Lactobacillus acidophilus Mitigates Osteoarthritis-Associated Pain, Cartilage Disintegration and Gut Microbiota Dysbiosis in an Experimental Murine OA Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surgical Induction of Murine OA and Probiotic Treatment
2.2. Longitudinal Behavioral Pain Measurements
2.3. Histopathology and Immuno-Fluorescence Microscopy
2.4. Reverse Transcription and qPCR Analysis
2.5. Microbiome Analysis
2.6. Statistical Analyses
3. Results
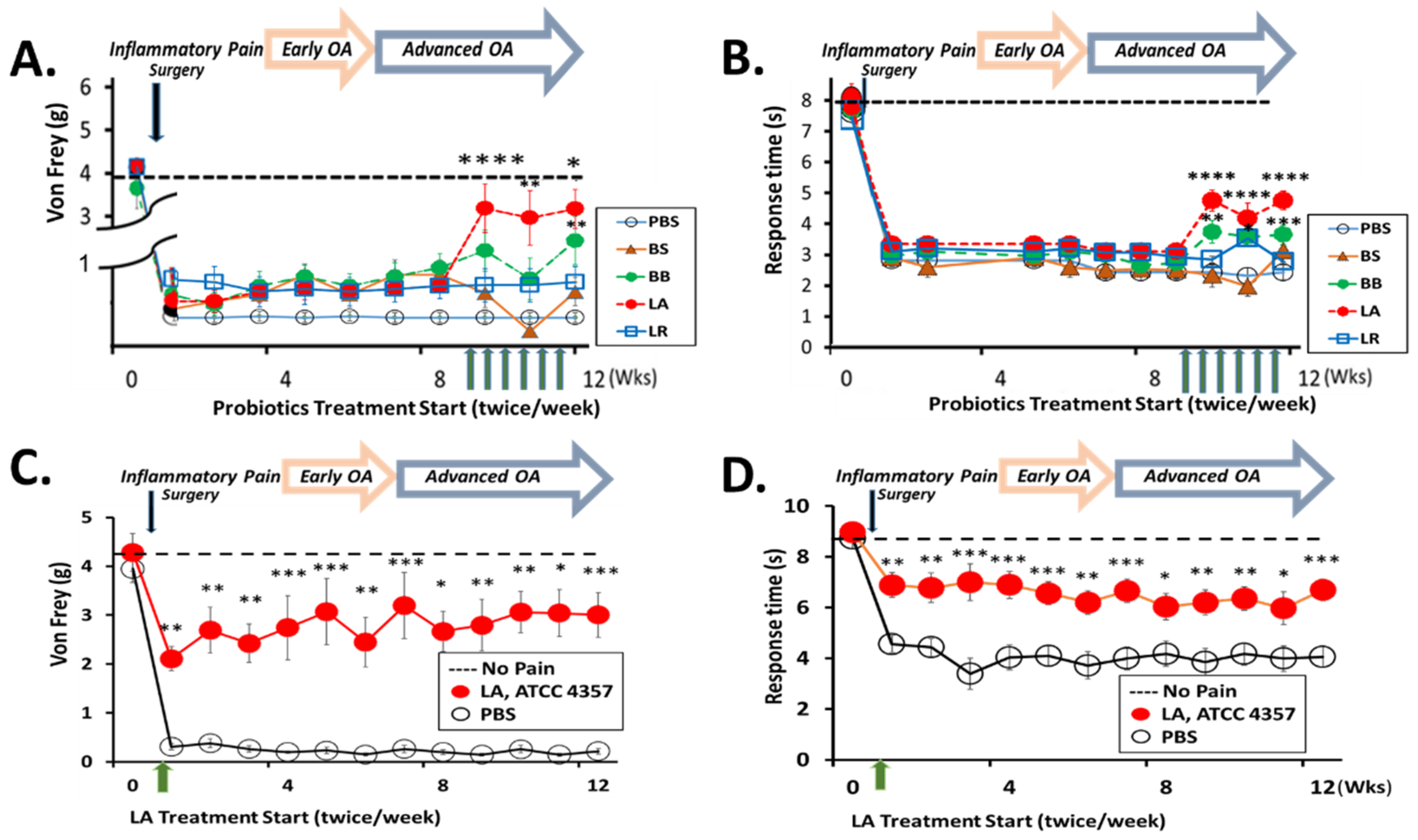
3.1. Oral Administration of LA Relieves Knee OA Pain
3.2. LA Treatment Not Only Rapidly Reduced Pain but Also Prevented Cartilage Degradation
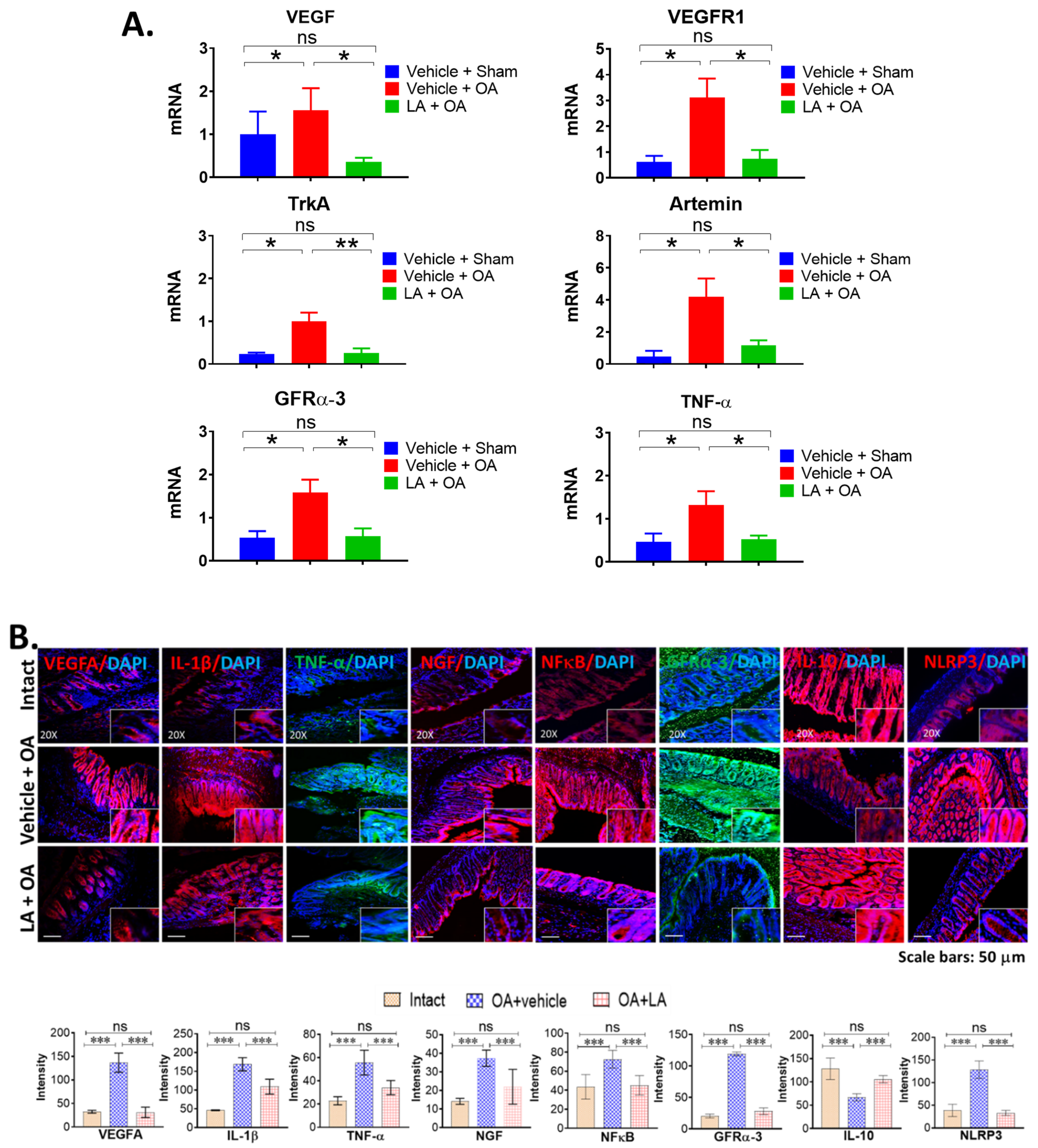
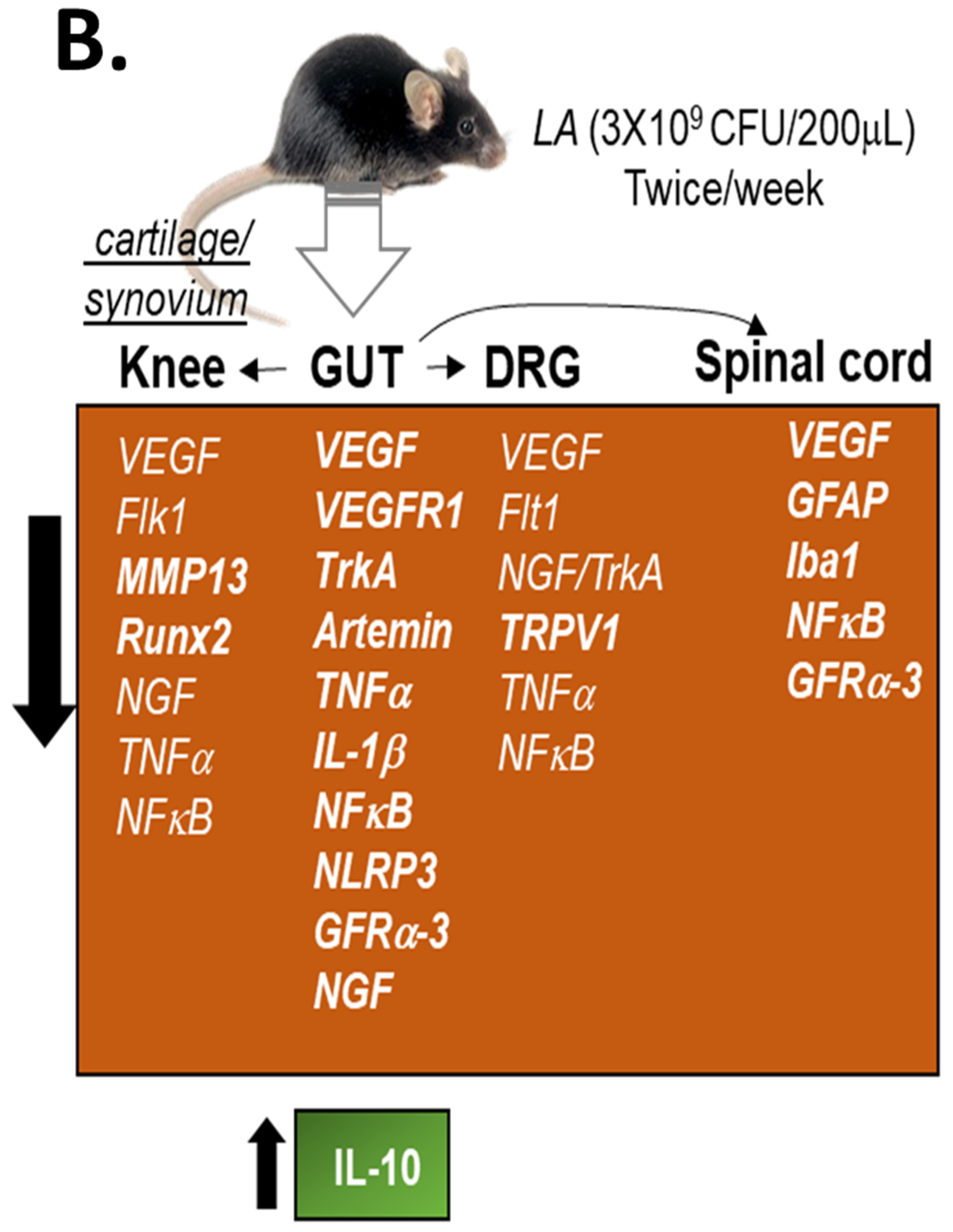
3.3. LA Treatment in Chronic OA Altered the Expression of Pain Markers, Neurotrophic Factors and Pro-Inflammatory Cytokines in the Distal Colon of OA Mice
3.4. LA Reduced Spinal Glial Activity and Inflammatory Markers in Spinal Cords of OA Mice
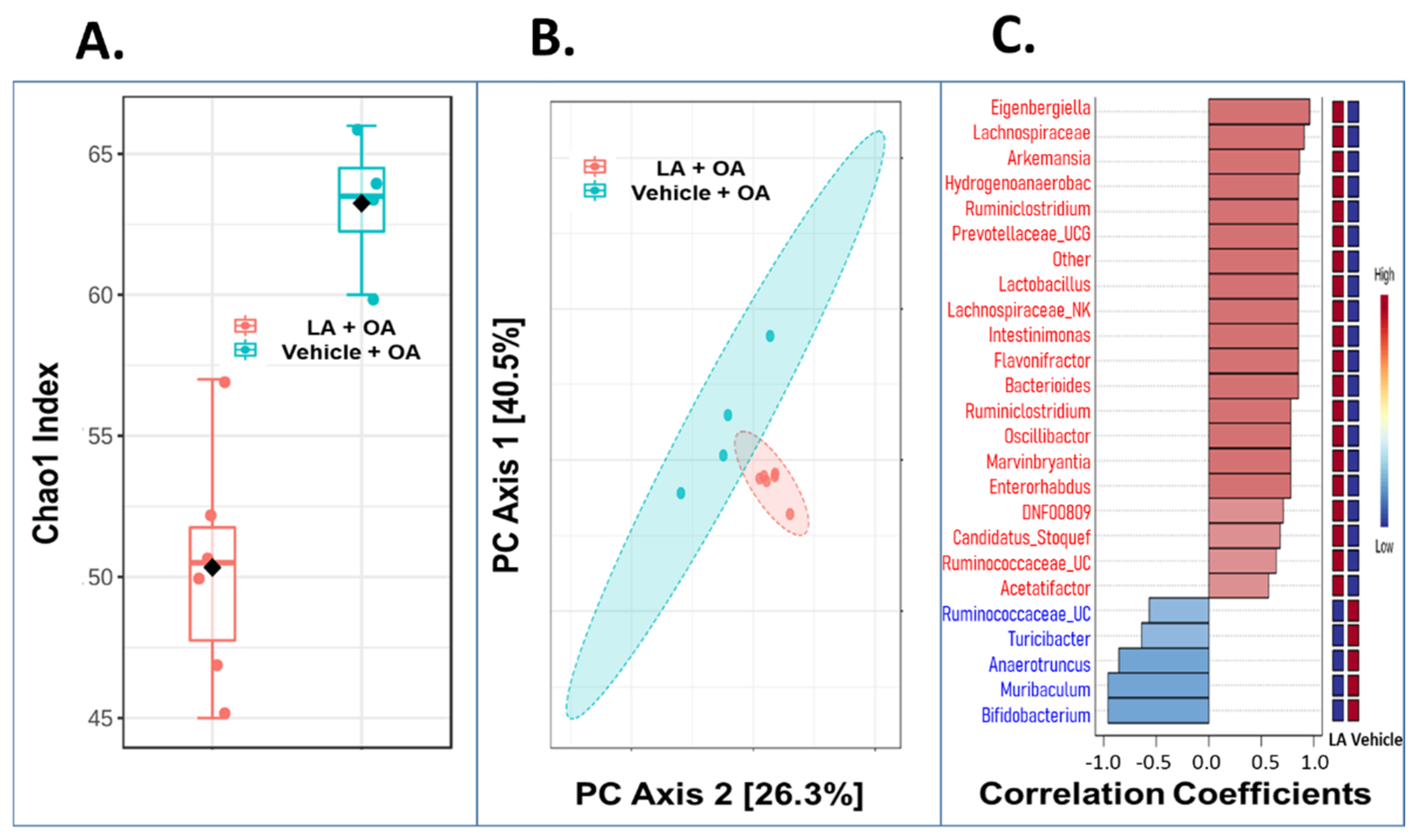
3.5. Oral Administration of LA Significantly Altered the Fecal Microbiome in OA Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ARTN | Artemin |
| CRP | C-Reactive Protein, DRG: Dorsal Root Ganglion |
| GDNF | Glial Cell-Derived Neurotrophic Factor |
| GFAP | Glial Fibrillary Acidic Protein |
| GFR α3 | Glial Cell-Derived Neurotrophic Factor Family Receptor α3 |
| IBA-1 | Ionized calcium Binding Adaptor molecule-1 |
| IF | Immuno-Fluorescence |
| IFN | Interferon |
| ILs | Interleukins |
| LA | Lactobacillus acidophilus |
| MIA | Monosodium Iodoacetate |
| MMP-13 | Matrix Metalloproteinase-13 |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NGF | Nerve Growth Factor |
| NLRP3 | Nucleotide-binding domain Leucine-rich Repeat and Pyrin domain containing receptor 3 |
| OA | Osteoarthritis |
| OADMD | OA Disease Modifying Drug |
| OARSI | score: Osteoarthritis Research Society International score |
| PMM | Partial Medial Meniscectomy |
| PCoA | Principal Coordinates Analysis |
| RUNX2 | Runt-related transcription factor 2 |
| RTqPCR | Real Time Quantitative Polymerase Chain Reaction |
| SC | Spinal Cord, SCFA: Short-Chain Fatty Acid |
| TrkA | Tropomyosin Receptor Kinase A |
References
- Wytt, L.A.; Nwosu, L.N.; Wilson, D. Molecular expression patterns in the synovium and their association with advanced symptomatic knee osteoarthritis. Osteoarthr. Cartil. 2019, 27, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gräss, S.; Zaucke, F.; Madry, H. Osteoarthritis: Novel Molecular Mechanisms Increase Our Understanding of the Disease Pathology. J. Clin. Med. 2021, 10, 1938. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Kraus, V.B. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat. Rev. Rheumatol. 2016, 12, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, C.M.; Jeffries, M.A. The Microbiome in Osteoarthritis: A Narrative Review of Recent Human and Animal Model Literature. Curr. Rheumatol. Rep. 2022, 24, 139–148. [Google Scholar] [CrossRef]
- Rushing, B.R.; McRitchie, S.; Arbeeva, L.; Nelson, A.E.; Azcarate-Peril, M.A.; Li, Y.Y.; Qian, Y.; Pathmasiri, W.; Sumner, S.C.J.; Loeser, R.F. Fecal metabolomics reveals products of dysregulated proteolysis and altered microbial metabolism in obesity-related osteoarthritis. Osteoarthr. Cartil. 2022, 30, 81–91. [Google Scholar] [CrossRef]
- Guss, J.D.; Ziemian, S.N.; Luna, M.; Sandoval, T.N.; Holyoak, D.T.; Guisado, G.G.; Roubert, S.; Callahan, R.L.; Brito, I.L.; van der Meulen, M.C.H.; et al. The effects of metabolic syndrome, obesity, and the gut microbiome on load-induced osteoarthritis. Osteoarthr. Cartil. 2019, 27, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Ulici, V.; Kelley, K.L.; Azcarate-Peril, M.A.; Cleveland, R.J.; Sartor, R.B.; Schwartz, T.A.; Loeser, R.F. Osteoarthritis induced by destabilization of the medial meniscus is reduced in germ-free mice. Osteoarthr. Cartil. 2018, 26, 1098–1109. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Chen, J.; Li, B.; Zeng, B.; Chou, C.H.; Zheng, X.; Xie, J.; Li, H.; Hao, Y.; Chen, G.; et al. Faecal microbiota transplantation from metabolically compromised human donors accelerates osteoarthritis in mice. Ann. Rheum. Dis. 2020, 79, 646–656. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. A cross talk between dysbiosis and gut-associated immune system governs the development of inflammatory arthropathies. Semin. Arthritis Rheum. 2019, 49, 474–484. [Google Scholar] [CrossRef]
- Schott, E.M.; Farnsworth, C.W.; Grier, A.; Lillis, J.A.; Soniwala, S.; Dadourian, G.H.; Bell, R.D.; Doolittle, M.L.; Villani, D.A.; Awad, H.; et al. Targeting the gut microbiome to treat the osteoarthritis of obesity. JCI Insight 2018, 3, 95997. [Google Scholar] [CrossRef]
- De Sire, A.; de Sire, R.; Curci, C.; Castiglione, F.; Wahli, W. Role of Dietary Supplements and Probiotics in Modulating Microbiota and Bone Health: The Gut-Bone Axis. Cells 2022, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Bacali, C.; Vulturar, R.; Buduru, S.; Cozma, A.; Fodor, A.; Chiș, A.; Lucaciu, O.; Damian, L.; Moldovan, M.L. Oral Microbiome: Getting to Know and Befriend Neighbors, a Biological Approach. Biomedicines 2022, 10, 671. [Google Scholar] [CrossRef] [PubMed]
- De Sire, A.; Marotta, N.; Marinaro, C.; Curci, C.; Invernizzi, M.; Ammendolia, A. Role of Physical Exercise and Nutraceuticals in Modulating Molecular Pathways of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 5722. [Google Scholar] [CrossRef] [PubMed]
- Zagato, E.; Mileti, E.; Massimiliano, L.; Fasano, F.; Budelli, A.; Penna, G.; Rescigno, M. Lactobacillus paracasei CBA L74 Metabolic Products and Fermented Milk for Infant Formula Have Anti-Inflammatory Activity on Dendritic Cells In Vitro and Protective Effects against Colitis and an Enteric Pathogen In Vivo. PLoS ONE 2014, 9, e87615. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kwon, J.Y.; Jhun, J.; Jung, K.; Park, S.H.; Yang, C.W.; Cho, Y.; Kim, S.J.; Cho, M.L. Lactobacillus acidophilus ameliorates pain and cartilage degradation in experimental osteoarthritis. Immunol. Lett. 2018, 203, 6–14. [Google Scholar] [CrossRef]
- Das, V.; Kc, R.; Li, X.; O-Sullivan, I.; van Wijnen, A.J.; Kroin, J.S.; Pytowski, B.; Applegate, D.T.; Votta-Velis, G.; Ripper, R.L.; et al. Blockade of Vascular Endothelial Growth Factor Receptor-1 (Flt-1), Reveals a Novel Analgesic For Osteoarthritis-Induced Joint Pain. Gene Rep. 2018, 11, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Kumar, A.; Raheja, G.; Anbazhagan, A.N.; Priyamvada, S.; Saksena, S.; Jhandier, M.N.; Gill, R.K.; Alrefai, W.A.; Borthakur, A.; et al. Lactobacillus acidophilus attenuates downregulation of DRA function and expression in inflammatory models. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 15, G623–G631. [Google Scholar] [CrossRef] [Green Version]
- Das, V.; Kc, R.; Li, X.; Varma, D.; Qiu, S.; Kroin, J.S.; Forsyth, C.B.; Keshavarzian, A.; van Wijnen, A.J.; Park, T.J.; et al. Pharmacological targeting of the mammalian clock reveals a novel analgesic for osteoarthritis-induced pain. Gene 2018, 655, 1–12. [Google Scholar] [CrossRef]
- Im, H.J.; Kim, J.S.; Li, X.; Kotwal, N.; Sumner, D.R.; Van Wijnen, A.J.; Davis, F.J.; Yan, D.; Levine, B.; Henry, J.L.; et al. Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum. 2010, 62, 2995–3005. [Google Scholar] [CrossRef] [Green Version]
- Tajerian, M.; Sahbaie, P.; Sun, Y.; Leu, D.; Yang, H.Y.; Li, W.; Huang, T.T.; Kingery, W.; David Clark, J. Sex differences in a Murine Model of Complex Regional Pain Syndrome. Neurobiol Learn. Mem. 2015, 123, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Kc, R.; Li, X.; Voigt, R.M.; Ellman, M.B.; Summa, K.C.; Vitaterna, M.H.; Keshavarizian, A.; Turek, F.W.; Meng, Q.J.; Stein, G.S.; et al. Environmental disruption of circadian rhythm predisposes mice to osteoarthritis-like changes in knee joint. J. Cell. Physiol. 2015, 230, 2174–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative-recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18, S17–S23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, S.J.; Venkatramanan, R.; Naqib, A. Deconstructing the polymerase chain reaction: Understanding and correcting bias associated with primer degeneracies and primer-template mismatches. PLoS ONE 2015, 10, e0128122. [Google Scholar]
- Collins, K.H.; Paul, H.A.; Reimer, R.A.; Seerattan, R.A.; Hart, D.A.; Herzog, W. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: Studies in a rat model. Osteoarthr. Cartil. 2015, 23, 1989–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, Z.; Jia, J.; Zhang, C.; Sun, T.; Zhang, W.; Yuan, W.; Leng, H.; Song, C. Gut microbiome dysbiosis alleviates the progression of osteoarthritis in mice. Clin. Sci. 2020, 134, 3159–3174. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Sig. Transduct. Target 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Minnema, L.; Wheeler, J.; Enomoto, M.; Pitake, S.; Mishra, S.; Lascelles, B. Correlation of Artemin and GFRα3 with Osteoarthritis Pain: Early Evidence from Naturally Occurring Osteoarthritis-Associated Chronic Pain in Dogs. Front. Neurosci. 2020, 14, 77. [Google Scholar] [CrossRef] [Green Version]
- Ren, F.; Zhang, H.; Qi, C.; Gao, M.L.; Wang, H.; Li, X.Q. Blockade of transient receptor potential cation channel subfamily V member 1 promotes regeneration after sciatic nerve injury. Neural. Regen. Res. 2015, 10, 1324–1331. [Google Scholar] [CrossRef]
- Komori, T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem. Cell Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O-Sullivan, I.; Natarajan Anbazhagan, A.; Singh, G.; Ma, K.; Green, S.J.; Singhal, M.; Wang, J.; Kumar, A.; Dudeja, P.K.; Unterman, T.G.; et al. Lactobacillus acidophilus Mitigates Osteoarthritis-Associated Pain, Cartilage Disintegration and Gut Microbiota Dysbiosis in an Experimental Murine OA Model. Biomedicines 2022, 10, 1298. https://doi.org/10.3390/biomedicines10061298
O-Sullivan I, Natarajan Anbazhagan A, Singh G, Ma K, Green SJ, Singhal M, Wang J, Kumar A, Dudeja PK, Unterman TG, et al. Lactobacillus acidophilus Mitigates Osteoarthritis-Associated Pain, Cartilage Disintegration and Gut Microbiota Dysbiosis in an Experimental Murine OA Model. Biomedicines. 2022; 10(6):1298. https://doi.org/10.3390/biomedicines10061298
Chicago/Turabian StyleO-Sullivan, InSug, Arivarasu Natarajan Anbazhagan, Gurjit Singh, Kaige Ma, Stefan J. Green, Megha Singhal, Jun Wang, Anoop Kumar, Pradeep K. Dudeja, Terry G. Unterman, and et al. 2022. "Lactobacillus acidophilus Mitigates Osteoarthritis-Associated Pain, Cartilage Disintegration and Gut Microbiota Dysbiosis in an Experimental Murine OA Model" Biomedicines 10, no. 6: 1298. https://doi.org/10.3390/biomedicines10061298
APA StyleO-Sullivan, I., Natarajan Anbazhagan, A., Singh, G., Ma, K., Green, S. J., Singhal, M., Wang, J., Kumar, A., Dudeja, P. K., Unterman, T. G., Votta-Velis, G., Bruce, B., van Wijnen, A. J., & Im, H.-J. (2022). Lactobacillus acidophilus Mitigates Osteoarthritis-Associated Pain, Cartilage Disintegration and Gut Microbiota Dysbiosis in an Experimental Murine OA Model. Biomedicines, 10(6), 1298. https://doi.org/10.3390/biomedicines10061298






