Abstract
The inoculum effect (IE) is a well-known phenomenon with beta-lactams. At the same time, the IE has not been extensively studied with carbapenem/carbapenemase inhibitor combinations. The antibiotic-to-inhibitor concentration ratio used in susceptibility testing can influence the in vitro activity of the combination. To explore the role of these factors, imipenem/relebactam and doripenem/relebactam MICs were estimated against six Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae strains at standard inocula (SI) and high inocula (HI) by two methods: with a fixed relebactam concentration and with a fixed, pharmacokinetic-based carbapenem-to-relebactam concentration ratio. The combination MICs at HI, compared to SI, increased with most of the tested strains. However, the IE occurred with only two K. pneumoniae strains regardless of the MIC testing method. The relationship between the MICs at SI and the respective inoculum-induced MIC changes was observed when the MICs were estimated at pharmacokinetic-based carbapenem-to-relebactam concentration ratios. Thus, (1) IE was observed with both carbapenem/relebactam combinations regardless of the MIC testing method; however, IE was not observed frequently among tested K. pneumoniae strains. (2) At HI, carbapenem/relebactam combination MICs increased to levels associated with carbapenem resistance. (3) Combination MICs determined at pharmacokinetic-based carbapenem-to-inhibitor concentration ratios predict susceptibility elevations at HI in KPC-producing K. pneumoniae.
1. Introduction
Beta-lactam/beta-lactamase inhibitor combinations are widely used to treat infections caused by Gram-negative bacteria that produce beta-lactamases. Imipenem/relebactam is a recently approved combination effective against carbapenem-resistant Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae [1,2,3]. As was shown in numerous in vitro studies, relebactam effectively restores the antibacterial activity of imipenem against carbapenem-resistant bacteria [4,5,6]. However, it is not yet clear whether the antibacterial activity of imipenem/relebactam is maintained at high bacterial inocula (HI, is about 107–108 CFU/mL), i.e., significantly higher than that used in standard in vitro susceptibility (MIC) testing experiments (SI, 5 × 105 CFU/mL), or whether an inoculum effect (IE) occurs. The IE describes a significant decrease in antibacterial activity of antibiotics at high bacterial inocula and is a well-known phenomenon for beta-lactams [7,8,9]. In Gram-negative bacteria, the prevalent mechanism of resistance that mediates the IE is the production of beta-lactamase enzymes. Knowledge about IE could be crucial in treating high-burden bacterial infections as lowered antibacterial activity may be responsible for unexpected antibiotic treatment failures. For example, treatment failure in patients with staphylococcal bacteremia has been reported with cefazolin due to the IE [10,11]. In another in vivo study with piperacillin/tazobactam, this combination was prone to the IE [12]. Of note, the IE has not been extensively studied with carbapenem/carbapenemase inhibitor combinations, particularly with imipenem/relebactam. In this respect, the combination of doripenem, another carbapenem antibiotic, with relebactam is also of interest.
Along with IE, another factor that can influence the in vitro activity of beta-lactam/beta-lactamase inhibitor combinations is the concentration ratio of drugs used in susceptibility testing experiments. Antibacterial activity of imipenem/relebactam is routinely determined by varying imipenem concentrations in the presence of a fixed relebactam concentration [13]. However, this traditional approach to MIC determinations for antibiotic/inhibitor combinations leads to arbitrary antibiotic-to-inhibitor concentration ratios that do not always correspond to those achieved in humans. Our group recently proposed a pharmacokinetic-based (PK-based) approach to determining MICs of antibiotic/inhibitor combinations [14]. The antibacterial effects of imipenem/relebactam and doripenem/relebactam combinations in time-kill experiments were accurately predicted by MICs determined using PK-based antibiotic-to-inhibitor concentration ratios. In addition, it was confirmed that the PK-based approach could predict the efficacy of antibiotic/antibiotic combinations in series of pharmacodynamic experiments using an in vitro dynamic model with Gram-positive [15,16,17] and Gram-negative [18] bacteria.
To explore if the IE occurs and if the carbapenem-to-inhibitor concentration ratio influences the in vitro activity of antibiotic/inhibitor combinations, we evaluated MICs of imipenem and doripenem used alone or in combination with relebactam against KPC-producing K. pneumoniae strains at standard and high-density inocula by the traditional method at a fixed relebactam concentration and using a pharmacokinetic-based carbapenem-to-inhibitor concentration ratio.
2. Materials and Methods
2.1. Antimicrobial Agents and Bacterial Strains
Imipenem monohydrate and doripenem hydrate powders were purchased from Acros Organics (Fair Lawn, NJ, USA). Relebactam was purchased from Invivochem (Libertyville, IL, USA). Six blaKPC-positive, by PCR, non-mucoid K. pneumoniae strains with different susceptibility to imipenem and doripenem were used in the study: two clinical isolates, K. pneumoniae 14 and 16, and four ATCC K. pneumoniae strains (KPC reference strains), BAA-1705, BAA-1902, BAA-1904, and BAA-1905. K. pneumoniae ATCC 700,603 was used as a negative control. Before each test, carbapenemase production was confirmed for each bacterial strain by a modified carbapenem-inactivation method [19].
2.2. Susceptibility Testing
Susceptibility testing for antibiotics and inhibitor used alone or in combination was performed using broth microdilution techniques with standard inocula of approximately 5 × 105 CFU/mL (SI) and at high inocula of 5 × 107 CFU/mL (HI). When used alone, MICs at SI were determined according to standard CLSI recommendations [13]. When the MICs (for single carbapenems and their combination with relebactam) were determined at HI, bacterial growth was quantified by optical density at 600 nm (OD). ODs were estimated before and after 18 h of incubation at 37 °C. The MIC was the dilution at which the 18 h OD was equal to or less than that at time 0. An inoculum effect was defined as an eight-fold or greater increase in MIC when tested with the HI relative to SI. For carbapenem/relebactam combinations, MIC testing was performed under two different conditions, as determined by method 1 or method 2 regarding the ratio of imipenem or doripenem to relebactam. Before reading, plates were incubated at 37 °C for 18 h. MIC values were obtained at least in triplicate, and the modal MICs were estimated.
Method 1 (standard, MIC1): MIC testing for imipenem/relebactam used a fixed relebactam concentration of 4 mg/L with doubling dilutions of imipenem according to CLSI recommendations [13]. With doripenem/relebactam, the susceptibility testing recommendations are absent, so the MIC testing procedure was the same as with imipenem/relebactam.
Method 2 (PK-based, MIC2): MIC testing for imipenem/relebactam and doripenem/relebactam combinations used a fixed PK-based carbapenem-to-relebactam concentration ratio of 1.5/1 by varying the carbapenem and relebactam concentrations in parallel for each subsequent dilution. This concentration ratio is equal to the therapeutic 24 h area under the concentration–time curve (AUC) ratio of imipenem or doripenem (for a 500 mg dose of each carbapenem every 6 h [20,21]) to the therapeutic AUC of relebactam (for a 250 mg dose every 6 h [21]). The PK-based ratio was equal for imipenem/relebactam and doripenem/relebactam combinations as both carbapenems are characterized by similar pharmacokinetic profiles.
The MIC breakpoints for imipenem and imipenem/relebactam susceptibility testing were used according to CLSI recommendations [22]. For the doripenem/relebactam combination, MICs were interpreted using CLSI breakpoints for doripenem. The carbapenem MIC breakpoints for HI were the same as for SI. In all cases, the interpretive criteria for susceptibility were as follows: susceptible, ≤1 mg/L; intermediate, 2 mg/L; resistant, ≥4 mg/L.
3. Results
3.1. Susceptibility Testing with Single Imipenem and Doripenem
Imipenem and doripenem MICs for carbapenemase-producing K. pneumoniae strains varied from 4 to 64 mg/L and from 4 to 128 mg/L, respectively, when estimated at SI (Table 1). At HI, MICs of both antibiotics were higher, and decreased susceptibility was more pronounced with imipenem (4- to 64-fold) than with doripenem (2- to 16-fold). The IE was observed in four of six K. pneumoniae strains exposed to imipenem and in three of six strains exposed to doripenem.

Table 1.
MICs (mg/L) of imipenem and doripenem at standard inoculum (SI) and high inoculum (HI) density against K. pneumoniae.
It is worth noting that 8- to 64-fold increases in carbapenem MICs at HI were observed against K. pneumoniae strains that were initially more susceptible to imipenem and doripenem (MICs at SI of 8 and 16 mg/L and of 4 and 8 mg/L, respectively). Less pronounced density-related MIC elevations (4-fold with imipenem and 2-fold or no increase with doripenem) occurred with two strains that were highly resistant to both carbapenems (MICs at SI of 64 or 128 mg/L).
3.2. Susceptibility Testing with Imipenem and Doripenem at Fixed Relebactam Concentration (Method 1)
At SI, all tested K. pneumoniae strains were susceptible to both carbapenems when relebactam was added, and respective MIC1s were 0.25 to 0.5 mg/L for imipenem and 0.06 to 1 mg/L for doripenem (Table 2).

Table 2.
MICs (mg/L) of imipenem and doripenem in combination with relebactam at standard inoculum (SI) and high inoculum (HI) density against K. pneumoniae.
At HI, the imipenem MICs against K. pneumoniae in the presence of relebactam increased 4- to 8-fold (MICs of 1–4 mg/L); as a result, for two K. pneumoniae strains (BAA-1904 and BAA-1905), the IE was observed. Similar results were obtained with doripenem: MICs increased 2- to 8-fold (MICs of 0.5–2 mg/L) and the IE was observed for K. pneumoniae strains BAA-1705 and BAA-1905. With an increase in MIC1s at HI, which was observed for all K. pneumoniae strains, two of six strains became imipenem-intermediate (16, BAA-1705 and BAA-1902; MIC of 2 mg/L) and one became imipenem-resistant (BAA-1904; MIC of 4 mg/L). The patterns of K. pneumoniae carbapenem susceptibility at HI in the presence of relebactam at fixed concentration placed alongside CLSI MIC breakpoints are shown in Figure 1a,b. With doripenem, two of six K. pneumoniae strains at HI had a MIC1 of 2 mg/L (16 and BAA-1902); therefore, intermediate carbapenem resistance was defined.
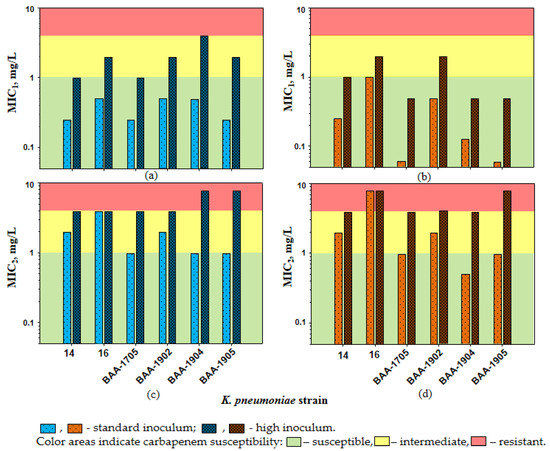
Figure 1.
The susceptibility of K. pneumoniae to imipenem (a,c) and doripenem (b,d) in the presence of relebactam. The interpretive criteria for susceptibility were as follows: susceptible, ≤1 mg/L; intermediate, 2 mg/L; resistant, ≥4 mg/L.
3.3. Susceptibility Testing at PK-Based Carbapenem-to-Relebactam Concentration Ratio (Method 2)
At SI, MIC2s were higher than MIC1s for all K. pneumoniae strains: 1–4 versus 0.25–0.5 mg/L with imipenem and 0.5–8 versus 0.06–1 mg/L with doripenem (Table 2). As seen in the Table, when exposed to imipenem or doripenem combined with relebactam, three of six K. pneumoniae strains had MIC2 values that were classified as carbapenem-intermediate (MIC of 2 mg/L for 14 and BAA-1902) or resistant (MIC of 4 and 8 mg/L for 16).
At HI, density-related MIC2 elevations led to even lower K. pneumoniae carbapenem susceptibility, and all strains became imipenem- or doripenem-intermediate or resistant (Figure 1c,d and Table 2). Similar to MIC1s, MIC2s at SI and HI reached an 8-fold difference and the IE was detected for two K. pneumoniae strains (BAA-1904 and BAA-1905) with both carbapenems.
4. Discussion
In the current study, carbapenemase-producing K. pneumoniae strains were exposed to imipenem and doripenem at HI (5 × 107 CFU/mL) and the IE was observed against strains with initial carbapenem MICs of 4 to 16 mg/L. MIC elevations were 8- to 64-fold. Carbapenem susceptibility of K. pneumoniae strains highly resistant to imipenem and doripenem (MICs 64 and 128 mg/L at SI) also decreased at HI, but the IE was not observed. It is possible that the carbapenemase enzymes may be potentiated more at increased inocula in strains with lower MICs than in highly resistant strains.
Assuming these patterns of carbapenem susceptibility at HI, we examined whether there is any relationship between the bacterial susceptibility at SI and the exhibited elevations in MIC at high inocula. Imipenem and doripenem MICs against K. pneumoniae strains at SI were compared with the respective inoculum-related MIC changes and were expressed as the ratio of antibiotic MIC at HI to MIC at SI—MICHI/MICSI. As seen in Figure 2, there was a clear relationship: the lower the imipenem and doripenem MICs at SI, the greater the respective MIC elevations at HI. In general, imipenem alone was more prone to the IE than doripenem, as imipenem MIC elevations associated with the IE were always higher. Similar imipenem inoculum-related MIC increases (32-fold and higher) for KPC-producing K. pneumoniae strains were described in several in vitro studies [23,24].
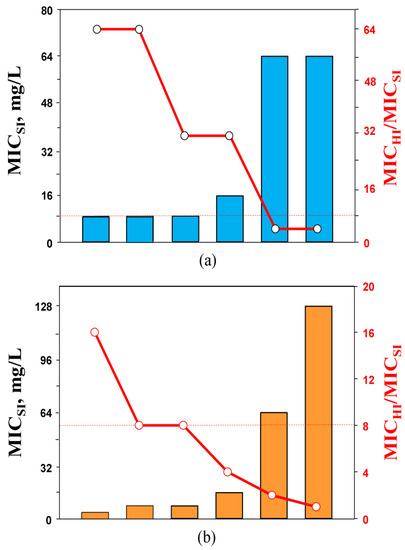
Figure 2.
The MICs of imipenem (a) and doripenem (b) against K. pneumoniae at standard inoculum density (bars) and respective changes in the susceptibility at high inoculum density (circles). The dashed lines indicate the lower level of MIC changes associated with the inoculum effect (IE, ≥8-fold).
Carbapenem MICs decreased significantly in the presence of relebactam both at SI and HI. At the same time, bacterial susceptibility to antibiotic/inhibitor combinations differed between these inocula. At HI, the 2- to 8-fold MIC elevations were detected compared to respective combination MICs at SI. However, IE was observed for only two K. pneumoniae strains regardless of the combination MIC testing method. In previous studies, diminished activity at high inocula was reported for piperacillin/tazobactam [25,26,27,28], amoxicillin/clavulanate [26], ampicillin/sulbactam and ticarcillin/clavulanate [25], ceftazidime/avibactam [29,30], aztreonam/avibactam [30], and several cephalosporin/clavulanate combinations [31] against Enterobacteriaceae, including K. pneumoniae. Piperacillin/tazobactam, ticarcillin/clavulanate, and aztreonam/avibactam combinations were prone to the inoculum effect, while for with other antibiotic/inhibitor combinations, the IE was not registered at all (ceftazidime/avibactam) or was observed only for a minority of tested isolates. Our data with imipenem/relebactam and doripenem/relebactam combinations demonstrated similar patterns.
Although in the current study the IE was observed only in some tested K. pneumoniae strains, density-related combination MIC elevations (2- to 8-fold) were found in most of them. Thus, at high inocula, carbapenem MICs in the presence of relebactam (especially when estimated at the pharmacokinetic-based concentration ratio) increased to levels associated with carbapenem resistance (according to the CLSI breakpoints [22]). Hence, even slightly diminished activity of carbapenem/relebactam combinations at high inocula (i.e., less than 8-fold) should be considered a risk factor for decreased treatment efficacy of high-burden bacterial infections and possible treatment failure.
It would be interesting to know if the decrease in carbapenem susceptibility in the presence of relebactam at increased inocula related to MICs at SI, similar to that observed with single imipenem and doripenem, could be found. To answer this question, the combination MICs at SI against K. pneumoniae strains determined by two methods were compared with the respective inoculum-related MIC changes (expressed as ratio of MIC1 or MIC2 at HI to MIC1 or MIC2 at SI—MIC1,HI/MIC1,SI or MIC2,HI/MIC2,SI, respectively). As seen in Figure 3a, consistency between the carbapenem/inhibitor MIC1s at SI and the MIC1,HI/MIC1,SI ratio was not observed. Similar observations were made in other in vitro studies with Gram-negative bacteria, and several combinations of cephalosporin/clavulanate [31] and piperacillin/tazobactam [26] (all had a fixed inhibitor concentration) were analyzed. There was no relationship between the density-related MIC elevations and the susceptibility of K. pneumoniae or Escherichia coli to tested beta-lactam/beta-lactamase inhibitor combinations at SI.
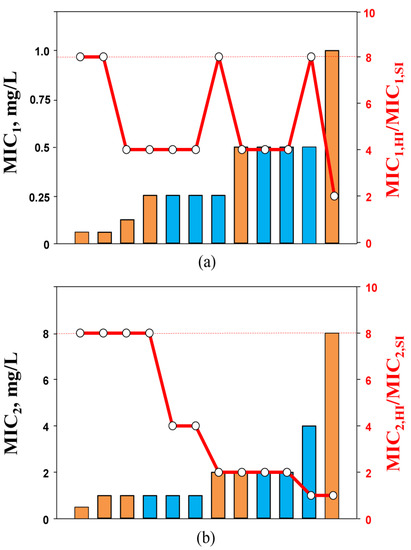
Figure 3.
MIC1 (a) and MIC2 (b) values of imipenem (blue bars) and doripenem (orange bars) in the presence of relebactam against K. pneumoniae at standard inoculum density and changes in the susceptibility at high inoculum density (circles). The dashed lines indicate the lower level of MIC changes associated with the inoculum effect (IE, ≥8-fold).
However, a relation was found between MIC2s (at SI) and the respective MIC2,HI/MIC2,SI ratio (Figure 3b): the higher the carbapenem/relebactam MIC2 at SI, the less likely that density-related MIC2 changes would be observed. With K. pneumoniae strains for which MIC2s at SI were highest (4 or 8 mg/L), no diminished activity of the combination was detected at HI. Therefore, with imipenem/relebactam and doripenem/relebactam combinations, similar susceptibility patterns at HI were observed as with imipenem and doripenem alone. This could likely be explained by the inoculum-potentiated beta-lactamases in low-MIC strains that are not blocked by the same inhibitor concentration at SI. It is possible that alternative resistance mechanisms could contribute to resistance to imipenem and doripenem combinations with relebactam at HI. For example, in another in vitro study with KPC-producing K. pneumoniae that exhibited a carbapenem-susceptibility phenotype, rapid inoculum-induced high-level imipenem resistance was mediated by the essential coordination between blaKPC and OmpK36 expression [23]. Similar resistance mechanisms with coordination of blaKPC and mutations affecting the genes encoding porins were found in KPC-producing K. pneumoniae isolates with decreased susceptibility to imipenem/relebactam combinations (MIC of 2 mg/L or higher). Moreover, the same mechanisms of K. pneumoniae resistance to combinations of imipenem with relebactam were also described in vivo. It was reported that the K. pneumoniae clinical isolate initially susceptible to imipenem/relebactam acquired resistance to this combination during antimicrobial therapy of a hematology patient with a bloodstream infection [32]. The resistance mechanism of this K. pneumoniae isolate was associated with an increased blaKPC-3 copy number and disruptions of porins (OmpK35 and OmpK36).
The relation between the MIC2 at SI and the MIC2,HI/MIC2,SI ratio indicates that the use of a PK-based approach to carbapenem/inhibitor combination MIC determination predicts MIC elevations at HI and the probability of the IE in carbapenemase-producing K. pneumoniae.
In our previous study [14], the predictive potential of MIC testing at PK-based carbapenem-to-relebactam concentration ratios regarding the antibacterial effectiveness of imipenem/relebactam and doripenem/relebactam combinations against KPC-producing K. pneumoniae was demonstrated. In contrast to the standard approach to MIC determination using a fixed relebactam concentration, the PK-based approach allowed a significant correlation between the C/MIC parameter (the ratio of carbapenem concentration in the time-kill experiments and the culture MIC) and the antibacterial effect. These data suggest that MIC determinations at PK-based carbapenem-to-relebactam concentration ratios might be better in vitro predictors of antibacterial effects than MICs determined at a fixed concentration of relebactam, i.e., at an arbitrary antibiotic/inhibitor ratio. We believe that the PK-based approach could be a reliable tool for susceptibility testing of carbapenemase-producing K. pneumoniae strains at both SI and HI. Pharmacodynamic experiments with imipenem/relebactam and doripenem/relebactam combinations at SI and HI in in vitro dynamic models are needed to further validate the PK-based approach to determine carbapenem/relebactam MIC and IE determinations with different bacterial inocula.
Our study has several limitations. It did not include many K. pneumoniae strains, and additional studies with other K. pneumoniae isolates and with other Gram-negative bacteria are necessary to fully evaluate the IE using standard methods and the PK-based approach. Moreover, we did not determine if the K. pneumoniae strains used in the study are biofilm producers. Previously, it was reported that this bacterial feature could significantly influence the antibiotic susceptibility and treatment outcome [33]. This limits the potential clinical relevance of our findings. In addition, the subsequent studies with a wide range of antibiotic/inhibitor combinations would enhance the generalizability of our results. We did not investigate the resistance mechanisms exhibited by K. pneumoniae strains at increased inocula to confirm our assumptions about prevalent mechanisms of imipenem/relebactam and doripenem/relebactam resistance at HI.
5. Conclusions
In the current study, we evaluated the IE with carbapenem/carbapenemase inhibitor combinations. Previously, the IE was shown to appear with beta-lactams and beta-lactam/beta-lactamase combinations. According to our findings, the IE was observed with both carbapenem/relebactam combinations regardless of the MIC testing method; however, IE was not prevalent among the tested KPC-producing K. pneumoniae strains. It seems promising that relebactam can decrease inoculum-related susceptibility reductions and minimize the impact of the IE. At the same time, although imipenem/relebactam and doripenem/relebactam combinations were less prone to the IE compared to single antibiotics, at the HI, carbapenem MICs in the presence of relebactam increased to levels associated with carbapenem resistance (especially with the PK-based concentration ratio). In the current study, we also reported that the use of a PK-based approach to carbapenem/inhibitor combination MIC determinations allows the prediction of MIC elevations at HI and the probability of the IE in carbapenemase-producing K. pneumoniae strains. Accordingly, it can be hypothesized that using the PK-based approach could allow a more realistic assessment of carbapenem susceptibility in KPC-producing K. pneumoniae strains and might be a helpful option to evaluate treatment failures due to IE with carbapenem/carbapenemase inhibitor combinations.
Author Contributions
Conceptualization, M.V.G., V.A.A., S.V.S. and S.H.Z.; methodology, M.V.G., K.N.A. and S.H.Z.; software, A.V.F.; validation, A.V.F., V.A.A., O.S.S. and A.A.A.; formal analysis, A.V.F., K.N.A., O.S.S. and A.A.A.; investigation, A.V.F., O.S.S. and A.A.A.; resources, V.A.A.; data curation, K.N.A., A.V.F. and A.A.A.; writing—original draft preparation, M.V.G., V.A.A., S.V.S. and S.H.Z.; writing—review and editing, K.N.A. and A.V.F.; visualization, A.V.F.; supervision, M.V.G. and V.A.A.; project administration, M.V.G., V.A.A. and S.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Russian Science Foundation (grant number 21-74-10090).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andrei, S.; Droc, G.; Stefan, G. FDA approved antibacterial drugs: 2018–2019. Discoveries 2019, 31, e102. [Google Scholar] [CrossRef]
- Thakare, R.; Dasgupta, A.; Chopra, S. Imipenem/cilastatin sodium/relebactam fixed combination to treat urinary infections and complicated intra-abdominal bacterial infections. Drugs Today 2020, 56, 241–255. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-antibiotic-treat-hospital-acquired-bacterial-pneumonia-and-ventilator-associated (accessed on 21 April 2022).
- Galani, I.; Souli, M.; Nafplioti, K.; Adamou, P.; Karaiskos, I.; Giamarellou, H.; Antoniadou, A. Study Collaborators. In vitro activity of imipenem-relebactam against non-MBL carbapenemase-producing Klebsiella pneumoniae isolated in Greek hospitals in 2015–2016. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1143–1150. [Google Scholar] [CrossRef]
- Kurihara, D.; Matsumoto, S.; Kishi, N.; Ishii, Y.; Mori, M. In vitro antibacterial activity of imipenem/relebactam against clinical isolates in Japan. Microbiol. Spectr. 2022, 13, e0223521. [Google Scholar] [CrossRef]
- Lob, S.; Hackel, M.; Chen, W.-T.; Khoo, Y.; Balwani, K.; Young, K.; Motyl, M.; Sahm, D.F. In vitro activity of imipenem/relebactam against Klebsiella pneumoniae and Pseudomonas aeruginosa from patients in icus in the Asia/Pacific region—SMART 2015–2018. Open Forum Infect. Dis. 2020, 7 (Suppl. S1), S789. [Google Scholar] [CrossRef]
- Lenhard, J.R.; Bulman, Z.P. Inoculum effect of β-lactam antibiotics. J. Antimicrob. Chemother. 2019, 74, 2825–2843. [Google Scholar] [CrossRef]
- Ogawa, Y.; Nakano, R.; Kasahara, K.; Mizuno, T.; Hirai, N.; Nakano, A.; Suzuki, Y.; Kakuta, N.; Masui, T.; Yano, H.; et al. Comparison of the inoculum size effects of antibiotics on IMP-6 β-lactamase-producing Enterobacteriaceae co-harboring plasmid-mediated quinolone resistance genes. PLoS ONE 2019, 14, e0225210. [Google Scholar] [CrossRef]
- Udekwu, K.I.; Parrish, N.; Ankomah, P.; Baquero, F.; Levin, B.R. Functional relationship between bacterial cell density and the efficacy of antibiotics. J. Antimicrob. Chemother. 2009, 63, 745–757. [Google Scholar] [CrossRef]
- Smelter, D.; McCrone, S.; Rose, W. Cefazolin inoculum effect predicts reduced susceptibility to other antibiotics and patient outcomes in MSSA endovascular infections. Open Forum Infect. Dis. 2020, 7 (Suppl. S1), S617. [Google Scholar] [CrossRef]
- Miller, W.R.; Seas, C.; Carvajal, L.P.; Diaz, L.; Echeverri, A.M.; Ferro, C.; Rios, R.; Porras, P.; Luna, C.; Gotuzzo, E.; et al. The cefazolin inoculum effect is associated with increased mortality in methicillin-susceptible Staphylococcus aureus bacteremia. Open Forum Infect. Dis. 2018, 5, ofy123. [Google Scholar] [CrossRef]
- Harada, Y.; Morinaga, Y.; Kaku, N.; Nakamura, S.; Uno, N.; Hasegawa, H.; Izumikawa, K.; Kohno, S.; Yanagihara, K. In vitro and in vivo activities of piperacillin-tazobactam and meropenem at different inoculum sizes of ESBL-producing Klebsiella pneumoniae. Clin. Microbiol. Infect. 2014, 20, O831–O839. [Google Scholar] [CrossRef] [PubMed]
- Clinical Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- Filimonova, A.V.; Golikova, M.V.; Strukova, E.N.; Portnoy, Y.A.; Kuznetsova, A.A.; Zinner, S.H. Predicting the effects of carbapenem/carbapenemase inhibitor combinations against KPC-producing Klebsiella pneumoniae in time-kill experiments: Alternative versus traditional approaches to MIC determination. Antibiotics 2021, 10, 1520. [Google Scholar] [CrossRef] [PubMed]
- Golikova, M.V.; Strukova, E.N.; Portnoy, Y.A.; Dovzhenko, S.A.; Kobrin, M.B.; Zinner, S.H.; Firsov, A.A. Predicting effects of antibiotic combinations using MICs determined at pharmacokinetically derived concentration ratios: In vitro model studies with linezolid- and rifampicin-exposed Staphylococcus aureus. J. Chemother. 2017, 5, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Golikova, M.V.; Strukova, E.N.; Portnoy, Y.A.; Zinner, S.H.; Firsov, A.A. Predicting the antistaphylococcal effects of daptomycin-rifampicin combinations in an in vitro dynamic model. J. Antibiot. 2020, 73, 101–107. [Google Scholar] [CrossRef]
- Golikova, M.V.; Strukova, E.N.; Portnoy, Y.A.; Zinner, S.H.; Firsov, A.A. Verification of a novel approach to predicting effects of antibiotic combinations: In vitro dynamic model study with daptomycin and gentamicin against Staphylococcus aureus. Antibiotics 2020, 9, 538. [Google Scholar] [CrossRef]
- Golikova, M.V.; Strukova, E.N.; Alieva, K.N.; Portnoy, Y.A.; Filimonova, A.V.; Zinner, S.H.; Firsov, A.A. A pharmacokinetically-based approach to predict anti-mutant efficacy of combined doripenem and levofloxacin therapy in in vitro model studies with Pseudomonas aeruginosa. In Proceedings of the 31th European Congress of Clinical Microbiology & Infectious Diseases, Vienna, Austria, 9–12 July 2021; p. #833. [Google Scholar]
- Van der Zwaluw, K.; de Haan, A.; Pluister, G.N.; Bootsma, H.J.; de Neeling, A.J.; Schouls, L.M. The carbapenem inactivation method (CIM), a simple and low-cost alternative for the Carba NP test to assess phenotypic carbapenemase activity in gram-negative rods. PLoS ONE 2015, 10, e0123690. [Google Scholar] [CrossRef]
- Cirillo, I.; Vaccaro, N.; Turner, K.; Solanki, B.; Natarajan, J.; Redman, R. Pharmacokinetics, safety, and tolerability of doripenem after 0.5-, 1-, and 4-hour infusions in healthy volunteers. J. Clin. Pharmacol. 2009, 49, 798–806. [Google Scholar] [CrossRef]
- Rhee, E.G.; Rizk, M.L.; Calder, N.; Nefliu, M.; Warrington, S.J.; Schwartz, M.S.; Mangin, E.; Boundy, K.; Bhagunde, P.; Colon-Gonzalez, F.; et al. Pharmacokinetics, safety, and tolerability of single and multiple doses of relebactam, a β-lactamase inhibitor, in combination with imipenem and cilastatin in healthy participants. Antimicrob. Agents Chemother. 2018, 62, e00280-18. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Adams-Sapper, S.; Nolen, S.; Donzelli, G.F.; Lal, M.; Chen, K.; Justo da Silva, L.H.; Moreira, B.M.; Riley, L.W. Rapid induction of high-level carbapenem resistance in heteroresistant KPC-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2015, 59, 3281–3289. [Google Scholar] [CrossRef]
- Roth, A.L.; Thomson, K.S.; Lister, P.D.; Hanson, N.D. Production of KPC-2 alone does not always result in β-lactam MICs representing resistance in gram-negative pathogens. J. Clin. Microbiol. 2012, 50, 4183–4184. [Google Scholar] [CrossRef]
- Goldstein, E.J.; Citron, D.M.; Cherubin, C.E. Comparison of the inoculum effects of members of the family Enterobacteriaceae on cefoxitin and other cephalosporins, beta-lactamase inhibitor combinations, and the penicillin-derived components of these combinations. Antimicrob. Agents Chemother. 1991, 35, 560–566. [Google Scholar] [CrossRef] [PubMed][Green Version]
- López-Cerero, L.; Picón, E.; Morillo, C.; Hernández, J.R.; Docobo, F.; Pachón, J.; Rodríguez-Baño, J.; Pascual, A. Comparative assessment of inoculum effects on the antimicrobial activity of amoxycillin-clavulanate and piperacillin-tazobactam with extended-spectrum beta-lactamase-producing and extended-spectrum beta-lactamase-non-producing Escherichia coli isolates. Clin. Microbiol. Infect. 2010, 16, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Thomson, K.S.; Moland, E.S. Cefepime, piperacillin-tazobactam, and the inoculum effect in tests with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2001, 45, 3548–3554. [Google Scholar] [CrossRef]
- Wu, N.; Chen, B.Y.; Tian, S.F.; Chu, Y.Z. The inoculum effect of antibiotics against CTX-M-extended-spectrum β-lactamase-producing Escherichia coli. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.P.; Kirby, J.E. The inoculum effect in the era of multidrug resistance: Minor differences in inoculum have dramatic effect on mic determination. Antimicrob. Agents Chemother. 2018, 62, e00433-18. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, T.; Park, J.H.; Bae, S.; Sung, H.; Kim, M.-N.; Jung, J.; Kim, M.J.; Kim, S.-H.; Lee, S.-O.; et al. In vitro activities of ceftazidime–avibactam and aztreonam–avibactam at different inoculum sizes of extended-spectrum β-lactam-resistant Enterobacterales blood isolates. Antibiotics 2021, 10, 1492. [Google Scholar] [CrossRef]
- Queenan, A.M.; Foleno, B.; Gownley, C.; Wira, E.; Bush, K. Effects of inoculum and beta-lactamase activity in AmpC- and extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae clinical isolates tested by using NCCLS ESBL methodology. J. Clin. Microbiol. 2004, 42, 269–275. [Google Scholar] [CrossRef]
- Gaibani, P.; Bussini, L.; Amadesi, S.; Bartoletti, M.; Bovo, F.; Lazzarotto, T.; Viale, P.; Ambretti, S. Successful treatment of bloodstream infection due to a KPC-producing Klebsiella pneumoniae resistant to imipenem/relebactam in a hematological patient. Microorganisms 2022, 10, 778. [Google Scholar] [CrossRef]
- Fasciana, T.; Ciammaruconi, A.; Gentile, B.; Di Carlo, P.; Virruso, R.; Tricoli, M.R.; Palma, D.M.; Pitarresi, G.L.; Lista, F.; Giammanco, A. Draft Genome Sequence and Biofilm Production of a Carbapenemase-Producing Klebsiella pneumoniae (KpR405) Sequence Type 405 Strain Isolated in Italy. Antibiotics 2021, 10, 560. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).