Why Does Psychotherapy Work and for Whom? Hormonal Answers
Abstract
:1. Introduction
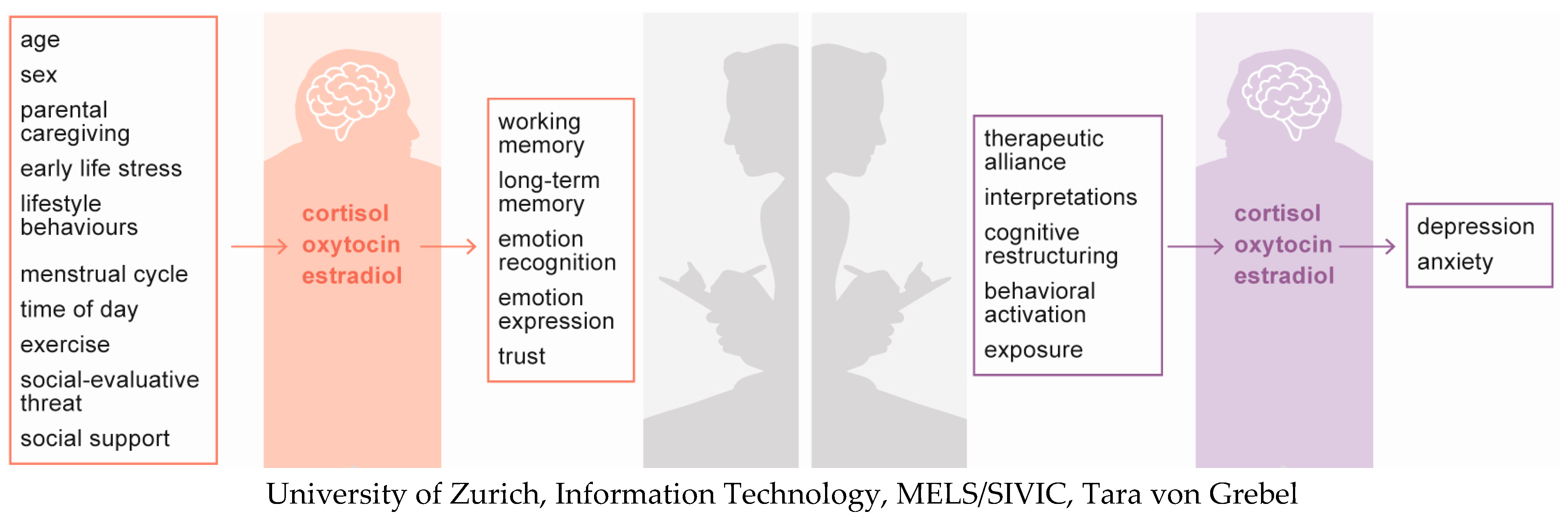
2. Cortisol
2.1. Main Determinants
2.2. Effects on Cognition, Emotion, and/or Behaviour
2.3. Role in Psychotherapy
2.3.1. Cortisol as Predictor of Psychotherapeutic Changes
- Treatment level
- Session level
- Interim summary
2.3.2. Cortisol as Indicator of Psychotherapeutic Changes
- Treatment level
- Session level
- Interim summary
3. Oxytocin
3.1. Main Determinants
3.2. Effects on Cognition, Emotions, and/or Behaviour
3.3. Role in Psychotherapy
3.3.1. Oxytocin as Predictor of Psychotherapeutic Changes
- Treatment level
- Session level
- Interim summary
3.3.2. Oxytocin as Indicator of Psychotherapeutic Changes
4. Oestradiol
4.1. Main Determinants
4.2. Effects on Cognition, Emotions, and/or Behaviour
4.3. Role in Psychotherapy
Oestradiol as Predictor of Psychotherapeutic Changes
- Treatment level
- Session level
- Interim summary
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Institute for Health and Care Excellence. Depression in Adults: Recognition and Management. Available online: https://www.nice.org.uk/guidance/CG90 (accessed on 5 June 2022).
- National Institute for Health and Care Excellence. Generalised Anxiety Disorder and Panic Disorder in Adults: Management. Available online: https://www.nice.org.uk/guidance/cg113 (accessed on 5 June 2022).
- National Institute for Health and Care Excellence. Social Anxiety Disorder: Recognition, Assessment and Treatment. Available online: https://www.nice.org.uk/guidance/cg159 (accessed on 5 June 2022).
- Cuijpers, P.; Karyotaki, E.; Weitz, E.; Andersson, G.; Hollon, S.D.; van Straten, A. The effects of psychotherapies for major depression in adults on remission, recovery and improvement: A meta-analysis. J. Affect. Disord. 2014, 159, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.K.; Andrews, L.A.; Witcraft, S.M.; Powers, M.B.; Smits, J.A.J.; Hofmann, S.G. Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depress. Anxiety 2018, 35, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Reijnders, M.; Huibers, M.J.H. The role of common factors in psychotherapy outcomes. Annu. Rev. Clin. Psychol. 2019, 15, 207–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, G.L. The need for a new medical model: A challenge for biomedicine. Science 1977, 196, 129–136. [Google Scholar] [CrossRef]
- Insel, T.; Cuthbert, B.; Garvey, M.; Heinssen, R.; Pine, D.S.; Quinn, K.; Sanislow, C.; Wang, P. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am. J. Psychiatry 2010, 167, 748–751. [Google Scholar] [CrossRef] [Green Version]
- Wolf, J.M.; Saucier, E. Psychoneuroendocrinology; Springer: New York, NY, USA, 2013. [Google Scholar]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Gaffey, A.E.; Bergeman, C.S.; Clark, L.A.; Wirth, M.M. Aging and the HPA axis: Stress and resilience in older adults. Neurosci. Biobehav. Rev. 2016, 68, 928–945. [Google Scholar] [CrossRef] [Green Version]
- Strahler, J.; Skoluda, N.; Kappert, M.B.; Nater, U.M. Simultaneous measurement of salivary cortisol and alpha-amylase: Application and recommendations. Neurosci. Biobehav. Rev. 2017, 83, 657–677. [Google Scholar] [CrossRef]
- Fogelman, N.; Canli, T. Early life stress and cortisol: A meta-analysis. Horm. Behav. 2018, 98, 63–76. [Google Scholar] [CrossRef]
- Miller, G.E.; Chen, E.; Zhou, E.S. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 2007, 133, 25–45. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.; Stalder, T.; Jarczok, M.; Almeida, D.M.; Badrick, E.; Bartels, M.; Boomsma, D.I.; Coe, C.L.; Dekker, M.C.; Donzella, B.; et al. The CIRCORT database: Reference ranges and seasonal changes in diurnal salivary cortisol derived from a meta-dataset comprised of 15 field studies. Psychoneuroendocrinology 2016, 73, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickerson, S.S.; Kemeny, M.E. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004, 130, 355–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoccola, P.M.; Dickerson, S.S. Assessing the relationship between rumination and cortisol: A review. J. Psychosom. Res. 2012, 73, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shields, G.S.; Bonner, J.C.; Moons, W.G. Does cortisol influence core executive functions? A meta-analysis of acute cortisol administration effects on working memory, inhibition, and set-shifting. Psychoneuroendocrinology 2015, 58, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Het, S.; Ramlow, G.; Wolf, O.T. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology 2005, 30, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Stetler, C.; Miller, G.E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef]
- O’Connor, D.B.; Ferguson, E.; Green, J.A.; O’Carroll, R.E.; O’Connor, R.C. Cortisol levels and suicidal behavior: A meta-analysis. Psychoneuroendocrinology 2016, 63, 370–379. [Google Scholar] [CrossRef]
- Elnazer, H.Y.; Baldwin, D.S. Investigation of cortisol levels in patients with anxiety disorders: A structured review. In Behavioral Neurobiology of Stress-Related Disorders; Springer: New York, NY, USA, 2014; Volume 18, pp. 191–216. [Google Scholar] [CrossRef]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders—Clinical Descriptions and Diagnostic Guidelines; World Health Organization: Geneva, Switzerland, 1992.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; APA: Washington, DC, USA, 2013. [Google Scholar]
- Fischer, S.; Strawbridge, R.; Vives, A.H.; Cleare, A.J. Cortisol as a predictor of psychological therapy response in depressive disorders: Systematic review and meta-analysis. Br. J. Psychiatry J. Ment. Sci. 2017, 210, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Fischer, S.; King, S.; Papadopoulos, A.; Hotopf, M.; Young, A.H.; Cleare, A.J. Hair cortisol and childhood trauma predict psychological therapy response in depression and anxiety disorders. Acta Psychiatr. Scand. 2018, 138, 526–535. [Google Scholar] [CrossRef] [Green Version]
- Fischer, S.; Cleare, A.J. Cortisol as a predictor of psychological therapy response in anxiety disorders-Systematic review and meta-analysis. J. Anxiety Disord. 2017, 47, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Masdrakis, V.G.; Legaki, E.M.; Papageorgiou, C.; Markianos, M. Stress hormones as predictors of response to cognitive behavior therapy in panic disorder. Neuropsychobiology 2021, 80, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, S.; Bornstein, S.R.; Lorenz, T.; Petrowski, K. Stress hormone response to the DEX-CRH test and its relation to psychotherapy outcome in panic disorder patients with and without agoraphobia. Transl. Psychiatry 2018, 8, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wichmann, S.; Kirschbaum, C.; Lorenz, T.; Petrowski, K. Effects of the cortisol stress response on the psychotherapy outcome of panic disorder patients. Psychoneuroendocrinology 2017, 77, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kuhlman, K.R.; Treanor, M.; Imbriano, G.; Craske, M.G. Endogenous in-session cortisol during exposure therapy predicts symptom improvement: Preliminary results from a scopolamine-augmentation trial. Psychoneuroendocrinology 2020, 116, 104657. [Google Scholar] [CrossRef] [PubMed]
- Meuret, A.E.; Rosenfield, D.; Bhaskara, L.; Auchus, R.; Liberzon, I.; Ritz, T.; Abelson, J.L. Timing matters: Endogenous cortisol mediates benefits from early-day psychotherapy. Psychoneuroendocrinology 2016, 74, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Soravia, L.M.; Heinrichs, M.; Aerni, A.; Maroni, C.; Schelling, G.; Ehlert, U.; Roozendaal, B.; de Quervain, D.J. Glucocorticoids reduce phobic fear in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 5585–5590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soravia, L.M.; Heinrichs, M.; Winzeler, L.; Fisler, M.; Schmitt, W.; Horn, H.; Dierks, T.; Strik, W.; Hofmann, S.G.; de Quervain, D.J. Glucocorticoids enhance in vivo exposure-based therapy of spider phobia. Depress. Anxiety 2014, 31, 429–435. [Google Scholar] [CrossRef] [PubMed]
- de Quervain, D.J.; Bentz, D.; Michael, T.; Bolt, O.C.; Wiederhold, B.K.; Margraf, J.; Wilhelm, F.H. Glucocorticoids enhance extinction-based psychotherapy. Proc. Natl. Acad. Sci. USA 2011, 108, 6621–6625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laufer, S.; Engel, S.; Knaevelsrud, C.; Schumacher, S. Cortisol and alpha-amylase assessment in psychotherapeutic intervention studies: A systematic review. Neurosci. Biobehav. Rev. 2018, 95, 235–262. [Google Scholar] [CrossRef] [PubMed]
- Field, T.; Deeds, O.; Diego, M.; Hernandez-Reif, M.; Gauler, A.; Sullivan, S.; Wilson, D.; Nearing, G. Benefits of combining massage therapy with group interpersonal psychotherapy in prenatally depressed women. J. Bodyw. Mov. Ther. 2009, 13, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Field, T.; Diego, M.; Delgado, J.; Medina, L. Peer support and interpersonal psychotherapy groups experienced decreased prenatal depression, anxiety and cortisol. Early Hum. Dev. 2013, 89, 621–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.; Lim, S.K.; Chung, E.J.; Woo, J.M. The effect of cognitive behavior therapy-based psychotherapy applied in a forest environment on physiological changes and remission of major depressive disorder. Psychiatry Investig. 2009, 6, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kundermann, B.; Strate, P.; Hemmeter-Spernal, J.; Huber, M.T.; Krieg, J.C.; Lautenbacher, S. Mid-term effects of serial sleep deprivation therapy implemented in cognitive-behavioral treatment on the neuroendocrine response to clomipramine in patients with major depression. J. Psychiatr. Res. 2009, 43, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Mcknight, D.L.; Nelsongray, R.O.; Barnhill, J. Dexamethasone Suppression Test and Response to Cognitive Therapy and Antidepressant Medication. Behav. Ther. 1992, 23, 99–111. [Google Scholar] [CrossRef]
- Taylor, C.B.; Conrad, A.; Wilhelm, F.H.; Strachowski, D.; Khaylis, A.; Neri, E.; Giese-Davis, J.; Roth, W.T.; Cooke, J.P.; Kraemer, H.; et al. Does improving mood in depressed patients alter factors that may affect cardiovascular disease risk? J. Psychiatr. Res. 2009, 43, 1246–1252. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, N.; Zhang, Y.L.; Zhang, J.; Yang, H.; Timothy, T.C. Comparison of the neurobiological effects of attribution retraining group therapy with those of selective serotonin reuptake inhibitors. Braz. J. Med. Biol. Res. 2013, 46, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Alpers, G.W.; Abelson, J.L.; Wilhelm, F.H.; Roth, W.T. Salivary cortisol response during exposure treatment in driving phobics. Psychosom. Med. 2003, 65, 679–687. [Google Scholar] [CrossRef]
- Faucher, J.; Koszycki, D.; Bradwejn, J.; Merali, Z.; Bielajew, C. Effects of CBT versus MBSR treatment on social stress reactions in social anxiety disorder. Mindfulness 2016, 7, 514–526. [Google Scholar] [CrossRef]
- Gaab, J.; Jucker, P.; Staub, F.; Ehlert, U. Mind over matter: Psychobiological effects of exposure therapy in arachnophobia. Z. Klin. Psychol. Psychother. 2005, 34, 121–132. [Google Scholar] [CrossRef]
- Rosnick, C.B.; Wetherell, J.L.; White, K.S.; Andreescu, C.; Dixon, D.; Lenze, E.J. Cognitive-behavioral therapy augmentation of SSRI reduces cortisol levels in older adults with generalized anxiety disorder: A randomized clinical trial. J. Consult. Clin. Psychol. 2016, 84, 345–352. [Google Scholar] [CrossRef]
- Shiban, Y.; Peperkorn, H.; Alpers, G.W.; Pauli, P.; Muhlberger, A. Influence of perceptual cues and conceptual information on the activation and reduction of claustrophobic fear. J. Behav. Ther. Exp. Psychiatry 2016, 51, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Tafet, G.E.; Feder, D.J.; Abulafia, D.P.; Roffman, S.S. Regulation of hypothalamic-pituitary-adrenal activity in response to cognitive therapy in patients with generalized anxiety disorder. Cogn. Affect. Behav. Neurosci. 2005, 5, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Plag, J.; Gaudlitz, K.; Schumacher, S.; Dimeo, F.; Bobbert, T.; Kirschbaum, C.; Strohle, A. Effect of combined cognitive-behavioural therapy and endurance training on cortisol and salivary alpha-amylase in panic disorder. J. Psychiatr. Res. 2014, 58, 12–19. [Google Scholar] [CrossRef]
- Schumacher, S.; Gaudlitz, K.; Plag, J.; Miller, R.; Kirschbaum, C.; Fehm, L.; Fydrich, T.; Strohle, A. Who is stressed? A pilot study of salivary cortisol and alpha-amylase concentrations in agoraphobic patients and their novice therapists undergoing in vivo exposure. Psychoneuroendocrinology 2014, 49, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, S.; Miller, R.; Fehm, L.; Kirschbaum, C.; Fydrich, T.; Strohle, A. Therapists’ and patients’ stress responses during graduated versus flooding in vivo exposure in the treatment of specific phobia: A preliminary observational study. Psychiatry Res. 2015, 230, 668–675. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Domes, G.; Kirsch, P.; Heinrichs, M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011, 12, 524–538. [Google Scholar] [CrossRef]
- Engel, S.; Laufer, S.; Miller, R.; Niemeyer, H.; Knaevelsrud, C.; Schumacher, S. Demographic, sampling- and assay-related confounders of endogenous oxytocin concentrations: A systematic review and meta-analysis. Front. Neuroendocrinol. 2019, 54, 100775. [Google Scholar] [CrossRef]
- Alves, E.; Fielder, A.; Ghabriel, N.; Sawyer, M.; Buisman-Pijlman, F.T. Early social environment affects the endogenous oxytocin system: A review and future directions. Front. Endocrinol. 2015, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Donadon, M.F.; Martin-Santos, R.; Osorio, F.L. The associations between oxytocin and trauma in humans: A systematic review. Front. Pharmacol. 2018, 9, 154. [Google Scholar] [CrossRef] [Green Version]
- Engel, S.; Klusmann, H.; Ditzen, B.; Knaevelsrud, C.; Schumacher, S. Menstrual cycle-related fluctuations in oxytocin concentrations: A systematic review and meta-analysis. Front. Neuroendocrinol. 2019, 52, 144–155. [Google Scholar] [CrossRef]
- Crockford, C.; Deschner, T.; Ziegler, T.E.; Wittig, R.M. Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: A review. Front. Behav. Neurosci. 2014, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Leppanen, J.; Ng, K.W.; Tchanturia, K.; Treasure, J. Meta-analysis of the effects of intranasal oxytocin on interpretation and expression of emotions. Neurosci. Biobehav. Rev. 2017, 78, 125–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brambilla, M.; Manenti, R.; de Girolamo, G.; Adenzato, M.; Bocchio-Chiavetto, L.; Cotelli, M. Effects of intranasal oxytocin on long-term memory in healthy humans: A systematic review. Drug Dev. Res. 2016, 77, 479–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van IJzendoorn, M.H.; Bakermans-Kranenburg, M.J. A sniff of trust: Meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology 2012, 37, 438–443. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.; Kim, Y.K. The role of the oxytocin system in anxiety disorders. In Anxiety Disorders; Springer: Singapore, 2020; Volume 1191, pp. 103–120. [Google Scholar] [CrossRef]
- Engel, S.; Laufer, S.; Knaevelsrud, C.; Schumacher, S. The endogenous oxytocin system in depressive disorders: A systematic review and meta-analysis. Psychoneuroendocrinology 2018, 101, 138–149. [Google Scholar] [CrossRef]
- Jobst, A.; Sabass, L.; Hall, D.; Brucklmeier, B.; Buchheim, A.; Hall, J.; Sarubin, N.; Zill, P.; Falkai, P.; Brakemeier, E.L.; et al. Oxytocin plasma levels predict the outcome of psychotherapy: A pilot study in chronic depression. J. Affect. Disord. 2018, 227, 206–213. [Google Scholar] [CrossRef]
- Zilcha-Mano, S.; Goldstein, P.; Dolev-Amit, T.; Shamay-Tsoory, S. Oxytocin synchrony between patients and therapists as a mechanism underlying effective psychotherapy for depression. J. Consult. Clin. Psychol. 2021, 89, 49–57. [Google Scholar] [CrossRef]
- Guastella, A.J.; Howard, A.L.; Dadds, M.R.; Mitchell, P.; Carson, D.S. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology 2009, 34, 917–923. [Google Scholar] [CrossRef]
- Acheson, D.T.; Feifel, D.; Kamenski, M.; McKinney, R.; Risbrough, V.B. Intranasal oxytocin administration prior to exposure therapy for arachnophobia impedes treatment response. Depress. Anxiety 2015, 32, 400–407. [Google Scholar] [CrossRef]
- MacDonald, K.; MacDonald, T.M.; Brune, M.; Lamb, K.; Wilson, M.P.; Golshan, S.; Feifel, D. Oxytocin and psychotherapy: A pilot study of its physiological, behavioral and subjective effects in males with depression. Psychoneuroendocrinology 2013, 38, 2831–2843. [Google Scholar] [CrossRef]
- Zilcha-Mano, S.; Porat, Y.; Dolev, T.; Shamay-Tsoory, S. Oxytocin as a neurobiological marker of ruptures in the working alliance. Psychother. Psychosom. 2018, 87, 126–127. [Google Scholar] [CrossRef] [PubMed]
- Zilcha-Mano, S.; Shamay-Tsoory, S.; Dolev-Amit, T.; Zagoory-Sharon, O.; Feldman, R. Oxytocin as a biomarker of the formation of therapeutic alliance in psychotherapy and counseling psychology. J. Couns. Psychol. 2020, 67, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Melcangi, R.C.; Giatti, S.; Garcia-Segura, L.M. Levels and actions of neuroactive steroids in the nervous system under physiological and pathological conditions: Sex-specific features. Neurosci. Biobehav. Rev. 2016, 67, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Fiacco, S.; Walther, A.; Ehlert, U. Steroid secretion in healthy aging. Psychoneuroendocrinology 2019, 105, 64–78. [Google Scholar] [CrossRef]
- Ennour-Idrissi, K.; Maunsell, E.; Diorio, C. Effect of physical activity on sex hormones in women: A systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res. 2015, 17, 139. [Google Scholar] [CrossRef] [Green Version]
- Hampson, E. A brief guide to the menstrual cycle and oral contraceptive use for researchers in behavioral endocrinology. Horm. Behav. 2020, 119, 104655. [Google Scholar] [CrossRef]
- Cano Sokoloff, N.; Misra, M.; Ackerman, K.E. Exercise, training, and the hypothalamic-pituitary-gonadal axis in men and women. Front. Horm. Res. 2016, 47, 27–43. [Google Scholar] [CrossRef]
- Beltz, A.M.; Moser, J.S. Ovarian hormones: A long overlooked but critical contributor to cognitive brain structures and function. Ann. N. Y. Acad. Sci. 2020, 1464, 156–180. [Google Scholar] [CrossRef]
- Hamson, D.K.; Roes, M.M.; Galea, L.A. Sex hormones and cognition: Neuroendocrine influences on memory and learning. Compr. Physiol. 2016, 6, 1295–1337. [Google Scholar] [CrossRef] [Green Version]
- Fischer, S.; Ehlert, U.; Amiel Castro, R. Hormones of the hypothalamic-pituitary-gonadal (HPG) axis in male depressive disorders—A systematic review and meta-analysis. Front. Neuroendocrinol. 2019, 55, 100792. [Google Scholar] [CrossRef]
- Amiel Castro, R.T.; Ehlert, U.; Fischer, S. Variation in genes and hormones of the hypothalamic-pituitary-ovarian axis in female mood disorders—A systematic review and meta-analysis. Front. Neuroendocrinol. 2021, 62, 100929. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.M.; Li, S.H.; Black, M.J.; Ost, L.G. The association between estradiol levels, hormonal contraceptive use, and responsiveness to one-session-treatment for spider phobia in women. Psychoneuroendocrinology 2018, 90, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Raeder, F.; Heidemann, F.; Schedlowski, M.; Margraf, J.; Zlomuzica, A. No pills, more skills: The adverse effect of hormonal contraceptive use on exposure therapy benefit. J. Psychiatr. Res. 2019, 119, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Graham, B.M. Progesterone levels predict reductions in behavioral avoidance following cognitive restructuring in women with spider phobia. J. Affect. Disord. 2020, 270, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hammen, C. Stress and depression. Annu. Rev. Clin. Psychol. 2005, 1, 293–319. [Google Scholar] [CrossRef] [Green Version]
- de Quervain, D.; Schwabe, L.; Roozendaal, B. Stress, glucocorticoids and memory: Implications for treating fear-related disorders. Nat. Rev. Neurosci. 2017, 18, 7–19. [Google Scholar] [CrossRef]
- Shamay-Tsoory, S.G.; Abu-Akel, A. The social salience hypothesis of oxytocin. Biol. Psychiatry 2016, 79, 194–202. [Google Scholar] [CrossRef]
- Olff, M.; Frijling, J.L.; Kubzansky, L.D.; Bradley, B.; Ellenbogen, M.A.; Cardoso, C.; Bartz, J.A.; Yee, J.R.; van Zuiden, M. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology 2013, 38, 1883–1894. [Google Scholar] [CrossRef] [Green Version]
- Bartz, J.A.; Zaki, J.; Bolger, N.; Ochsner, K.N. Social effects of oxytocin in humans: Context and person matter. Trends Cogn. Sci. 2011, 15, 301–309. [Google Scholar] [CrossRef]
- Castonguay, L.G.; Hill, C.E. Transformation in Psychotherapy: Corrective Experiences across Cognitive Behavioral, Humanistic, and Psychodynamic Approaches; American Psychological Association Press: Washington, DC, USA, 2012. [Google Scholar]
- Soares, C.N.; Zitek, B. Reproductive hormone sensitivity and risk for depression across the female life cycle: A continuum of vulnerability? J. Psychiatry Neurosci. 2008, 33, 331–343. [Google Scholar]
- Studd, J.; Nappi, R.E. Reproductive depression. Gynecol. Endocrinol. 2012, 28 (Suppl. S1), 42–45. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Graham, B.M. The role of hormonal and reproductive status in the treatment of anxiety disorders in women. In Anxiety Disorders; Springer: Singapore, 2020; Volume 1191, pp. 523–541. [Google Scholar] [CrossRef]
- Levi, E.; Fischer, S.; Fisher, H.; Admon, R.; Zilcha-Mano, S. Patient and Therapist In-Session Cortisol as Predictor of Post-Session Patient Reported Affect. Brain Sci. 2021, 11, 1483. [Google Scholar] [CrossRef]
- Viau, V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J. Neuroendocr. 2002, 14, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Rodriguez, A.; Kauffman, A.S.; Cherrington, B.D.; Borges, C.S.; Roepke, T.A.; Laconi, M. Emerging insights into hypothalamic-pituitary-gonadal axis regulation and interaction with stress signalling. J. Neuroendocrinol. 2018, 30, e12590. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Hellhammer, D.H. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 1994, 19, 313–333. [Google Scholar] [CrossRef]
- Stalder, T.; Kirschbaum, C. Analysis of cortisol in hair–state of the art and future directions. Brain Behav. Immun. 2012, 26, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
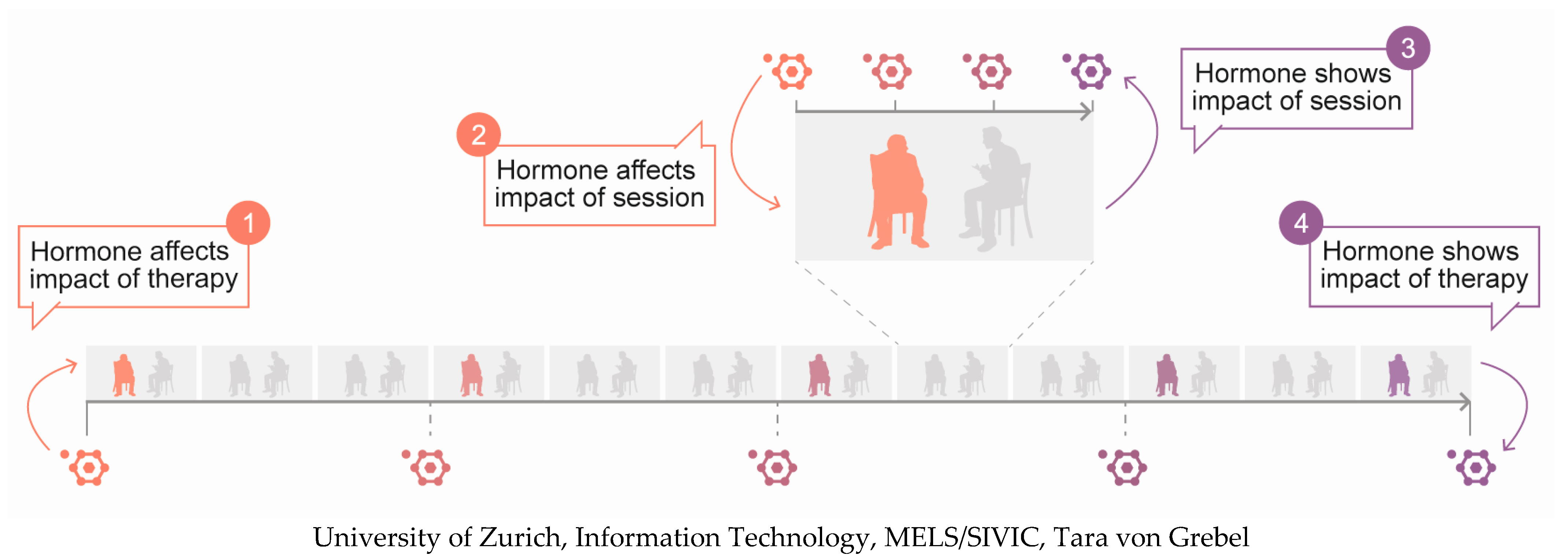
| Predictor of Outcomes | Indicator of Outcomes | |||
|---|---|---|---|---|
| Treatment | Session | Treatment | Session | |
| Hormone | ||||
| Cortisol | High levels predict worse outcomes [25,26] | - | Levels decrease over treatment [37,38,39,40,41,42,43] | - |
| Oxytocin | Low levels predict worse outcomes [64] Low synchrony with therapists predicts worse outcomes [65] | Low levels predict lower state anxiety and increased non-verbal flight behaviour during disclosure of personal information [68] | - | Levels increase with conflict and confrontational ruptures [69] Levels increase with reduced proximity seeking [70] |
| Oestradiol | - | - | - | - |
| Hormone as Predictor of Therapeutic Change | Hormone as Indicator of Therapeutic Change | |||
|---|---|---|---|---|
| Treatment Level | Session Level | Treatment Level | Session Level | |
| Hormone | ||||
| Cortisol | Phobias: Low levels during exposure predict worse outcomes [27,31,32,33,34,35] | Social anxiety disorder: Low levels predict greater fear during exposure [33] | Generalised anxiety disorder: Levels decrease over treatment [43,44,45,46,47,48,49,50] Social anxiety disorder: No change [31,45] | Phobias: No change with exposure [27,31,44,45,48] |
| Oxytocin | Phobias: High levels predict worse outcomes [67] Social anxiety disorder: No effects on outcomes, but high levels predict better evaluation of appearance and speech performance [66] | - | - | - |
| Oestradiol | Phobias: Low levels predict worse outcomes to exposure therapy but not cognitive therapy [80,81,82] | Phobias: Low levels predict slower improvement during exposure [80] | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, S.; Zilcha-Mano, S. Why Does Psychotherapy Work and for Whom? Hormonal Answers. Biomedicines 2022, 10, 1361. https://doi.org/10.3390/biomedicines10061361
Fischer S, Zilcha-Mano S. Why Does Psychotherapy Work and for Whom? Hormonal Answers. Biomedicines. 2022; 10(6):1361. https://doi.org/10.3390/biomedicines10061361
Chicago/Turabian StyleFischer, Susanne, and Sigal Zilcha-Mano. 2022. "Why Does Psychotherapy Work and for Whom? Hormonal Answers" Biomedicines 10, no. 6: 1361. https://doi.org/10.3390/biomedicines10061361
APA StyleFischer, S., & Zilcha-Mano, S. (2022). Why Does Psychotherapy Work and for Whom? Hormonal Answers. Biomedicines, 10(6), 1361. https://doi.org/10.3390/biomedicines10061361






