [CrossRef]
- Masuda, I.; Koike, M.; Nakashima, S.; Mizutani, Y.; Ozawa, Y.; Watanabe, K.; Sawada, Y.; Sugiyama, H.; Sugimoto, A.; Nojiri, H.; et al. Apple procyanidins promote mitochondrial biogenesis and proteoglycan biosynthesis in chondrocytes. Sci. Rep. 2018, 8, 7229. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Butein Activates Autophagy Through AMPK/TSC2/ULK1/mTOR Pathway to Inhibit IL-6 Expression in IL-1beta Stimulated Human Chondrocytes. Cell Physiol. Biochem. 2018, 49, 932–946. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Jiang, K.; Han, W.; Zhang, J.; Xie, L.; Liu, Y.; Xiao, J.; Wang, X. Mangiferin Prevents TBHP-Induced Apoptosis and ECM Degradation in Mouse Osteoarthritic Chondrocytes via Restoring Autophagy and Ameliorates Murine Osteoarthritis. Oxid. Med. Cell. Longev. 2019, 2019, 8783197. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K. Progress of research in osteoarthritis. Involvement of reactive oxygen species in the pathogenesis of osteoarthritis. Clin. Calcium 2009, 19, 1602–1606. [Google Scholar] [PubMed]
- Li, Y.-S.; Xiao, W.-F.; Luo, W. Cellular aging towards osteoarthritis. Mech. Ageing Dev. 2017, 162, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Hartmann, A.; Bechmann, V.; Graf, B.; Nerlich, M.; Angele, P. Oxidative stress induces senescence in chondrocytes. J. Orthop. Res. 2011, 29, 1114–1120. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.-W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef]
- Jeon, O.H.; Kim, C.; Laberge, R.-M.; DeMaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef]
- Diekman, B.O.; Collins, J.A.; Loeser, R.F. Does Joint Injury Make Young Joints Old? J. Am. Acad. Orthop. Surg. 2018, 26, e455–e456. [Google Scholar] [CrossRef]
- Xu, M.; Bradley, E.W.; Weivoda, M.M.; Hwang, S.M.; Pirtskhalava, T.; Decklever, T.; Curran, G.L.; Ogrodnik, M.; Jurk, D.; Johnson, K.O.; et al. Transplanted Senescent Cells Induce an Osteoarthritis-Like Condition in Mice. J. Gerontol. Ser. A 2016, 72, 780–785. [Google Scholar] [CrossRef]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2015, 23, 303–314. [Google Scholar] [CrossRef]
- McCulloch, K.; Litherland, G.J.; Rai, T.S. Cellular senescence in osteoarthritis pathology. Aging Cell 2017, 16, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Narita, M. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 2011, 332, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, V.; Bohensky, J.; Shapiro, I.M. Autophagy: A new phase in the maturation of growth plate chondrocytes is regulated by HIF, mTOR and AMP kinase. Cells Tissues Organs 2009, 189, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Caramés, B.; Taniguchi, N.; Otsuki, S.; Blanco, F.J.; Lotz, M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Care Res. 2010, 62, 791–801. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Trojel-Hansen, C.; Kroemer, G. Mitochondrial Control of Cellular Life, Stress, and Death. Circ. Res. 2012, 111, 1198–1207. [Google Scholar] [CrossRef]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef]
- Carames, B.; Olmer, M.; Kiosses, W.B.; Lotz, M.K. The Relationship of Autophagy Defects to Cartilage Damage During Joint Aging in a Mouse Model. Arthritis Rheumatol. 2015, 67, 1568–1576. [Google Scholar] [CrossRef]
- Francisco, J.; Blanco, R.L.O.; Schwarz, T.H.; Lotz, M. Chondrocyte apoptosis induced by nitric oxide. Am. J. Patol. 1995, 146, 75. [Google Scholar]
- Zhong, Z. NF-kappaB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef]
- Caramés, B.; de Figueroa, P.L.; Lotz, M.; Blanco, F. Autophagy activation protects from mitochondrial dysfunction in human chondrocytes. Osteoarthr. Cartil. 2014, 22, 966–976. [Google Scholar] [CrossRef][Green Version]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Kim, H.A. Cell Death and Apoptosis in Ostearthritic Cartilage. Curr. Drug Targets 2007, 8, 333–345. [Google Scholar]
- Maneiro, E.; López-Armada, M.J.; De Andres, M.C.; Caramés, B.; Martín, M.A.; Bonilla, A.; Del Hoyo, P.; Galdo, F.; Arenas, J.; Blanco, F.J. Effect of nitric oxide on mitochondrial respiratory activity of human articular chondrocytes. Ann. Rheum. Dis. 2004, 64, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Loening, A.; James, I.E.; Levenston, M.; Badger, A.M.; Frank, E.H.; Kurz, B.; Nuttall, M.E.; Hung, H.-H.; Blake, S.M.; Grodzinsky, A.J.; et al. Injurious Mechanical Compression of Bovine Articular Cartilage Induces Chondrocyte Apoptosis. Arch. Biochem. Biophys. 2000, 381, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.S. Mitochondrial calcium uptake. Proc. Natl. Acad. Sci. USA 2013, 110, 10479–10486. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhou, Y.; Chen, X.; Han, L.; Wang, L.; Lu, X.L. Calcium signaling of in situ chondrocytes in articular cartilage under compressive loading: Roles of calcium sources and cell membrane ion channels. J. Orthop. Res. 2017, 36, 730–738. [Google Scholar] [CrossRef]
- Shapiro, I.M.; S Kakuta, E.E.G.; Hazelgrove, J.; Havery, J.; Chance, B.; Frasca, P. Initiation of Endochondral Calcification Is Related to Changes in the Redox State of Hypertrophic Chondrocytes. Science 1982, 217, 950–952. [Google Scholar] [CrossRef]
- Matsumoto, H.; Debolt, K.; Shapiro, I.M. Adenine, guanine, and inosine nucleotides of chick growth cartilage relationship between energy status and the mineralization process. J. Bone Miner. Res. 1988, 3, 347–352. [Google Scholar] [CrossRef]
- Johnson, K.A.J.; Murphy, A.; Andreyev, A.; Dykens, J.; Terkeltaub, R. Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2000, 43, 1560–1570. [Google Scholar] [CrossRef]
- D’Andrea, P.; Calabrese, A.; Capozzi, I.; Grandolfo, M.; Tonon, R.; Vittur, F. Intercellular Ca2+ waves in mechanically stimulated articular chondrocytes. Biorheology 2000, 37, 75–83. [Google Scholar] [CrossRef]
- Huser, C.A.M.; Davies, M.E. Calcium signaling leads to mitochondrial depolarization in impact-induced chondrocyte death in equine articular cartilage explants. Arthritis Care Res. 2007, 56, 2322–2334. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Ockinger, J.; Yu, J.; Byles, V.; McColl, A.; Hofer, A.M.; Horng, T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. USA 2012, 109, 11282–11287. [Google Scholar] [CrossRef] [PubMed]
- Wann, A.K.T.; Zuo, N.; Haycraft, C.J.; Jensen, C.G.; Poole, C.A.; McGlashan, S.R.; Knight, M.M. Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB J. 2011, 26, 1663–1671. [Google Scholar] [CrossRef]
- Duchen, M.R. Contributions of mitochondria to animal physiology: From homeostatic sensor to calcium signalling and cell death. J. Physiol. 1999, 516, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Borutaite, V.; Brown, C.G. Release of cytochrome c from heart mitochondria is induced by high Ca2+ and peroxynitrite and is responsible for Ca2+-induced inhibition of substrate oxidation. Biochim. Biophys. Acta Mol. Basis Dis. 1999, 1453, 41–48. [Google Scholar] [CrossRef]
- Sato, M.; Sato, K. Maternal inheritance of mitochondrial DNA by diverse mechanisms to eliminate paternal mitochondrial DNA. Biochim. Biophys. Acta 2013, 1833, 1979–1984. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef]
- Lü, H.B.; Zhou, Y.; Hu, J.Z. Mitochondrial DNA deletion mutations in articular chondrocytes of cartilage affected by osteoarthritis. Yi Xue Ban J. Cent. South Univ. Med. Sci. 2006, 31, 640–644. [Google Scholar]
- Ruiz-Pesini, E.; Mishmar, D.; Brandon, M.; Procaccio, V.; Wallace, D.C. Effects of Purifying and Adaptive Selection on Regional Variation in Human mtDNA. Science 2004, 303, 223–226. [Google Scholar] [CrossRef]
- Wallace, D.C. Maternal genes: Mitochondrial diseases. Birth Defects Orig. Artic. Ser. 1987, 23, 137–190. [Google Scholar]
- Blanco, F.J.; Valdes, A.; Rego-Pérez, I. Mitochondrial DNA variation and the pathogenesis of osteoarthritis phenotypes. Nat. Rev. Rheumatol. 2018, 14, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Rego, I.; Fernández-Moreno, M.; Fernández-López, C.; Gómez-Reino, J.J.; González, A.; Arenas, J.; Blanco, F.J. Role of European mitochondrial DNA haplogroups in the prevalence of hip osteoarthritis in Galicia, Northern Spain. Ann. Rheum. Dis. 2009, 69, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Rego-Pérez, I.; Fernández-Moreno, M.; Fernández-López, C.; Arenas, J.; Blanco, F.J. Mitochondrial DNA haplogroups: Role in the prevalence and severity of knee osteoarthritis. Arthritis Care Res. 2008, 58, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Soto-Hermida, A.; Fernandez-Moreno, M.; Oreiro, N.; Fernández-López, C.; Rego-Pérez, I.; Blanco, F. mtDNA haplogroups and osteoarthritis in different geographic populations. Mitochondrion 2014, 15, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Moreno, M.; Soto-Hermida, A.; Mosquera, M.E.V.; Cortés-Pereira, E.; Relaño, S.; Gómez, T.H.; Pértega, S.; Oreiro-Villar, N.; Fernández-López, C.; Garesse, R.; et al. Mitochondrial DNA haplogroups influence the risk of incident knee osteoarthritis in OAI and CHECK cohorts. A meta-analysis and functional study. Ann. Rheum. Dis. 2016, 76, 1114–1122. [Google Scholar] [CrossRef]
- Fang, H.; Liu, X.; Shen, L.; Li, F.; Liu, Y.; Chi, H.; Miao, H.; Lu, J.; Bai, Y. Role of mtDNA Haplogroups in the Prevalence of Knee Osteoarthritis in a Southern Chinese Population. Int. J. Mol. Sci. 2014, 15, 2646–2659. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Wang, M.; Jin, Y.; Liao, W.; Zhao, Z.; Fang, J. Mitochondrial DNA haplogroups participate in osteoarthritis: Current evidence based on a meta-analysis. Clin. Rheumatol. 2020, 39, 1027–1037. [Google Scholar] [CrossRef]
- Fernandez-Moreno, M.; Soto-Hermida, A.; Mosquera, M.E.V.; Cortés-Pereira, E.; Pértega, S.; Relaño, S.; Oreiro-Villar, N.; Fernández-López, C.; Blanco, F.J.; Rego-Pérez, I. A replication study and meta-analysis of mitochondrial DNA variants in the radiographic progression of knee osteoarthritis. Rheumatology 2016, 56, 263–270. [Google Scholar] [CrossRef]
- Soto-Hermida, A.; Fernández-Moreno, M.; Pértega-Díaz, S.; Oreiro, N.; Fernández-López, C.; Blanco, F.J.; Rego-Pérez, I. Mitochondrial DNA haplogroups modulate the radiographic progression of Spanish patients with osteoarthritis. Rheumatol. Int. 2014, 35, 337–344. [Google Scholar] [CrossRef]
- Vaamonde-García, C.; López-Armada, M.J. Role of mitochondrial dysfunction on rheumatic diseases. Biochem. Pharmacol. 2019, 165, 181–195. [Google Scholar] [CrossRef]
- Kenney, M.C.; Chwa, M.; Atilano, S.R.; Falatoonzadeh, P.; Ramirez, C.; Malik, D.; Tarek, M.; Cáceres-Del-Carpio, J.; Nesburn, A.B.; Boyer, D.S.; et al. Inherited mitochondrial DNA variants can affect complement, inflammation and apoptosis pathways: Insights into mitochondrial-nuclear interactions. Hum. Mol. Genet. 2014, 23, 3537–3551. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Redondo, D.; Marcuello, A.; Casajús, J.A.; Ara, I.; Dahmani, Y.; Montoya, J.; Ruiz-Pesini, E.; López-Pérez, M.J.; Díez-Sánchez, C. Human mitochondrial haplogroup H: The highest VO2max consumer—Is it a paradox? Mitochondrion 2010, 10, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Zole, E.; Ranka, R. Mitochondria, its DNA and telomeres in ageing and human population. Biogerontology 2018, 19, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Auwerx, J. Calorie restriction: Is AMPK a key sensor and effector? Physiology 2011, 26, 214–224. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell. Mol. Life Sci. 2010, 67, 3407–3423. [Google Scholar] [CrossRef]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Xu, J.; Ji, J.; Yan, X.-H. Cross-Talk between AMPK and mTOR in Regulating Energy Balance. Crit. Rev. Food Sci. Nutr. 2012, 52, 373–381. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Li, X. SIRT1 and energy metabolism. Acta Biochim. Biophys. Sin. 2013, 45, 51–60. [Google Scholar] [CrossRef]
- Chang, H.-C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2013, 25, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E.; Hirschey, M.; Finley, L.W.; Haigis, M.C. Sirtuin regulation of mitochondria: Energy production, apoptosis, and signaling. Trends Biochem. Sci. 2010, 35, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Xie, S.; Qiu, X.; Mohrin, M.; Shin, J.; Liu, Y.; Zhang, D.; Scadden, D.T.; Chen, D. SIRT3 Reverses Aging-Associated Degeneration. Cell Rep. 2013, 3, 319–327. [Google Scholar] [CrossRef]
- Salinas, D.; Mumey, B.M.; June, R.K. Physiological dynamic compression regulates central energy metabolism in primary human chondrocytes. Biomech. Model. Mechanobiol. 2018, 18, 69–77. [Google Scholar] [CrossRef]
- Wolff, K.J.; Ramakrishnan, P.S.; Brouillette, M.J.; Journot, B.J.; McKinley, T.O.; Buckwalter, J.A.; Martin, J.A. Mechanical stress and ATP synthesis are coupled by mitochondrial oxidants in articular cartilage. J. Orthop. Res. 2013, 31, 191–196. [Google Scholar] [CrossRef] [PubMed]
- He, D.-S.; Hu, X.-J.; Yan, Y.-Q.; Liu, H. Underlying mechanism of Sirt1 on apoptosis and extracellular matrix degradation of osteoarthritis chondrocytes. Mol. Med. Rep. 2017, 16, 845–850. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Zhang, Y.; Liu, J.; Yao, Z.; Zhang, C. Protective effects of metformin against osteoarthritis through upregulation of SIRT3-mediated PINK1/Parkin-dependent mitophagy in primary chondrocytes. Biosci. Trends 2019, 12, 605–612. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Lotz, M.; Terkeltaub, R.; Liu-Bryan, R. Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor gamma coactivator 1alpha. Arthritis Rheumatol. 2015, 67, 2141–2153. [Google Scholar] [CrossRef]
- Zhao, X.; Petursson, F.; Viollet, B.; Lotz, M.; Terkeltaub, R.; Liu-Bryan, R. Peroxisome proliferator-activated receptor gamma coactivator 1alpha and FoxO3A mediate chondroprotection by AMP-activated protein kinase. Arthritis Rheumatol. 2014, 66, 3073–3082. [Google Scholar] [CrossRef]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. 2007. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef]
- Gleyzer, N.; Vercauteren, K.; Scarpulla, R.C. Control of Mitochondrial Transcription Specificity Factors (TFB1M and TFB2M) by Nuclear Respiratory Factors (NRF-1 and NRF-2) and PGC-1 Family Coactivators. Mol. Cell. Biol. 2005, 25, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Larsson, N.G.; Wang, J.; Wilhelmsson, H.; Oldfors, A.; Rustin, P.; Lewandoski, M.; Barsh, G.S.; Clayton, D.A. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in micepdf. Nat. Genet. 1998, 18, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Poulet, B.; Staines, K.A. New developments in osteoarthritis and cartilage biology. Curr. Opin. Pharmacol. 2016, 28, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Wei, Z.; Tao, L.; Wang, S.; Zhang, F.; Shen, C.; Wu, H.; Liu, Z.; Zhu, P.; Wang, A.; et al. Prophylaxis of Diallyl Disulfide on Skin Carcinogenic Model via p21-dependent Nrf2 stabilization. Sci. Rep. 2016, 6, 35676. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.A.; Diekman, B.; Loeser, R.F. Targeting aging for disease modification in osteoarthritis. Curr. Opin. Rheumatol. 2018, 30, 101–107. [Google Scholar] [CrossRef]
- Marchev, A.S.; Dimitrova, P.A.; Burns, A.J.; Kostov, R.V.; Dinkova-Kostova, A.T.; Georgiev, M.I. Oxidative stress and chronic inflammation in osteoarthritis: Can NRF2 counteract these partners in crime? Ann. N. Y. Acad. Sci. 2017, 1401, 114–135. [Google Scholar] [CrossRef]
- Wang, L.; Shan, H.; Wang, B.; Wang, N.; Zhou, Z.; Pan, C.; Wang, F. Puerarin Attenuates Osteoarthritis via Upregulating AMP-Activated Protein Kinase/Proliferator-Activated Receptor-gamma Coactivator-1 Signaling Pathway in Osteoarthritis Rats. Pharmacology 2018, 102, 117–125. [Google Scholar] [CrossRef]
- Bento, C.F.; Renna, M.; Ghislat, G.; Puri, C.; Ashkenazi, A.; Vicinanza, M.; Menzies, F.M.; Rubinsztein, D.C. Mammalian Autophagy: How Does It Work? Annu. Rev. Biochem. 2016, 85, 685–713. [Google Scholar] [CrossRef]
- Kubli, D.A.; Gustafsson, A.B. Mitochondria and mitophagy: The yin and yang of cell death control. Circ. Res. 2012, 111, 1208–1221. [Google Scholar] [CrossRef]
- Inoki, K.; Zhu, T.; Guan, K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Mair, W.; Vasquez, D.S.; Joshi, A.; Gwinn, D.M.; Taylor, R.; et al. Phosphorylation of ULK1 (hATG1) by AMP-Activated Protein Kinase Connects Energy Sensing to Mitophagy. Science 2011, 331, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Laker, R.C.; Drake, J.C.; Wilson, R.J.; Lira, V.A.; Lewellen, B.M.; Ryall, K.A.; Fisher, C.C.; Zhang, M.; Saucerman, J.J.; Goodyear, L.J.; et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat. Commun. 2017, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Rego-Pérez, I. Mitochondria and mitophagy: Biosensors for cartilage degradation and osteoarthritis. Osteoarthr. Cartil. 2018, 26, 989–991. [Google Scholar] [CrossRef]
- Yu, W.; Gao, B.; Li, N.; Wang, J.; Qiu, C.; Zhang, G.; Liu, M.; Zhang, R.; Li, C.; Ji, G.; et al. Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-Parkin-mediated mitophagy. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 1973–1983. [Google Scholar] [CrossRef]
- Greer, E.; Oskoui, P.R.; Banko, M.R.; Maniar, J.M.; Gygi, M.P.; Gygi, S.P.; Brunet, A. The Energy Sensor AMP-activated Protein Kinase Directly Regulates the Mammalian FOXO3 Transcription Factor. J. Biol. Chem. 2007, 282, 30107–30119. [Google Scholar] [CrossRef]
- Zhao, J.; Brault, J.J.; Schild, A.; Cao, P.; Sandri, M.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. FoxO3 Coordinately Activates Protein Degradation by the Autophagic/Lysosomal and Proteasomal Pathways in Atrophying Muscle Cells. Cell Metab. 2007, 6, 472–483. [Google Scholar] [CrossRef]
- Bowman, C.J.; Ayer, D.; Dynlacht, B.D. Foxk proteins repress the initiation of starvation-induced atrophy and autophagy programs. Nat. Cell Biol. 2014, 16, 1202–1214. [Google Scholar] [CrossRef]
- Sarraf, S.; Raman, M.; Guarani-Pereira, V.; Sowa, M.E.; Huttlin, E.; Gygi, S.P.; Harper, J.W. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature 2013, 496, 372–376. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Padman, B.S.; Lazarou, M. Deciphering the Molecular Signals of PINK1/Parkin Mitophagy. Trends Cell Biol. 2016, 26, 733–744. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Khan, N.M.; Ahmad, I.; Haqqi, T.M. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthr. Cartil. 2018, 26, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in Human Health and Disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Caramés, B.; Hasegawa, A.; Taniguchi, N.; Miyaki, S.; Blanco, F.J.; Lotz, M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann. Rheum. Dis. 2011, 71, 575–581. [Google Scholar] [CrossRef]
- Ansari, A.; Rahman, M.S.; Saha, S.K.; Saikot, F.K.; Deep, A.; Kim, K.-H. Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell 2017, 16, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Wang, Y.; Terkeltaub, R.; Liu-Bryan, R. Activation of AMPK-SIRT3 signaling is chondroprotective by preserving mitochondrial DNA integrity and function. Osteoarthr. Cartil. 2018, 26, 1539–1550. [Google Scholar] [CrossRef]
- Olmos, Y.; Valle, I.; Borniquel, S.; Tierrez, A.; Soria, E.; Lamas, S.; Monsalve, M. Mutual Dependence of Foxo3a and PGC-1α in the Induction of Oxidative Stress Genes. J. Biol. Chem. 2009, 284, 14476–14484. [Google Scholar] [CrossRef]
- Kincaid, B.; Bossy-Wetzel, E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front. Aging Neurosci. 2013, 5, 48. [Google Scholar] [CrossRef]
- Yu, W.; Dittenhafer-Reed, K.; Denu, J.M. SIRT3 Protein Deacetylates Isocitrate Dehydrogenase 2 (IDH2) and Regulates Mitochondrial Redox Status. J. Biol. Chem. 2012, 287, 14078–14086. [Google Scholar] [CrossRef]
- Zhu, S.; Makosa, D.; Miller, B.F.; Griffin, T.M. Glutathione as a mediator of cartilage oxidative stress resistance and resilience during aging and osteoarthritis. Connect. Tissue Res. 2019, 61, 34–47. [Google Scholar] [CrossRef]
- Cheng, Y.; Ren, X.; Gowda, A.S.; Shan, Y.; Zhang, L.; Yuan, Y.-S.; Patel, R.; Wu, H.; Huber-Keener, K.; Yang, J.W.; et al. Interaction of Sirt3 with OGG1 contributes to repair of mitochondrial DNA and protects from apoptotic cell death under oxidative stress. Cell Death Dis. 2013, 4, e731. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res. Rev. 2012, 11, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Bu, R.; Yang, Q.; Jia, J.; Li, T.; Wang, Q.; Chen, Y. Exendin-4 Protects against Hyperglycemia-Induced Cardiomyocyte Pyroptosis via the AMPK-TXNIP Pathway. J. Diabetes Res. 2019, 2019, 8905917. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.K.; Lee, S.-J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P.; et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Ishida, K.; Matsushita, T.; Fujita, N.; Hayashi, S.; Sasaki, K.; Tei, K.; Kubo, S.; Matsumoto, T.; Fujioka, H.; et al. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Care Res. 2009, 60, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Makunin, I.V. Non-coding RNA. Hum. Mol. Genet. 2006, 15, R17–R29. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhang, R. Regulatory non-coding RNAs: Revolutionizing the RNA world. Mol. Biol. Rep. 2014, 41, 3915–3923. [Google Scholar] [CrossRef]
- Wei, J.W.; Huang, K.; Yang, C.; Kang, C.S. Non-coding RNAs as regulators in epigenetics (Review). Oncol. Rep. 2017, 37, 3–9. [Google Scholar] [CrossRef]
- Yao, R.-W.; Wang, Y.; Chen, L.-L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019, 21, 542–551. [Google Scholar] [CrossRef]
- Su, W.; Xie, W.; Shang, Q.; Su, B. The Long Noncoding RNA MEG3 Is Downregulated and Inversely Associated with VEGF Levels in Osteoarthritis. BioMed Res. Int. 2015, 2015, 356893. [Google Scholar] [CrossRef]
- Xing, D.; Liang, J.-Q.; Li, Y.; Lu, J.; Jia, H.-B.; Xu, L.-Y.; Ma, X.-L. Identification of Long Noncoding RNA Associated with Osteoarthritis in Humans. Orthop. Surg. 2014, 6, 288–293. [Google Scholar] [CrossRef]
- Svoboda, M.; Slyskova, J.; Schneiderová, M.; Makovicky, P.; Bielik, L.; Levy, M.; Lipska, L.; Hemmelova, B.; Kala, Z.; Protivankova, M.; et al. HOTAIR long non-coding RNA is a negative prognostic factor not only in primary tumors, but also in the blood of colorectal cancer patients. Carcinogenesis 2014, 35, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Z.; Pan, Y.; Ma, J.; Miao, X.; Qi, X.; Zhou, H.; Jia, L. MiR-26a and miR-26b mediate osteoarthritis progression by targeting FUT4 via NF-kappaB signaling pathway. Int. J. Biochem. Cell Biol. 2018, 94, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Z.; Shan, Y.; Pan, Y.; Ma, J.; Jia, L. Long non-coding RNA HOTAIR promotes osteoarthritis progression via miR-17-5p/FUT2/beta-catenin axis. Cell Death Dis. 2018, 9, 711. [Google Scholar] [CrossRef] [PubMed]
- Dou, P.D.P.; Hu, R.H.R.; Zhu, W.Z.W.; Tang, Q.T.Q.; Li, D.L.D.; Li, H.L.H.; Wang, W.W.W. Long non-coding RNA HOTAIR promotes expression of ADAMTS-5 in human osteoarthritic articular chondrocytes. Pharmazie 2017, 72, 113–117. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, J.; Luo, T.; Chen, Q.; Lu, M.; Meng, D. LncRNA PACER is down-regulated in osteoarthritis and regulates chondrocyte apoptosis and lncRNA HOTAIR expression. Biosci. Rep. 2019, 39, BSR20190404. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, X.; Tian, X.; Tian, J.; Zhang, Y.; Ma, J.; Xu, H.; Wang, S. LncRNA MALAT1 negatively regulates MDSCs in patients with lung cancer. J. Cancer 2018, 9, 2436–2442. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Chen, G.; He, R.; Yang, L. LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3 axis. Cell Biosci. 2019, 9, 54. [Google Scholar] [CrossRef]
- Nanus, D.E.; Wijesinghe, S.N.; Pearson, M.; Hadjicharalambous, M.R.; Rosser, A.; Davis, E.T.; Lindsay, M.A.; Jones, S.W. Regulation of the Inflammatory Synovial Fibroblast Phenotype by Metastasis-Associated Lung Adenocarcinoma Transcript 1 Long Noncoding RNA in Obese Patients with Osteoarthritis. Arthritis Rheumatol. 2019, 72, 609–619. [Google Scholar] [CrossRef]
- Song, J.; Ahn, C.; Chun, C.-H.; Jin, E.-J. A long non-coding RNA, GAS5, plays a critical role in the regulation of miR-21 during osteoarthritis. J. Orthop. Res. 2014, 32, 1628–1635. [Google Scholar] [CrossRef]
- Hu, Y.; Li, S.; Zou, Y. Knockdown of LncRNA H19 Relieves LPS-Induced Damage by Modulating miR-130a in Osteoarthritis. Yonsei Med. J. 2019, 60, 381–388. [Google Scholar] [CrossRef]
- Steck, E.; Boeuf, S.; Gabler, J.; Werth, N.; Schnatzer, P.; Diederichs, S.; Richter, W. Regulation of H19 and its encoded microRNA-675 in osteoarthritis and under anabolic and catabolic in vitro conditions. Klin. Wochenschr. 2012, 90, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhu, H.; Zheng, M.Q.; Dong, Q.R. LncRNA MEG3 Inhibits the Degradation of the Extracellular Matrix of Chondrocytes in Osteoarthritis via Targeting miR-93/TGFBR2 Axis. Cartilage 2021, 13 (Suppl. S2), 1274S–1284S. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wu, G.; Ma, X.; Xiao, J.; Yu, G.; Yang, C.; Xu, N.; Zhang, B.; Zhou, J.; Ye, Z.; et al. Attenuation of TGFBR2 expression and tumour progression in prostate cancer involve diverse hypoxia-regulated pathways. J. Exp. Clin. Cancer Res. 2018, 37, 89. [Google Scholar] [CrossRef]
- Wang, Z.; Chi, X.; Liu, L.; Wang, Y.; Mei, X.; Yang, Y.; Jia, T. RETRACTED: Long noncoding RNA maternally expressed gene 3 knockdown alleviates lipopolysaccharide-induced inflammatory injury by up-regulation of miR-203 in ATDC5 cells. Biomed. Pharmacother. 2018, 100, 240–249. [Google Scholar] [CrossRef]
- Zhu, J.-K.; He, T.-D.; Wei, Z.-X.; Wang, Y.-M. LncRNA FAS-AS1 promotes the degradation of extracellular matrix of cartilage in osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2966–2972. [Google Scholar] [PubMed]
- Wang, Y.; Cao, L.; Wang, Q.; Huang, J.; Xu, S. LncRNA FOXD2-AS1 induces chondrocyte proliferation through sponging miR-27a-3p in osteoarthritis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1241–1247. [Google Scholar] [CrossRef]
- Cao, L.; Wang, Y.; Wang, Q.; Huang, J. LncRNA FOXD2-AS1 regulates chondrocyte proliferation in osteoarthritis by acting as a sponge of miR-206 to modulate CCND1 expression. Biomed. Pharmacother. 2018, 106, 1220–1226. [Google Scholar] [CrossRef]
- Malemud, C.J. MicroRNAs and Osteoarthritis. Cells 2018, 7, 92. [Google Scholar] [CrossRef]
- Al-Modawi, R.N.; Brinchmann, J.E.; Karlsen, T.A. Multi-pathway Protective Effects of MicroRNAs on Human Chondrocytes in an In Vitro Model of Osteoarthritis. Mol. Ther.—Nucleic Acids 2019, 17, 776–790. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Sun, Q.; Wang, Y.; Yang, J.; Yang, J.; Zhang, T.; Luo, S.; Wang, L.; Jiang, Y.; et al. Intra-articular Delivery of Antago-miR-483-5p Inhibits Osteoarthritis by Modulating Matrilin 3 and Tissue Inhibitor of Metalloproteinase 2. Mol. Ther. 2017, 25, 715–727. [Google Scholar] [CrossRef]
- Hu, G.; Zhao, X.; Wang, C.; Geng, Y.; Zhao, J.; Xu, J.; Zuo, B.; Zhao, C.; Wang, C.; Zhang, X. MicroRNA-145 attenuates TNF-alpha-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis. 2017, 8, e3140. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jia, J.; Liu, X.; Yang, S.; Ye, S.; Yang, W.; Zhang, Y. MicroRNA-16-5p Controls Development of Osteoarthritis by Targeting SMAD3 in Chondrocytes. Curr. Pharm. Des. 2015, 21, 5160–5167. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, J.; Yang, S.; Liu, X.; Ye, S.; Tian, H. MicroRNA-21 controls the development of osteoarthritis by targeting GDF-5 in chondrocytes. Exp. Mol. Med. 2014, 46, e79. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, J.; Dai, L.; Yu, D.; Chen, Q.; Zhang, X.; Dai, K. miR-146a, an IL-1β responsive miRNA, induces vascular endothelial growth factor and chondrocyte apoptosis by targeting Smad4. Arthritis Res. Ther. 2012, 14, R75. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Zhang, H.; Shao, Y.; Chen, Z.; Feng, X.; Fang, H.; Zhao, C.; Pan, J.; Zhang, H.; et al. MiR-146b accelerates osteoarthritis progression by targeting alpha-2-macroglobulin. Aging 2019, 11, 6014–6028. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Meng, R.; Li, H.; Li, J.; Jing, L.; Qin, L.; Gao, Y. miR-181a Modulates Chondrocyte Apoptosis by Targeting Glycerol-3-Phosphate Dehydrogenase 1-Like Protein (GPD1L) in Osteoarthritis. Med. Sci. Monit. 2017, 23, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Meng, D.; Li, G.; Xu, J.; Tian, K.; Li, Y. Overexpression of microRNA-210 promotes chondrocyte proliferation and extracellular matrix deposition by targeting HIF-3α in osteoarthritis. Mol. Med. Rep. 2016, 13, 2769–2776. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Watkins, G.; Le Good, N.; Roberts, S.; Murphy, C.; Brockbank, S.; Needham, M.; Read, S.; Newham, P. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-α and MMP13. Osteoarthr. Cartil. 2009, 17, 464–472. [Google Scholar] [CrossRef]
- Bazzoni, F.; Rossato, M.; Fabbri, M.; Gaudiosi, D.; Mirolo, M.; Mori, L.; Tamassia, N.; Mantovani, A.; Cassatella, M.A.; Locati, M. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc. Natl. Acad. Sci. USA 2009, 106, 5282–5287. [Google Scholar] [CrossRef]
- Park, S.J.; Cheon, E.J.; Kim, H.A. MicroRNA-558 regulates the expression of cyclooxygenase-2 and IL-1beta-induced catabolic effects in human articular chondrocytes. Osteoarthr. Cartil. 2013, 21, 981–989. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.-Y.; Chang, C.-F.; Lu, C.-C.; Wu, S.-C.; Huang, B.; Cheng, T.-L.; Lin, S.-Y.; Ho, C.-J.; Lee, M.-J.; Yang, C.-D.; et al. The Role of Mitochondrial Metabolism, AMPK-SIRT Mediated Pathway, LncRNA and MicroRNA in Osteoarthritis. Biomedicines 2022, 10, 1477. https://doi.org/10.3390/biomedicines10071477
Liu H-Y, Chang C-F, Lu C-C, Wu S-C, Huang B, Cheng T-L, Lin S-Y, Ho C-J, Lee M-J, Yang C-D, et al. The Role of Mitochondrial Metabolism, AMPK-SIRT Mediated Pathway, LncRNA and MicroRNA in Osteoarthritis. Biomedicines. 2022; 10(7):1477. https://doi.org/10.3390/biomedicines10071477
Chicago/Turabian StyleLiu, Hao-Yu, Chi-Fen Chang, Cheng-Chang Lu, Shun-Cheng Wu, Bin Huang, Tsung-Lin Cheng, Sung-Yen Lin, Cheng-Jung Ho, Mon-Juan Lee, Chung-Da Yang, and et al. 2022. "The Role of Mitochondrial Metabolism, AMPK-SIRT Mediated Pathway, LncRNA and MicroRNA in Osteoarthritis" Biomedicines 10, no. 7: 1477. https://doi.org/10.3390/biomedicines10071477
APA StyleLiu, H.-Y., Chang, C.-F., Lu, C.-C., Wu, S.-C., Huang, B., Cheng, T.-L., Lin, S.-Y., Ho, C.-J., Lee, M.-J., Yang, C.-D., Wang, Y.-C., Li, J.-Y., Liu, P.-C., Wei, C.-W., Kang, L., & Chen, C.-H. (2022). The Role of Mitochondrial Metabolism, AMPK-SIRT Mediated Pathway, LncRNA and MicroRNA in Osteoarthritis. Biomedicines, 10(7), 1477. https://doi.org/10.3390/biomedicines10071477









