Joint Cartilage in Long-Duration Spaceflight
Abstract
:1. Introduction
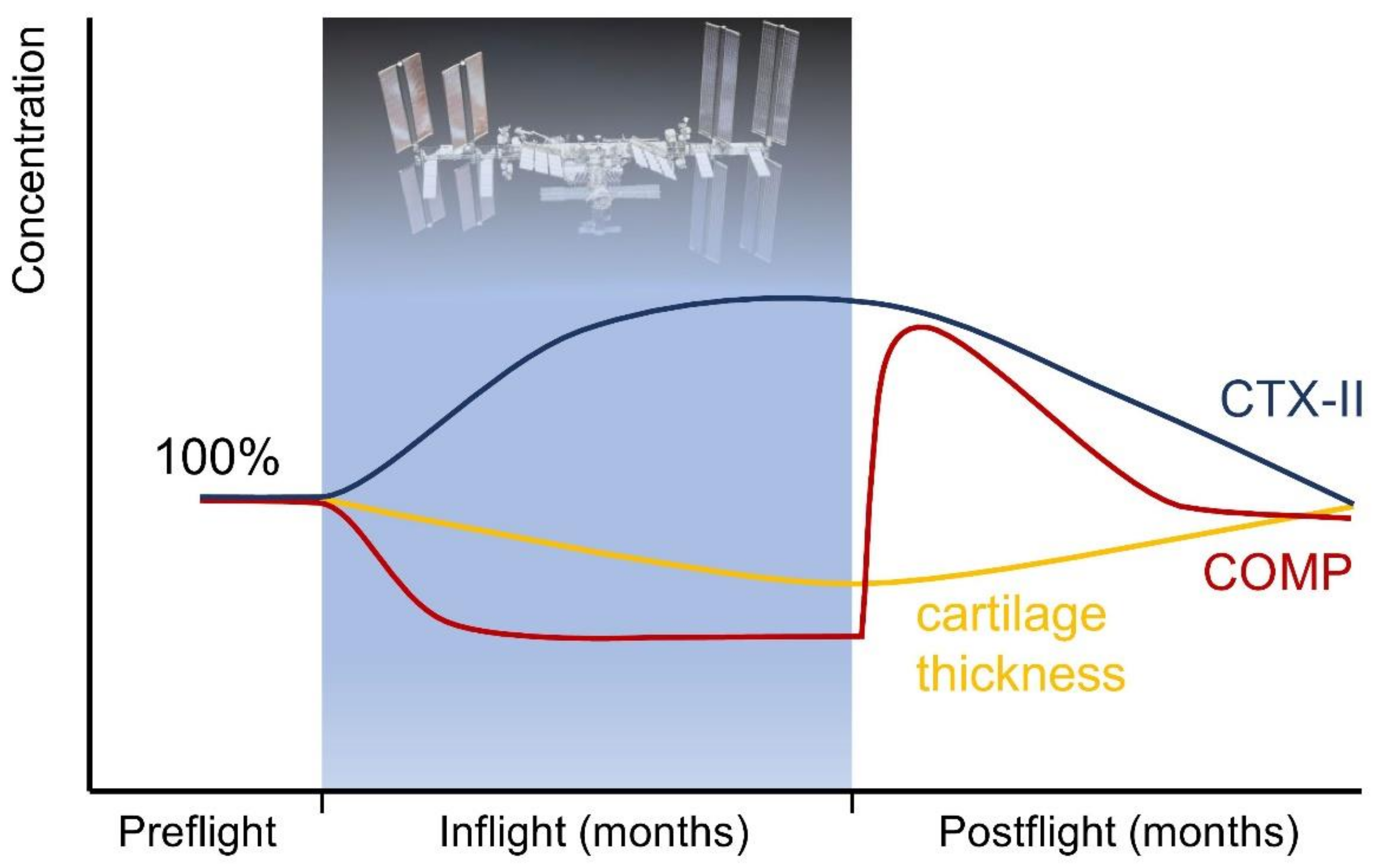
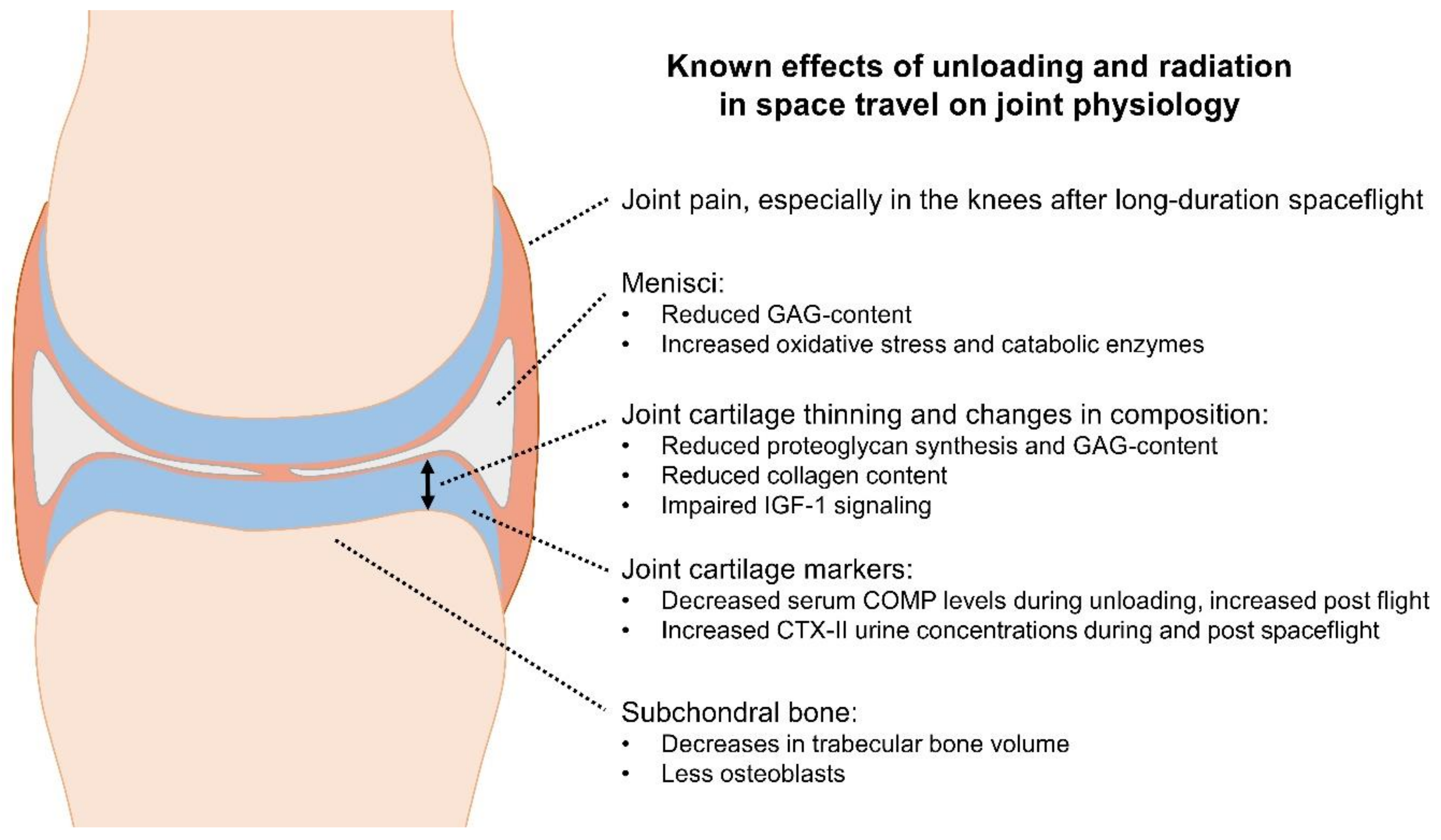
2. Pathophysiology and Biomarkers of Joint Cartilage Unloading
3. Bed Rest and Other Analogue Study Settings
4. Radiation Effects on Joint Cartilage
5. Findings from Spaceflight
5.1. Human Research
5.2. Rodent Research
5.3. Cell and Tissue Experiments
5.4. Deep-Space Missions
6. Countermeasures
6.1. Human Centrifugation and Reactive Jumping
6.2. Nutritional Countermeasures and Medications
6.3. Vibration Training, Ultrasound, Shock Waves, and Magnetic Fields
6.4. Radiation Protection
6.5. The Role of Skeletal Muscle
7. Discussion
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Comfort, P.; McMahon, J.J.; Jones, P.A.; Cuthbert, M.; Kendall, K.; Lake, J.P.; Haff, G.G. Effects of Spaceflight on Musculoskeletal Health: A Systematic Review and Meta-Analysis, Considerations for Interplanetary Travel. Sports Med. 2021, 51, 2097–2114. [Google Scholar] [CrossRef]
- Juhl, O.J.; Buettmann, E.G.; Friedman, M.A.; DeNapoli, R.C.; Hoppock, G.A.; Donahue, H.J. Update on the effects of microgravity on the musculoskeletal system. NPJ Microgravity 2021, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Richardson, K.K.; Krager, K.J.; Ling, W.; Simmons, P.; Allen, A.R.; Aykin-Burns, N. Simulated Galactic Cosmic Rays Modify Mitochondrial Metabolism in Osteoclasts, Increase Osteoclastogenesis and Cause Trabecular Bone Loss in Mice. Int. J. Mol. Sci. 2021, 22, 11711. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Mercuri, J. Microgravity and Radiation Effects on Astronaut Intervertebral Disc Health. Aerosp. Med. Hum. Perform. 2021, 92, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Van Ombergen, A.; Demertzi, A.; Tomilovskaya, E.; Jeurissen, B.; Sijbers, J.; Kozlovskaya, I.B.; Parizel, P.M.; Van de Heyning, P.H.; Sunaert, S.; Laureys, S.; et al. The effect of spaceflight and microgravity on the human brain. J. Neurol. 2017, 264 (Suppl. S1), 18–22. [Google Scholar] [CrossRef] [Green Version]
- Zwart, S.R.; Mulavara, A.P.; Williams, T.J.; George, K.; Smith, S.M. The role of nutrition in space exploration: Implications for sensorimotor, cognition, behavior and the cerebral changes due to the exposure to radiation, altered gravity, and isolation/confinement hazards of spaceflight. Neurosci. Biobehav. Rev. 2021, 127, 307–331. [Google Scholar] [CrossRef]
- Buchheim, J.I.; Matzel, S.; Rykova, M.; Vassilieva, G.; Ponomarev, S.; Nichiporuk, I.; Hörl, M.; Moser, D.; Biere, K.; Feuerecker, M.; et al. Stress Related Shift toward Inflammaging in Cosmonauts after Long-Duration Space Flight. Front. Physiol. 2019, 10, 85. [Google Scholar] [CrossRef] [Green Version]
- Ponomarev, S.; Kalinin, S.; Sadova, A.; Rykova, M.; Orlova, K.; Crucian, B. Immunological Aspects of Isolation and Confinement. Front. Immunol. 2021, 12, 697435. [Google Scholar] [CrossRef]
- Pavei, G.; Minetti, A.E. Hopping locomotion at different gravity: Metabolism and mechanics in humans. J. Appl. Physiol. 2016, 120, 1223–1229. [Google Scholar] [CrossRef] [Green Version]
- Weber, T.; Green, D.A.; Attias, J.; Sies, W.; Frechette, A.; Braunstein, B.; Rittweger, J. Hopping in hypogravity—A rationale for a plyometric exercise countermeasure in planetary exploration missions. PLoS ONE 2019, 14, e0211263. [Google Scholar] [CrossRef] [Green Version]
- Coulombe, J.C.; Senwar, B.; Ferguson, V.L. Spaceflight-Induced Bone Tissue Changes That Affect Bone Quality and Increase Fracture Risk. Curr. Osteoporos. Rep. 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Rolvien, T.; Amling, M. Disuse Osteoporosis: Clinical and Mechanistic Insights. Calcif. Tissue Int. 2021, 110, 592–604. [Google Scholar] [CrossRef]
- Fitts, R.H.; Riley, D.R.; Widrick, J.J. Functional and structural adaptations of skeletal muscle to microgravity. J. Exp. Biol. 2001, 204 Pt 18, 3201–3208. [Google Scholar] [CrossRef]
- Marusic, U.; Narici, M.; Simunic, B.; Pisot, R.; Ritzmann, R. Nonuniform loss of muscle strength and atrophy during bed rest: A systematic review. J. Appl. Physiol. 2021, 131, 194–206. [Google Scholar] [CrossRef]
- Fitzgerald, J. Cartilage breakdown in microgravity—A problem for long-term spaceflight? NPJ Regen. Med. 2017, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Lazzari, Z.T.; Aria, K.M.; Menger, R. Neurosurgery and spinal adaptations in spaceflight: A literature review. Clin. Neurol. Neurosurg. 2021, 207, 106755. [Google Scholar] [CrossRef]
- Von Kroge, S.; Wölfel, E.M.; Buravkova, L.B.; Atiakshin, D.A.; Markina, E.A.; Schinke, T.; Rolvien, T.; Busse, B.; Jähn-Rickert, K. Bone loss recovery in mice following microgravity with concurrent bone-compartment-specific osteocyte characteristics. Eur. Cells Mater. 2021, 42, 220–231. [Google Scholar] [CrossRef]
- Sibonga, J.D.; Spector, E.R.; Keyak, J.H.; Zwart, S.R.; Smith, S.M.; Lang, T.F. Use of Quantitative Computed Tomography to Assess for Clinically-Relevant Skeletal Effects of Prolonged Spaceflight on Astronaut Hips. J. Clin. Densitom. 2020, 23, 155–164. [Google Scholar] [CrossRef]
- Kerstman, E.L.; Scheuring, R.A.; Barnes, M.G.; DeKorse, T.B.; Saile, L.G. Space adaptation back pain: A retrospective study. Aviat. Space Environ. Med. 2012, 83, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Penchev, R.; Scheuring, R.A.; Soto, A.T.; Miletich, D.M.; Kerstman, E.; Cohen, S.P. Back Pain in Outer Space. Anesthesiology 2021, 135, 384–395. [Google Scholar] [CrossRef]
- Scheuring, R.A.; Mathers, C.H.; Jones, J.A.; Wear, M.L. Musculoskeletal injuries and minor trauma in space: Incidence and injury mechanisms in U. S. astronauts. Aviat. Space Environ. Med. 2009, 80, 117–124. [Google Scholar] [CrossRef]
- Howle, L.E.; Weber, P.W.; Hada, E.A.; Vann, R.D.; Denoble, P.J. The probability and severity of decompression sickness. PLoS ONE 2017, 12, e0172665. [Google Scholar] [CrossRef]
- Sharareh, B.; Schwarzkopf, R. Dysbaric osteonecrosis: A literature review of pathophysiology, clinical presentation, and management. Clin. J. Sport Med. 2015, 25, 153–161. [Google Scholar] [CrossRef]
- Johnston, R.S.; Dietlein, L.F. Biomedical Results from Skylab; Scientific and Technical Information Office, National Aeronautics and Space Administration: Washington, DC, USA, 1977; Volume 377.
- Patel, Z.S.; Brunstetter, T.J.; Tarver, W.J.; Whitmire, A.M.; Zwart, S.R.; Smith, S.M.; Huff, J.L. Red risks for a journey to the red planet: The highest priority human health risks for a mission to Mars. NPJ Microgravity 2020, 6, 33. [Google Scholar] [CrossRef]
- Griffin, T.M.; Guliak, F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc. Sport Sci. Rev. 2005, 33, 195–200. [Google Scholar] [CrossRef]
- Kwok, A.T.; Mohamed, N.S.; Plate, J.F.; Yammani, R.R.; Rosas, S.; Bateman, T.A.; Livingston, E.; Moore, J.E.; Kerr, B.A.; Lee, J.; et al. Spaceflight and hind limb unloading induces an arthritic phenotype in knee articular cartilage and menisci of rodents. Sci. Rep. 2021, 11, 10469. [Google Scholar] [CrossRef]
- Fitzgerald, J.; Endicott, J.; Hansen, U.; Janowitz, C. Articular cartilage and sternal fibrocartilage respond differently to extended microgravity. NPJ Microgravity 2019, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Vincent, T.L.; Wann, A.K.T. Mechanoadaptation: Articular cartilage through thick and thin. J. Physiol. 2019, 597, 1271–1281. [Google Scholar] [CrossRef]
- Eckstein, F.; Hudelmaier, M.; Putz, R. The effects of exercise on human articular cartilage. J. Anat. 2006, 208, 491–512. [Google Scholar] [CrossRef]
- Hinterwimmer, S.; Krammer, M.; Krötz, M.; Gläser, C.; Baumgart, R.; Reiser, M.; Eckstein, F. Cartilage atrophy in the knees of patients after seven weeks of partial load bearing. Arthr. Rheum. 2004, 50, 2516–2520. [Google Scholar] [CrossRef]
- Liphardt, A.M.; Mündermann, A.; Koo, S.; Baecker, N.; Andriacchi, T.P.; Zange, J.; Mester, J.; Heer, M. Vibration training intervention to maintain cartilage thickness and serum concentrations of cartilage oligometric matrix protein (COMP) during immobilization. Osteoarthr. Cartil. 2009, 17, 1598–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanwanseele, B.; Eckstein, F.; Knecht, H.; Stussi, E.; Spaepen, A. Knee cartilage of spinal cord-injured patients displays progressive thinning in the absence of normal joint loading and movement. Arthr. Rheum. 2002, 46, 2073–2078. [Google Scholar] [CrossRef] [PubMed]
- Vanwanseele, B.; Eckstein, F.; Knecht, H.; Spaepen, A.; Stussi, E. Longitudinal analysis of cartilage atrophy in the knees of patients with spinal cord injury. Arthr. Rheum. 2003, 48, 3377–3381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zheng, Y.P.; Wang, X.Y.; Huang, Y.P.; Liu, M.Q.; Wang, S.Z.; Zhang, Z.K.; Guo, X. Ultra-sound evaluation of site-specific effect of simulated microgravity on articular cartilage. Ultrasound Med. Biol. 2010, 36, 1089–1097. [Google Scholar] [CrossRef]
- Bashir, A.; Gray, M.L.; Burstein, D. Gd-DTPA2- as a measure of cartilage degradation. Magn. Reson. Med. 1996, 36, 665–673. [Google Scholar] [CrossRef]
- Bashir, A.; Gray, M.L.; Hartke, J.; Burstein, D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn. Reson. Med. 1999, 41, 857–865. [Google Scholar] [CrossRef]
- Takahashi, I.; Matsuzaki, T.; Kuroki, H.; Hoso, M. Disuse Atrophy of Articular Cartilage Induced by Unloading Condition Accelerates Histological Progression of Osteoarthritis in a Post-Traumatic Rat Model. Cartilage 2020, 13, 1522S–1529S. [Google Scholar] [CrossRef]
- Grässel, S.; Zaucke, F.; Madry, H. Osteoarthritis: Novel Molecular Mechanisms Increase Our Understanding of the Disease Pathology. J. Clin. Med. 2021, 10, 1938. [Google Scholar] [CrossRef]
- Campbell, T.M.; Reilly, K.; Laneuville, O.; Uhthoff, H.; Trudel, G. Bone replaces articular cartilage in the rat knee joint after prolonged immobilization. Bone 2018, 106, 42–51. [Google Scholar] [CrossRef]
- Watanabe, M.; Campbell, T.M.; Reilly, K.; Uhthoff, H.K.; Laneuville, O.; Trudel, G. Bone replaces unloaded articular cartilage during knee immobilization. A longitudinal study in the rat. Bone 2021, 142, 115694. [Google Scholar] [CrossRef]
- Madry, H.; van Dijk, C.N.; Mueller-Gerbl, M. The basic science of the subchondral bone. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 419–433. [Google Scholar] [CrossRef]
- Ratneswaran, A.; Kapoor, M. Osteoarthritis year in review: Genetics, genomics, epigenetics. Osteoarthr. Cartil. 2021, 29, 151–160. [Google Scholar] [CrossRef]
- Roberts, H.M.; Law, R.J.; Thom, J.M. The time course and mechanisms of change in biomarkers of joint metabolism in response to acute exercise and chronic training in physiologic and pathological conditions. Eur. J. Appl. Physiol. 2019, 119, 2401–2420. [Google Scholar] [CrossRef] [Green Version]
- Liphardt, A.M.; Mündermann, A.; Heer, M.; Achtzehn, S.; Niehoff, A.; Mester, J. Locomotion replacement exercise cannot counteract cartilage biomarker response to 5 days of immobilization in healthy adults. J. Orthop. Res. 2020, 38, 2373–2382. [Google Scholar] [CrossRef]
- Posey, K.L.; Hecht, J.T. The role of cartilage oligomeric matrix protein (COMP) in skeletal disease. Curr. Drug Targets 2008, 9, 869–877. [Google Scholar] [CrossRef]
- Tseng, S.; Reddy, A.H.; Di Cesare, P.E. Cartilage Oligomeric Matrix Protein (COMP): A Biomarker of Arthritis. Biomark. Insights 2009, 4, 33–44. [Google Scholar] [CrossRef]
- Andersson, M.L.; Thorstensson, C.A.; Roos, E.M.; Petersson, I.F.; Heinegard, D.; Saxne, T. Serum levels of cartilage oligomeric matrix protein (COMP) increase temporarily after physical exercise in patients with knee osteoarthritis. BMC Musculoskelet. Disord. 2006, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Milaras, C.; Lepetsos, P.; Dafou, D.; Potoupnis, M.; Tsiridis, E. Association of Matrix Metalloproteinase (MMP) Gene Polymorphisms with Knee Osteoarthritis: A Review of the Literature. Cureus 2021, 13, e18607. [Google Scholar] [CrossRef]
- Cauwe, B.; Van den Steen, P.E.; Opdenakker, G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 113–185. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Hao, B.; Sun, J.; Yin, M. C-Terminal Cross-Linked Telopeptides of Type II Collagen as Biomarker for Radiological Knee Osteoarthritis: A Meta-Analysis. Cartilage 2020, 11, 512–520. [Google Scholar] [CrossRef]
- Hu, N.; Zhang, J.; Wang, J.; Wang, P.; Wang, J.; Qiang, Y.; Li, Z.; Wu, T.; Wang, X.; Wang, Y.; et al. Biomarkers of joint metabolism and bone mineral density are associated with early knee osteoarthritis in premenopausal females. Clin. Rheumatol. 2022, 41, 819–829. [Google Scholar] [CrossRef]
- Kumm, J.; Tamm, A.; Lintrop, M.; Tamm, A. Association between ultrasonographic findings and bone/cartilage biomarkers in patients with early-stage knee osteoarthritis. Calcif. Tissue Int. 2009, 85, 514–522. [Google Scholar] [CrossRef]
- Emanuel, K.S.; Kellner, L.J.; Peters, M.J.M.; Haartmans, M.J.J.; Hooijmans, M.T.; Emans, P.J. The relation between the biochemical composition of knee articular cartilage and quantitative MRI: A systematic review and meta-analysis. Osteoarthr. Cartil. 2021, 30, 650–662. [Google Scholar] [CrossRef]
- Bruno, F.; Arrigoni, F.; Palumbo, P.; Natella, R.; Maggialetti, N.; Reginelli, A.; Splendiani, A.; Di Cesare, E.; Brunese, L.; Guglielmi, G.; et al. New advances in MRI diagnosis of degenerative osteoarthropathy of the peripheral joints. Radiol. Med. 2019, 124, 1121–1127. [Google Scholar] [CrossRef]
- Ganse, B.; Zange, J.; Weber, T.; Pohle-Fröhlich, R.; WJohannes, B.; Hackenbroch, M.; Rittweger, J.; Eysel, P.; Koy, T. Muscular forces affect the glycosaminoglycan content of joint cartilage: Unloading in human volunteers with the HEPHAISTOS lower leg orthosis. Acta Orthop. 2015, 86, 388–392. [Google Scholar] [CrossRef]
- Bousson, V.; Lowitz, T.; Laouisset, L.; Engelke, K.; Laredo, J.D. CT imaging for the investigation of subchondral bone in knee osteoarthritis. Osteoporos. Int. 2012, 23 (Suppl. S8), S861–S865. [Google Scholar] [CrossRef]
- McDonnell, A.C.; Eiken, O.; Mekjavic, I.B.; Žlak, N.; Drobnič, M. The influence of a sustained 10-day hypoxic bed rest on cartilage biomarkers and subchondral bone in females: The FemHab study. Physiol. Rep. 2020, 8, e14413. [Google Scholar] [CrossRef]
- Hargens, A.R.; Vico, L. Long-duration bed rest as an analog to microgravity. J. Appl. Physiol. 2016, 120, 891–903. [Google Scholar] [CrossRef] [Green Version]
- Liphardt, A.M.; Mündermann, A.; Andriacchi, T.P.; Achtzehn, S.; Heer, M.; Mester, J. Sensitivity of serum concentration of cartilage biomarkers to 21-days of bed rest. J. Orthop. Res. 2018, 36, 1465–1471. [Google Scholar] [CrossRef]
- Stamenković, V.; Keller, G.; Nesic, D.; Cogoli, A.; Grogan, S.P. Neocartilage formation in 1 g, simulated, and microgravity environments: Implications for tissue engineering. Tissue Eng. Part A 2010, 16, 1729–1736. [Google Scholar] [CrossRef]
- Grimm, D.; Egli, M.; Krüger, M.; Riwaldt, S.; Corydon, T.J.; Kopp, S.; Wehland, M.; Wise, P.; Infanger, M.; Mann, V.; et al. Tissue Engineering under Microgravity Conditions—Use of Stem Cells and Specialized Cells. Stem Cells Dev. 2018, 27, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Vunjak-Novakovic, G.; Searby, N.; De Luis, J.; Freed, L.E. Microgravity studies of cells and tissues. Ann. N. Y. Acad. Sci. 2002, 974, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Tomita, N.; Nakajima, M.; Ikeuchi, K.; Wakitani, S. Effect of low loading and joint immobilization for spontaneous repair of osteochondral defect in the knees of weightless (tail suspension) rats. J. Orthop. Sci. 2005, 10, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, I.; Matsuzaki, T.; Kuroki, H.; Hoso, M. Physiological Reloading Recovers Histologically Disuse Atrophy of the Articular Cartilage and Bone by Hindlimb Suspension in Rat Knee Joint. Cartilage 2021, 13 (Suppl. S2), 1530S–1539S. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, J.C.; Scott, G.B.I.; Sutton, J.P. Space Radiation: The Number One Risk to Astronaut Health beyond Low Earth Orbit. Life 2014, 4, 491–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willey, J.S.; Long, D.L.; Vanderman, K.S.; Loeser, R.F. Ionizing radiation causes active degradation and reduces matrix synthesis in articular cartilage. Int. J. Radiat. Biol. 2013, 89, 268–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willey, J.S.; Kwok, A.T.; Moore, J.E.; Payne, V.; Lindburg, C.A.; Balk, S.A.; Olson, J.; Black, P.J.; Walb, M.C.; Yammani, R.R.; et al. Spaceflight-Relevant Challenges of Radiation and/or Reduced Weight Bearing Cause Arthritic Responses in Knee Articular Cartilage. Radiat. Res. 2016, 186, 333–344. [Google Scholar] [CrossRef]
- Kwok, A.T.; Moore, J.E.; Rosas, S.; Kerr, B.A.; Andrews, R.N.; Nguyen, C.M.; Lee, J.; Furdui, C.M.; Collins, B.E.; Munley, M.T.; et al. Knee and Hip Joint Cartilage Damage from Combined Spaceflight Hazards of Low-Dose Radiation Less Than 1 Gy and Prolonged Hindlimb Unloading. Radiat. Res. 2019, 191, 497–506. [Google Scholar] [CrossRef]
- Lloyd, S.A.; Bandstra, E.R.; Willey, J.S.; Riffle, S.E.; Tirado-Lee, L.; Nelson, G.A.; Pecaut, M.J.; Bateman, T.A. Effect of proton irradiation followed by hindlimb unloading on bone in mature mice: A model of long-duration spaceflight. Bone 2012, 51, 756–764. [Google Scholar] [CrossRef] [Green Version]
- Niehoff, A.; Brüggemann, G.P.; Zaucke, F.; Eckstein, F.; Bloch, W.; Mündermann, A.; Koo, S.; Mester, J.; Liphardt, A.M. Long-duration space flight and cartilage adaptation: First results on changes in tissue metabolism. Osteoarthr. Cartil. 2016, 24, S144–S145. [Google Scholar] [CrossRef] [Green Version]
- Niehoff, A.; Dreiner, M.; Mündermann, A.; Zaucke, F.; Liphardt, A.M. The effect of micro- and hypergravity on serum comp levels in healthy adults. Osteoarthr. Cartil. 2019, 27, S169–S170. [Google Scholar] [CrossRef] [Green Version]
- Liphardt, A.M.; Djalal, N.; Smith, S.M.; Zwart, S.R.; Mündermann, A.; Zaucke, F.; Schett, G.; Niehoff, A. Urine Concentration of Cartilage Degradation Marker CTX-II is Sensitive to Microgravity. Abstract. In Proceedings of the NASA Human Research Program Investigators’ Workshop, Virtual; 2022. [Google Scholar]
- Jee, W.S.; Wronski, T.J.; Morey, E.R.; Kimmel, D.B. Effects of spaceflight on trabecular bone in rats. Am. J. Physiol. 1983, 244, R310–R314. [Google Scholar] [CrossRef]
- Duke, P.J.; Durnova, G.; Montufar-Solis, D. Histomorphometric and electron microscopic analyses of tibial epiphyseal plates from Cosmos 1887 rats. FASEB J. 1990, 4, 41–46. [Google Scholar] [CrossRef]
- Freed, L.E.; Langer, R.; Martin, I.; Pellis, N.R.; Vunjak-Novakovic, G. Tissue engineering of cartilage in space. Proc. Natl. Acad. Sci. USA 1997, 94, 13885–13890. [Google Scholar] [CrossRef] [Green Version]
- Volova, L.T.; Rossinskaya, V.V.; Milyakova, M.N.; Boltovskaya, V.V.; Nefedova, I.E.; Kulagina, L.N.; Pugachev, E.I. Studies of Spaceflight Effects on a 3D Model of Chondroblast Culture. Aviakosm. Ekol. Med. 2016, 50, 11–17. [Google Scholar] [CrossRef]
- Wehland, M.; Aleshcheva, G.; Schulz, H.; Saar, K.; Hübner, N.; Hemmersbach, R.; Braun, M.; Ma, X.; Frett, T.; Warnke, E.; et al. Differential gene expression of human chondrocytes cultured under short-term altered gravity conditions during parabolic flight maneuvers. Cell Commun. Signal. 2015, 13, 18. [Google Scholar] [CrossRef] [Green Version]
- Grimm, D.; Wehland, M.; Corydon, T.J.; Richter, P.; Prasad, B.; Bauer, J.; Egli, M.; Kopp, S.; Lebert, M.; Krüger, M. The effects of microgravity on differentiation and cell growth in stem cells and cancer stem cells. Stem Cells Transl. Med. 2020, 9, 882–894. [Google Scholar] [CrossRef]
- Walsh, L.; Schneider, U.; Fogtman, A.; Kausch, C.; McKenna-Lawlor, S.; Narici, L.; Ngo-Anh, J.; Reitz, G.; Sabatier, L.; Santin, G.; et al. Research plans in Europe for radiation health hazard assessment in exploratory space missions. Life Sci. Space Res. 2019, 21, 73–82. [Google Scholar] [CrossRef]
- Restier-Verlet, J.; El-Nachef, L.; Ferlazzo, M.L.; Al-Choboq, J.; Granzotto, A.; Bouchet, A.; Foray, N. Radiation on Earth or in Space: What Does It Change? Int. J. Mol. Sci. 2021, 22, 3739. [Google Scholar] [CrossRef]
- Person, P.; Eversole, L.R.; Shklar, G.; Johnson, L.C.; Moss, M.L. The effects of cosmic particle radiation on pocket mice aboard Apollo XVII: Appendix II. Evaluation of oral, dental, and skeletal tissues. Aviat. Space Environ. Med. 1975, 46, 634–638. [Google Scholar]
- Ramkumar, P.N.; Navarro, S.M.; Becker, J.; Ahmad, F.; Minkara, A.A.; Haeberle, H.S.; Mont, M.A.; Williams, R.J. The Effects of Space Microgravity on Hip and Knee Cartilage: A New Frontier in Orthopaedics. Surg. Technol. Int. 2019, 35, 421–425. [Google Scholar]
- Qaisar, R.; Karim, A.; Elmoselhi, A.B. Muscle unloading: A comparison between spaceflight and ground-based models. Acta Physiol. 2020, 228, e13431. [Google Scholar] [CrossRef]
- Koy, T.; Zange, J.; Rittweger, J.; Pohle-Fröhlich, R.; Hackenbroch, M.; Eysel, P.; Ganse, B. Assessment of lumbar intervertebral disc glycosaminoglycan content by gadolinium-enhanced MRI before and after 21-days of head-down-tilt bedrest. PLoS ONE 2014, 9, e112104. [Google Scholar] [CrossRef] [Green Version]
- Koy, T.; Ganse, B.; Zange, J.; Rittweger, J.; Pohle-Fröhlich, R.; Fings-Meuthen, P.; Johannes, B.; Felsenberg, D.; Eysel, P.; Bansmann, P.M.; et al. T2-relaxation time increases in lumbar intervertebral discs after 21d head-down tilt bed-rest. J. Musculoskelet. Neuronal Interact. 2017, 17, 140–145. [Google Scholar]
- Ramachandran, V.; Wang, R.; Ramachandran, S.S.; Ahmed, A.S.; Phan, K.; Antonsen, E.L. Effects of spaceflight on cartilage: Implications on spinal physiology. J. Spine Surg. 2018, 4, 433–445. [Google Scholar] [CrossRef]
- Petersen, N.; Jaekel, P.; Rosenberger, A.; Weber, T.; Scott, J.; Castrucci, F.; Lambrecht, G.; Ploutz-Snyder, L.; Damann, V.; Kozlovskaya, I.; et al. Exercise in space: The European Space Agency approach to in-flight exercise countermeasures for long-duration missions on ISS. Extreme Physiol. Med. 2016, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Burton, R.R.; Meeker, L.J. Physiologic validation of a short-arm centrifuge for space application. Aviat. Space Environ. Med. 1992, 63, 476–481. [Google Scholar]
- Clément, G.; Pavy-Le Traon, A. Centrifugation as a countermeasure during actual and simulated microgravity: A review. Eur. J. Appl. Physiol. 2004, 92, 235–248. [Google Scholar] [CrossRef]
- Martin, K.M.; Landau, D.F.; Longuski, J.M. Method to maintain artificial gravity during transfer maneuvers for tethered spacecraft. Acta Astronaut. 2016, 120, 138–153. [Google Scholar] [CrossRef]
- Spilker, T.R. Engineering Challenges of Artificial Gravity Stations. AIAA 2020, 4111. [Google Scholar] [CrossRef]
- Dreiner, M.; Willwacher, S.; Kramer, A.; Kümmel, J.; Frett, T.; Zaucke, F.; Liphardt, A.M.; Gruber, M.; Niehoff, A. Short-Term Response of Serum Cartilage Oligomeric Matrix Protein to Different Types of Impact Loading under Normal and Artificial Gravity. Front. Physiol. 2020, 11, 1032. [Google Scholar] [CrossRef] [PubMed]
- Ganse, B.; Bosutti, A.; Drey, M.; Degens, H. Sixty days of head-down tilt bed rest with or without artificial gravity do not affect the neuromuscular secretome. Exp. Cell Res. 2021, 399, 112463. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Venegas-Carro, M.; Zange, J.; Sies, W.; Maffiuletti, N.A.; Gruber, M.; Degens, H.; Moreno-Villanueva, M.; Mulder, E. Daily 30-min exposure to artificial gravity during 60 days of bed rest does not maintain aerobic exercise capacity but mitigates some deteriorations of muscle function: Results from the AGBRESA RCT. Eur. J. Appl. Physiol. 2021, 121, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Chatani, M.; Higashitani, A.; Higashibata, A.; Kawano, F.; Nikawa, T.; Numaga-Tomita, T.; Ogura, T.; Sato, F.; Sehara-Fujisawa, A.; et al. Findings from recent studies by the Japan Aerospace Exploration Agency examining musculoskeletal atrophy in space and on Earth. NPJ Microgravity 2021, 7, 18. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Guermazi, A.; Guehring, H.; Aydemir, A.; Wax, S.; Fleuranceau-Morel, P.; Reinstrup Bihlet, A.; Byrjalsen, I.; Ragnar Andersen, J.; Eckstein, F. Effect of Intra-Articular Sprifermin vs. Placebo on Femorotibial Joint Cartilage Thickness in Patients with Osteoarthritis: The FORWARD Randomized Clinical Trial. JAMA 2019, 322, 1360–1370. [Google Scholar] [CrossRef]
- Conaghan, P.G.; Bowes, M.A.; Kingsbury, S.R.; Brett, A.; Guillard, G.; Rizoska, B.; Sjögren, N.; Graham, P.; Jansson, Å.; Wadell, C.; et al. Disease-Modifying Effects of a Novel Cathepsin K Inhibitor in Osteoarthritis: A Randomized Controlled Trial. Ann. Intern. Med. 2020, 172, 86–95. [Google Scholar] [CrossRef]
- Yazici, Y.; Mcalindon, T.E.; Gibofsky, A.; Lane, N.E.; Lattermann, C.; Skrepnik, N.; Swearingen, C.J.; Simsek, I.; Ghandehari, H.; DiFrancesco, A.; et al. A Phase 2b randomized trial of lorecivivint, a novel intra-articular CLK2/DYRK1A inhibitor and Wnt pathway modulator for knee osteoarthritis. Osteoarthr. Cartil. 2021, 29, 654–666. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Sun, M. The application of whole-body vibration training in knee osteoarthritis. Jt. Bone Spine 2022, 89, 105276. [Google Scholar] [CrossRef]
- Uddin, S.M.Z.; Komatsu, D.E. Therapeutic Potential Low-Intensity Pulsed Ultrasound for Osteoarthritis: Pre-clinical and Clinical Perspectives. Ultrasound Med. Biol. 2020, 46, 909–920. [Google Scholar] [CrossRef]
- Moretti, L.; Bizzoca, D.; Giancaspro, G.A.; Cassano, G.D.; Moretti, F.; Setti, S.; Moretti, B. Biophysical Stimulation in Athletes’ Joint Degeneration: A Narrative Review. Medicina 2021, 57, 1206. [Google Scholar] [CrossRef]
- Li, M.; Gonon, G.; Buonanno, M.; Autsavapromporn, N.; de Toledo, S.M.; Pain, D.; Azzam, E.I. Health risks of space exploration: Targeted and nontargeted oxidative injury by high-charge and high-energy particles. Antioxid. Redox Signal. 2014, 20, 1501–1523. [Google Scholar] [CrossRef] [Green Version]
- Shavers, M.R.; Zapp, N.; Barber, R.E.; Wilson, J.W.; Qualls, G.; Toupes, L.; Ramsey, S.; Vinci, V.; Smith, G.; Cucinotta, F.A. Implementation of ALARA radiation protection on the ISS through polyethylene shielding augmentation of the Service Module Crew Quarters. Adv. Space Res. 2004, 34, 1333–1337. [Google Scholar] [CrossRef]
- Washburn, S.A.; Blattnig, S.R.; Singleterry, R.C.; Westover, S.C. Active magnetic radiation shielding system analysis and key technologies. Life Sci. Space Res. 2015, 4, 22–34. [Google Scholar] [CrossRef]
- Herrmann, M.; Engelke, K.; Ebert, R.; Müller-Deubert, S.; Rudert, M.; Ziouti, F.; Jundt, F.; Felsenberg, D.; Jakob, F. Interactions between Muscle and Bone—Where Physics Meets Biology. Biomolecules 2020, 10, 432. [Google Scholar] [CrossRef] [Green Version]
- Debevec, T.; Ganse, B.; Mittag, U.; Eiken, O.; Mekjavic, I.B.; Rittweger, J. Hypoxia Aggravates Inactivity-Related Muscle Wasting. Front. Physiol. 2018, 9, 494. [Google Scholar] [CrossRef] [Green Version]
- Trappe, T.A.; Burd, N.A.; Louis, E.S.; Lee, G.A.; Trappe, S.W. Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol. 2007, 191, 147–159. [Google Scholar] [CrossRef]
- Alkner, B.A.; Tesch, P.A. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur. J. Appl. Physiol. 2004, 93, 294–305. [Google Scholar] [CrossRef]
- LeBlanc, A.; Lin, C.; Shackelford, L.; Sinitsyn, V.; Evans, H.; Belichenko, O.; Schenkman, B.; Kozlovskaya, I.; Oganov, V.; Bakulin, A.; et al. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J. Appl. Physiol. 2000, 89, 2158–2164. [Google Scholar] [CrossRef]
- Bloomberg, J.J.; Peters, B.T.; Smith, S.L.; Huebner, W.P.; Reschke, M.F. Locomotor head-trunk coordination strategies following space flight. J. Vestib. Res. 1997, 7, 161–177. [Google Scholar] [CrossRef]
- Miller, C.A.; Peters, B.T.; Brady, R.R.; Richards, J.R.; Ploutz-Snyder, R.J.; Mulavara, A.P.; Bloomberg, J.J. Changes in toe clearance during treadmill walking after long-duration spaceflight. Aviat. Space Environ. Med. 2010, 81, 919–928. [Google Scholar] [CrossRef]
- Speers, R.A.; Paloski, W.H.; Kuo, A.D. Multivariate changes in coordination of postural control following spaceflight. J. Biomech. 1998, 31, 883–889. [Google Scholar] [CrossRef]
- Ackermann, M.; van den Bogert, A.J. Predictive simulation of gait at low gravity reveals skipping as the preferred locomotion strategy. J. Biomech. 2012, 45, 1293–1298. [Google Scholar] [CrossRef] [Green Version]
- Pavei, G.; Biancardi, C.M.; Minetti, A.E. Skipping vs. running as the bipedal gait of choice in hypogravity. J. Appl. Physiol. 2015, 119, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Richter, C.; Braunstein, B.; Staeudle, B.; Attias, J.; Suess, A.; Weber, T.; Mileva, K.N.; Rittweger, J.; Green, D.A.; Albracht, K. Gastrocnemius medialis contractile behavior during running differs between simulated Lunar and Martian gravities. Sci. Rep. 2021, 11, 22555. [Google Scholar] [CrossRef]
- Richter, C.; Braunstein, B.; Winnard, A.; Nasser, M.; Weber, T. Human Biomechanical and Cardiopulmonary Responses to Partial Gravity—A Systematic Review. Front. Physiol. 2017, 8, 583. [Google Scholar] [CrossRef] [Green Version]
- Hassler, D.M.; Zeitlin, C.; Wimmer-Schweingruber, R.F.; Ehresmann, B.; Rafkin, S.; Eigenbrode, J.L.; Brinza, D.E.; Weigle, G.; Böttcher, S.; Böhm, E.; et al. Mars’ surface radiation environment measured with the Mars Science Laboratory’s Curiosity rover. Science 2014, 343, 1244797. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganse, B.; Cucchiarini, M.; Madry, H. Joint Cartilage in Long-Duration Spaceflight. Biomedicines 2022, 10, 1356. https://doi.org/10.3390/biomedicines10061356
Ganse B, Cucchiarini M, Madry H. Joint Cartilage in Long-Duration Spaceflight. Biomedicines. 2022; 10(6):1356. https://doi.org/10.3390/biomedicines10061356
Chicago/Turabian StyleGanse, Bergita, Magali Cucchiarini, and Henning Madry. 2022. "Joint Cartilage in Long-Duration Spaceflight" Biomedicines 10, no. 6: 1356. https://doi.org/10.3390/biomedicines10061356
APA StyleGanse, B., Cucchiarini, M., & Madry, H. (2022). Joint Cartilage in Long-Duration Spaceflight. Biomedicines, 10(6), 1356. https://doi.org/10.3390/biomedicines10061356







